Prognostic Modelling of Mortality in Chronic Critical Illness After Traumatic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Statistical Analysis
3. Results
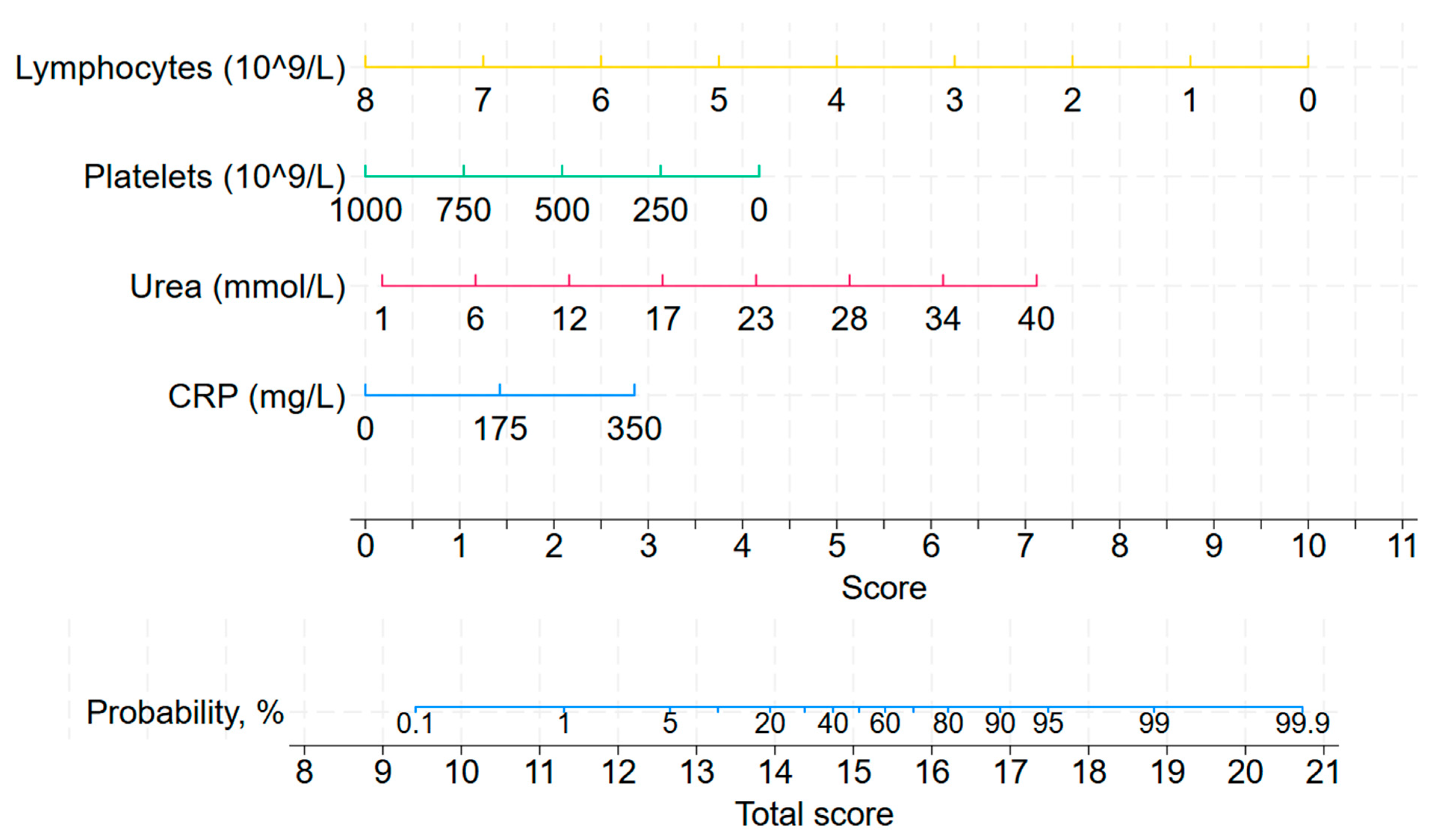
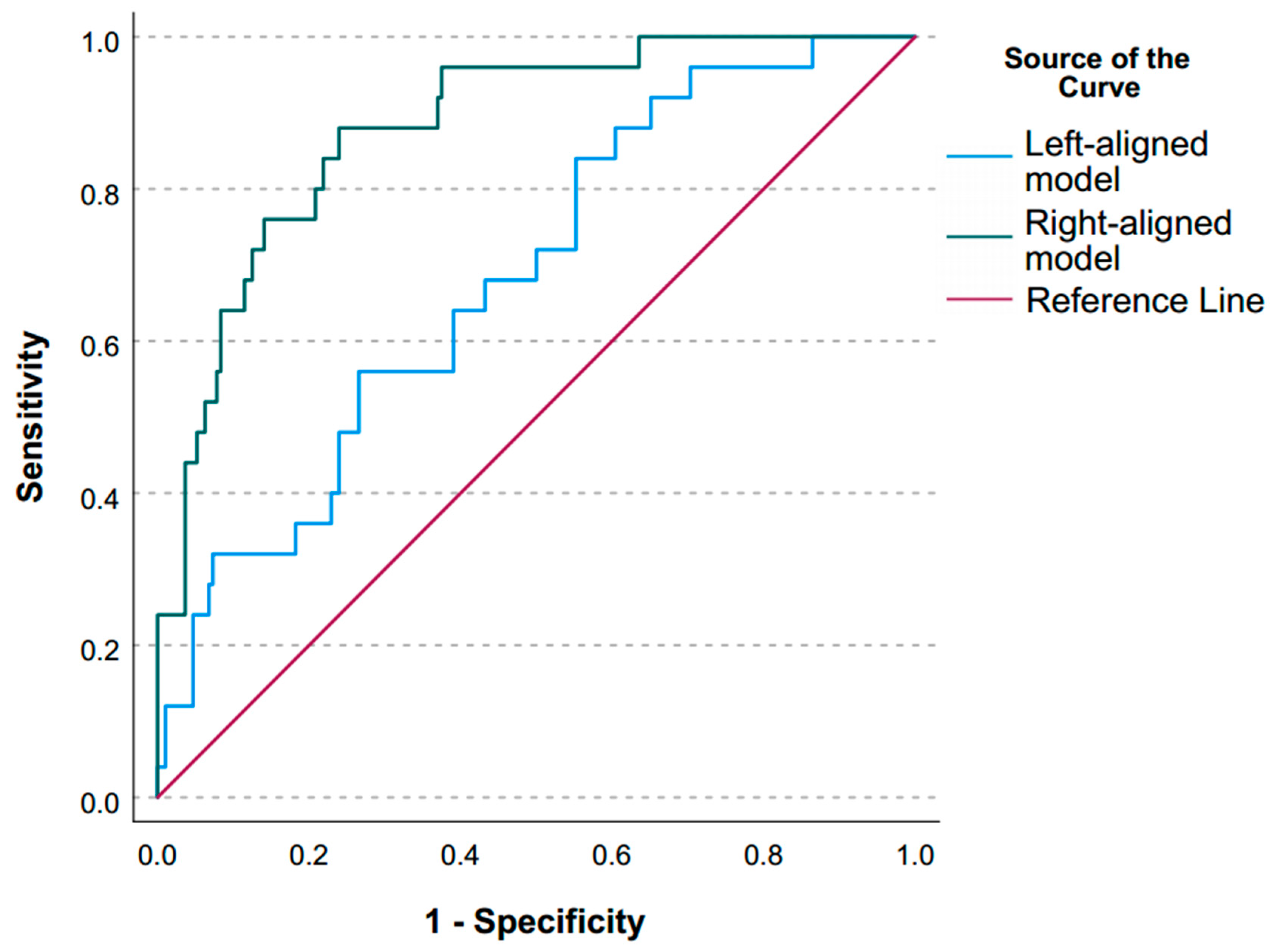
3.1. Left-Aligned Model
3.2. Right-Aligned Model
4. Discussion
4.1. Key Findings
4.2. Relationship with Previous Studies
4.3. Significance of Study Findings and Clinical Utility
4.4. Strengths and Limitations
4.5. Future Studies and Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beij, A.; Verdonk, R.C.; van Santvoort, H.C.; de-Madaria, E.; Voermans, R.P. Acute Pancreatitis: An Update of Evidence-Based Management and Recent Trends in Treatment Strategies. United Eur. Gastroenterol. J. 2025, 13, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Zavala, E.; Tomás, R.; Fernandez, R. Clinical factors associated with success of proportional assist ventilation in the acute phase of critical illness: Pilot study. Med. Intensiv. 2014, 38, 65–72. [Google Scholar] [CrossRef]
- Allum, L.; Apps, C.; Pattison, N.; Connolly, B.; Rose, L. Informing the standardising of care for prolonged stay patients in the intensive care unit: A scoping review of quality improvement tools. Intensive Crit. Care Nurs. 2022, 73, 103302. [Google Scholar] [CrossRef]
- Martin, C.M.; Hill, A.D.; Burns, K.; Chen, L.M. Characteristics and outcomes for critically ill patients with prolonged intensive care unit stays. Crit. Care Med. 2005, 33, 1922–1927, quiz 1936. [Google Scholar] [CrossRef]
- Kahn, J.M.; Le, T.; Angus, D.C.; Cox, C.E.; Hough, C.L.; White, D.B.; Yende, S.; Carson, S.S. The epidemiology of chronic critical illness in the United States. Crit. Care Med. 2015, 43, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, N.R. Chronic critical illness: The growing challenge to health care. Respir. Care 2012, 57, 1021–1027. [Google Scholar] [CrossRef]
- Voiriot, G.; Oualha, M.; Pierre, A.; Salmon-Gandonnière, C.; Gaudet, A.; Jouan, Y.; Kallel, H.; Radermacher, P.; Vodovar, D.; Sarton, B.; et al. Chronic critical illness and post-intensive care syndrome: From pathophysiology to clinical challenges. Ann. Intensive Care 2022, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.D.; Vanzant, E.L.; Moore, F.A. Chronic Critical Illness and PICS Nutritional Strategies. J. Clin. Med. 2021, 10, 2294. [Google Scholar] [CrossRef]
- Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Horiguchi, H.; Brakenridge, S.C.; Gardner, A.; Efron, P.A.; Bihorac, A.; Segal, M.; Moore, F.A.; et al. Chronic Critical Illness and the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Front. Immunol. 2018, 9, 1511. [Google Scholar] [CrossRef]
- Madrid, R.A.; McGee, W. Value, Chronic Critical Illness, and Choosing Wisely. J. Intensive Care Med. 2019, 34, 609–614. [Google Scholar] [CrossRef]
- Rousseau, A.-F.F.; Prescott, H.C.; Brett, S.J.; Weiss, B.; Azoulay, E.; Creteur, J.; Latronico, N.; Hough, C.L.; Weber-Carstens, S.; Vincent, J.-L.L.; et al. Long-term outcomes after critical illness: Recent insights. Crit. Care 2021, 25, 108. [Google Scholar] [CrossRef]
- Marchioni, A.; Fantini, R.; Antenora, F.; Clini, E.; Fabbri, L. Chronic critical illness: The price of survival. Eur. J. Clin. Investig. 2015, 45, 1341–1349. [Google Scholar] [CrossRef]
- Likhvantsev, V.V.; Berikashvili, L.B.; Yadgarov, M.Y.; Yakovlev, A.A.; Kuzovlev, A.N. The Tri-Steps Model of Critical Conditions in Intensive Care: Introducing a New Paradigm for Chronic Critical Illness. J. Clin. Med. 2024, 13, 3683. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I. Critical illness-based chronic disease: A new framework for intensive metabolic support. Curr. Opin. Crit. Care 2025, 31, 417–427. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Hodgson, C.L.; Pilcher, D.; Bailey, M.; van Lint, A.; Chavan, S.; Bellomo, R. Timing of onset and burden of persistent critical illness in Australia and New Zealand: A retrospective, population-based, observational study. Lancet Respir. Med. 2016, 4, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Grechko, A.V.; Yadgarov, M.Y.; Yakovlev, A.A.; Berikashvili, L.B.; Kuzovlev, A.N.; Polyakov, P.A.; Kuznetsov, I.V.; Likhvantsev, V.V. Russian database of intensive care patients—RICD. Gen. Reanimatol. 2024, 20, 22–31. [Google Scholar] [CrossRef]
- RICD—An Open-Access Database. Available online: https://fnkcrr-database.ru/ru_main.html (accessed on 26 August 2025).
- Heinze, G.; Wallisch, C.; Dunkler, D. Variable selection—A review and recommendations for the practicing statistician. Biom. J. 2018, 60, 431–449. [Google Scholar] [CrossRef]
- Carson, S.S.; Bach, P.B. Predicting mortality in patients suffering from prolonged critical illness: An assessment of four severity-of-illness measures. Chest 2001, 120, 928–933. [Google Scholar] [CrossRef]
- Carson, S.S.; Garrett, J.; Hanson, L.C.; Lanier, J.; Govert, J.; Brake, M.C.; Landucci, D.L.; Cox, C.E.; Carey, T.S. A prognostic model for one-year mortality in patients requiring prolonged mechanical ventilation. Crit. Care Med. 2008, 36, 2061–2069. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Jo, E.-J.; Eom, J.S.; Mok, J.; Kim, M.-H.; Kim, K.U.; Park, H.-K.; Lee, M.K.; Lee, K. Validation of the Prognosis for Prolonged Ventilation (ProVent) score in patients receiving 14days of mechanical ventilation. J. Crit. Care 2018, 44, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.K.; Lee, Y.M.; Sun, X.; van Essen, T.A.; Elguindy, M.M.; Belton, P.J.; Pisică, D.; Mikolic, A.; Deng, H.; Kanter, J.H.; et al. Performance of the IMPACT and CRASH prognostic models for traumatic brain injury in a contemporary multicenter cohort: A TRACK-TBI study. J. Neurosurg. 2024, 141, 417–429. [Google Scholar] [CrossRef] [PubMed]
- George, N.; Moseley, E.; Eber, R.; Siu, J.; Samuel, M.; Yam, J.; Huang, K.; Celi, L.A.; Lindvall, C. Deep learning to predict long-term mortality in patients requiring 7 days of mechanical ventilation. PLoS ONE 2021, 16, e0253443. [Google Scholar] [CrossRef] [PubMed]


| Parameter | All, n = 430 | Survivors, n = 387 | Non-Survivors, n = 43 | p (Mann–Whitney/Fisher) |
|---|---|---|---|---|
| Sex, male | 315 (73.3%) | 282 (72.9%) | 33 (76.7%) | 0.717 |
| Age, years | 42.5 (32; 57) | 42 (31; 56) | 45 (34; 70) | 0.088 |
| BMI, kg/m2 | 21.5 (18.8; 24.7) | 21.9 (18.8; 24.8) | 20.8 (18.8; 24.7) | 0.431 |
| Comorbidity | ||||
| Atrial Fibrillation | 2 (0.5%) | 1 (0.3%) | 1 (2.3%) | 0.190 |
| Coronary Artery Disease | 50 (11.6%) | 38 (9.8%) | 12 (27.9%) | 0.002 |
| Valvular Heart Disease | 5 (1.2%) | 4 (1.0%) | 1 (2.3%) | 0.411 |
| Arterial Hypertension | 147 (34.2%) | 129 (33.3%) | 18 (41.9%) | 0.309 |
| Type 2 Diabetes | 11 (2.6%) | 10 (2.6%) | 1 (2.3%) | >0.9 |
| Type 1 Diabetes | 4 (0.9%) | 3 (0.8%) | 1 (2.3%) | 0.345 |
| CKD | 9 (2.1%) | 5 (1.3%) | 4 (9.3%) | 0.008 |
| COPD | 1 (0.2%) | 1 (0.3%) | 0 (0.0%) | >0.9 |
| Polytrauma | 53 (12.3%) | 47 (12.1%) | 6 (14.0%) | 0.806 |
| Multiorgan Failure on Admission | 219 (65.4%) | 186 (62.6%) | 33 (86.8%) | 0.003 |
| Malignant Tumor | 1 (0.2%) | 1 (0.3%) | 0 (0.0%) | >0.9 |
| Laboratory parameters (first 48 h in the ICU) | ||||
| RBC (1012/L) | 3.6 (3.2; 4.1) | 3.6 (3.2; 4.1) | 3.3 (2.8; 3.8) | 0.009 |
| Hemoglobin (g/L) | 104.0 (93.0; 118.0) | 105.0 (94.0; 119.5) | 94.0 (84.0; 107.0) | <0.001 |
| WBC (109/L) | 9.1 (6.8; 11.5) | 8.9 (6.8; 11.5) | 9.5 (7.2; 11.7) | 0.459 |
| Neutrophils (109/L) | 6.6 (4.5; 9.0) | 6.5 (4.5; 9.1) | 7.0 (5.2; 8.4) | 0.537 |
| Eosinophils (109/L) | 0.1 (0.1; 0.3) | 0.2 (0.1; 0.3) | 0.1 (0.1; 0.4) | 0.430 |
| Basophils (109/L) | 0.1 (0.0; 0.1) | 0.1 (0.0; 0.1) | 0.0 (0.0; 0.1) | 0.037 |
| Lymphocytes (109/L) | 1.4 (1.0; 1.8) | 1.4 (1.0; 1.8) | 1.4 (1.0; 1.9) | 0.797 |
| Platelets (109/L) | 330.5 (247.0; 425.0) | 332.0 (249.0; 429.0) | 311.0 (220.0; 409.0) | 0.259 |
| Creatinine (µmol/L) | 66.3 (53.3; 80.8) | 66.3 (53.3; 80.4) | 67.1 (56.0; 84.7) | 0.444 |
| Urea (mmol/L) | 4.6 (3.1; 7.4) | 4.5 (3.0; 7.3) | 5.4 (4.0; 8.3) | 0.028 |
| Potassium (mmol/L) | 3.9 (3.7; 4.2) | 3.9 (3.7; 4.2) | 4.0 (3.6; 4.3) | 0.518 |
| Sodium (mmol/L) | 136.6 (134.5; 139.8) | 136.8 (134.6; 139.9) | 135.6 (131.6; 137.8) | 0.055 |
| Chloride (mmol/L) | 101.9 (98.9; 105.4) | 101.9 (99.0; 105.3) | 101.1 (98.2; 106.0) | 0.840 |
| Bilirubin Total (µmol/L) | 10.1 (7.4; 13.2) | 10.0 (7.4; 13.2) | 11.2 (8.7; 13.1) | 0.306 |
| Bilirubin Direct (µmol/L) | 2.1 (1.5; 3.1) | 2.0 (1.5; 3.0) | 2.3 (1.8; 3.7) | 0.246 |
| ALT (U/L) | 30.0 (16.4; 58.6) | 29.8 (16.5; 61.3) | 30.3 (13.6; 50.7) | 0.495 |
| AST (U/L) | 28.9 (19.0; 48.2) | 28.5 (18.8; 47.1) | 30.4 (19.7; 54.0) | 0.617 |
| LDH (U/L) | 260.5 (186.7; 360.2) | 258.0 (184.0; 360.2) | 287.8 (214.6; 397.2) | 0.501 |
| Alpha-Amylase (U/L) | 52.4 (39.1; 92.6) | 53.9 (41.6; 100.6) | 41.0 (26.8; 53.1) | 0.021 |
| Lactate (mmol/L) | 1.2 (0.9; 1.5) | 1.1 (0.9; 1.5) | 1.4 (1.1; 1.9) | 0.137 |
| CRP (mg/L) | 43.9 (18.5; 88.3) | 41.5 (18.0; 78.2) | 79.5 (35.5; 142.7) | 0.003 |
| Total Protein (g/L) | 62.2 (56.5; 67.9) | 62.8 (56.8; 68.2) | 58.6 (53.4; 64.8) | 0.010 |
| Albumin (g/L) | 31.3 (26.3; 35.6) | 32.0 (27.3; 35.7) | 26.7 (21.4; 31.5) | <0.001 |
| Glucose (mmol/L) | 5.4 (4.9; 6.2) | 5.4 (4.9; 6.1) | 6.0 (4.6; 6.9) | 0.086 |
| Procalcitonin (ng/mL) | 0.3 (0.1; 1.4) | 0.3 (0.1; 0.8) | 1.0 (0.1; 5.0) | 0.661 |
| Scales (first 48 h in the ICU) | ||||
| SOFA | 3 (2; 5) | 3 (2; 5) | 4 (3; 5) | 0.052 |
| GCS | 10 (8; 13) | 10 (8; 13) | 9 (6; 11) | 0.011 |
| FOUR | 13 (11; 15) | 13 (11; 16) | 12 (8; 14) | 0.015 |
| Parameters | All, n = 430 | Survivors, n = 387 | Non-Survivors, n = 43 | p (Mann–Whitney/Fisher) |
|---|---|---|---|---|
| Laboratory parameters | ||||
| RBC (1012/L) | 3.51 (3.08; 3.89) | 3.53 (3.12; 3.92) | 3.15 (2.78; 3.55) | <0.001 |
| Hemoglobin (g/L) | 101 (91; 114) | 102 (92; 115) | 89 (80; 101) | <0.001 |
| WBC (109/L) | 7.5 (5.8; 9.8) | 7.4 (5.8; 9.5) | 9.6 (6.5; 13.3) | 0.019 |
| Neutrophils (109/L) | 4.9 (3.5; 7.2) | 4.8 (3.4; 6.8) | 7.3 (5.0; 12.2) | <0.001 |
| Eosinophils (109/L) | 0.2 (0.1; 0.3) | 0.2 (0.1; 0.3) | 0.1 (0.0; 0.2) | 0.005 |
| Basophils (109/L) | 0.1 (0.0; 0.1) | 0.1 (0.0; 0.1) | 0.0 (0.0; 0.1) | <0.001 |
| Lymphocytes (109/L) | 1.6 (1.1; 2.0) | 1.6 (1.2; 2.1) | 0.9 (0.7; 1.3) | <0.001 |
| Platelets (109/L) | 322.5 (236; 399) | 329 (246; 406) | 211 (137; 296) | <0.001 |
| Creatinine (µmol/L) | 60.8 (51.0; 75.1) | 60.8 (51.2; 75.1) | 60.9 (48.6; 82.9) | 0.819 |
| Urea (mmol/L) | 4.1 (2.9; 6.0) | 3.8 (2.8; 5.5) | 6.9 (4.9; 13.3) | <0.001 |
| Potassium (mmol/L) | 3.9 (3.6; 4.3) | 3.9 (3.6; 4.3) | 4.0 (3.3; 4.3) | 0.705 |
| Sodium (mmol/L) | 136.8 (133.8; 138.9) | 136.8 (134.0; 138.8) | 136.4 (129.6; 144.7) | 0.939 |
| Chloride (mmol/L) | 101.3 (98.0; 104.2) | 101.3 (98.1; 104.2) | 100.0 (96.8; 106.2) | 0.941 |
| Bilirubin Total (µmol/L) | 8.2 (6.4; 10.6) | 8.0 (6.2; 10.0) | 12.1 (8.8; 15.4) | <0.001 |
| Bilirubin Indirect (µmol/L) | 9.3 (7.2; 11.2) | 9.3 (7.2; 11.2) | ND | NA |
| Bilirubin Direct (µmol/L) | 1.7 (1.1; 2.3) | 1.6 (1.1; 2.1) | 3.1 (2.1; 5.8) | <0.001 |
| ALT (U/L) | 21.5 (12.5; 38.8) | 21.4 (12.4; 36.4) | 28.6 (14.7; 70.1) | 0.052 |
| AST (U/L) | 20.9 (14.8; 33.4) | 20.6 (14.8; 31.7) | 27.8 (14.5; 84.5) | 0.046 |
| LDH (U/L) | 196.4 (141.2; 286.3) | 196.5 (138.9; 286.3) | 188.0 (143.0; 623.9) | 0.677 |
| Alpha-Amylase (U/L) | 44.5 (33.9; 65.1) | 45.4 (36.1; 66.4) | 31.0 (23.7; 43.4) | 0.011 |
| Lactate (mmol/L) | 1.2 (0.9; 1.9) | 1.1 (0.9; 1.2) | 1.9 (1.3; 2.3) | 0.004 |
| CRP (mg/L) | 33.1 (10.2; 71.9) | 29.4 (8.4; 61.3) | 112.1 (61.8; 152.8) | <0.001 |
| Total Protein (g/L) | 59.5 (54.3; 65.1) | 60.0 (54.8; 65.3) | 54.7 (50.2; 61.3) | 0.003 |
| Albumin (g/L) | 30.4 (26.7; 33.9) | 30.7 (27.1; 34.3) | 26.6 (22.4; 28.5) | <0.001 |
| Glucose (mmol/L) | 4.9 (4.5; 5.6) | 4.9 (4.5; 5.6) | 4.8 (4.2; 5.7) | 0.723 |
| Procalcitonin (ng/mL) | 0.4 (0.2; 3.2) | 0.3 (0.1; 1.0) | 0.9 (0.2; 8.1) | 0.113 |
| Scales | ||||
| SOFA | 3 (2; 4) | 3 (2; 4) | 6 (3; 9) | <0.001 |
| GCS | 11 (9; 15) | 11 (9; 15) | 14 (10; 15) | 0.423 |
| FOUR | 15 (13; 16) | 15 (12; 16) | 14 (14; 16) | 0.822 |
| Complications | ||||
| Anemia | 59 (13.7%) | 49 (12.7%) | 10 (23.3%) | 0.063 |
| Coagulopathy | 2 (0.5%) | 1 (0.3%) | 1 (2.3%) | 0.19 |
| Heart Failure | 20 (4.7%) | 19 (4.9%) | 1 (2.3%) | 0.708 |
| Pneumonia | 181 (42.1%) | 158 (40.8%) | 23 (53.5%) | 0.142 |
| Sepsis | 92 (35.7%) | 74 (33.3%) | 18 (50.0%) | 0.062 |
| Septic Shock | 12 (2.8%) | 4 (1.0%) | 8 (18.6%) | <0.001 |
| Polyneuropathy | 10 (2.3%) | 8 (2.1%) | 2 (4.7%) | 0.263 |
| Central Nervous System Inflammation | 11 (2.6%) | 9 (2.3%) | 2 (4.7%) | 0.303 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Likhvantsev, V.; Kolesov, D.; Berikashvili, L.; Korolenok, E.; Yadgarov, M.; Kadantseva, K.; Kuznetsov, I.; Polyakov, P.; Kuzovlev, A.; Grechko, A. Prognostic Modelling of Mortality in Chronic Critical Illness After Traumatic Brain Injury. J. Clin. Med. 2025, 14, 8202. https://doi.org/10.3390/jcm14228202
Likhvantsev V, Kolesov D, Berikashvili L, Korolenok E, Yadgarov M, Kadantseva K, Kuznetsov I, Polyakov P, Kuzovlev A, Grechko A. Prognostic Modelling of Mortality in Chronic Critical Illness After Traumatic Brain Injury. Journal of Clinical Medicine. 2025; 14(22):8202. https://doi.org/10.3390/jcm14228202
Chicago/Turabian StyleLikhvantsev, Valery, Dmitriy Kolesov, Levan Berikashvili, Elizaveta Korolenok, Mikhail Yadgarov, Kristina Kadantseva, Ivan Kuznetsov, Petr Polyakov, Artem Kuzovlev, and Andrey Grechko. 2025. "Prognostic Modelling of Mortality in Chronic Critical Illness After Traumatic Brain Injury" Journal of Clinical Medicine 14, no. 22: 8202. https://doi.org/10.3390/jcm14228202
APA StyleLikhvantsev, V., Kolesov, D., Berikashvili, L., Korolenok, E., Yadgarov, M., Kadantseva, K., Kuznetsov, I., Polyakov, P., Kuzovlev, A., & Grechko, A. (2025). Prognostic Modelling of Mortality in Chronic Critical Illness After Traumatic Brain Injury. Journal of Clinical Medicine, 14(22), 8202. https://doi.org/10.3390/jcm14228202





