The Impact of Orthodontic Treatment on Masseter Muscle Development in Pediatric Patients: A One-Year Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Design and Participants
2.3. Sample Size Calculation
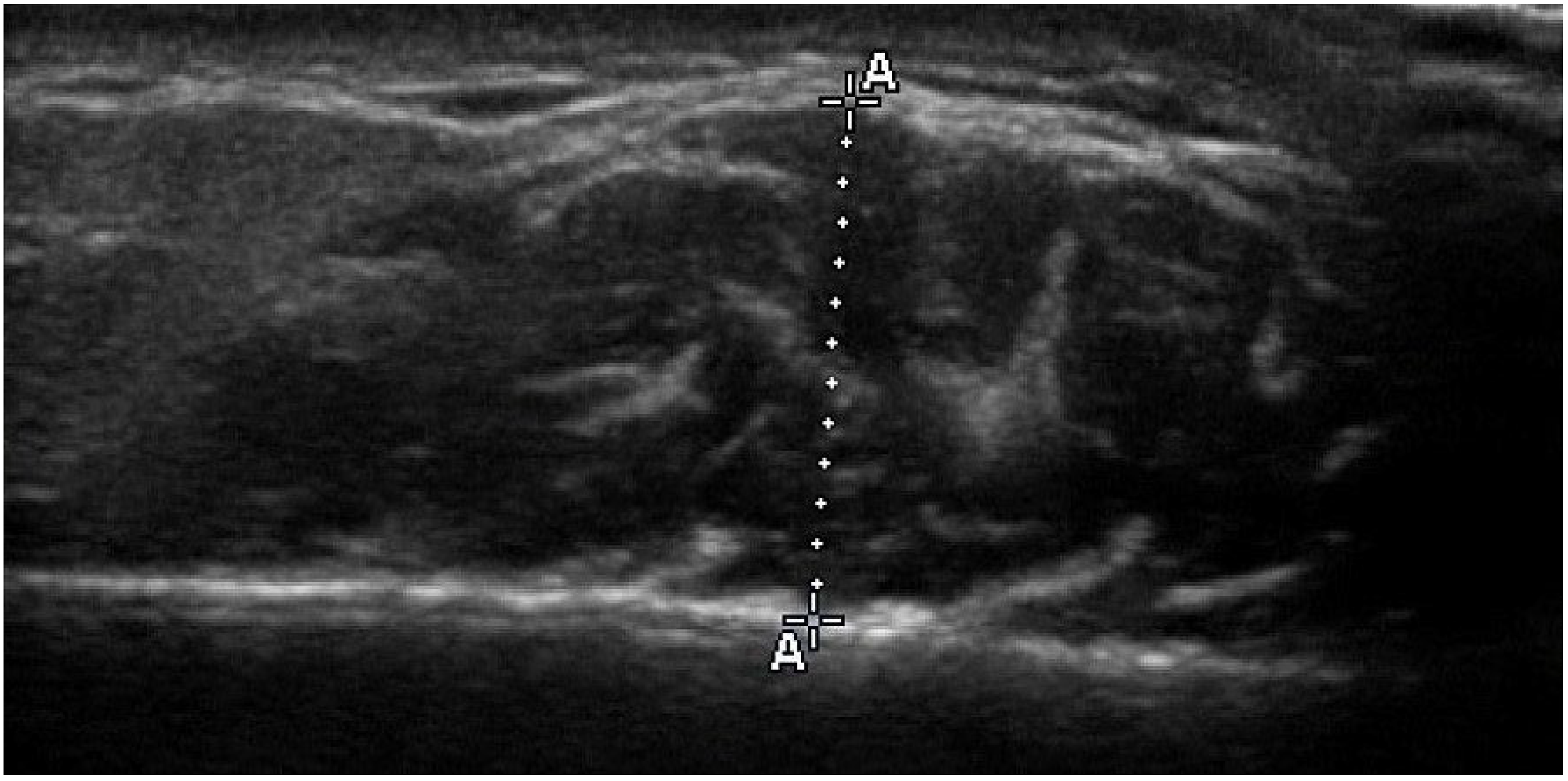
2.4. Ultrasonographic Thickness of Masseter Muscle
2.5. Cephalometric Analysis
2.6. Body Mass Index Assessment
2.7. Cervical Vertebral Maturation Assessment
2.8. Statistical Methods
2.9. Error of Method
3. Results
3.1. Demographics
3.1.1. Orthodontic Treatment Group
3.1.2. Control Group
3.2. Masseter Muscle Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, G.P.; Throckmorton, G.S.; Ellis, E.; Sinn, D.P. The Effects of Orthodontic Treatment on Isometric Bite Forces and Mandibular Motion in Patients before Orthognathic Surgery. J. Oral Maxillofac. Surg. 1995, 53, 673–678; discussion 678–679. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, I.B.; Pereira, L.J.; Andrade, A.S.; Gouvea, D.B.; Gameiro, G.H. The Influence of Fixed Orthodontic Appliances on Masticatory and Swallowing Threshold Performances. J. Oral Rehabil. 2014, 41, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Prema, A.; Vimala, G.; Rao, U.; Shameer, A. Gayathri Occlusal Bite Force Changes during Fixed Orthodontic Treatment in Patients with Different Vertical Facial Morphology. Saudi Dent. J. 2019, 31, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Therkildsen, N.M.; Sonnesen, L. Bite Force, Occlusal Contact and Pain in Orthodontic Patients during Fixed-Appliance Treatment. Dent. J. 2022, 10, 14. [Google Scholar] [CrossRef]
- Ghislanzoni, L.T.H.; Toll, D.E.; Defraia, E.; Baccetti, T.; Franchi, L. Treatment and Posttreatment Outcomes Induced by the Mandibular Advancement Repositioning Appliance; a Controlled Clinical Study. Angle Orthod. 2011, 81, 684–691. [Google Scholar] [CrossRef]
- WoŸniak, K.; Piątkowska, D.; Lipski, M.; Mehr, K. Surface Electromyography in Orthodontics—A Literature Review. Med. Sci. Monit. 2013, 19, 416–423. [Google Scholar] [CrossRef]
- Zhan, Y.; Yang, M.; Bai, S.; Zhang, S.; Huang, Y.; Gong, F.; Nong, X. Effects of Orthodontic Treatment on Masticatory Muscles Activity: A Meta-Analysis. Ann. Hum. Biol. 2023, 50, 465–471. [Google Scholar] [CrossRef]
- Lekavičiūtė, R.; Paldauskaitė, S.; Stučinskaitė, S.; Trakinienė, G. The Effect of Clear Aligner Treatment on Masticatory Muscles (Masseter, Temporalis) Activity in Adults: A Systematic Review and Meta-Analysis. Eur. J. Orthod. 2024, 46, cjae030. [Google Scholar] [CrossRef]
- Goldreich, H.; Gazit, E.; Lieberman, M.A.; Rugh, J.D. The Effect of Pain from Orthodontic Arch Wire Adjustment on Masseter Muscle Electromyographic Activity. Am. J. Orthod. Dentofac. Orthop. 1994, 106, 365–370. [Google Scholar] [CrossRef]
- Scheurer, P.A.; Firestone, A.R.; Bürgin, W.B. Perception of Pain as a Result of Orthodontic Treatment with Fixed Appliances. Eur. J. Orthod. 1996, 18, 349–357. [Google Scholar] [CrossRef]
- Al-Khateeb, S.N.; Abu Alhaija, E.S.; Majzoub, S. Occlusal Bite Force Change after Orthodontic Treatment with Andresen Functional Appliance. Eur. J. Orthod. 2015, 37, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.P.; Donga, R.; Widmer, C.G.; Stohler, C.S. The Pain-Adaptation Model: A Discussion of the Relationship between Chronic Musculoskeletal Pain and Motor Activity. Can. J. Physiol. Pharmacol. 1991, 69, 683–694. [Google Scholar] [CrossRef]
- Michelotti, A.; Farella, M.; Martina, R. Sensory and Motor Changes of the Human Jaw Muscles during Induced Orthodontic Pain. Eur. J. Orthod. 1999, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cui, C.; Hu, Z.; Li, J.; Wang, J. Changes of Neuroplasticity in Cortical Motor Control of Human Masseter Muscle Related to Orthodontic Treatment. J. Oral Rehabil. 2022, 49, 258–264. [Google Scholar] [CrossRef]
- Weijs, W.A.; Hillen, B. Relationship between the Physiological Cross-Section of the Human Jaw Muscles and Their Cross-Sectional Area in Computer Tomograms. Acta Anat. 1984, 118, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Weijs, W.A.; Hillen, B. Physiological Cross-Section of the Human Jaw Muscles. Acta Anat. 1985, 121, 31–35. [Google Scholar] [CrossRef]
- Kiliaridis, S.; Kälebo, P. Masseter Muscle Thickness Measured by Ultrasonography and Its Relation to Facial Morphology. J. Dent. Res. 1991, 70, 1262–1265. [Google Scholar] [CrossRef]
- Raadsheer, M.C.; Van Eijden, T.M.; Van Spronsen, P.H.; Van Ginkel, F.C.; Kiliaridis, S.; Prahl-Andersen, B. A Comparison of Human Masseter Muscle Thickness Measured by Ultrasonography and Magnetic Resonance Imaging. Arch. Oral Biol. 1994, 39, 1079–1084. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Kiliaridis, S.; Mills, C.M.; Antonarakis, G.S. Masseter Muscle Thickness as a Predictive Variable in Treatment Outcome of the Twin-Block Appliance and Masseteric Thickness Changes during Treatment. Orthod. Craniofac. Res. 2010, 13, 203–213. [Google Scholar] [CrossRef]
- Weijs, W.A.; Hillen, B. Relationships between Masticatory Muscle Cross-Section and Skull Shape. J. Dent. Res. 1984, 63, 1154–1157. [Google Scholar] [CrossRef]
- Kubota, M.; Nakano, H.; Sanjo, I.; Satoh, K.; Sanjo, T.; Kamegai, T.; Ishikawa, F. Maxillofacial Morphology and Masseter Muscle Thickness in Adults. Eur. J. Orthod. 1998, 20, 535–542. [Google Scholar] [CrossRef]
- Benington, P.C.; Gardener, J.E.; Hunt, N.P. Masseter Muscle Volume Measured Using Ultrasonography and Its Relationship with Facial Morphology. Eur. J. Orthod. 1999, 21, 659–670. [Google Scholar] [CrossRef]
- Satiroğlu, F.; Arun, T.; Işik, F. Comparative Data on Facial Morphology and Muscle Thickness Using Ultrasonography. Eur. J. Orthod. 2005, 27, 562–567. [Google Scholar] [CrossRef]
- Hasund, B. Clinical Cephalometry for the Bergen-Technique; Dental Institute, University of Bergen: Bergen, Norway, 1977. [Google Scholar]
- Baccetti, T.; Franchi, L.; McNamara, J.A. An Improved Version of the Cervical Vertebral Maturation (CVM) Method for the Assessment of Mandibular Growth. Angle Orthod. 2002, 72, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Comparisons against Baseline within Randomised Groups Are Often Used and Can Be Highly Misleading. Trials 2011, 12, 264. [Google Scholar] [CrossRef]
- Dahlberg, G. Statistical Methods for Medical and Biological Students. Br. Med. J. 1940, 2, 358–359. [Google Scholar]
- Houston, W.J. The Analysis of Errors in Orthodontic Measurements. Am. J. Orthod. 1983, 83, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Raadsheer, M.C.; Kiliaridis, S.; Van Eijden, T.M.; Van Ginkel, F.C.; Prahl-Andersen, B. Masseter Muscle Thickness in Growing Individuals and Its Relation to Facial Morphology. Arch. Oral Biol. 1996, 41, 323–332. [Google Scholar] [CrossRef]
- Antonarakis, G.S.; Kiliaridis, S. Predictive Value of Masseter Muscle Thickness and Bite Force on Class II Functional Appliance Treatment: A Prospective Controlled Study. Eur. J. Orthod. 2015, 37, 570–577. [Google Scholar] [CrossRef]
- Paes-Souza, S.D.A.; Garcia, M.A.C.; Souza, V.H.; Morais, L.S.; Nojima, L.I.; Nojima, M.D.C.G. Response of Masticatory Muscles to Treatment with Orthodontic Aligners: A Preliminary Prospective Longitudinal Study. Dent. Press J. Orthod. 2023, 28, e232198. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, S.; Shen, L.; Pei, Y.; Zhang, Y.; Xu, T. Thickness Change of Masseter Muscles and the Surrounding Soft Tissues in Female Patients during Orthodontic Treatment: A Retrospective Study. BMC Oral Health 2020, 20, 181. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Ahmad, N.; Tajik, I.; Javaid, A.; Khan, A.M. The Impact of Orthodontic Treatment on Body Weight Due to Change in Dietary Habits. Cureus 2024, 16, e75764. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, M.; Ilhan, A.; Gorucu-Coskuner, H.; Taner, T.; Bilgic, P. Assessment of Food Consumption Changes in Adolescents during Orthodontic Treatment. Am. J. Orthod. Dentofac. Orthop. 2021, 159, 604–612. [Google Scholar] [CrossRef]
- Gnanasambandam, V.; Gnaneswar, S.M. Effects of Orthodontic Treatment on Body Mass Index, Food Habits and Self-Esteem of Patients: A Prospective Single-Arm Cohort Study. J. Taibah Univ. Med. Sci. 2022, 17, 818–825. [Google Scholar] [CrossRef]
- Soltis, J.E.; Nakfoor, P.R.; Bowman, D.C. Changes in Ability of Patients to Differentiate Intensity of Forces Applied to Maxillary Central Incisors during Orthodontic Treatment. J. Dent. Res. 1971, 50, 590–596. [Google Scholar] [CrossRef]
- Kiliaridis, S.; Tzakis, M.G.; Carlsson, G.E. Effects of Fatigue and Chewing Training on Maximal Bite Force and Endurance. Am. J. Orthod. Dentofac. Orthop. 1995, 107, 372–378. [Google Scholar] [CrossRef]
- Aslan, E.M.; Artaş, A. Ultrasonographic Assessment of Masseter and Anterior Temporal Muscle Thickness and Internal Structure in Young Adult Patients With Bruxism. J. Clin. Ultrasound 2025, 53, 286–293. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chun, Y.-H.; Bae, H.; Lee, J.-W.; Kim, H.-J. Comparison of Ultrasonography-Based Masticatory Muscle Thickness between Temporomandibular Disorders Bruxers and Temporomandibular Disorders Non-Bruxers. Sci. Rep. 2024, 14, 6923. [Google Scholar] [CrossRef]
- Tatlı, E.C.; Arslan, Z.B. Probable Bruxism Effects on Masseter Muscle Thickness in Children: Ultrasonographic Evaluation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 135, 456–461. [Google Scholar] [CrossRef]
- Booth, F.W. Effect of Limb Immobilization on Skeletal Muscle. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982, 52, 1113–1118. [Google Scholar] [CrossRef]
- Fazzini, B.; Märkl, T.; Costas, C.; Blobner, M.; Schaller, S.J.; Prowle, J.; Puthucheary, Z.; Wackerhage, H. The Rate and Assessment of Muscle Wasting during Critical Illness: A Systematic Review and Meta-Analysis. Crit. Care 2023, 27, 2. [Google Scholar] [CrossRef]
| Variable (at T0) | Treatment (n = 20) | Control (n = 18) | p Value |
|---|---|---|---|
| Age (yr) | 10.4 ± 2.1 | 9.9 ± 2.0 | 0.422 |
| BMI (kg/m2) | 18.5 ± 2.8 | 19.1 ± 4.3 | 0.573 |
| ANB angle (°) | 4.2 ± 2.0 | 3.6 ± 3.3 | 0.470 |
| NSL/ML angle (°) | 35.1 ± 4.2 | 33.4 ± 4.9 | 0.275 |
| NL/ML angle (°) | 25.7 ± 4.1 | 25.8 ± 4.8 | 0.930 |
| Treatment Group | Control Group | Difference Between the Groups (Control—Treatment Group) | |||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | 95% CI | p Value | |
| T0 | 11.7 ± 1.4 | 11.4 ± 1.3 | −0.3 ± 0.4 | −1.241 to 0.50 | 0.403 (n.s.) |
| T1 | 11.1 ± 1.1 | 11.9 ± 1.3 | 0.8 ± 0.4 | 0.003 to 0.57 | 0.049 |
| T1 − T0 | −0.6 ± 0.7 | 0.5 ± 0.6 | |||
| p value | <0.001 | <0.001 | |||
| Variables | Coefficient b | Standard Error | Significance (p Value) |
|---|---|---|---|
| Group | −1.079 | 0.171 | <0.001 |
| Age T0 | −0.015 | 0.043 | 0.722 (n.s.) |
| Gender | 0.030 | 0.181 | 0.869 (n.s.) |
| MMT0 | −0.178 | 0.066 | 0.011 |
- Regression equation: (Y) = b0 + b1 × GROUP + b2 × AGE T0 + b3 × GENDER + b4 × MMT0;
- MMT CHANGE = 2.687 − 1.079 × GROUP − 0.015 × AGE T0 + 0.030 × GENDER − 0.178 × MMT0;
- Dependent variable (Y): Masseter thickness changes (mm) during in 1 year (MMT CHANGE).
- b0 = constant, b1, b2, b3, b4 = regression coefficients.
- Independent variables: GROUP, AGE T0, GENDER, MMT0;
- Significance of the model: R = 0.792 (correlation coefficient), R2 = 62.7% (percentage of explained variance), p < 0.001.
- GROUP—control (=0) or treatment group (=1), AGE T0—age of subjects at the beginning of the study, GENDER—male (=0) or female (=1), MMT0—Mean masseter muscle thickness at T0, n.s.—not statistically significant, MMT CHANGE—change in masseter muscle thickness.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiliaridis, S.; Frasiola, A.; Georgiakaki, I.; Charalampidou, M.; Antonarakis, G.S. The Impact of Orthodontic Treatment on Masseter Muscle Development in Pediatric Patients: A One-Year Follow-Up Study. J. Clin. Med. 2025, 14, 8175. https://doi.org/10.3390/jcm14228175
Kiliaridis S, Frasiola A, Georgiakaki I, Charalampidou M, Antonarakis GS. The Impact of Orthodontic Treatment on Masseter Muscle Development in Pediatric Patients: A One-Year Follow-Up Study. Journal of Clinical Medicine. 2025; 14(22):8175. https://doi.org/10.3390/jcm14228175
Chicago/Turabian StyleKiliaridis, Stavros, Aikaterini Frasiola, Ioanna Georgiakaki, Maria Charalampidou, and Gregory S. Antonarakis. 2025. "The Impact of Orthodontic Treatment on Masseter Muscle Development in Pediatric Patients: A One-Year Follow-Up Study" Journal of Clinical Medicine 14, no. 22: 8175. https://doi.org/10.3390/jcm14228175
APA StyleKiliaridis, S., Frasiola, A., Georgiakaki, I., Charalampidou, M., & Antonarakis, G. S. (2025). The Impact of Orthodontic Treatment on Masseter Muscle Development in Pediatric Patients: A One-Year Follow-Up Study. Journal of Clinical Medicine, 14(22), 8175. https://doi.org/10.3390/jcm14228175








