Automated Artificial Intelligence Mapping of Coronary Plaque Calcification: A Comparison with Manual Intravascular Image Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Ground Truth Annotation
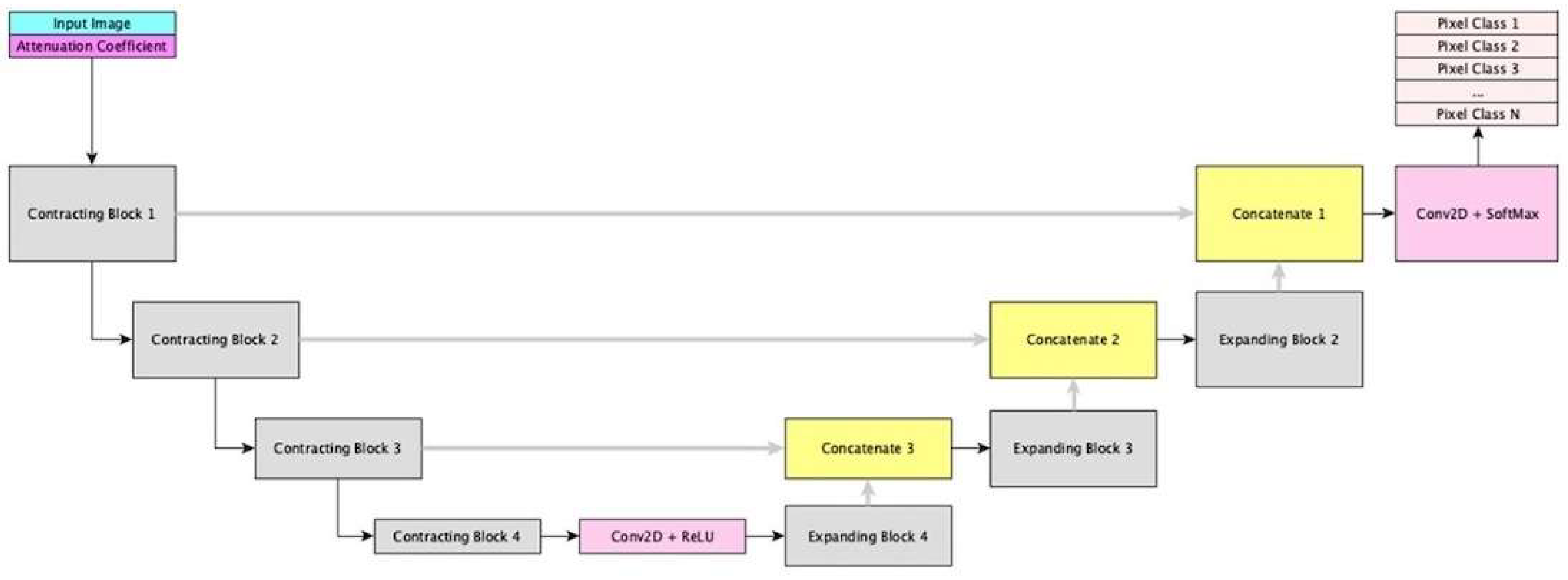
2.3. Deep Network Architecture Development and Training
2.4. Independent External Validation
2.5. Statistical Analysis
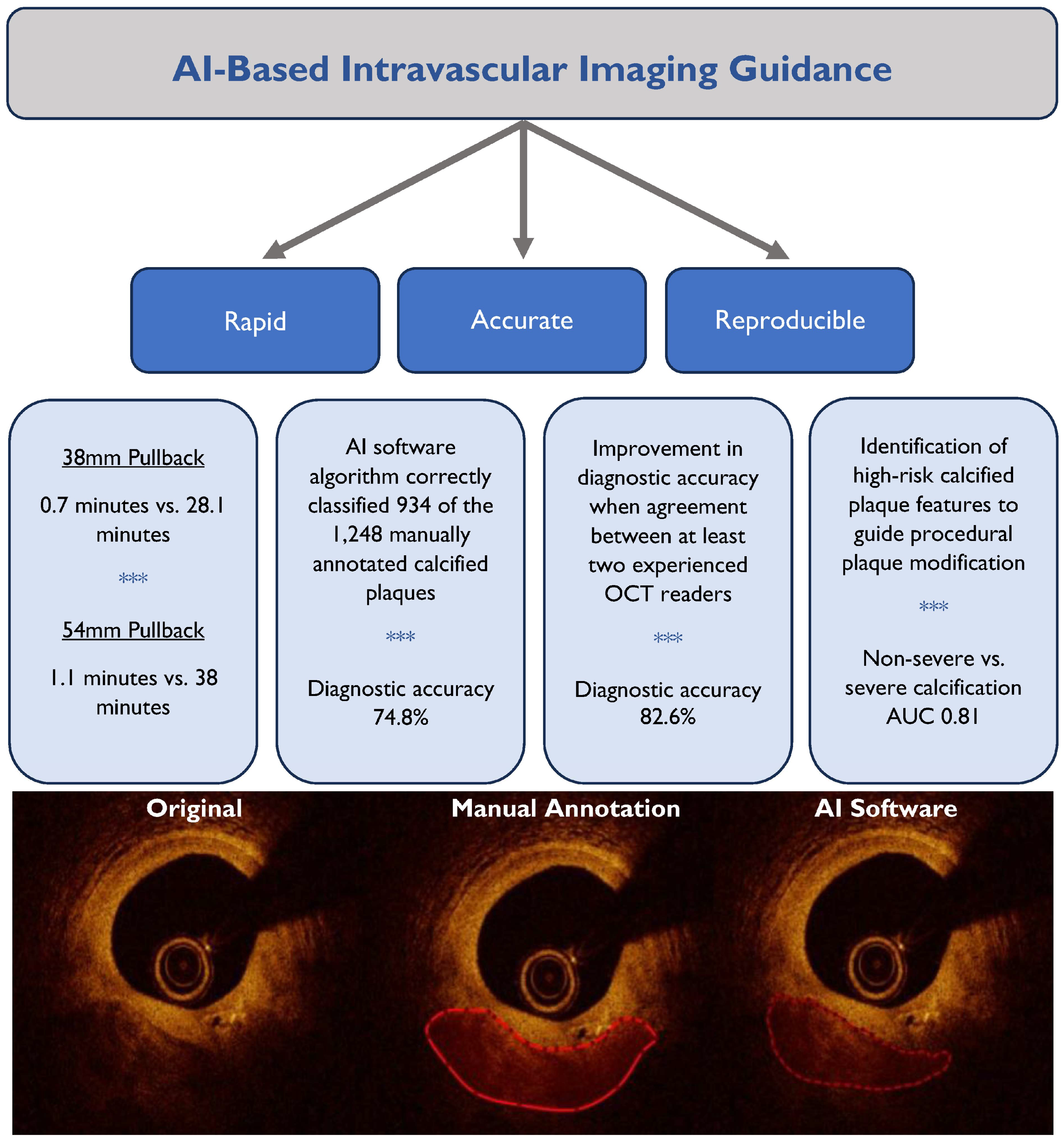
3. Results
3.1. OCT Pullback Characteristics
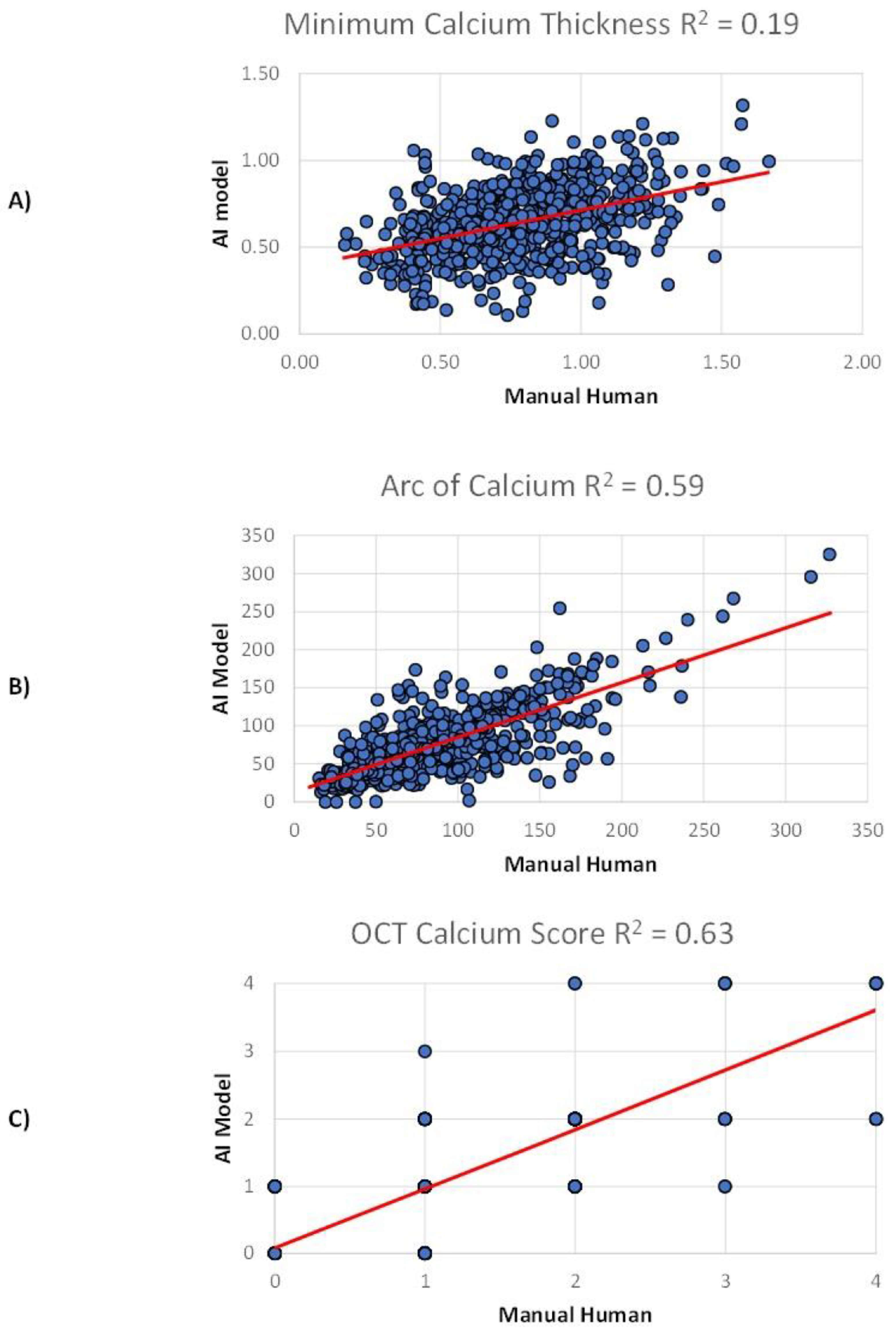
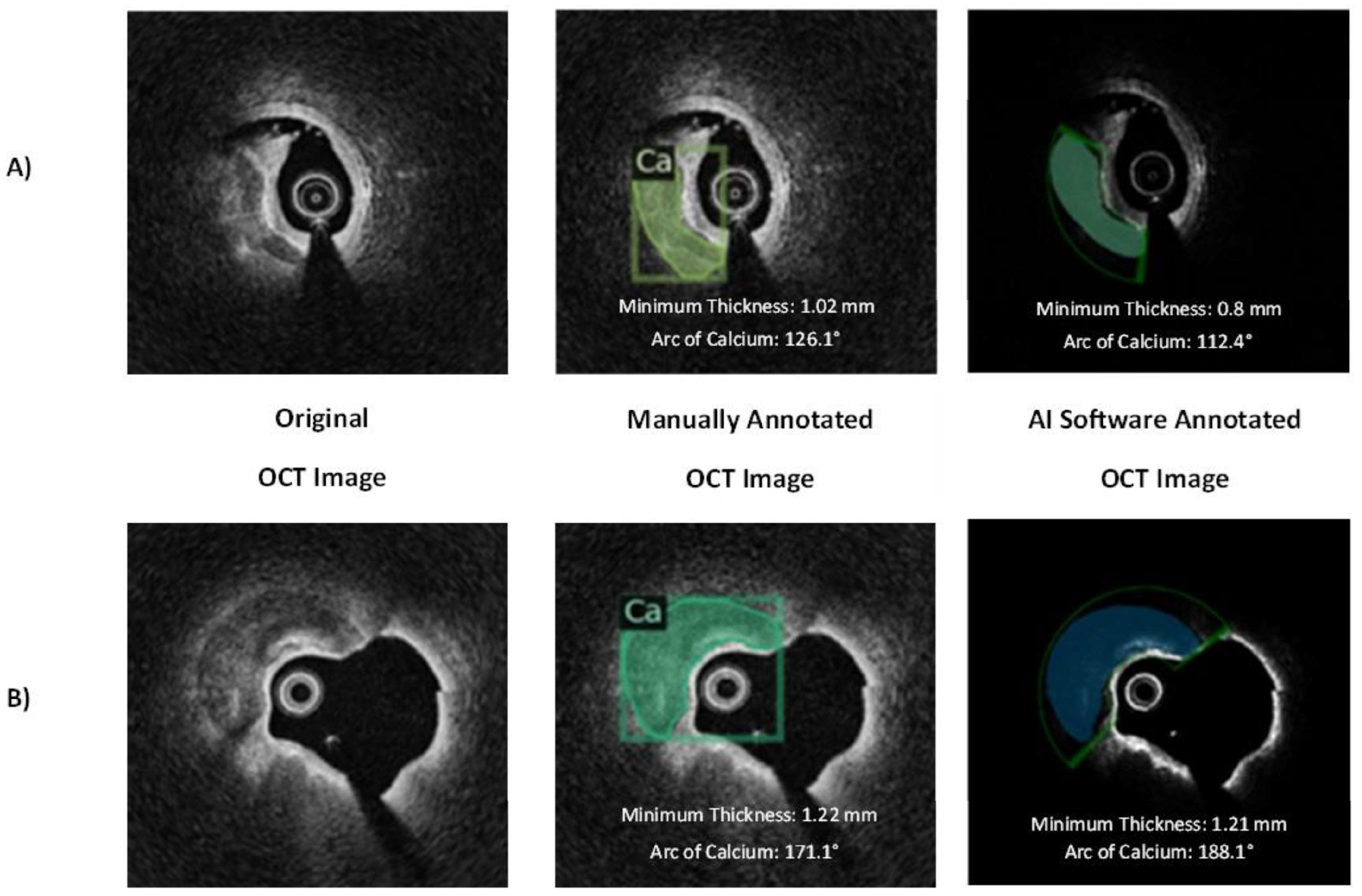
3.2. Internal Validation
3.3. Independent External Validation
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCI | Percutaneous Coronary Intervention. |
| OCT | Optical Coherence Tomography. |
| AI | Artificial Intelligence. |
| AUC | Area under the Receiving Operator Characteristic Curve. |
| NURD | Non-uniform Rotational Distortion. |
| ROC | Receiver Operating Characteristic Curve. |
References
- Truesdell, A.G.; Alasnag, M.A.; Kaul, P.; Rab, S.T.; Riley, R.F.; Young, M.N.; Batchelor, W.B.; Maehara, A.; Welt, F.G.; Kirtane, A.J. Intravascular Imaging During Percutaneous Coronary Intervention: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 81, 590–605. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Kan, J.; Ge, Z.; Han, L.; Lu, S.; Tian, N.; Lin, S.; Lu, Q.; Wu, X.; et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J. Am. Coll. Cardiol. 2018, 72, 3126–3137. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, B.K.; Shin, D.H.; Nam, C.M.; Kim, J.S.; Ko, Y.G.; Choi, D.; Kang, T.S.; Kang, W.C.; Her, A.Y.; et al. Effect of Intravascular Ultrasound-Guided vs Angiography-Guided Everolimus-Eluting Stent Implantation: The IVUS-XPL Randomized Clinical Trial. Jama 2015, 314, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Choi, K.H.; Song, Y.B.; Lee, J.Y.; Lee, S.J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J.; et al. Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef]
- Ali, Z.A.; Landmesser, U.; Maehara, A.; Matsumura, M.; Shlofmitz, R.A.; Guagliumi, G.; Price, M.J.; Hill, J.M.; Akasaka, T.; Prati, F.; et al. Optical Coherence Tomography-Guided versus Angiography-Guided Pci. N. Engl. J. Med. 2023, 389, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Bergmark, B.A.; Osborn, E.A.; Ali, Z.A.; Gupta, A.; Kolli, K.K.; Prillinger, J.B.; Hasegawa, J.; West, N.E.J.; Croce, K.; Secemsky, E. Association Between Intracoronary Imaging During PCI and Clinical Outcomes in a Real-World US Medicare Population. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100556. [Google Scholar] [CrossRef]
- Patel, B.; Makaryus, A.N. Artificial Intelligence Advances in the World of Cardiovascular Imaging. Healthcare 2022, 10, 154. [Google Scholar] [CrossRef]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.M.; Chowdhary, S.; et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Fujino, A.; Mintz, G.S.; Matsumura, M.; Lee, T.; Kim, S.Y.; Hoshino, M.; Usui, E.; Yonetsu, T.; Haag, E.S.; Shlofmitz, R.A.; et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention 2018, 13, e2182–e2189. [Google Scholar] [CrossRef] [PubMed]
- Mintz, G.S. Intravascular Imaging of Coronary Calcification and Its Clinical Implications. JACC Cardiovasc. Imaging 2015, 8, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Shlofmitz, E.; Ali, Z.A.; Maehara, A.; Mintz, G.S.; Shlofmitz, R.; Jeremias, A. Intravascular Imaging-Guided Percutaneous Coronary Intervention: A Universal Approach for Optimization of Stent Implantation. Circ. Cardiovasc. Interv. 2020, 13, e008686. [Google Scholar] [CrossRef]
- Gao, X.F.; Ge, Z.; Kong, X.Q.; Kan, J.; Han, L.; Lu, S.; Tian, N.L.; Lin, S.; Lu, Q.H.; Wang, X.Y.; et al. 3-Year Outcomes of the ULTIMATE Trial Comparing Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. JACC Cardiovasc. Interv. 2021, 14, 247–257. [Google Scholar] [CrossRef]
- Liu, M.H.; Zhao, C.; Wang, S.; Jia, H.; Yu, B. Artificial Intelligence-a Good Assistant to Multi-Modality Imaging in Managing Acute Coronary Syndrome. Front. Cardiovasc. Med. 2021, 8, 782971. [Google Scholar] [CrossRef]
- Seetharam, K.; Shrestha, S.; Sengupta, P.P. Cardiovascular Imaging And Intervention Through the Lens of Artificial Intelligence. Interv. Cardiol. 2021, 16, e31. [Google Scholar] [CrossRef]
- Zhang, J.; Han, R.; Shao, G.; Lv, B.; Sun, K. Artificial Intelligence in Cardiovascular Atherosclerosis Imaging. J. Pers. Med. 2022, 12, 420. [Google Scholar] [CrossRef]
- Desai, R.; Patel, U.; Fong, H.K.; Sadolikar, A.; Zalavadia, D.; Gupta, S.; Doshi, R.; Sachdeva, R.; Kumar, G. Modern-Day Nationwide Utilization of Intravascular Ultrasound and Its Impact on the Outcomes of Percutaneous Coronary Intervention with Coronary Atherectomy in the United States. J. Ultrasound Med. 2019, 38, 2295–2304. [Google Scholar] [CrossRef]
- Hill, J.M.; Kereiakes, D.J.; Shlofmitz, R.A.; Klein, A.J.; Riley, R.F.; Price, M.J.; Herrmann, H.C.; Bachinsky, W.; Waksman, R.; Stone, G.W. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 76, 2635–2646. [Google Scholar] [CrossRef]
- Ali, Z.A.; Nef, H.; Escaned, J.; Werner, N.; Banning, A.P.; Hill, J.M.; De Bruyne, B.; Montorfano, M.; Lefevre, T.; Stone, G.W.; et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Stenoses: The Disrupt Cad II Study. Circ. Cardiovasc. Interv. 2019, 12, e008434. [Google Scholar] [CrossRef]
- Chambers, J.W.; Feldman, R.L.; Himmelstein, S.I.; Bhatheja, R.; Villa, A.E.; Strickman, N.E.; Shlofmitz, R.A.; Dulas, D.D.; Arab, D.; Khanna, P.K.; et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc. Interv. 2014, 7, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Kassimis, G.; Raina, T.; Kontogiannis, N.; Patri, G.; Abramik, J.; Zaphiriou, A.; Banning, A.P. How Should We Treat Heavily Calcified Coronary Artery Disease in Contemporary Practice? From Atherectomy to Intravascular Lithotripsy. Cardiovasc. Revasc Med. 2019, 20, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Zhang, J.J.; Mintz, G.S.; Hong, S.J.; Ahn, C.M.; Kim, J.S.; Kim, B.K.; Ko, Y.G.; Choi, D.; Jang, Y.; et al. Impact of Intravascular Ultrasound-Guided Optimal Stent Expansion on 3-Year Hard Clinical Outcomes. Circ. Cardiovasc. Interv. 2021, 14, e011124. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Baraki, T.G.; Kim, B.G.; Lee, Y.J.; Lee, S.J.; Hong, S.J.; Ahn, C.M.; Shin, D.H.; Kim, B.K.; Ko, Y.G.; et al. stent expansion evaluated by optical coherence tomography and subsequent outcomes. Sci. Rep. 2023, 13, 3781. [Google Scholar] [CrossRef]
- Fujimura, T.; Matsumura, M.; Witzenbichler, B.; Metzger, D.C.; Rinaldi, M.J.; Duffy, P.L.; Weisz, G.; Stuckey, T.D.; Ali, Z.A.; Zhou, Z.; et al. Stent Expansion Indexes to Predict Clinical Outcomes: An IVUS Substudy from Adapt-Des. JACC Cardiovasc. Interv. 2021, 14, 1639–1650. [Google Scholar] [CrossRef]
- Ughi, G.J.; Adriaenssens, T.; Sinnaeve, P.; Desmet, W.; D’Hooge, J. Automated tissue characterization of in vivo atherosclerotic plaques by intravascular optical coherence tomography images. Biomed. Opt. Express 2013, 4, 1014–1030. [Google Scholar] [CrossRef]
- Gargesha, M.; Shalev, R.; Prabhu, D.; Tanaka, K.; Rollins, A.M.; Costa, M.; Bezerra, H.G.; Wilson, D.L. Parameter estimation of atherosclerotic tissue optical properties from three-dimensional intravascular optical coherence tomography. J. Med. Imaging (Bellingham) 2015, 2, 016001. [Google Scholar] [CrossRef]
- Shalev, R.; Bezerra, H.G.; Ray, S.; Prabhu, D.; Wilson, D.L. Classification of calcium in intravascular OCT images for the purpose of intervention planning. Proc. SPIE Int. Soc. Opt. Eng. 2016, 9786, 978605. [Google Scholar]
- Lu, H.; Lee, J.; Jakl, M.; Wang, Z.; Cervinka, P.; Bezerra, H.G.; Wilson, D.L. Application and Evaluation of Highly Automated Software for Comprehensive Stent Analysis in Intravascular Optical Coherence Tomography. Sci. Rep. 2020, 10, 2150. [Google Scholar] [CrossRef]
- Shalev, R.; Gargesha, M.; Prabhu, D.; Tanaka, K.; Rollins, A.M.; Lamouche, G.; Bisaillon, C.E.; Bezerra, H.G.; Ray, S.; Wilson, D.L. Processing to determine optical parameters of atherosclerotic disease from phantom and clinical intravascular optical coherence tomography three-dimensional pullbacks. J. Med. Imaging 2016, 3, 024501. [Google Scholar] [CrossRef]
- Abdolmanafi, A.; Duong, L.; Dahdah, N.; Cheriet, F. Deep feature learning for automatic tissue classification of coronary artery using optical coherence tomography. Biomed. Opt. Express 2017, 8, 1203–1220. [Google Scholar] [CrossRef]
- Gessert, N.; Lutz, M.; Heyder, M.; Latus, S.; Leistner, D.M.; Abdelwahed, Y.S.; Schlaefer, A. Automatic Plaque Detection in ivoct Pullbacks Using Convolutional Neural Networks. IEEE Trans. Med. Imaging 2019, 38, 426–434. [Google Scholar] [CrossRef]
- Li, L.; Jia, T. Optical Coherence Tomography Vulnerable Plaque Segmentation Based on Deep Residual u-Net. Rev. Cardiovasc. Med. 2019, 20, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Jia, H.; Gutiérrez-Chico, J.L.; Maehara, A.; Ali, Z.A.; Zeng, X.; He, L.; Zhao, C.; Matsumura, M.; Wu, P.; et al. Artificial intelligence and optical coherence tomography for the automatic characterisation of human atherosclerotic plaques. EuroIntervention 2021, 17, 41–50. [Google Scholar] [CrossRef]
- Bartuś, S.; Siłka, W.; Kasprzycki, K.; Sabatowski, K.; Malinowski, K.P.; Rzeszutko, Ł.; Chyrchel, M.; Bryniarski, L.; Surdacki, A.; Bartuś, K.; et al. Experience with Optical Coherence Tomography Enhanced by a Novel Software (Ultreon™ 1.0 Software)-the First One Hundred Cases. Medicina 2022, 58, 1227. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, Y.; Hosoi, Y.; Bota, H.; Kuroda, K.; Kasai, Y.; Ishikawa, K.; Semba, N.; Yamasaki, K.; Tani, T.; Yamazaki, S. Artificial Intelligence vs Visual Assessment of Calcified Plaque in Coronary Artery Using Optical Coherence Tomography. JACC Adv. 2022, 1, 100080. [Google Scholar] [CrossRef] [PubMed]
- Januszek, R.; Siłka, W.; Sabatowski, K.; Malinowski, K.P.; Heba, G.; Surowiec, S.; Chyrchel, M.; Rzeszutko, Ł.; Bryniarski, L.; Surdacki, A.; et al. Procedure-Related Differences and Clinical Outcomes in Patients Treated with Percutaneous Coronary Intervention Assisted by Optical Coherence Tomography Between New and Earlier Generation Software (Ultreon™ 1.0 Software vs. AptiVue™ Software). J. Cardiovasc. Dev. Dis. 2022, 9, 218. [Google Scholar] [CrossRef]
| Characteristic | Total (N = 50) |
|---|---|
| Sex—No. (%) | |
| Male | 37 (74) |
| Age—years | |
| Median | 64.5 |
| Range | [35, 89] |
| Race—No. (%) | |
| Caucasian | 38 (76) |
| African American | 6 (12) |
| Other | 6 (12) |
| Smoking—No. (%) | |
| Current | 9 (18) |
| Former | 19 (38) |
| Comorbidities—No. (%) | |
| Hypertension | 34 (68) |
| Dyslipidemia | 37 (74) |
| Diabetes Mellitus | 13 (26) |
| Atrial Fibrillation | 3 (6) |
| Peripheral Arterial Disease | 1 (2) |
| Coronary artery disease | 19 (38) |
| Prior MI | 10 (20) |
| Prior PCI | 11 (22) |
| Prior CABG | 5 (1) |
| Renal function—median (range) | |
| Creatinine (g/dL) | 1.0 (0.6, 10.9) |
| eGFR (mL/min/1.73 m2) | 74.25 (4.3, 136.1) |
| Dialysis (No., %) | 1 (2) |
| LVEF—% | |
| Median | 57.1 |
| Range | 30, 75 |
| Medications—No. (%) | |
| Aspirin | 28 (56) |
| P2Y12 inhibitor | 7 (14) |
| DOAC | 2 (4) |
| Warfarin | 1 (2) |
| Statin | 29 (58) |
| ACE inhibitor/ARB/ARNI | 15 (30) |
| Beta blocker | 24 (48) |
| Calcium channel blocker | 13 (26) |
| Insulin | 3 (6) |
| Oral diabetic agent | 8 (16) |
| Clinical presentation—No. (%) | |
| Silent ischemia | 2 (4) |
| Stable angina | 19 (38) |
| Unstable angina | 11 (22) |
| NSTEMI | 12 (24) |
| STEMI | 6 (12) |
| Characteristic | Total (N = 50) |
|---|---|
| Duration—median (range) | |
| Procedure (mins) | 91 (33, 191) |
| Fluoroscopy (mins) | 18.2 (9, 60.8) |
| Radiation dose—mGy | |
| Median | 667 |
| Range | [239, 2772] |
| Contrast volume—mL | |
| Median | 147.5 |
| Range | (80, 360) |
| Access—No. (%) | |
| Radial | 44 (88) |
| Ulnar | 1 (2) |
| Femoral | 5 (10) |
| PCI—No. (%) | 46 (92) |
| OCT—No. (%) | |
| Pre-PCI | 50 (100) |
| Post-PCI | 40 (80) |
| Vessel OCT performed—No. (%) | |
| LAD | 37 (74) |
| LCx | 4 (8) |
| RCA | 8 (16) |
| Other | 1 (2) |
| Minimum lumen area—mm2 | |
| Median | 1.79 |
| Range | [0.5, 4.8] |
| Calcified plaque characteristics | |
| Minimum thickness, mm—median [range] | 0.72 [0.16, 1.66] |
| Arc of calcium, °—median [range] | 66.95 [9.30, 327.20] |
| OCT-calcium score—median [range] | 1 [0, 4] |
| Calcium modification performed—No. (%) | |
| Rotational atherectomy | 5 (10) |
| Laser atherectomy | 0 (0) |
| Intravascular lithotripsy | 2 (4) |
| Cutting/Scoring balloon angioplasty | 2 (4) |
| Minimum stent area post PCI—mm2 | |
| Median | 5.12 |
| Range | [2.91, 9.95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCarthy, K.J.; Larnard, E.A.; Anderson, C.K.; Chowdhury, M.; Shalev, R.; Hakim, D.A.; Croce, K.J.; Osborn, E.A. Automated Artificial Intelligence Mapping of Coronary Plaque Calcification: A Comparison with Manual Intravascular Image Analysis. J. Clin. Med. 2025, 14, 8166. https://doi.org/10.3390/jcm14228166
McCarthy KJ, Larnard EA, Anderson CK, Chowdhury M, Shalev R, Hakim DA, Croce KJ, Osborn EA. Automated Artificial Intelligence Mapping of Coronary Plaque Calcification: A Comparison with Manual Intravascular Image Analysis. Journal of Clinical Medicine. 2025; 14(22):8166. https://doi.org/10.3390/jcm14228166
Chicago/Turabian StyleMcCarthy, Killian J., Emily A. Larnard, Christina K. Anderson, Mohsin Chowdhury, Ronny Shalev, Diaa A. Hakim, Kevin J. Croce, and Eric A. Osborn. 2025. "Automated Artificial Intelligence Mapping of Coronary Plaque Calcification: A Comparison with Manual Intravascular Image Analysis" Journal of Clinical Medicine 14, no. 22: 8166. https://doi.org/10.3390/jcm14228166
APA StyleMcCarthy, K. J., Larnard, E. A., Anderson, C. K., Chowdhury, M., Shalev, R., Hakim, D. A., Croce, K. J., & Osborn, E. A. (2025). Automated Artificial Intelligence Mapping of Coronary Plaque Calcification: A Comparison with Manual Intravascular Image Analysis. Journal of Clinical Medicine, 14(22), 8166. https://doi.org/10.3390/jcm14228166





