Assessing Venous Congestion in Acute and Chronic Heart Failure: A Review of Splanchnic, Cardiac and Pulmonary Ultrasound: Part 1: Conventional B-Mode, Colordoppler, and Vexus Protocol
Abstract
1. Introduction
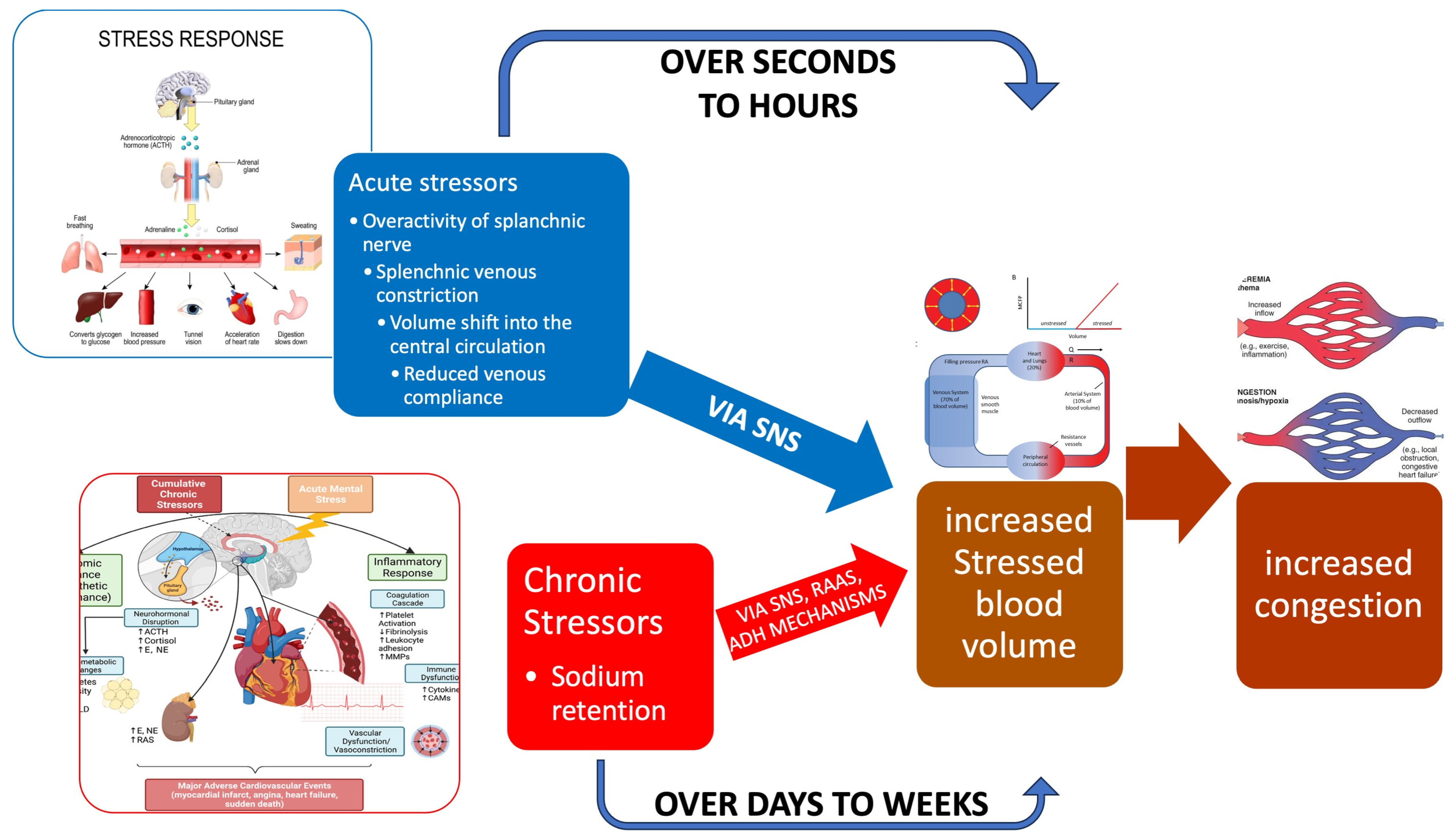
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
Risk of Bias and Certainty Assessment
2.4. Data Synthesis and Analysis Framework
- -
- What are the specific ultrasonographic parameters (B-mode, Doppler) used to assess the splanchnic, cardiac, and pulmonary circulations in heart failure?
- -
- What is the evidence for the association between these parameters and objective measures of haemodynamic congestion (e.g., right atrial pressure, pulmonary capillary wedge pressure)?
- -
- What is the prognostic value of these parameters for clinical outcomes (e.g., mortality, heart failure hospitalization, worsening renal function)?
- -
- How are these parameters integrated into multiparametric protocols (e.g., VExUS), and what is the evidence for the utility of such integrated approaches?
- -
- B-Mode Parameters: Bowel wall thickness, inferior vena cava (IVC) diameter and collapsibility, hepatic vein dilation.
- -
- Doppler Parameters: Hepatic vein waveform patterns (triphasic, biphasic, monophasic), portal vein pulsatility and flow direction, intra-renal venous flow patterns.
- -
- Multiparametric Scoring Systems: Synthesizing evidence on the development, validation, and clinical application of the Venous Excess Ultrasound (VExUS) score and its extended (eVExUS) applications.
- -
- Cardiac Evaluation: Synthesizing evidence on the specific echocardiographic parameters (e.g., E/e′ ratio, TAPSE, RVSP) most consistently linked to systemic congestion in the context of the included studies.
- -
- Pulmonary Congestion: Synthesizing evidence on the use of lung ultrasound (B-lines) for diagnosing and monitoring congestion, and its correlation with other systemic congestion markers.
3. Results
3.1. Ultrasound Measurements of Splanchnic Circulation
3.1.1. B-Mode
Bowel-Wall Thickening
Venous Congestion
3.1.2. Ecocolordoppler and Spectral Velocity Variations
Portal Vein
- 1.
- Increased Pulsatility (Pulsatile Waveform): A pulsatile portal venous flow occurs when there is a significant difference between peak systolic and end-diastolic velocities. This is due to abnormal transmission of pressure through the hepatic sinusoids, often caused by conditions like tricuspid regurgitation, right-sided heart failure (CHF), or arteriovenous shunting (as seen in cirrhosis or hereditary haemorrhagic telangiectasia (Figure 9A) [105]. Pulsatility can be differentiated clinically, with right-sided CHF and tricuspid regurgitation identifiable through the hepatic venous waveform and grayscale US showing dilated hepatic veins, unlike in cirrhosis, where hepatic veins are compressed.
- 2.
- Slow Portal Venous Flow: Slow flow occurs when back pressure restricts forward flow, typically indicating portal hypertension. In these cases, peak velocity is less than 16 cm/s [106]. Causes of portal hypertension include cirrhosis, portal vein thrombosis (prehepatic), and right-sided heart failure (posthepatic). The most specific findings include the development of portosystemic shunts (like a recanalized umbilical vein) and slow or reversed (hepatofugal) flow.
- 3.
- Hepatofugal (Retrograde) Flow: Hepatofugal flow happens when the pressure in the portal vein exceeds that of the liver, causing flow to reverse and appear below the baseline. This is another indicator of portal hypertension, which can be caused by various conditions, including cirrhosis, right-sided heart failure and other portal vein obstructions [107] (Figure 9).Figure 9. (A) Increased pulsatility due to arteriovenous shunting in a case of hereditary haemorrhagic telangiectasia; (B) Reduced Portal Flow in a case of cirrhosis (C) Hepatofugal Flow of Portal Vein is a late sign of Portal Hypertension. It happens when the pressure in the portal vein exceeds that of the liver, causing flow to reverse and appear below the baseline. This is another indicator of portal hypertension, which can be caused by various conditions, including cirrhosis, right-sided heart failure and other portal vein obstructions.Figure 9. (A) Increased pulsatility due to arteriovenous shunting in a case of hereditary haemorrhagic telangiectasia; (B) Reduced Portal Flow in a case of cirrhosis (C) Hepatofugal Flow of Portal Vein is a late sign of Portal Hypertension. It happens when the pressure in the portal vein exceeds that of the liver, causing flow to reverse and appear below the baseline. This is another indicator of portal hypertension, which can be caused by various conditions, including cirrhosis, right-sided heart failure and other portal vein obstructions.
- 4.
- Absent (Aphasic) Portal Venous Flow:
Hepatic Veins
- The a wave: Caused by increased RAP during atrial contraction, this upward wave peaks with maximal retrograde flow. It is wider and taller than the v wave in normal states.
- The S wave: Generated by a decrease in RAP during systole, this downward wave represents antegrade flow. It reaches its lowest point at midsystole and is the largest downward wave in the cycle.
- The v wave: This upward wave is produced by increased RAP due to systemic venous return. Its peak marks the transition from systole to diastole, and the wave slopes downward as pressure is relieved during early diastolic right ventricular filling.
3.1.3. Synopsis of the Study of Splanchnic System Congestion: Venous Excess Ultrasound Score (VExUS) and Extended VExUS
- 1.
- Screening:
- 2.
- Grading
- Hepatic Vein Doppler: In normal conditions, hepatic vein (HV) flow is pulsatile, corresponding to the RAP waveform. Pathologies like right ventricular dysfunction or tricuspid regurgitation can alter HV waveforms, and increasing RAP can reduce venous return during systole, leading to distinct changes in the waveform.
- Portal Vein Doppler: Normal portal vein flow is continuous, but severe venous congestion can cause pulsatility in the portal circulation. The pulsatility fraction (PVPF: [(Vmax − Vmin)/Vmax] × 100) quantifies this, with values above 30% indicating mild abnormalities and above 50% suggesting severe congestion [57]. Elevated PVPF is a strong predictor of acute kidney injury in post-cardiac surgery patients.
- Intra-Renal Vein Doppler: Similar to the portal vein, intra-renal veins show continuous flow under normal conditions, but congestion leads to a pulsatile pattern. This can manifest as a biphasic pattern in moderate congestion and a monophasic pattern in severe cases [114]. Altered intra-renal flow is associated with poor outcomes in heart failure and pulmonary hypertension patients [56,114].
- Grading Venous Congestion: Venous congestion is categorized into grades 0–3 based on waveform alterations, a system known as VExUS (Venous Excess Ultrasound Score) (Figure 14) [63]. This grading system provides a practical method for assessing the severity of venous congestion in clinical settings (Figure 15).
Extended Venous Ultrasound (eVExUS) as a Complementary Haemodynamic Paradigm
3.1.4. Arterial Hypovascularization
B-Mode Renal Ultrasound and Renal Artery
Color-Doppler: Renal Resistive Index (RRI)
Clinical Use of Conventional US in Splanchnic Evaluation in Heart Failure
The Importance of VExUS Grading and Clinical Interpretation Is Founded from Diagnosis to Management
Clinical Scenarios
3.2. Cardiac Evaluation: Echocardiography
3.2.1. The Evolving Role of Echocardiography of Left Heart in Heart Failure
3.2.2. The Evolving Role of Echocardiography in Heart Failure: A Focus on the Right Heart and Pulmonary Hypertension (PH)
3.2.3. Echocardiography in Advanced Heart Failure
3.3. Lung Ultrasound and Splanchnic Circulation in Heart Failure
3.3.1. Methodological Principles and Protocols
3.3.2. LUS in the Acute Heart Failure Setting
Diagnostic Application
Monitoring Therapeutic Efficacy
Prognostic Stratification
3.3.3. LUS in the Chronic Ambulatory Heart Failure Setting
3.3.4. LUS During Stress Echocardiography
3.4. Guiding Decongestive Therapy: The Role of Serial Ultrasound Assessment
3.4.1. Establishing a Baseline and Informing the Decongestive Prescription
3.4.2. Monitoring Efficacy and Guiding Titration
3.4.3. The Pragmatic Cycle: A Bedside Workflow for Ultrasound-Guided Decongestion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen-Izu, Y.; Banyasz, T.; Shaw, J.A.; Izu, L.T. The Heart is a Smart Pump: Mechanotransduction Mechanisms of the Frank-Starling Law and the Anrep Effect. Annu. Rev. Physiol. 2024, 87, 53–77. [Google Scholar] [CrossRef]
- Swenne, C.A.; Shusterman, V. Neurocardiology: Major mechanisms and effects. J. Electrocardiol. 2024, 88, 153836. [Google Scholar] [CrossRef]
- Manolis, A.A.; Manolis, T.A.; Manolis, A.S. Neurohumoral Activation in Heart Failure. Int. J. Mol. Sci. 2023, 24, 15472. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.W.; Piper, H.; Starling, E.H. The regulation of the heart beat. J. Physiol. 1914, 48, 465–513. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.G.; Hanck, D.A.; Jewell, B.R. The Anrep effect: An intrinsic myocardial mechanism. Can. J. Physiol. Pharmacol. 1988, 66, 924–929. [Google Scholar] [CrossRef]
- Hornby-Foster, I.; Richards, C.T.; Drane, A.L.; Lodge, F.M.; Stembridge, M.; Lord, R.N.; Davey, H.; Yousef, Z.; Pugh, C.J.A. Resistance- and endurance-trained young men display comparable carotid artery strain parameters that are superior to untrained men. Eur. J. Appl. Physiol. 2024, 125, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Herring, N.; Paterson, D. Haemodynamics: Flow, pressure and resistance. In Levick’s Introduction to Cardiovascular Physiology; Herring, N., Paterson, D., Eds.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- O’Rourke, M.F.; Hashimoto, J. Mechanical factors in arterial aging: A clinical perspective. J. Am. Coll. Cardiol. 2007, 50, 1–13. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.; Feletou, M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009, 196, 193–222. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Vascular remodeling in hypertension: Mechanisms and treatment. Hypertension 2012, 59, 367–374. [Google Scholar] [CrossRef]
- Shirwany, N.A.; Zou, M.H. Arterial stiffness: A brief review. Acta Pharmacol. Sin. 2010, 31, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F. Arterial stiffness and hypertension. Hypertension 2014, 64, 13–18. [Google Scholar] [CrossRef]
- Guyton, A.C.; Polizo, D.; Armstrong, G.G. Mean circulatory filling pressure measured immediately after cessation of heart pumping. Am. J. Physiol. 1954, 179, 261–267. [Google Scholar] [CrossRef]
- Versprille, A.; Jansen, J.R. Mean systemic filling pressure as a characteristic pressure for venous return. Pflugers Arch. 1985, 405, 226–233. [Google Scholar] [CrossRef]
- Guyton, A.C.; Lindsey, A.W.; Abernathy, B.; Richardson, T. Venous return at various right atrial pressures and the normal venous return curve. Am. J. Physiol. 1957, 189, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Aya, H.D.; Cecconi, M. Mean Systemic Filling Pressure Is an Old Concept but a New Tool for Fluid Management. In Perioperative Fluid Management; Springer: Berlin/Heidelberg, Germany, 2016; pp. 171–188. [Google Scholar]
- Spiegel, R. Stressed vs. unstressed volume and its relevance to critical care practitioners. Clin. Exp. Emerg. Med. 2016, 3, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, J.D.; Boyle, C.M.; Schwartz, L.B. The mesenteric circulation. Anatomy and physiology. Surg. Clin. N. Am. 1997, 77, 289–306. [Google Scholar] [CrossRef]
- Netter, F.H. Overview of lower digestive tract. In Netter Collection of Medical Illustrations: Digestive System, Part II—Lower Digestive Tract; Elsevier, Inc.: Amsterdam, The Netherlands, 2025; Volume II, pp. 1–30. [Google Scholar]
- Yaku, H.; Fudim, M.; Shah, S.J. Role of splanchnic circulation in the pathogenesis of heart failure: State-of-the-art review. J. Cardiol. 2024, 83, 330–337. [Google Scholar] [CrossRef]
- Rutlen, D.L.; Supple, E.W.; Powell, W.J., Jr. The role of the liver in the adrenergic regulation of blood flow from the splanchnic to the central circulation. Yale J. Biol. Med. 1979, 52, 99–106. [Google Scholar]
- Parks, D.A.; Jacobson, E.D. Physiology of the splanchnic circulation. Arch. Intern. Med. 1985, 145, 1278–1281. [Google Scholar] [CrossRef]
- Mocan, D.; Lala, R.I.; Puschita, M.; Pilat, L.; Darabantiu, D.A.; Pop Moldovan, A. The congestion “pandemic” in acute heart failure patients. Biomedicines 2024, 12, 951. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Congestion/decongestion in heart failure: What does it mean, how do we assess it, and what are we missing?-is there utility in measuring volume? Heart Fail. Rev. 2024, 29, 1187–1199. [Google Scholar] [CrossRef]
- Rubio-Gracia, J.; Demissei, B.G.; Ter Maaten, J.M.; Cleland, J.G.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int. J. Cardiol. 2018, 258, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lala, A.; McNulty, S.E.; Mentz, R.J.; Dunlay, S.M.; Vader, J.M.; AbouEzzeddine, O.F.; DeVore, A.D.; Khazanie, P.; Redfield, M.M.; Goldsmith, S.R.; et al. Relief and Recurrence of Congestion During and After Hospitalization for Acute Heart Failure: Insights From Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ. Heart Fail. 2015, 8, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Drazner, M.H.; Hellkamp, A.S.; Leier, C.V.; Shah, M.R.; Miller, L.W.; Russell, S.D.; Young, J.B.; Califf, R.M.; Nohria, A. Value of clinician assessment of hemodynamics in advanced heart failure: The ESCAPE trial. Circ. Heart Fail. 2008, 1, 170–177. [Google Scholar] [CrossRef]
- Narang, N.; Chung, B.; Nguyen, A.; Kalathiya, R.J.; Laffin, L.J.; Holzhauser, L.; Ebong, I.A.; Besser, S.A.; Imamura, T.; Smith, B.A.; et al. Discordance Between Clinical Assessment and Invasive Hemodynamics in Patients With Advanced Heart Failure. J. Card. Fail. 2020, 26, 128–135. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Ostrominski, J.W.; DeFilippis, E.M.; Bansal, K.; Riello, R.J., 3rd; Bozkurt, B.; Heidenreich, P.A.; Vaduganathan, M. Contemporary American and European Guidelines for Heart Failure Management: JACC: Heart Failure Guideline Comparison. JACC Heart Fail. 2024, 12, 810–825. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Bohm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2024, 26, 5–17. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, M.; Chen, T.; Zhou, Y. Correlation Between Liver Stiffness and Diastolic Function, Left Ventricular Hypertrophy, and Right Cardiac Function in Patients With Ejection Fraction Preserved Heart Failure. Front. Cardiovasc. Med. 2021, 8, 748173. [Google Scholar] [CrossRef] [PubMed]
- Samsky, M.D.; Patel, C.B.; DeWald, T.A.; Smith, A.D.; Felker, G.M.; Rogers, J.G.; Hernandez, A.F. Cardiohepatic interactions in heart failure: An overview and clinical implications. J. Am. Coll. Cardiol. 2013, 61, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschope, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 391–412. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef]
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr. Physiol. 2015, 6, 187–214. [Google Scholar] [CrossRef]
- Fudim, M.; Neuzil, P.; Malek, F.; Engelman, Z.J.; Reddy, V.Y. Greater Splanchnic Nerve Stimulation in Heart Failure With Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 1952–1953. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, 1810–1852. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Bauersachs, J.; Brugts, J.J.; Ezekowitz, J.A.; Lam, C.S.P.; Lund, L.H.; Ponikowski, P.; Voors, A.A.; Zannad, F.; Zieroth, S.; et al. Worsening Heart Failure: Nomenclature, Epidemiology, and Future Directions: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 81, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Jimenez, S.; Garcia Sebastian, C.; Vela Martin, P.; Garcia Magallon, B.; Martin Centellas, A.; de Castro, D.; Mitroi, C.; Del Prado Diaz, S.; Hernandez-Perez, F.J.; Jimenez-Blanco Bravo, M.; et al. Prevalence and prognostic impact of subclinical venous congestion in patients hospitalized for acute heart failure. Eur. Heart J. Acute Cardiovasc. Care 2025, zuaf097. [Google Scholar] [CrossRef] [PubMed]
- Saadi, M.P.; Silvano, G.P.; Machado, G.P.; Almeida, R.F.; Scolari, F.L.; Biolo, A.; Aboumarie, H.S.; Telo, G.H.; Donelli da Silveira, A. Modified VExUS: A Dynamic Tool to Predict Mortality in Acute Decompensated Heart Failure. J. Am. Soc. Echocardiogr. 2025, in press. [Google Scholar] [CrossRef]
- Gamarra, A.; Salamanca, J.; Diez-Villanueva, P.; Cuenca, S.; Vazquez, J.; Aguilar, R.J.; Diego, G.; Rodriguez, A.P.; Alfonso, F. Ultrasound imaging of congestion in heart failure: A narrative review. Cardiovasc. Diagn. Ther. 2025, 15, 233–250. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.U.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.A.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 13 April 2025).
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Fede, G.; Privitera, G.; Tomaselli, T.; Spadaro, L.; Purrello, F. Cardiovascular dysfunction in patients with liver cirrhosis. Ann. Gastroenterol. 2015, 28, 31–40. [Google Scholar]
- Mukhtar, A.; Dabbous, H. Modulation of splanchnic circulation: Role in perioperative management of liver transplant patients. World J. Gastroenterol. 2016, 22, 1582–1592. [Google Scholar] [CrossRef]
- Moriyasu, F.; Nishida, O.; Ban, N.; Nakamura, T.; Sakai, M.; Miyake, T.; Uchino, H. “Congestion index” of the portal vein. AJR Am. J. Roentgenol. 1986, 146, 735–739. [Google Scholar] [CrossRef]
- Wells, M.L.; Venkatesh, S.K. Congestive hepatopathy. Abdom. Radiol. 2018, 43, 2037–2051. [Google Scholar] [CrossRef]
- Iida, N.; Seo, Y.; Sai, S.; Machino-Ohtsuka, T.; Yamamoto, M.; Ishizu, T.; Kawakami, Y.; Aonuma, K. Clinical Implications of Intrarenal Hemodynamic Evaluation by Doppler Ultrasonography in Heart Failure. JACC Heart Fail. 2016, 4, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Beaubien-Souligny, W.; Benkreira, A.; Robillard, P.; Bouabdallaoui, N.; Chasse, M.; Desjardins, G.; Lamarche, Y.; White, M.; Bouchard, J.; Denault, A. Alterations in Portal Vein Flow and Intrarenal Venous Flow Are Associated With Acute Kidney Injury After Cardiac Surgery: A Prospective Observational Cohort Study. J. Am. Heart Assoc. 2018, 7, e009961. [Google Scholar] [CrossRef]
- Kuwahara, N.; Honjo, T.; Sone, N.; Imanishi, J.; Nakayama, K.; Kamemura, K.; Iwahashi, M.; Ohta, S.; Kaihotsu, K. Clinical impact of portal vein pulsatility on the prognosis of hospitalized patients with acute heart failure. World J. Cardiol. 2023, 15, 599–608. [Google Scholar] [CrossRef]
- McNaughton, D.A.; Abu-Yousef, M.M. Doppler US of the liver made simple. Radiographics 2011, 31, 161–188. [Google Scholar] [CrossRef]
- Pivetta, E.; Goffi, A.; Lupia, E.; Tizzani, M.; Porrino, G.; Ferreri, E.; Volpicelli, G.; Balzaretti, P.; Banderali, A.; Iacobucci, A.; et al. Lung Ultrasound-Implemented Diagnosis of Acute Decompensated Heart Failure in the ED: A SIMEU Multicenter Study. Chest 2015, 148, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Ishii, S.; Fujita, T.; Iida, Y.; Kaida, T.; Nabeta, T.; Maekawa, E.; Yanagisawa, T.; Koitabashi, T.; Takeuchi, I.; et al. Prognostic impact of intestinal wall thickening in hospitalized patients with heart failure. Int. J. Cardiol. 2017, 230, 120–126. [Google Scholar] [CrossRef]
- Platz, E.; Lewis, E.F.; Uno, H.; Peck, J.; Pivetta, E.; Merz, A.A.; Hempel, D.; Wilson, C.; Frasure, S.E.; Jhund, P.S.; et al. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur. Heart J. 2016, 37, 1244–1251. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- Prowle, J.R.; Bellomo, R. Fluid administration and the kidney. Curr. Opin. Crit. Care 2013, 19, 308–314. [Google Scholar] [CrossRef]
- Yoshihisa, A.; Ishibashi, S.; Matsuda, M.; Yamadera, Y.; Ichijo, Y.; Sato, Y.; Yokokawa, T.; Misaka, T.; Oikawa, M.; Kobayashi, A.; et al. Clinical Implications of Hepatic Hemodynamic Evaluation by Abdominal Ultrasonographic Imaging in Patients With Heart Failure. J. Am. Heart Assoc. 2020, 9, e016689. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Beaubien-Souligny, W.; Oussaid, E.; Henri, C.; Racine, N.; Denault, A.Y.; Rouleau, J.L. Assessing Splanchnic Compartment Using Portal Venous Doppler and Impact of Adding It to the EVEREST Score for Risk Assessment in Heart Failure. CJC Open 2020, 2, 311–320. [Google Scholar] [CrossRef]
- Hao, R.; Zheng, Y.; Zhao, Q.; Chen, J.; Fan, R.; Chen, P.; Yin, N.; Qin, H. Evaluation value of ultrasound on gastrointestinal function in patients with acute heart failure. Front. Cardiovasc. Med. 2024, 11, 1475920. [Google Scholar] [CrossRef]
- Goncalvesova, E.; Lesny, P.; Luknar, M.; Solik, P.; Varga, I. Changes of portal flow in heart failure patients with liver congestion. Bratisl. Lek. Listy 2010, 111, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.; Vikneswaran, G.; Rola, P.; Raju, S.; Bhat, R.S.; Jayakumar, A.; Alva, A. Combination of Inferior Vena Cava Diameter, Hepatic Venous Flow, and Portal Vein Pulsatility Index: Venous Excess Ultrasound Score (VEXUS Score) in Predicting Acute Kidney Injury in Patients with Cardiorenal Syndrome: A Prospective Cohort Study. Indian J. Crit. Care Med. 2020, 24, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Gargani, L.; Pang, P.S.; Frassi, F.; Miglioranza, M.H.; Dini, F.L.; Landi, P.; Picano, E. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: A lung ultrasound study. Cardiovasc. Ultrasound 2015, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Ahmad, M.; Soloway, R.D. Duplex Doppler ultrasound examination of the portal venous system: An emerging novel technique for the estimation of portal vein pressure. Dig. Dis. Sci. 2010, 55, 1230–1240. [Google Scholar] [CrossRef]
- Rengo, C.; Brevetti, G.; Sorrentino, G.; D’Amato, T.; Imparato, M.; Vitale, D.F.; Acanfora, D.; Rengo, F. Portal vein pulsatility ratio provides a measure of right heart function in chronic heart failure. Ultrasound Med. Biol. 1998, 24, 327–332. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Iacoviello, M.; Gesualdo, L.; Puzzovivo, A.; Antoncecchi, V.; Doronzo, A.; Monitillo, F.; Citarelli, G.; Paradies, V.; Favale, S. The renal arterial resistance index: A marker of renal function with an independent and incremental role in predicting heart failure progression. Eur. J. Heart Fail. 2014, 16, 210–216. [Google Scholar] [CrossRef]
- Argaiz, E.R. VExUS Nexus: Bedside Assessment of Venous Congestion. Adv. Chronic Kidney Dis. 2021, 28, 252–261. [Google Scholar] [CrossRef]
- Denault, A.; Canty, D.; Azzam, M.; Amir, A.; Gebhard, C.E. Whole body ultrasound in the operating room and intensive care unit. Korean J. Anesthesiol. 2019, 72, 413–428. [Google Scholar] [CrossRef]
- Valentova, M.; von Haehling, S.; Bauditz, J.; Doehner, W.; Ebner, N.; Bekfani, T.; Elsner, S.; Sliziuk, V.; Scherbakov, N.; Murin, J.; et al. Intestinal congestion and right ventricular dysfunction: A link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur. Heart J. 2016, 37, 1684–1691. [Google Scholar] [CrossRef]
- Sandek, A.; Swidsinski, A.; Schroedl, W.; Watson, A.; Valentova, M.; Herrmann, R.; Scherbakov, N.; Cramer, L.; Rauchhaus, M.; Grosse-Herrenthey, A.; et al. Intestinal blood flow in patients with chronic heart failure: A link with bacterial growth, gastrointestinal symptoms, and cachexia. J. Am. Coll. Cardiol. 2014, 64, 1092–1102. [Google Scholar] [CrossRef]
- Pellicori, P.; Zhang, J.; Cuthbert, J.; Urbinati, A.; Shah, P.; Kazmi, S.; Clark, A.L.; Cleland, J.G.F. High-sensitivity C-reactive protein in chronic heart failure: Patient characteristics, phenotypes, and mode of death. Cardiovasc. Res. 2020, 116, 91–100. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ishii, S.; Maemura, K.; Oki, T.; Yazaki, M.; Fujita, T.; Nabeta, T.; Maekawa, E.; Koitabashi, T.; Ako, J. Association between intestinal oedema and oral loop diuretic resistance in hospitalized patients with acute heart failure. ESC Heart Fail. 2021, 8, 4067–4076. [Google Scholar] [CrossRef]
- Ciozda, W.; Kedan, I.; Kehl, D.W.; Zimmer, R.; Khandwalla, R.; Kimchi, A. The efficacy of sonographic measurement of inferior vena cava diameter as an estimate of central venous pressure. Cardiovasc. Ultrasound 2016, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, G. Inferior Vena Cava Collapsibility Index is a Valuable and Non-Invasive Index for Elevated General Heart End-Diastolic Volume Index Estimation in Septic Shock Patients. Med. Sci. Monit. 2016, 22, 3843–3848. [Google Scholar] [CrossRef] [PubMed]
- Aspromonte, N.; Fumarulo, I.; Petrucci, L.; Biferali, B.; Liguori, A.; Gasbarrini, A.; Massetti, M.; Miele, L. The Liver in Heart Failure: From Biomarkers to Clinical Risk. Int. J. Mol. Sci. 2023, 24, 15665. [Google Scholar] [CrossRef]
- Wells, M.L.; Fenstad, E.R.; Poterucha, J.T.; Hough, D.M.; Young, P.M.; Araoz, P.A.; Ehman, R.L.; Venkatesh, S.K. Imaging Findings of Congestive Hepatopathy. Radiographics 2016, 36, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Yokosuka, O. Ultrasonography for Noninvasive Assessment of Portal Hypertension. Gut Liver 2017, 11, 464–473. [Google Scholar] [CrossRef]
- Gerstenmaier, J.F.; Gibson, R.N. Ultrasound in chronic liver disease. Insights Imaging 2014, 5, 441–455. [Google Scholar] [CrossRef]
- Weinreb, J.; Kumari, S.; Phillips, G.; Pochaczevsky, R. Portal vein measurements by real-time sonography. AJR Am. J. Roentgenol. 1982, 139, 497–499. [Google Scholar] [CrossRef]
- Procopet, B.; Berzigotti, A. Diagnosis of cirrhosis and portal hypertension: Imaging, non-invasive markers of fibrosis and liver biopsy. Gastroenterol. Rep. 2017, 5, 79–89. [Google Scholar] [CrossRef]
- Allen, L.A.; Felker, G.M.; Pocock, S.; McMurray, J.J.; Pfeffer, M.A.; Swedberg, K.; Wang, D.; Yusuf, S.; Michelson, E.L.; Granger, C.B.; et al. Liver function abnormalities and outcome in patients with chronic heart failure: Data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur. J. Heart Fail. 2009, 11, 170–177. [Google Scholar] [CrossRef]
- Brankovic, M.; Lee, P.; Pyrsopoulos, N.; Klapholz, M. Cardiac Syndromes in Liver Disease: A Clinical Conundrum. J. Clin. Transl. Hepatol. 2023, 11, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.P.; Mullens, W.; Banaszewski, M.; Bauersachs, J.; Brunner-La Rocca, H.P.; Chioncel, O.; Collins, S.P.; Doehner, W.; Filippatos, G.S.; Flammer, A.J.; et al. Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2017, 19, 821–836. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Feng, G.; Tian, X. Association Between the Albumin-Bilirubin (ALBI) Score and All-cause Mortality Risk in Intensive Care Unit Patients with Heart Failure. Glob. Heart 2024, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- Tessler, F.N.; Gehring, B.J.; Gomes, A.S.; Perrella, R.R.; Ragavendra, N.; Busuttil, R.W.; Grant, E.G. Diagnosis of portal vein thrombosis: Value of color Doppler imaging. AJR Am. J. Roentgenol. 1991, 157, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, T.; Arisawa, J.; Marukawa, T.; Tokunaga, K.; Kuroda, C.; Kozuka, T.; Nakano, S. Portal blood flow in congestive heart failure: Pulsed duplex sonographic findings. Radiology 1990, 174, 733–736. [Google Scholar] [CrossRef]
- Nelson, R.C.; Lovett, K.E.; Chezmar, J.L.; Moyers, J.H.; Torres, W.E.; Murphy, F.B.; Bernardino, M.E. Comparison of pulsed Doppler sonography and angiography in patients with portal hypertension. AJR Am. J. Roentgenol. 1987, 149, 77–81. [Google Scholar] [CrossRef]
- Gaiani, S.; Bolondi, L.; Li Bassi, S.; Santi, V.; Zironi, G.; Barbara, L. Effect of meal on portal hemodynamics in healthy humans and in patients with chronic liver disease. Hepatology 1989, 9, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, B.R.; Balthazar, E.J.; Madamba, M.R.; Raghavendra, B.N.; Horii, S.C.; Lefleur, R.S. Sonography of portosystemic venous collaterals in portal hypertension. Radiology 1983, 146, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.N.; Gibson, P.R.; Donlan, J.D.; Clunie, D.A. Identification of a patent paraumbilical vein by using Doppler sonography: Importance in the diagnosis of portal hypertension. AJR Am. J. Roentgenol. 1989, 153, 513–516. [Google Scholar] [CrossRef]
- Iranpour, P.; Lall, C.; Houshyar, R.; Helmy, M.; Yang, A.; Choi, J.I.; Ward, G.; Goodwin, S.C. Altered Doppler flow patterns in cirrhosis patients: An overview. Ultrasonography 2016, 35, 3–12. [Google Scholar] [CrossRef]
- Afif, A.M.; Chang, J.P.; Wang, Y.Y.; Lau, S.D.; Deng, F.; Goh, S.Y.; Pwint, M.K.; Ooi, C.C.; Venkatanarasimha, N.; Lo, R.H. A sonographic Doppler study of the hepatic vein, portal vein and hepatic artery in liver cirrhosis: Correlation of hepatic hemodynamics with clinical Child Pugh score in Singapore. Ultrasound 2017, 25, 213–221. [Google Scholar] [CrossRef]
- Baikpour, M.; Ozturk, A.; Dhyani, M.; Mercaldo, N.D.; Pierce, T.T.; Grajo, J.R.; Samir, A.E. Portal Venous Pulsatility Index: A Novel Biomarker for Diagnosis of High-Risk Nonalcoholic Fatty Liver Disease. AJR Am. J. Roentgenol. 2020, 214, 786–791. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, J.; Duan, Y.; Yang, Y.; Yuan, L.; Cao, T. Assessment of intrahepatic blood flow by Doppler ultrasonography: Relationship between the hepatic vein, portal vein, hepatic artery and portal pressure measured intraoperatively in patients with portal hypertension. BMC Gastroenterol. 2011, 11, 84. [Google Scholar] [CrossRef]
- Abu-Yousef, M.M.; Milam, S.G.; Farner, R.M. Pulsatile portal vein flow: A sign of tricuspid regurgitation on duplex Doppler sonography. AJR Am. J. Roentgenol. 1990, 155, 785–788. [Google Scholar] [CrossRef]
- Gallix, B.P.; Taourel, P.; Dauzat, M.; Bruel, J.M.; Lafortune, M. Flow pulsatility in the portal venous system: A study of Doppler sonography in healthy adults. AJR Am. J. Roentgenol. 1997, 169, 141–144. [Google Scholar] [CrossRef]
- Caselitz, M.; Bahr, M.J.; Bleck, J.S.; Chavan, A.; Manns, M.P.; Wagner, S.; Gebel, M. Sonographic criteria for the diagnosis of hepatic involvement in hereditary hemorrhagic telangiectasia (HHT). Hepatology 2003, 37, 1139–1146. [Google Scholar] [CrossRef]
- Abou-Arab, O.; Beyls, C.; Moussa, M.D.; Huette, P.; Beaudelot, E.; Guilbart, M.; De Broca, B.; Yzet, T.; Dupont, H.; Bouzerar, R.; et al. Portal Vein Pulsatility Index as a Potential Risk of Venous Congestion Assessed by Magnetic Resonance Imaging: A Prospective Study on Healthy Volunteers. Front. Physiol. 2022, 13, 811286. [Google Scholar] [CrossRef]
- Gorg, C.; Seifart, U.; Zugmaier, G. Color Doppler sonographic signs of respiration-dependent hepatofugal portal flow. J. Clin. Ultrasound 2004, 32, 62–68. [Google Scholar] [CrossRef]
- Hidajat, N.; Stobbe, H.; Griesshaber, V.; Felix, R.; Schroder, R.J. Imaging and radiological interventions of portal vein thrombosis. Acta Radiol. 2005, 46, 336–343. [Google Scholar] [CrossRef]
- Rossi, S.; Ghittoni, G.; Ravetta, V.; Torello Viera, F.; Rosa, L.; Serassi, M.; Scabini, M.; Vercelli, A.; Tinelli, C.; Dal Bello, B.; et al. Contrast-enhanced ultrasonography and spiral computed tomography in the detection and characterization of portal vein thrombosis complicating hepatocellular carcinoma. Eur. Radiol. 2008, 18, 1749–1756. [Google Scholar] [CrossRef]
- Altinkaya, N.; Koc, Z.; Ulusan, S.; Demir, S.; Gurel, K. Effects of respiratory manoeuvres on hepatic vein Doppler waveform and flow velocities in a healthy population. Eur. J. Radiol. 2011, 79, 60–63. [Google Scholar] [CrossRef]
- Piscaglia, F.; Donati, G.; Serra, C.; Muratori, R.; Solmi, L.; Gaiani, S.; Gramantieri, L.; Bolondi, L. Value of splanchnic Doppler ultrasound in the diagnosis of portal hypertension. Ultrasound Med. Biol. 2001, 27, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Hirsch, M.; Schneider, D.; Gonzalez, D. Congestive hepatopathy: The role of the radiologist in the diagnosis. Diagn. Interv. Radiol. 2020, 26, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Catalano, D.; Caruso, G.; DiFazzio, S.; Carpinteri, G.; Scalisi, N.; Trovato, G.M. Portal vein pulsatility ratio and heart failure. J. Clin. Ultrasound 1998, 26, 27–31. [Google Scholar] [CrossRef]
- Husain-Syed, F.; Birk, H.W.; Ronco, C.; Schormann, T.; Tello, K.; Richter, M.J.; Wilhelm, J.; Sommer, N.; Steyerberg, E.; Bauer, P.; et al. Doppler-Derived Renal Venous Stasis Index in the Prognosis of Right Heart Failure. J. Am. Heart Assoc. 2019, 8, e013584. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ishii, S.; Yazaki, M.; Fujita, T.; Iida, Y.; Kaida, T.; Nabeta, T.; Nakatani, E.; Maekawa, E.; Yanagisawa, T.; et al. Portal congestion and intestinal edema in hospitalized patients with heart failure. Heart Vessel. 2018, 33, 740–751. [Google Scholar] [CrossRef]
- Galindo, P.; Gasca, C.; Argaiz, E.R.; Koratala, A. Point of care venous Doppler ultrasound: Exploring the missing piece of bedside hemodynamic assessment. World J. Crit. Care Med. 2021, 10, 310–322. [Google Scholar] [CrossRef]
- Rola, P.; Miralles-Aguiar, F.; Argaiz, E.; Beaubien-Souligny, W.; Haycock, K.; Karimov, T.; Dinh, V.A.; Spiegel, R. Clinical applications of the venous excess ultrasound (VExUS) score: Conceptual review and case series. Ultrasound J. 2021, 13, 32. [Google Scholar] [CrossRef]
- Turk, M.; Koratala, A.; Robertson, T.; Kalagara, H.K.P.; Bronshteyn, Y.S. Demystifying Venous Excess Ultrasound (VExUS): Image Acquisition and Interpretation. J. Vis. Exp. JoVE 2025, 219, e68107. [Google Scholar] [CrossRef] [PubMed]
- Istrail, L.; Kiernan, J.; Stepanova, M. A Novel Method for Estimating Right Atrial Pressure With Point-of-Care Ultrasound. J. Am. Soc. Echocardiogr. 2023, 36, 278–283. [Google Scholar] [CrossRef]
- Deschamps, J.; Denault, A.; Galarza, L.; Rola, P.; Ledoux-Hutchinson, L.; Huard, K.; Gebhard, C.E.; Calderone, A.; Canty, D.; Beaubien-Souligny, W. Venous Doppler to Assess Congestion: A Comprehensive Review of Current Evidence and Nomenclature. Ultrasound Med. Biol. 2023, 49, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Goldhammer, E.; Mesnick, N.; Abinader, E.G.; Sagiv, M. Dilated inferior vena cava: A common echocardiographic finding in highly trained elite athletes. J. Am. Soc. Echocardiogr. 1999, 12, 988–993. [Google Scholar] [CrossRef]
- Vivier, E.; Metton, O.; Piriou, V.; Lhuillier, F.; Cottet-Emard, J.M.; Branche, P.; Duperret, S.; Viale, J.P. Effects of increased intra-abdominal pressure on central circulation. Br. J. Anaesth. 2006, 96, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Aznarez, S.; Campos-Saenz de Santamaria, A.; Sanchez-Marteles, M.; Garces-Horna, V.; Josa-Laorden, C.; Gimenez-Lopez, I.; Perez-Calvo, J.I.; Rubio-Gracia, J. The Association Between Intra-abdominal Pressure and Diuretic Response in Heart Failure. Curr. Heart Fail. Rep. 2023, 20, 390–400. [Google Scholar] [CrossRef]
- Rora Bertovic, M.; Trkulja, V.; Curcic Karabaic, E.; Sundalic, S.; Bielen, L.; Ivicic, T.; Radonic, R. Influence of Increased Intra-Abdominal Pressure on the Validity of Ultrasound-Derived Inferior Vena Cava Measurements for Estimating Central Venous Pressure. J. Clin. Med. 2025, 14, 3684. [Google Scholar] [CrossRef]
- Davison, R.; Cannon, R. Estimation of central venous pressure by examination of jugular veins. Am. Heart J. 1974, 87, 279–282. [Google Scholar] [CrossRef]
- Chayapinun, V.; Koratala, A.; Assavapokee, T. Seeing beneath the surface: Harnessing point-of-care ultrasound for internal jugular vein evaluation. World J. Cardiol. 2024, 16, 73–79. [Google Scholar] [CrossRef]
- Leal-Villarreal, M.A.J.; Aguirre-Villarreal, D.; Vidal-Mayo, J.J.; Argaiz, E.R.; Garcia-Juarez, I. Correlation of Internal Jugular Vein Collapsibility With Central Venous Pressure in Patients With Liver Cirrhosis. Am. J. Gastroenterol. 2023, 118, 1684–1687. [Google Scholar] [CrossRef]
- Bauman, Z.; Coba, V.; Gassner, M.; Amponsah, D.; Gallien, J.; Blyden, D.; Killu, K. Inferior vena cava collapsibility loses correlation with internal jugular vein collapsibility during increased thoracic or intra-abdominal pressure. J. Ultrasound 2015, 18, 343–348. [Google Scholar] [CrossRef]
- Benkreira, A.; Beaubien-Souligny, W.; Mailhot, T.; Bouabdallaoui, N.; Robillard, P.; Desjardins, G.; Lamarche, Y.; Cossette, S.; Denault, A. Portal Hypertension Is Associated With Congestive Encephalopathy and Delirium After Cardiac Surgery. Can. J. Cardiol. 2019, 35, 1134–1141. [Google Scholar] [CrossRef]
- Croquette, M.; Puyade, M.; Montani, D.; Jutant, E.M.; De Gea, M.; Laneelle, D.; Thollot, C.; Trihan, J.E. Diagnostic Performance of Pulsed Doppler Ultrasound of the Common Femoral Vein to Detect Elevated Right Atrial Pressure in Pulmonary Hypertension. J. Cardiovasc. Transl. Res. 2023, 16, 141–151. [Google Scholar] [CrossRef]
- Croquette, M.; Larrieu Ardilouze, E.; Beaufort, C.; Jutant, E.M.; Puyade, M.; Montani, D.; Thollot, C.; Laneelle, D.; De Gea, M.; Trihan, J.E. Femoral venous stasis index predicts elevated right atrial pressure and mortality in pulmonary hypertension. ERJ Open Res. 2025, 11, 01027-2024. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Rola, P.; Denault, A.; Vikneswaran, G.; Spiegel, R. Femoral vein pulsatility: A simple tool for venous congestion assessment. Ultrasound J. 2023, 15, 24. [Google Scholar] [CrossRef]
- Murayama, M.; Kaga, S.; Okada, K.; Iwano, H.; Nakabachi, M.; Yokoyama, S.; Nishino, H.; Tsujinaga, S.; Chiba, Y.; Ishizaka, S.; et al. Clinical Utility of Superior Vena Cava Flow Velocity Waveform Measured from the Subcostal Window for Estimating Right Atrial Pressure. J. Am. Soc. Echocardiogr. 2022, 35, 727–737. [Google Scholar] [CrossRef]
- Murayama, M.; Kaga, S.; Onoda, A.; Nishino, H.; Yokoyama, S.; Goto, M.; Suzuki, Y.; Yanagi, Y.; Shimono, Y.; Nakamura, K.; et al. Head-to-Head Comparison of Hepatic Vein and Superior Vena Cava Flow Velocity Waveform Analyses for Predicting Elevated Right Atrial Pressure. Ultrasound Med. Biol. 2024, 50, 1352–1360. [Google Scholar] [CrossRef]
- Lee, J.H.; Denault, A.Y.; Beaubien-Souligny, W.; Cho, S.A.; Ji, S.H.; Jang, Y.E.; Kim, E.H.; Kim, H.S.; Kim, J.T. Evaluation of Portal, Splenic, and Hepatic Vein Flows in Children Undergoing Congenital Heart Surgery. J. Cardiothorac. Vasc. Anesth. 2023, 37, 1456–1468. [Google Scholar] [CrossRef]
- Gonzalez, C.; Chamberland, M.E.; Aldred, M.P.; Couture, E.; Beaubien-Souligny, W.; Calderone, A.; Lamarche, Y.; Denault, A. Constrictive pericarditis: Portal, splenic, and femoral venous Doppler pulsatility: A case series. Can. J. Anaesth. J. Can. Anesth. 2022, 69, 119–128. [Google Scholar] [CrossRef]
- Martinez-Noguera, A.; Montserrat, E.; Torrubia, S.; Villalba, J. Doppler in hepatic cirrhosis and chronic hepatitis. Semin. Ultrasound CT MR 2002, 23, 19–36. [Google Scholar] [CrossRef]
- Kim, M.Y.; Baik, S.K.; Park, D.H.; Lim, D.W.; Kim, J.W.; Kim, H.S.; Kwon, S.O.; Kim, Y.J.; Chang, S.J.; Lee, S.S. Damping index of Doppler hepatic vein waveform to assess the severity of portal hypertension and response to propranolol in liver cirrhosis: A prospective nonrandomized study. Liver Int. 2007, 27, 1103–1110. [Google Scholar] [CrossRef]
- Dodd, G.D., 3rd; Memel, D.S.; Zajko, A.B.; Baron, R.L.; Santaguida, L.A. Hepatic artery stenosis and thrombosis in transplant recipients: Doppler diagnosis with resistive index and systolic acceleration time. Radiology 1994, 192, 657–661. [Google Scholar] [CrossRef]
- Lemmer, A.; VanWagner, L.; Ganger, D. Congestive hepatopathy: Differentiating congestion from fibrosis. Clin. Liver Dis. 2017, 10, 139–143. [Google Scholar] [CrossRef]
- Schneider, A.W.; Kalk, J.F.; Klein, C.P. Hepatic arterial pulsatility index in cirrhosis: Correlation with portal pressure. J. Hepatol. 1999, 30, 876–881. [Google Scholar] [CrossRef]
- Bolognesi, M.; Sacerdoti, D.; Merkel, C.; Gerunda, G.; Maffei-Faccioli, A.; Angeli, P.; Jemmolo, R.M.; Bombonato, G.; Gatta, A. Splenic Doppler impedance indices: Influence of different portal hemodynamic conditions. Hepatology 1996, 23, 1035–1040. [Google Scholar] [CrossRef]
- Bolognesi, M.; Quaglio, C.; Bombonato, G.; Gaiani, S.; Pesce, P.; Bizzotto, P.; Favaretto, E.; Gatta, A.; Sacerdoti, D. Splenic Doppler impedance indices estimate splenic congestion in patients with right-sided or congestive heart failure. Ultrasound Med. Biol. 2012, 38, 21–27. [Google Scholar] [CrossRef]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef]
- Boddi, M.; Natucci, F.; Ciani, E. The internist and the renal resistive index: Truths and doubts. Intern. Emerg. Med. 2015, 10, 893–905. [Google Scholar] [CrossRef]
- George, S.M.; Kalantarinia, K. The role of imaging in the management of cardiorenal syndrome. Int. J. Nephrol. 2011, 2011, 245241. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, W.H.; Choi, B.I.; Kim, C.W. Duplex Doppler US in patients with medical renal disease: Resistive index vs serum creatinine level. Clin. Radiol. 1992, 45, 85–87. [Google Scholar] [CrossRef]
- Radermacher, J.; Chavan, A.; Bleck, J.; Vitzthum, A.; Stoess, B.; Gebel, M.J.; Galanski, M.; Koch, K.M.; Haller, H. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N. Engl. J. Med. 2001, 344, 410–417. [Google Scholar] [CrossRef]
- Kim, H.L.; Jo, S.H. Arterial Stiffness and Heart Failure With Preserved Ejection Fraction. J. Korean Med. Sci. 2024, 39, e195. [Google Scholar] [CrossRef]
- Doyle, A.; Mark, P.B.; Johnston, N.; Foster, J.; Connell, J.M.; Dargie, H.; Jardine, A.; Padmanabhan, N. Aortic stiffness and diastolic flow abnormalities in end-stage renal disease assessed by magnetic resonance imaging. Nephron Clin. Pract. 2008, 109, c1–c8. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Townsend, R.R. Systemic arterial hemodynamics and the “renal resistive index”: What is in a name? J. Clin. Hypertens. 2014, 16, 170–171. [Google Scholar] [CrossRef]
- O’Neill, W.C. Renal resistive index: A case of mistaken identity. Hypertension 2014, 64, 915–917. [Google Scholar] [CrossRef]
- Naesens, M.; Heylen, L.; Lerut, E.; Claes, K.; De Wever, L.; Claus, F.; Oyen, R.; Kuypers, D.; Evenepoel, P.; Bammens, B.; et al. Intrarenal resistive index after renal transplantation. N. Engl. J. Med. 2013, 369, 1797–1806. [Google Scholar] [CrossRef]
- Tamayo-Gutierrez, A.; Ibrahim, H.N. The Kidney in Heart Failure: The Role of Venous Congestion. Methodist. Debakey Cardiovasc. J. 2022, 18, 4–10. [Google Scholar] [CrossRef]
- Pena, D.L.; Iliesiu, A.M.; Aurelian, J.; Grigore, M.; Hodorogea, A.S.; Ciobanu, A.; Weiss, E.; Badila, E.; Balahura, A.M. Assessment of Decongestion Status Before Discharge in Acute Decompensated Heart Failure: A Review of Clinical, Biochemical, and Imaging Tools and Their Impact on Management Decisions. Medicina 2025, 61, 816. [Google Scholar] [CrossRef]
- Boddi, M.; Bonizzoli, M.; Chiostri, M.; Begliomini, D.; Molinaro, A.; Tadini Buoninsegni, L.; Gensini, G.F.; Peris, A. Renal Resistive Index and mortality in critical patients with acute kidney injury. Eur. J. Clin. Investig. 2016, 46, 242–251. [Google Scholar] [CrossRef]
- Toledo, C.; Thomas, G.; Schold, J.D.; Arrigain, S.; Gornik, H.L.; Nally, J.V.; Navaneethan, S.D. Renal resistive index and mortality in chronic kidney disease. Hypertension 2015, 66, 382–388. [Google Scholar] [CrossRef]
- Darabont, R.; Mihalcea, D.; Vinereanu, D. Current Insights into the Significance of the Renal Resistive Index in Kidney and Cardiovascular Disease. Diagnostics 2023, 13, 1687. [Google Scholar] [CrossRef]
- Mahfoud, F.; Cremers, B.; Janker, J.; Link, B.; Vonend, O.; Ukena, C.; Linz, D.; Schmieder, R.; Rump, L.C.; Kindermann, I.; et al. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension 2012, 60, 419–424. [Google Scholar] [CrossRef]
- Tedesco, M.A.; Natale, F.; Mocerino, R.; Tassinario, G.; Calabro, R. Renal resistive index and cardiovascular organ damage in a large population of hypertensive patients. J. Hum. Hypertens. 2007, 21, 291–296. [Google Scholar] [CrossRef]
- Viazzi, F.; Leoncini, G.; Derchi, L.E.; Pontremoli, R. Ultrasound Doppler renal resistive index: A useful tool for the management of the hypertensive patient. J. Hypertens. 2014, 32, 149–153. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Redfield, M.M. Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2016, 375, 1868–1877. [Google Scholar] [CrossRef]
- Sanders-van Wijk, S.; van Empel, V.; Davarzani, N.; Maeder, M.T.; Handschin, R.; Pfisterer, M.E.; Brunner-La Rocca, H.P.; TIME-CHF investigators. Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur. J. Heart Fail. 2015, 17, 1006–1014. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Li, X.N.; Liu, Y.T.; Kang, S.; Qu Yang, D.Z.; Xiao, H.Y.; Ma, W.K.; Shen, C.X.; Pan, J.W. Interdependence between myocardial deformation and perfusion in patients with T2DM and HFpEF: A feature-tracking and stress perfusion CMR study. Cardiovasc. Diabetol. 2024, 23, 303. [Google Scholar] [CrossRef]
- Lin, M.; Guo, J.; Tao, H.; Gu, Z.; Tang, W.; Zhou, F.; Jiang, Y.; Zhang, R.; Jia, D.; Sun, Y.; et al. Circulating mediators linking cardiometabolic diseases to HFpEF: A mediation Mendelian randomization analysis. Cardiovasc. Diabetol. 2025, 24, 201. [Google Scholar] [CrossRef]
- Carluccio, E.; Biagioli, P.; Reboldi, G.; Mengoni, A.; Lauciello, R.; Zuchi, C.; D’Addario, S.; Bardelli, G.; Ambrosio, G. Left ventricular remodeling response to SGLT2 inhibitors in heart failure: An updated meta-analysis of randomized controlled studies. Cardiovasc. Diabetol. 2023, 22, 235. [Google Scholar] [CrossRef] [PubMed]
- Correale, M.; D’Alessandro, D.; Tricarico, L.; Ceci, V.; Mazzeo, P.; Capasso, R.; Ferrara, S.; Barile, M.; Di Nunno, N.; Rossi, L.; et al. Left ventricular reverse remodeling after combined ARNI and SGLT2 therapy in heart failure patients with reduced or mildly reduced ejection fraction. Int. J. Cardiol. Heart Vasc. 2024, 54, 101492. [Google Scholar] [CrossRef]
- Veltmann, C.; Duncker, D.; Doering, M.; Gummadi, S.; Robertson, M.; Wittlinger, T.; Colley, B.J.; Perings, C.; Jonsson, O.; Bauersachs, J.; et al. Therapy duration and improvement of ventricular function in de novo heart failure: The Heart Failure Optimization study. Eur. Heart J. 2024, 45, 2771–2781. [Google Scholar] [CrossRef]
- Ichimura, K.; Boehm, M.; Andruska, A.M.; Zhang, F.; Schimmel, K.; Bonham, S.; Kabiri, A.; Kheyfets, V.O.; Ichimura, S.; Reddy, S.; et al. 3D Imaging Reveals Complex Microvascular Remodeling in the Right Ventricle in Pulmonary Hypertension. Circ. Res. 2024, 135, 60–75. [Google Scholar] [CrossRef]
- Mendiola, E.A.; da Silva Goncalves Bos, D.; Leichter, D.M.; Vang, A.; Zhang, P.; Leary, O.P.; Gilbert, R.J.; Avazmohammadi, R.; Choudhary, G. Right Ventricular Architectural Remodeling and Functional Adaptation in Pulmonary Hypertension. Circ. Heart Fail. 2023, 16, e009768. [Google Scholar] [CrossRef]
- Ito, K.; Kato, S.; Yasuda, N.; Sawamura, S.; Fukui, K.; Iwasawa, T.; Ogura, T.; Utsunomiya, D. Integrating CT-Based Lung Fibrosis and MRI-Derived Right Ventricular Function for the Detection of Pulmonary Hypertension in Interstitial Lung Disease. J. Clin. Med. 2025, 14, 5329. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, D.; Wang, J.; Gong, J.; Hu, H.; Zhang, X.; Wang, Y.; Yang, Y.; Lv, X.; Li, Y. Effects of right ventricular remodeling in chronic thromboembolic pulmonary hypertension on the outcomes of balloon pulmonary angioplasty: A 2D-speckle tracking echocardiography study. Respir. Res. 2024, 25, 164. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, R.; Hayashi, O.; Horio, T.; Fujiwara, R.; Matsuoka, Y.; Yokouchi, G.; Sakamoto, Y.; Matsumoto, N.; Fukuda, K.; Shimizu, M.; et al. The E/e’ ratio on echocardiography as an independent predictor of the improvement of left ventricular contraction in patients with heart failure with reduced ejection fraction. J. Clin. Ultrasound 2023, 51, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, B.; Rose, G.A.; Stacey, R.B.; Palma, R.A.; Ryan, T.; Pendyal, A.; Kelsey, A.M. The role of echocardiography in the diagnosis of heart failure with preserved ejection fraction. Heart Fail. Rev. 2025, 30, 899–922. [Google Scholar] [CrossRef]
- Pender, A.; Lewis-Owona, J.; Ekiyoyo, A.; Stoddard, M. Echocardiography and Heart Failure: An Echocardiographic Decision Aid for the Diagnosis and Management of Cardiomyopathies. Curr. Cardiol. Rep. 2025, 27, 64. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Grigore, A.M.; Grigore, M.; Balahura, A.M.; Uscoiu, G.; Verde, I.; Nicolae, C.; Badila, E.; Iliesiu, A.M. The Role of the Estimated Plasma Volume Variation in Assessing Decongestion in Patients with Acute Decompensated Heart Failure. Biomedicines 2025, 13, 88. [Google Scholar] [CrossRef]
- Henry, J.A.; Couch, L.S.; Rider, O.J. Myocardial Metabolism in Heart Failure with Preserved Ejection Fraction. J. Clin. Med. 2024, 13, 1195. [Google Scholar] [CrossRef]
- Sun, Q.; Wagg, C.S.; Wong, N.; Wei, K.; Ketema, E.B.; Zhang, L.; Fang, L.; Seubert, J.M.; Lopaschuk, G.D. Alterations of myocardial ketone metabolism in heart failure with preserved ejection fraction (HFpEF). ESC Heart Fail. 2025, 12, 3179–3182. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Sanborn, D.Y.; Oh, J.K.; Anderson, B.; Billick, K.; Derumeaux, G.; Klein, A.; Koulogiannis, K.; Mitchell, C.; Shah, A.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography and for Heart Failure With Preserved Ejection Fraction Diagnosis: An Update From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2025, 38, 537–569. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Morris, D.A.; Cardim, N.; Cikes, M.; Delgado, V.; Donal, E.; Flachskampf, F.A.; Galderisi, M.; Gerber, B.L.; Gimelli, A.; et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e34–e61. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.P.; Mebazaa, A.; Celutkiene, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef]
- Mukherjee, M.; Rudski, L.G.; Addetia, K.; Afilalo, J.; D’Alto, M.; Freed, B.H.; Friend, L.B.; Gargani, L.; Grapsa, J.; Hassoun, P.M.; et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults and Special Considerations in Pulmonary Hypertension: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2025, 38, 141–186. [Google Scholar] [CrossRef]
- Cordina, R.L.; Playford, D.; Lang, I.; Celermajer, D.S. State-of-the-Art Review: Echocardiography in Pulmonary Hypertension. Heart Lung Circ. 2019, 28, 1351–1364. [Google Scholar] [CrossRef]
- Labrada, L.; Vaidy, A.; Vaidya, A. Right ventricular assessment in pulmonary hypertension. Curr. Opin. Pulm. Med. 2023, 29, 348–354. [Google Scholar] [CrossRef]
- Tsipis, A.; Petropoulou, E. Echocardiography in the Evaluation of the Right Heart. US Cardiol. 2022, 16, e08. [Google Scholar] [CrossRef]
- D’Alto, M.; Di Maio, M.; Romeo, E.; Argiento, P.; Blasi, E.; Di Vilio, A.; Rea, G.; D’Andrea, A.; Golino, P.; Naeije, R. Echocardiographic probability of pulmonary hypertension: A validation study. Eur. Respir. J. 2022, 60, 2102548. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure With Preserved Ejection Fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.C.; Mandoli, G.E.; Aboumarie, H.S.; Santoro, C.; Bandera, F.; D’Andrea, A.; Benfari, G.; Esposito, R.; Evola, V.; Sorrentino, R.; et al. Basic and advanced echocardiography in advanced heart failure: An overview. Heart Fail. Rev. 2020, 25, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, Y.; Chen, F. Evaluating the impact of Sacubitril/valsartan on diastolic function in patients with heart failure: A systematic review and meta-analysis. Medicine 2024, 103, e37965. [Google Scholar] [CrossRef]
- Galzerano, D.; Savo, M.T.; Castaldi, B.; Kholaif, N.; Khaliel, F.; Pozza, A.; Aljheish, S.; Cattapan, I.; Martini, M.; Lassandro, E.; et al. Transforming Heart Failure Management: The Power of Strain Imaging, 3D Imaging, and Vortex Analysis in Echocardiography. J. Clin. Med. 2024, 13, 5759. [Google Scholar] [CrossRef]
- Nuzzi, V.; Manca, P.; Mule, M.; Leone, S.; Fazzini, L.; Cipriani, M.G.; Faletra, F.F. Contemporary clinical role of echocardiography in patients with advanced heart failure. Heart Fail. Rev. 2024, 29, 1247–1260. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Colonna, P.; Pinto, F.J.; Sorino, M.; Bovenzi, F.; D’Agostino, C.; de Luca, I. The emerging role of echocardiography in the screening of patients at risk of heart failure. Am. J. Cardiol. 2005, 96, 42L–51L. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.F.; Campbell, D.J.; Prior, D.L. Noninvasive Cardiac Imaging and the Prediction of Heart Failure Progression in Preclinical Stage A/B Subjects. JACC Cardiovasc. Imaging 2017, 10, 1504–1519. [Google Scholar] [CrossRef] [PubMed]
- Writing Group, M.; Doherty, J.U.; Kort, S.; Mehran, R.; Schoenhagen, P.; Soman, P.; Rating Panel, M.; Dehmer, G.J.; Doherty, J.U.; Schoenhagen, P.; et al. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 Appropriate Use Criteria for Multimodality Imaging in the Assessment of Cardiac Structure and Function in Nonvalvular Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and the Society of Thoracic Surgeons. J. Am. Soc. Echocardiogr. 2019, 32, 553–579. [Google Scholar] [CrossRef]
- Edvardsen, T.; Asch, F.M.; Davidson, B.; Delgado, V.; DeMaria, A.; Dilsizian, V.; Gaemperli, O.; Garcia, M.J.; Kamp, O.; Lee, D.C.; et al. Non-Invasive Imaging in Coronary Syndromes: Recommendations of The European Association of Cardiovascular Imaging and the American Society of Echocardiography, in Collaboration with The American Society of Nuclear Cardiology, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2022, 35, 329–354. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Jone, P.N.; Chamsi-Pasha, M.A.; Chen, T.; Collins, K.A.; Desai, M.Y.; Grayburn, P.; Groves, D.W.; Hahn, R.T.; Little, S.H.; et al. Guidelines for the Evaluation of Prosthetic Valve Function With Cardiovascular Imaging: A Report From the American Society of Echocardiography Developed in Collaboration With the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2024, 37, 2–63. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Chioncel, O.; Mebazaa, A.; Harjola, V.P.; Coats, A.J.; Piepoli, M.F.; Crespo-Leiro, M.G.; Laroche, C.; Seferovic, P.M.; Anker, S.D.; Ferrari, R.; et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1242–1254. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Mazzola, M.; Bandini, G.; Barbieri, G.; Spinelli, S.; De Biase, N.; Masi, S.; Moggi-Pignone, A.; Ghiadoni, L.; Taddei, S.; et al. Prognostic Role of Sonographic Decongestion in Patients with Acute Heart Failure with Reduced and Preserved Ejection Fraction: A Multicentre Study. J. Clin. Med. 2023, 12, 773. [Google Scholar] [CrossRef]
- Wang, C.S.; FitzGerald, J.M.; Schulzer, M.; Mak, E.; Ayas, N.T. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005, 294, 1944–1956. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Meziere, G.; Biderman, P.; Gepner, A.; Barre, O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1640–1646. [Google Scholar] [CrossRef]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef]
- Picano, E.; Frassi, F.; Agricola, E.; Gligorova, S.; Gargani, L.; Mottola, G. Ultrasound lung comets: A clinically useful sign of extravascular lung water. J. Am. Soc. Echocardiogr. 2006, 19, 356–363. [Google Scholar] [CrossRef]
- Gargani, L. Lung ultrasound: A new tool for the cardiologist. Cardiovasc. Ultrasound 2011, 9, 6. [Google Scholar] [CrossRef]
- Chouihed, T.; Coiro, S.; Zannad, F.; Girerd, N. Lung ultrasound: A diagnostic and prognostic tool at every step in the pathway of care for acute heart failure. Am. J. Emerg. Med. 2016, 34, 656–657. [Google Scholar] [CrossRef] [PubMed]
- Mottola, C.; Girerd, N.; Coiro, S.; Lamiral, Z.; Rossignol, P.; Frimat, L.; Girerd, S. Evaluation of Subclinical Fluid Overload Using Lung Ultrasound and Estimated Plasma Volume in the Postoperative Period Following Kidney Transplantation. Transplant. Proc. 2018, 50, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A.; Meziere, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef]
- Dubon-Peralta, E.E.; Lorenzo-Villalba, N.; Garcia-Klepzig, J.L.; Andres, E.; Mendez-Bailon, M. Prognostic value of B lines detected with lung ultrasound in acute heart failure. A systematic review. J. Clin. Ultrasound 2022, 50, 273–283. [Google Scholar] [CrossRef]
- Gargani, L.; Volpicelli, G. How I do it: Lung ultrasound. Cardiovasc. Ultrasound 2014, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Yuriditsky, E.; Horowitz, J.M.; Panebianco, N.L.; Sauthoff, H.; Saric, M. Lung Ultrasound Imaging: A Primer for Echocardiographers. J. Am. Soc. Echocardiogr. 2021, 34, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A. Lung ultrasound in the critically ill. Ann. Intensive Care 2014, 4, 1. [Google Scholar] [CrossRef]
- Biswas, A.; Lascano, J.E.; Mehta, H.J.; Faruqi, I. The Utility of the “Shred Sign” in the Diagnosis of Acute Respiratory Distress Syndrome Resulting from Multifocal Pneumonia. Am. J. Respir. Crit. Care Med. 2017, 195, e20–e22. [Google Scholar] [CrossRef]
- Soldati, G.; Demi, M.; Inchingolo, R.; Smargiassi, A.; Demi, L. On the Physical Basis of Pulmonary Sonographic Interstitial Syndrome. J. Ultrasound Med. 2016, 35, 2075–2086. [Google Scholar] [CrossRef]
- Soldati, G.; Demi, M. The use of lung ultrasound images for the differential diagnosis of pulmonary and cardiac interstitial pathology. J. Ultrasound 2017, 20, 91–96. [Google Scholar] [CrossRef]
- Laursen, C.B.; Clive, A.; Hallifax, R.; Pietersen, P.I.; Asciak, R.; Davidsen, J.R.; Bhatnagar, R.; Bedawi, E.O.; Jacobsen, N.; Coleman, C.; et al. European Respiratory Society statement on thoracic ultrasound. Eur. Respir. J. 2021, 57, 2001519. [Google Scholar] [CrossRef]
- Marini, T.J.; Rubens, D.J.; Zhao, Y.T.; Weis, J.; O’Connor, T.P.; Novak, W.H.; Kaproth-Joslin, K.A. Lung Ultrasound: The Essentials. Radiol. Cardiothorac. Imaging 2021, 3, e200564. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Scali, M.C.; Ciampi, Q.; Lichtenstein, D. Lung Ultrasound for the Cardiologist. JACC Cardiovasc. Imaging 2018, 11, 1692–1705. [Google Scholar] [CrossRef]
- Jambrik, Z.; Monti, S.; Coppola, V.; Agricola, E.; Mottola, G.; Miniati, M.; Picano, E. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am. J. Cardiol. 2004, 93, 1265–1270. [Google Scholar] [CrossRef]
- Volpicelli, G.; Mussa, A.; Garofalo, G.; Cardinale, L.; Casoli, G.; Perotto, F.; Fava, C.; Frascisco, M. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am. J. Emerg. Med. 2006, 24, 689–696. [Google Scholar] [CrossRef]
- Buessler, A.; Chouihed, T.; Duarte, K.; Bassand, A.; Huot-Marchand, M.; Gottwalles, Y.; Penine, A.; Andre, E.; Nace, L.; Jaeger, D.; et al. Accuracy of Several Lung Ultrasound Methods for the Diagnosis of Acute Heart Failure in the ED: A Multicenter Prospective Study. Chest 2020, 157, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Liteplo, A.S.; Marill, K.A.; Villen, T.; Miller, R.M.; Murray, A.F.; Croft, P.E.; Capp, R.; Noble, V.E. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): Sonographic B-lines and N-terminal pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad. Emerg. Med. 2009, 16, 201–210. [Google Scholar] [CrossRef]
- Platz, E.; Jhund, P.S.; Girerd, N.; Pivetta, E.; McMurray, J.J.V.; Peacock, W.F.; Masip, J.; Martin-Sanchez, F.J.; Miro, O.; Price, S.; et al. Expert consensus document: Reporting checklist for quantification of pulmonary congestion by lung ultrasound in heart failure. Eur. J. Heart Fail. 2019, 21, 844–851. [Google Scholar] [CrossRef]
- Pivetta, E.; Goffi, A.; Nazerian, P.; Castagno, D.; Tozzetti, C.; Tizzani, P.; Tizzani, M.; Porrino, G.; Ferreri, E.; Busso, V.; et al. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: A randomized controlled trial. Eur. J. Heart Fail. 2019, 21, 754–766. [Google Scholar] [CrossRef]
- Frassi, F.; Gargani, L.; Gligorova, S.; Ciampi, Q.; Mottola, G.; Picano, E. Clinical and echocardiographic determinants of ultrasound lung comets. Eur. J. Echocardiogr. 2007, 8, 474–479. [Google Scholar] [CrossRef]
- Volpicelli, G.; Caramello, V.; Cardinale, L.; Mussa, A.; Bar, F.; Frascisco, M.F. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am. J. Emerg. Med. 2008, 26, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Cortellaro, F.; Ceriani, E.; Spinelli, M.; Campanella, C.; Bossi, I.; Coen, D.; Casazza, G.; Cogliati, C. Lung ultrasound for monitoring cardiogenic pulmonary edema. Intern. Emerg. Med. 2017, 12, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Ohman, J.; Harjola, V.P.; Karjalainen, P.; Lassus, J. Assessment of early treatment response by rapid cardiothoracic ultrasound in acute heart failure: Cardiac filling pressures, pulmonary congestion and mortality. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 311–320. [Google Scholar] [CrossRef]
- Facchini, C.; Malfatto, G.; Giglio, A.; Facchini, M.; Parati, G.; Branzi, G. Lung ultrasound and transthoracic impedance for noninvasive evaluation of pulmonary congestion in heart failure. J. Cardiovasc. Med. 2016, 17, 510–517. [Google Scholar] [CrossRef]
- Coiro, S.; Porot, G.; Rossignol, P.; Ambrosio, G.; Carluccio, E.; Tritto, I.; Huttin, O.; Lemoine, S.; Sadoul, N.; Donal, E.; et al. Prognostic value of pulmonary congestion assessed by lung ultrasound imaging during heart failure hospitalisation: A two-centre cohort study. Sci. Rep. 2016, 6, 39426. [Google Scholar] [CrossRef]
- Platz, E.; Campbell, R.T.; Claggett, B.; Lewis, E.F.; Groarke, J.D.; Docherty, K.F.; Lee, M.M.Y.; Merz, A.A.; Silverman, M.; Swamy, V.; et al. Lung Ultrasound in Acute Heart Failure: Prevalence of Pulmonary Congestion and Short- and Long-Term Outcomes. JACC Heart Fail. 2019, 7, 849–858. [Google Scholar] [CrossRef]
- Coiro, S.; Rossignol, P.; Ambrosio, G.; Carluccio, E.; Alunni, G.; Murrone, A.; Tritto, I.; Zannad, F.; Girerd, N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur. J. Heart Fail. 2015, 17, 1172–1181. [Google Scholar] [CrossRef]
- Rivas-Lasarte, M.; Maestro, A.; Fernandez-Martinez, J.; Lopez-Lopez, L.; Sole-Gonzalez, E.; Vives-Borras, M.; Montero, S.; Mesado, N.; Pirla, M.J.; Mirabet, S.; et al. Prevalence and prognostic impact of subclinical pulmonary congestion at discharge in patients with acute heart failure. ESC Heart Fail. 2020, 7, 2621–2628. [Google Scholar] [CrossRef]
- Rastogi, T.; Bozec, E.; Pellicori, P.; Bayes-Genis, A.; Coiro, S.; Domingo, M.; Gargani, L.; Palazzuoli, A.; Girerd, N. Prognostic Value and Therapeutic Utility of Lung Ultrasound in Acute and Chronic Heart Failure: A Meta-Analysis. JACC Cardiovasc. Imaging 2022, 15, 950–952. [Google Scholar] [CrossRef]
- Miglioranza, M.H.; Gargani, L.; Sant’Anna, R.T.; Rover, M.M.; Martins, V.M.; Mantovani, A.; Weber, C.; Moraes, M.A.; Feldman, C.J.; Kalil, R.A.; et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: A comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc. Imaging 2013, 6, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Pellicori, P.; Shah, P.; Cuthbert, J.; Urbinati, A.; Zhang, J.; Kallvikbacka-Bennett, A.; Clark, A.L.; Cleland, J.G.F. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. Eur. J. Heart Fail. 2019, 21, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, K.H.; Merz, A.A.; Lewis, E.F.; Claggett, B.L.; Crousillat, D.R.; Lau, E.S.; Silverman, M.B.; Peck, J.; Rivero, J.; Cheng, S.; et al. Pulmonary Congestion by Lung Ultrasound in Ambulatory Patients With Heart Failure With Reduced or Preserved Ejection Fraction and Hypertension. J. Card. Fail. 2018, 24, 219–226. [Google Scholar] [CrossRef]
- Domingo, M.; Conangla, L.; Lupon, J.; de Antonio, M.; Moliner, P.; Santiago-Vacas, E.; Codina, P.; Zamora, E.; Cediel, G.; Gonzalez, B.; et al. Prognostic value of lung ultrasound in chronic stable ambulatory heart failure patients. Rev. Esp. Cardiol. 2021, 74, 862–869. [Google Scholar] [CrossRef]
- Morvai-Illes, B.; Polestyuk-Nemeth, N.; Szabo, I.A.; Monoki, M.; Gargani, L.; Picano, E.; Varga, A.; Agoston, G. The Prognostic Value of Lung Ultrasound in Patients With Newly Diagnosed Heart Failure With Preserved Ejection Fraction in the Ambulatory Setting. Front. Cardiovasc. Med. 2021, 8, 758147. [Google Scholar] [CrossRef]
- Rivas-Lasarte, M.; Alvarez-Garcia, J.; Fernandez-Martinez, J.; Maestro, A.; Lopez-Lopez, L.; Sole-Gonzalez, E.; Pirla, M.J.; Mesado, N.; Mirabet, S.; Fluvia, P.; et al. Lung ultrasound-guided treatment in ambulatory patients with heart failure: A randomized controlled clinical trial (LUS-HF study). Eur. J. Heart Fail. 2019, 21, 1605–1613. [Google Scholar] [CrossRef]
- Araiza-Garaygordobil, D.; Gopar-Nieto, R.; Martinez-Amezcua, P.; Cabello-Lopez, A.; Alanis-Estrada, G.; Luna-Herbert, A.; Gonzalez-Pacheco, H.; Paredes-Paucar, C.P.; Sierra-Lara, M.D.; Briseno-De la Cruz, J.L.; et al. A randomized controlled trial of lung ultrasound-guided therapy in heart failure (CLUSTER-HF study). Am. Heart J. 2020, 227, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Obokata, M.; Wiley, B.; Koepp, K.E.; Jorgenson, C.C.; Egbe, A.; Melenovsky, V.; Carter, R.E.; Borlaug, B.A. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur. Heart J. 2019, 40, 3721–3730. [Google Scholar] [CrossRef]
- Simonovic, D.; Coiro, S.; Carluccio, E.; Girerd, N.; Deljanin-Ilic, M.; Cattadori, G.; Ambrosio, G. Exercise elicits dynamic changes in extravascular lung water and haemodynamic congestion in heart failure patients with preserved ejection fraction. Eur. J. Heart Fail. 2018, 20, 1366–1369. [Google Scholar] [CrossRef]
- Scali, M.C.; Cortigiani, L.; Simionuc, A.; Gregori, D.; Marzilli, M.; Picano, E. Exercise-induced B-lines identify worse functional and prognostic stage in heart failure patients with depressed left ventricular ejection fraction. Eur. J. Heart Fail. 2017, 19, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Coiro, S.; Simonovic, D.; Deljanin-Ilic, M.; Duarte, K.; Carluccio, E.; Cattadori, G.; Girerd, N.; Ambrosio, G. Prognostic Value of Dynamic Changes in Pulmonary Congestion During Exercise Stress Echocardiography in Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2020, 13, e006769. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Masi, S. The emerging role of endothelial function in cardiovascular oncology. Eur. J. Prev. Cardiol. 2020, 27, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Kaye, D.M.; Borlaug, B.A.; Shah, S.J.; Rich, S.; Kapur, N.K.; Costanzo, M.R.; Brener, M.I.; Sunagawa, K.; Burkhoff, D. Venous Tone and Stressed Blood Volume in Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 1858–1869. [Google Scholar] [CrossRef]
- Fudim, M.; Hernandez, A.F.; Felker, G.M. Role of Volume Redistribution in the Congestion of Heart Failure. J. Am. Heart Assoc. 2017, 6, e006817. [Google Scholar] [CrossRef]
- Kanitkar, S.; Soni, K.; Vaishnav, B. Venous Excess Ultrasound for Fluid Assessment in Complex Cardiac Patients With Acute Kidney Injury. Cureus 2024, 16, e66003. [Google Scholar] [CrossRef]
- Melo, R.H.; Gioli-Pereira, L.; Melo, E.; Rola, P. Venous excess ultrasound score association with acute kidney injury in critically ill patients: A systematic review and meta-analysis of observational studies. Ultrasound J. 2025, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, S.; Antonopoulos, M. Portal vein pulsatility: An important sonographic tool assessment of systemic congestion for critical ill patients. World J. Cardiol. 2024, 16, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.C.; Reddy, Y.N.V. Approach to Echocardiography in Heart Failure with Preserved Ejection Fraction. Cardiol. Clin. 2022, 40, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Torres-Arrese, M.; Mata-Martinez, A.; Luordo-Tedesco, D.; Garcia-Casasola, G.; Alonso-Gonzalez, R.; Montero-Hernandez, E.; Cobo-Marcos, M.; Sanchez-Sauce, B.; Cuervas-Mons, V.; Tung-Chen, Y. Usefulness of Systemic Venous Ultrasound Protocols in the Prognosis of Heart Failure Patients: Results from a Prospective Multicentric Study. J. Clin. Med. 2023, 12, 1281. [Google Scholar] [CrossRef]
- Longino, A.A.; Martin, K.C.; Leyba, K.R.; McCormack, L.; Siegel, G.; Sharma, V.M.; Riscinti, M.; Lopez, C.O.; Douglas, I.S.; Gill, E.A. Reliability and reproducibility of the venous excess ultrasound (VExUS) score, a multi-site prospective study: Validating a novel ultrasound technique for comprehensive assessment of venous congestion. Crit. Care 2024, 28, 197. [Google Scholar] [CrossRef]
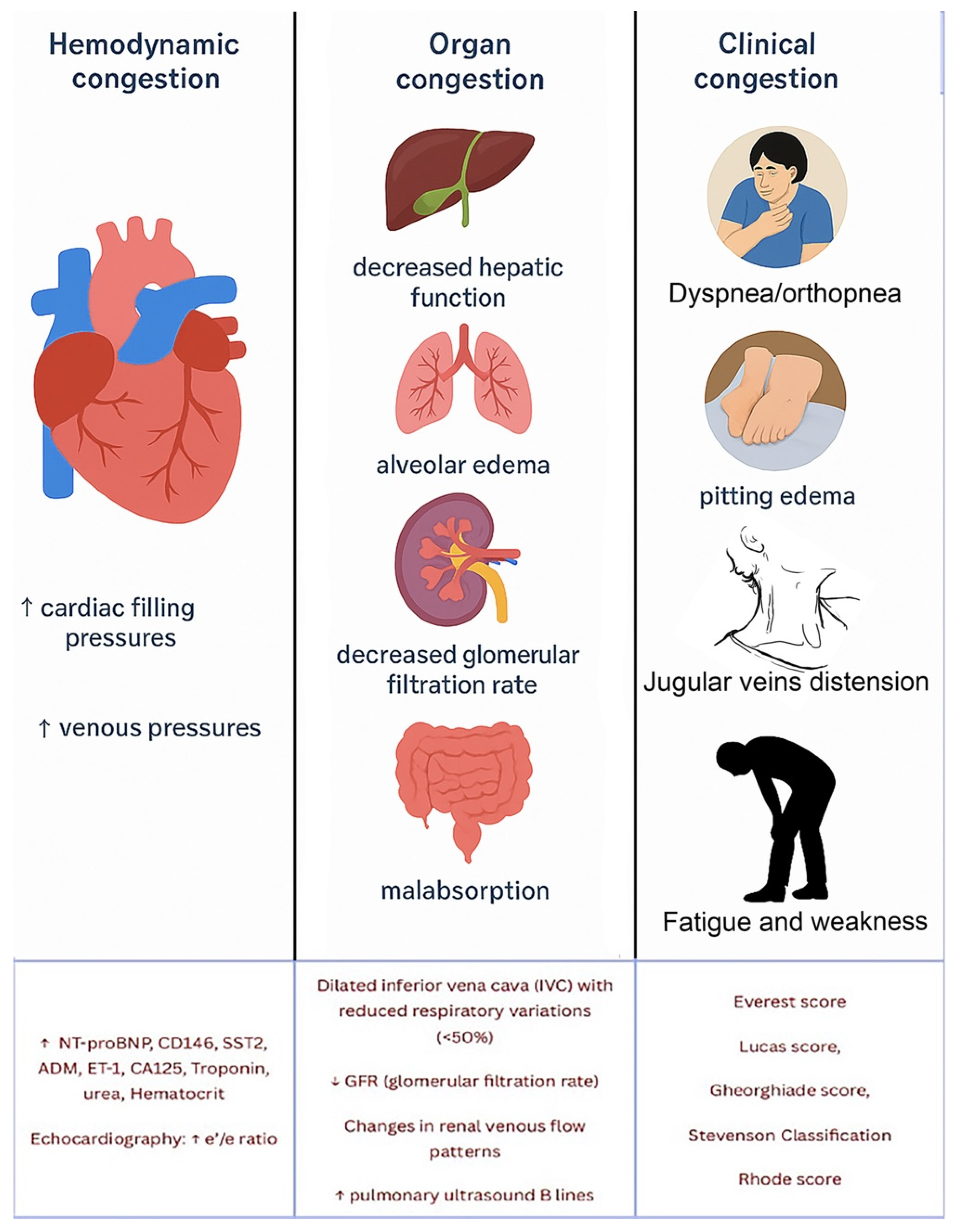
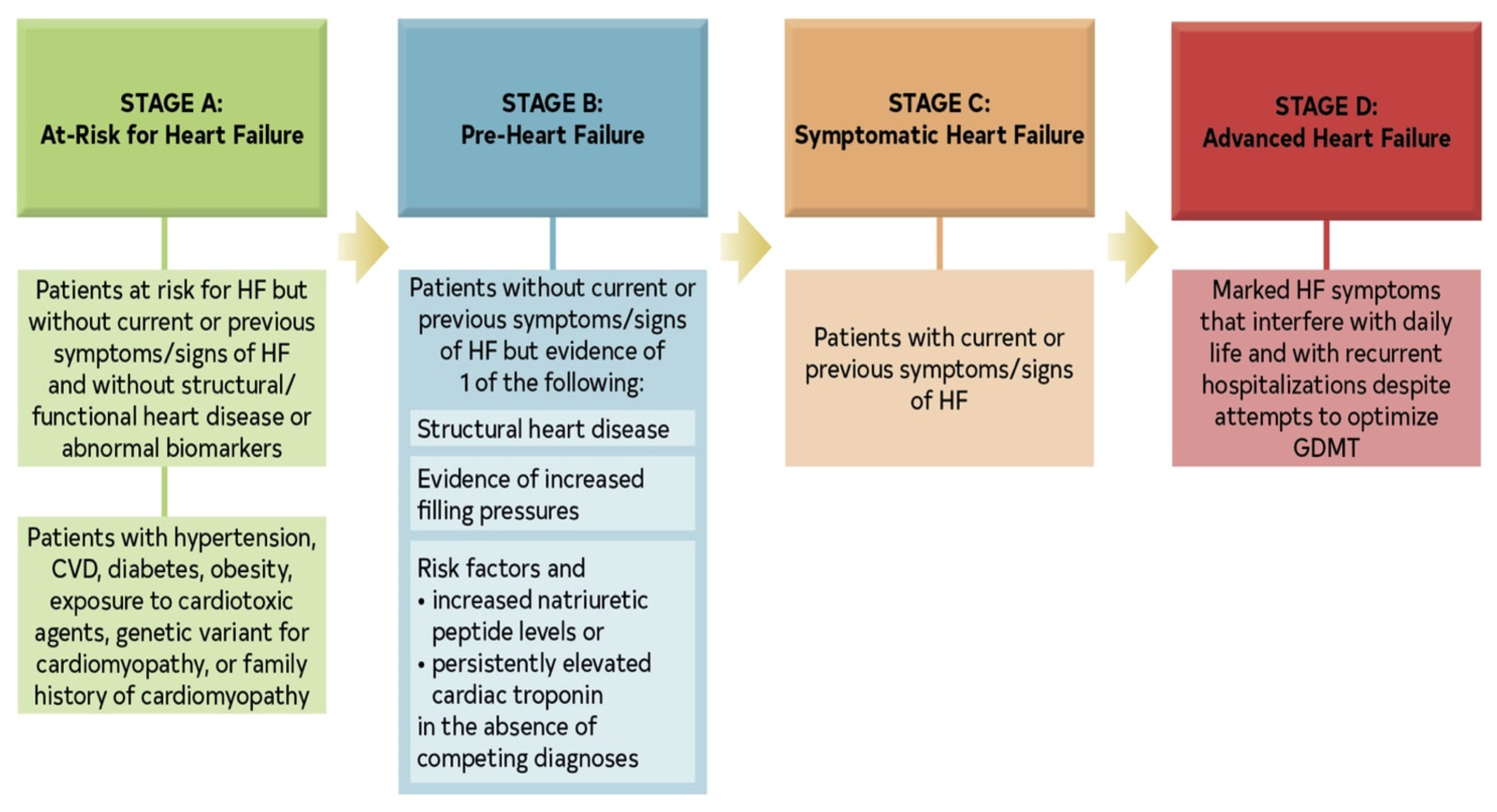


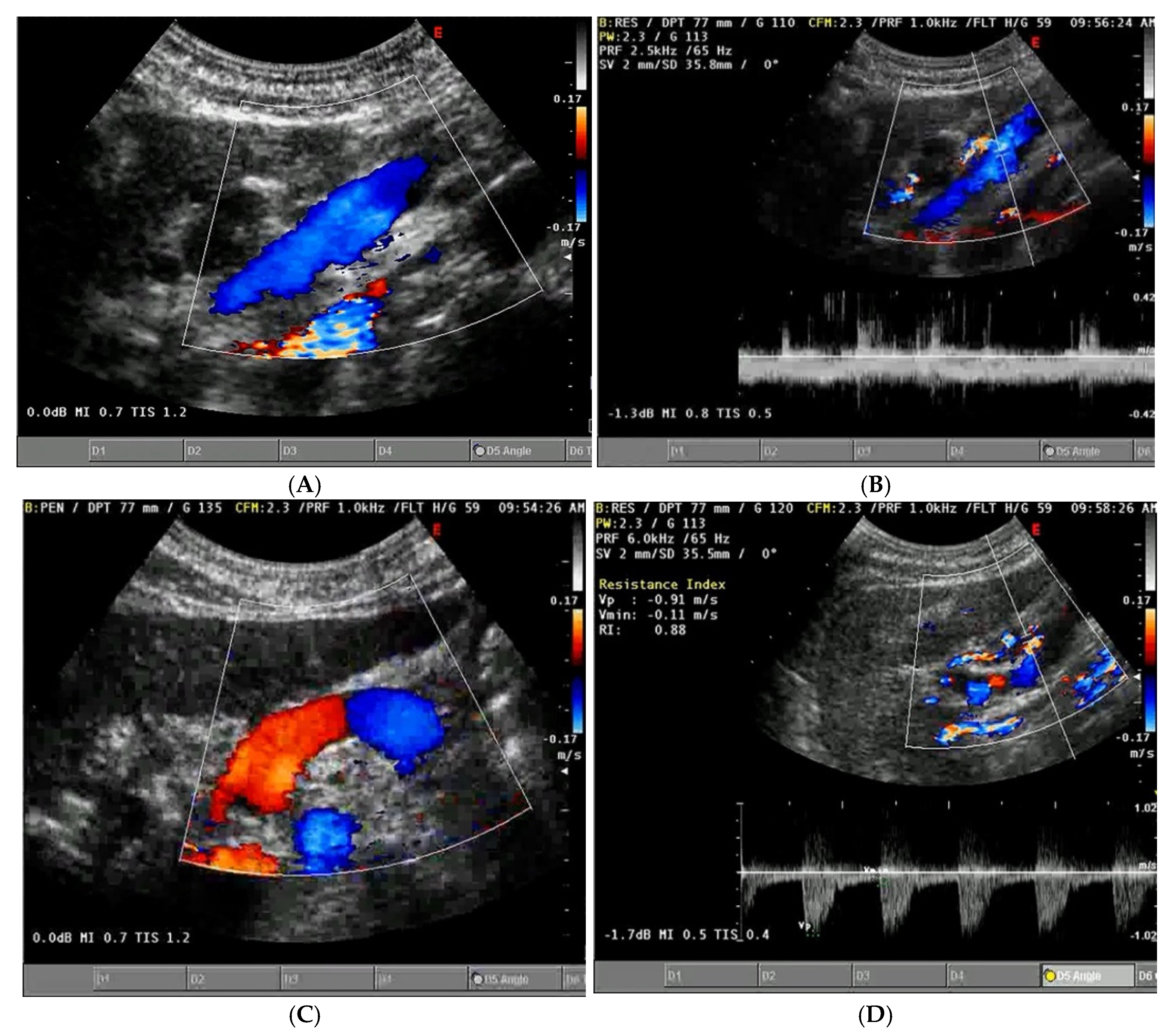
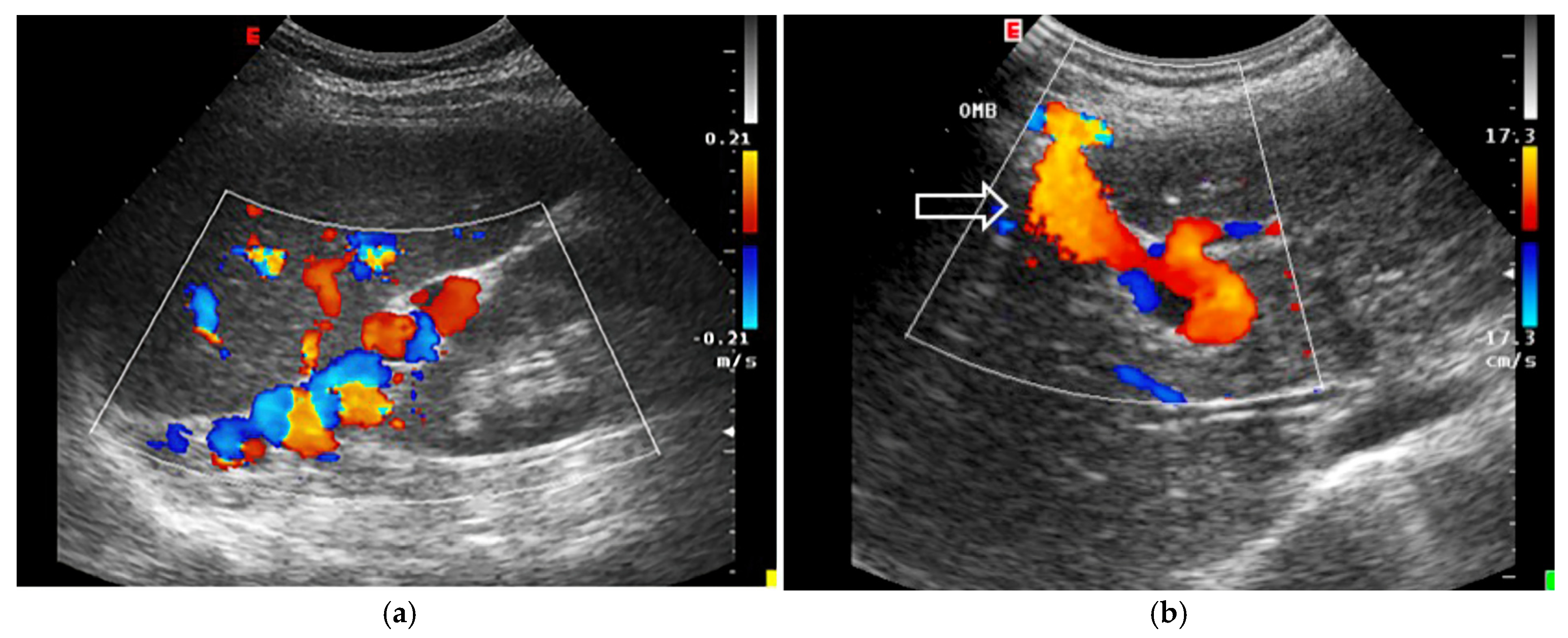
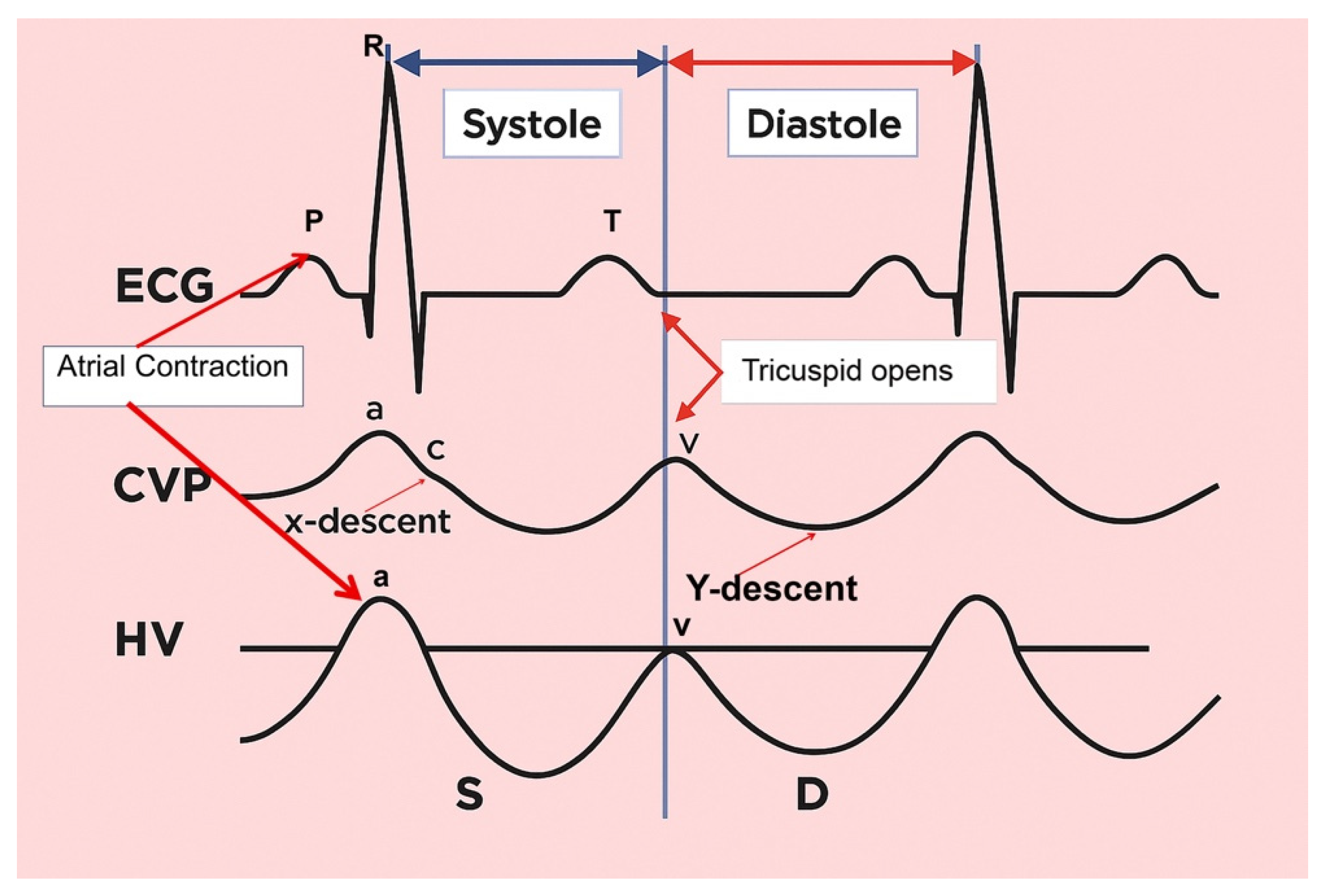
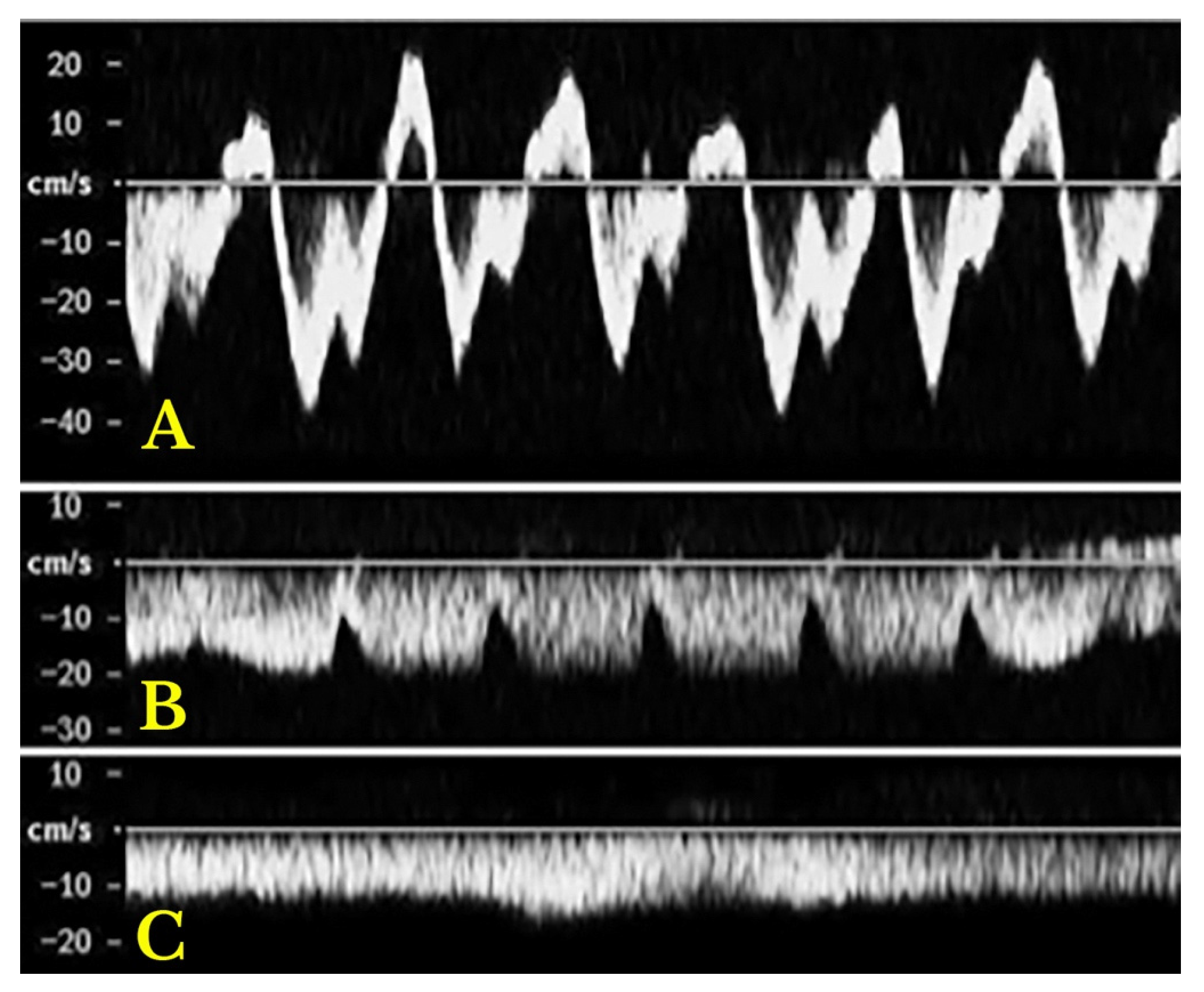



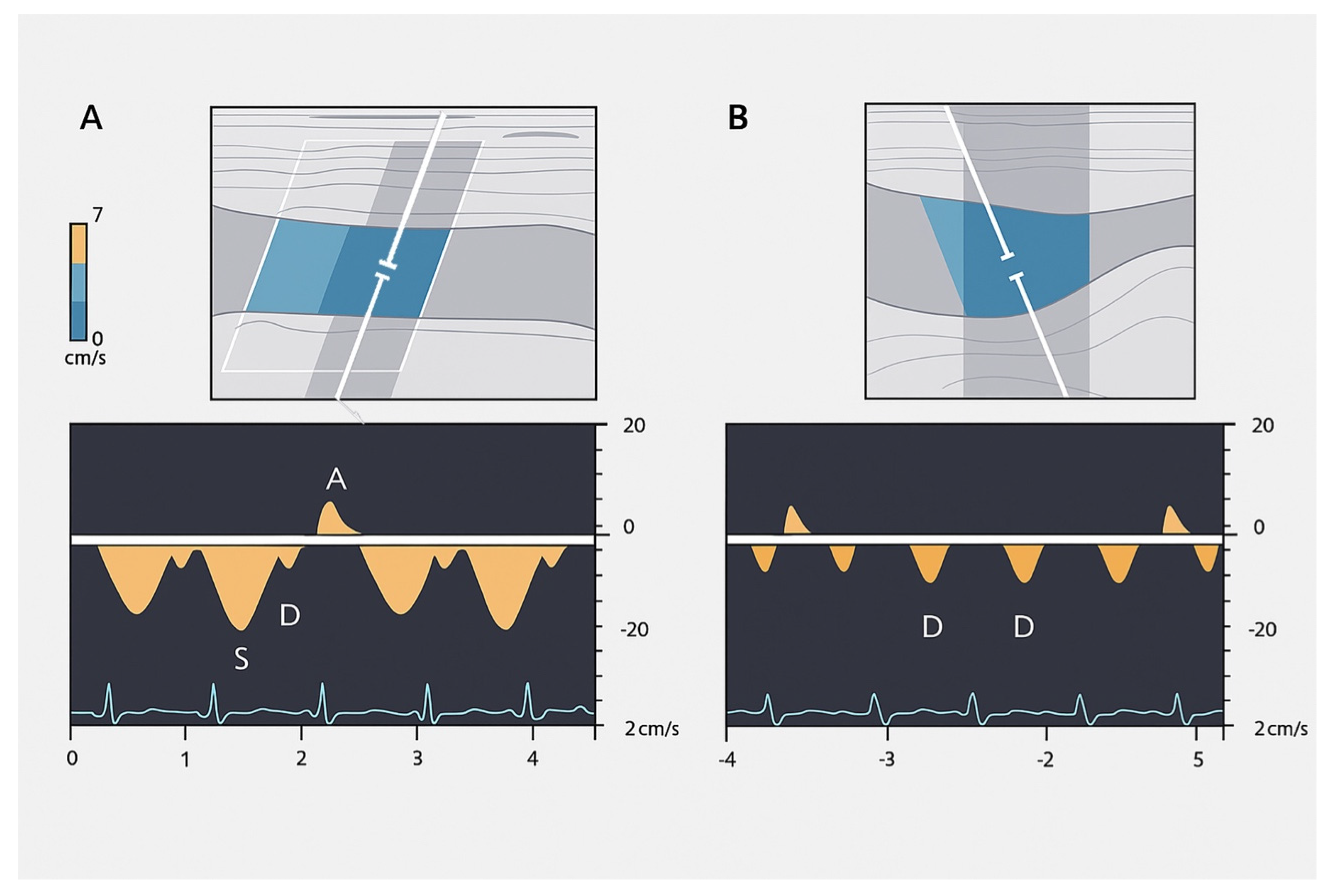
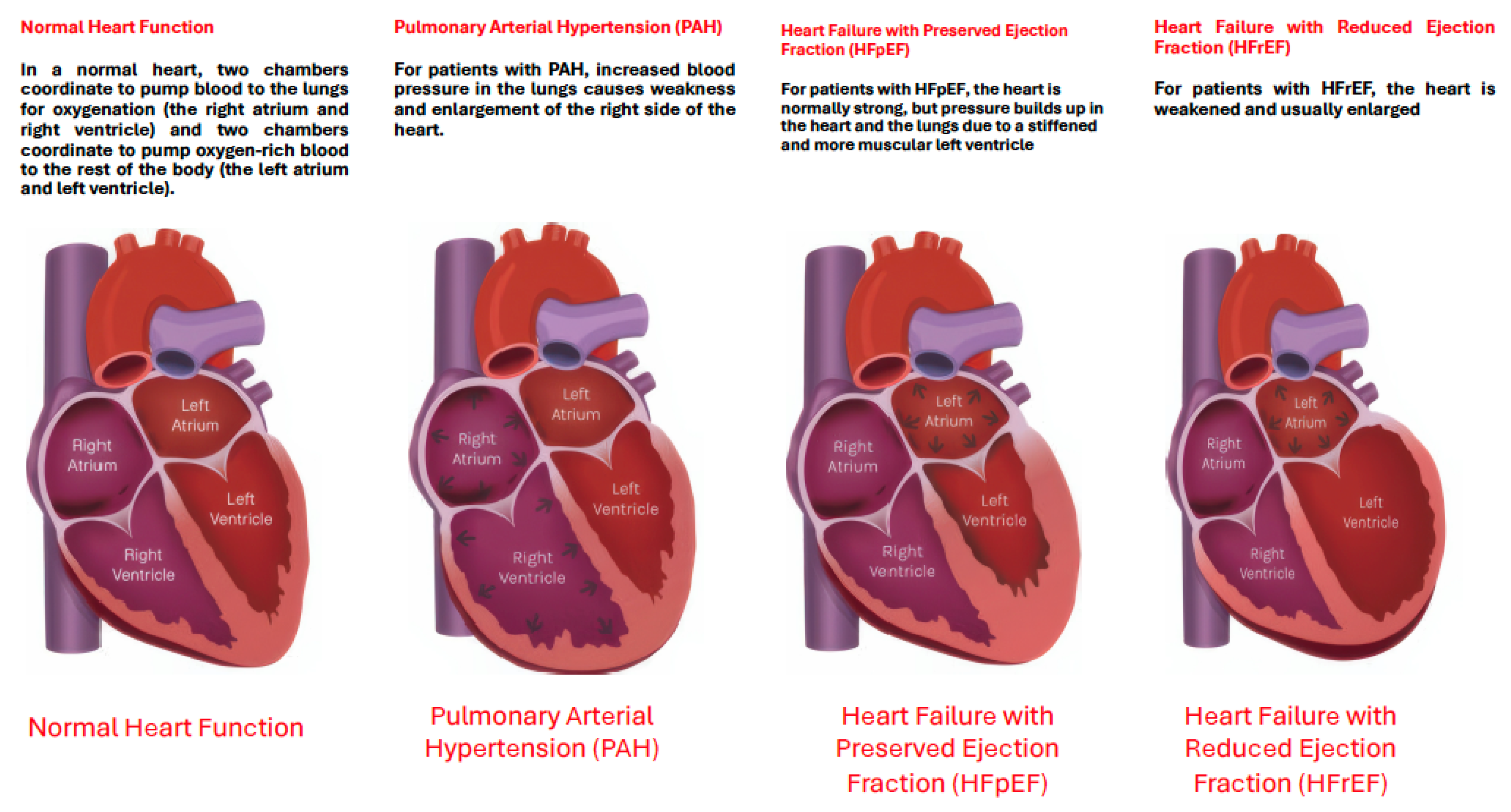
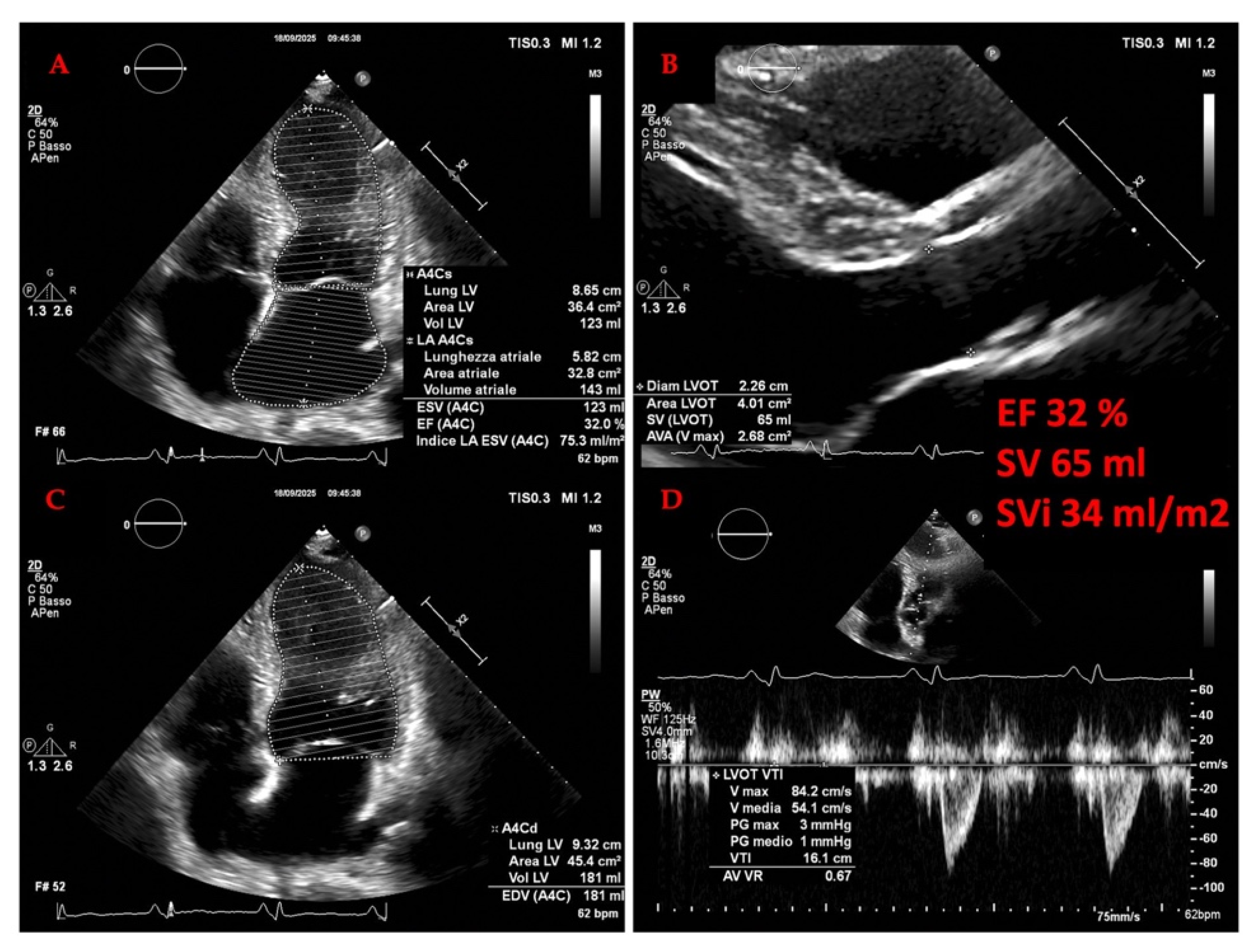
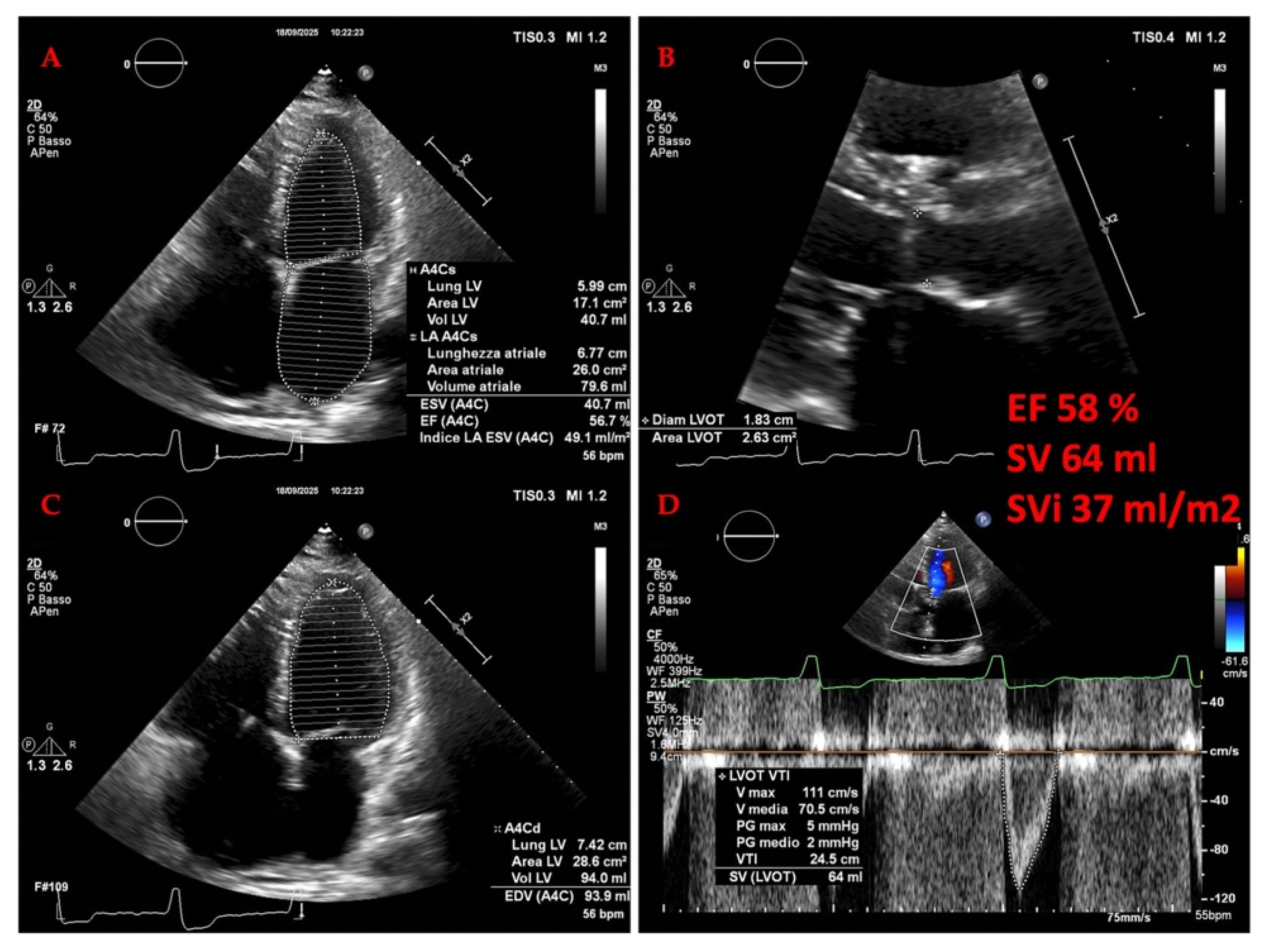
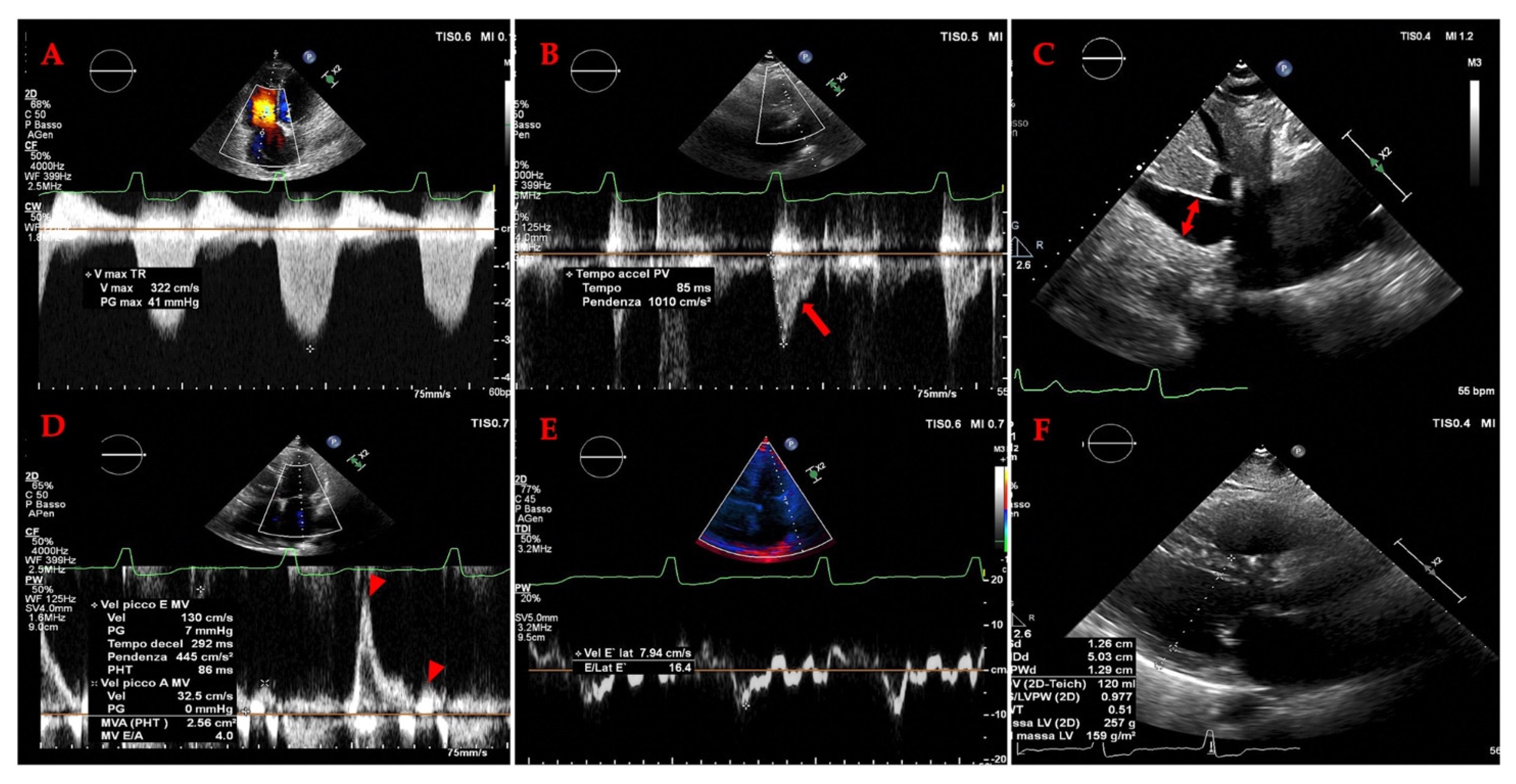
| Study Feature | Description (Number of Studies or Range) | Representative Examples (Citations) |
|---|---|---|
| Total Studies/Patients | 148 studies/~7000 patients | |
| Publication Years | 1986–2025 | Moriyasu et al., 1986 [54]; Saadi et al., 2025 [46] |
| Study Designs | • Prospective Cohort: e.g., [55] studies • Retrospective Cohort: e.g., [44] studies • Cross-Sectional: e.g., [30] studies • Case-Control: e.g., [5] studies • Systematic Reviews: e.g., [3] studies | • Prospective: Iida et al., 2016 [56], Beaubien-Souligny et al., 2018 [57] • Retrospective: Kuwahara et al., 2023 [58] |
| Patient Populations | • Acute Decompensated HF: e.g., [59] studies • Chronic Ambulatory HF: e.g., [38] studies • Post-Cardiac Surgery/Critically Ill: e.g., [25] studies • Mixed/Unspecified HF: e.g., [13] studies | • Acute: Pivetta et al., 2015 [60], Ikeda et al., 2017 [61] • Chronic: Platz et al., 2016 [62] • Post-Surgical: Beaubien-Souligny et al., 2020 [63] |
| HF Phenotypes | • HFrEF: e.g., [64] studies • HFpEF: e.g., [25] studies • Mixed/All Phenotypes: e.g., [65] studies | • HFpEF: Yoshihisa et al., 2020 [66] • Mixed: Bouabdallaoui et al., 2020 [67] |
| Ultrasdomatic Modalities Used | • Splanchnic B-mode/Doppler (isolated): e.g., [68] studies • VExUS Protocol: e.g., [15] studies • Echocardiography (for congestion): e.g., [49] studies • Lung Ultrasound (LUS): e.g., [38] studies • Multi-organ POCUS Protocol: e.g., [20] studies | • Splanchnic: Goncalvesova et al., 2010 [69] • VExUS: Bhardwaj et al., 2020 [70] • LUS: Gargani et al., 2015 [71] |
| Primary Outcomes Measured | • Correlation with Invasive Haemodynamics: e.g., [30] studies • Prognosis (Mortality/Hospitalization): e.g., [72] studies • Acute Kidney Injury (AKI): e.g., [20] studies • Diuretic Response/Therapy Guidance: e.g., [15] studies • Technical Feasibility/Diagnostic Accuracy: e.g., [38] studies | • Haemodynamics: Rengo et al., 1998 [73] • Prognosis: Ciccone et al., 2014 [74] • AKI: Beaubien-Souligny et al., 2018 [57] |
| Sample Size (Range) | Small (<100 patients): e.g., [75] studies Medium (100–500 patients): e.g., [49] studies Large (>500 patients): e.g., [8] studies | • Small: Denault et al., 2020 [76] case series • Large: Pivetta et al., 2015 [60] (n = 1005) |
| Hepatic Veins | Portal Venous System | ||
|---|---|---|---|
| Normal | IVC collapsibility >50% IVC diameter ≤ 21 mm IV diameter ≤ 10 mm | PV diameter < 13 mm Preserved collapsibility | |
| Other | |||
| Congestive hepatopathy | Increased diameter Reduced collapsibility <50% | Increased vessels diameter (PV > 13 mm) Reduced collapsibility | Hepatomegaly Ascites Splenomegaly Porto-systemic collaterals |
| Liver cirrhosis | Reduced diameter Serpiginous aspect | Signs of Portale Hypertension | |
| Intrahepatic Veins | Portal Vein | Hepatic Artery | |
|---|---|---|---|
| Normal | Triphasic pattern: “a” wave: positive, “a” > “v” “S” wave: negative, “S” > “D” “v” wave: positive; “v” < “a” “D” wave: negative, “D” < “S” | Anterograde flow Smoothly undulating venous waveforms Systolic speed 16–40 cm/s Pulsatility index > 0.5 | Maximum systolic speed: 30–60 cm/s Resistive index 0.55–0.7 |
| Heart failure | Increased anterograde and retrograde speeds | All cases of hepatic congestion | |
| Increased pulsatility | |||
| Right heart | Higher “a” and “v” waves | ||
| failure | Adequate ratio maintained between S and D waves | Pulsatility index < 0.5 | Resistive index > 0.7 |
| High and both positive “a” and “v” waves | Reduced systolic speed | ||
| Tricuspid regurgitation | Reduced “S” wave “S” wave < “D” wave | (more common in LC) | |
| Severe TR: “S” wave retrograde (“a-S-v complex”) | |||
| Liver cirrhosis | Loss of triphasic pattern | Systolic speed < 12.8 cm/s till reversal flow and thrombosis | |
| Venous Site | Ease of Acquisition | Key Advantages | Key Limitations & Pitfalls | Key Doppler Findings in Congestion |
|---|---|---|---|---|
| Internal Jugular (IJ) Vein | Moderate to High • Superficial, easily visualized with linear probe. • Patient positioning (Trendelenburg) can facilitate. | • Minimal respiratory variation. Less influenced by intra-abdominal pressure (IAP) than portal/hepatic veins. • Rapidly responsive to haemodynamic changes. • Useful for trending over time. | • Highly sensitive to transducer pressure. Can easily occlude the vein. • Can be difficult in patients with short necks, obesity, or cervical collars. • Not validated for formal VExUS grading. Best used as a qualitative “congestion marker.” | • Loss of phasicity: The normal, pulsatile waveform becomes monophasic or severely blunted. • Continuous, high-velocity flow indicates significantly elevated right atrial pressure. |
| Femoral Vein | High • Large, superficial, and easy to locate with a linear probe. • Consistent anatomical location. | • Excellent surrogate for IVC/right atrial pressure. • Waveform closely mirrors the hepatic vein pattern. • Well-suited for formal VExUS grading (can be classified as Grade 0–3). • Less affected by ascites or liver morphology. | • Highly influenced by IAP. A tense abdomen or ascites can artificially alter the waveform. • Can be affected by iliac vein thrombosis or compression. • Influenced by cirrhosis: Portal hypertension can cause pre-sinusoidal resistance, potentially altering waveforms. | • Progressive blunting of systolic wave (S). • S < D (Diastolic) flow. • Systolic Flow Reversal (severe congestion, VExUS Grade 3). |
| Superior Vena Cava (SVC)/Brachiocephalic Vein | Low • Requires a low-frequency phased array or curvilinear probe with a suprasternal or supraclavicular view. • Technically challenging and operator-dependent. | • Directly pre-cardiac. Provides the most direct assessment of central venous pressure short of a catheter. • Unaffected by IAP or liver disease. | • Acquisition is difficult and often not feasible in unselected patients (e.g., COPD, obesity, mechanical ventilation). • Not a practical routine site. • Limited data on its use in VExUS protocols. | • Severe blunting of the systolic wave. • Prominent diastolic wave. • Systolic Flow Reversal indicates profound congestion. |
| Feature | HFpEF | HFrEF | Pulmonary Hypertension |
|---|---|---|---|
| Primary dysfunction | Diastolic impairment (impaired relaxation, stiff ventricle) | Systolic impairment (reduced contractility, LVEF ≤ 40%) | Increased RV afterload due to elevated pulmonary vascular resistance |
| Structural remodeling | Concentric LV hypertrophy, LA enlargement, interstitial fibrosis | Eccentric LV hypertrophy, dilatation, secondary MR | RV hypertrophy (early), RV dilatation (late), septal shift |
| Metabolic features | Impaired substrate flexibility, reduced ketone oxidation [1,2] | Reverse remodelling under SGLT2i/ARNI therapy [5,6] | RV microvascular remodelling (shorter, tortuous vessels, preserved density) [8] |
| Microvascular/Perfusion | Reduced myocardial perfusion reserve, LA functional changes [3] | Partially reversible LV microvascular dysfunction | RV perfusion and microarchitecture linked to coupling [9] |
| Neurohormonal/Inflammatory role | Inflammatory mediators (IL-1R1, fibrosis pathways) [4] | Strong neurohormonal activation (RAAS, sympathetic system) | Endothelial dysfunction, smooth muscle proliferation, in situ thrombosis |
| Imaging markers | Strain analysis shows impaired LA reservoir and LV diastolic strain [3] | Global longitudinal strain improves with therapy [6] | RV strain by MRI/echo, CT-MRI integration improves PH detection [10,11] |
| Reversibility | Limited, comorbidity-driven phenotype | Partially reversible with optimal therapy [7] | Some vascular and RV changes reversible after unloading [8] |
| POCUS Application | Clinical Relevance | Advantages | Limitations |
|---|---|---|---|
| IVC ultrasound | Provides an estimation of | Relatively easy to perform; able to use | Unreliable in many clinical scenarios |
| RAP | handheld ultrasound devices | (e.g., mechanical ventilation, | |
| pulmonary embolism, PH, cardiac | |||
| tamponade, intra-abdominal | |||
| hypertension, chronic TR, athletes); | |||
| unable to distinguish between | |||
| hypovolemia, euvolemia, and high- | |||
| output cardiac states; collapsibility | |||
| influenced by strength of breath | |||
| Internal jugular vein | Provides an estimation of | Relatively easy to perform; able to use | Operator variability (bed angle, |
| ultrasound | RAP | handheld ultrasound devices; useful | transducer pressure, off-axis views); |
| when IVC is inaccessible or unreliable | protocol variability (e.g., column | ||
| (e.g., cirrhosis, obesity) | height, change with Valsalva, | ||
| respiratory variation); incorrect | |||
| assumptions (e.g., RA depth is 5.0 cm) | |||
| Hepatic vein Doppler | Aids in the assessment of | Same window used for assessing the | Need ECG tracing; unreliable in atrial |
| systemic venous | IVC; supplemental information (e.g., | fibrillation, right ventricular systolic | |
| congestion | right ventricular systolic function, | dysfunction, chronic PH, TR, cirrhosis | |
| constriction and tamponade); exhibits | |||
| dynamic change in response to | |||
| decongestive | |||
| treatment | |||
| Portal vein Doppler | Aids in the assessment of | Do not need ECG; exhibits dynamic | Operator variability (Doppler sampling |
| systemic venous | change in response to decongestive | location); unreliable in athletes (e.g., | |
| congestion | treatment (pulsatility may | pulsatility without high RAP) and | |
| improve even in chronic TR) | cirrhosis (e.g., no pulsatility with high | ||
| RAP or pulsatility due to arterioportal | |||
| shunts) | |||
| Intrarenal venous | Aids in the assessment of | Simultaneous arterial Doppler allows | Technically challenging (especially when |
| Doppler | systemic venous | identification of cardiac cycle; exhibits | patients unable to hold breath); |
| congestion | dynamic change in response to | operator variability (e.g., misinterpret | |
| decongestive treatment | pulsatility of main renal vessel as renal | ||
| parenchymal vessel); change in | |||
| response to decongestive treatment | |||
| may be delayed in the presence of | |||
| interstitial edema; no available data for | |||
| patients with advanced chronic kidney | |||
| disease | |||
| Femoral Vein Doppler (FVD) | Aids in the assessment of | Relatively easy to perform; feasible in | Operator variability (misaligned Doppler |
| systemic venous | patients unable to hold their breath | tracings, overreliance on absolute | |
| congestion | velocities or percent pulsatility); unable | ||
| to rule out venous congestion; | |||
| individual variability (cyclical variation | |||
| limits use of the stasis index) | |||
| Superior vena cava | Aids in the assessment of | Useful when hepatic or renal vessels are | Need ECG tracing; technically |
| Doppler | systemic venous | inaccessible or unreliable (e.g., | challenging transthoracic windows |
| congestion | cirrhosis, advanced kidney disease) | (especially in obese individuals) | |
| Lung ultrasound | Provides an assessment of | Relatively easy to perform; able to use | Operator variability (transducer angle); |
| extravascular lung water | handheld ultrasound devices; may | technically challenging in obese | |
| (e.g., pulmonary edema, | reduce need for serial chest x-ray to | individuals; protocol variability; B lines | |
| pleural effusions) | monitor response to decongestive | lack specificity for pulmonary edema; | |
| treatment | unreliable in preexisting lung disease | ||
| Mitral E/A ratio and E/e′ | Provides an estimation of | Reproducible; prognostic; useful to | Unreliable in many clinical scenarios |
| ratio | left atrial pressure | distinguish cardiogenic versus | (e.g., atrial fibrillation; mitral annular |
| noncardiogenic pulmonary edema | calcification; mitral valve and | ||
| pericardial disease); operator | |||
| variability | |||
| (Doppler cursor angle; sample volume placement); indeterminate E/e′ values are common | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giangregorio, F.; Centenara, E.; Mazzocchi, S.; Gerra, L.; Tursi, F.; Imberti, D.; Aschieri, D. Assessing Venous Congestion in Acute and Chronic Heart Failure: A Review of Splanchnic, Cardiac and Pulmonary Ultrasound: Part 1: Conventional B-Mode, Colordoppler, and Vexus Protocol. J. Clin. Med. 2025, 14, 8147. https://doi.org/10.3390/jcm14228147
Giangregorio F, Centenara E, Mazzocchi S, Gerra L, Tursi F, Imberti D, Aschieri D. Assessing Venous Congestion in Acute and Chronic Heart Failure: A Review of Splanchnic, Cardiac and Pulmonary Ultrasound: Part 1: Conventional B-Mode, Colordoppler, and Vexus Protocol. Journal of Clinical Medicine. 2025; 14(22):8147. https://doi.org/10.3390/jcm14228147
Chicago/Turabian StyleGiangregorio, Francesco, Ester Centenara, Samanta Mazzocchi, Luigi Gerra, Francesco Tursi, Davide Imberti, and Daniela Aschieri. 2025. "Assessing Venous Congestion in Acute and Chronic Heart Failure: A Review of Splanchnic, Cardiac and Pulmonary Ultrasound: Part 1: Conventional B-Mode, Colordoppler, and Vexus Protocol" Journal of Clinical Medicine 14, no. 22: 8147. https://doi.org/10.3390/jcm14228147
APA StyleGiangregorio, F., Centenara, E., Mazzocchi, S., Gerra, L., Tursi, F., Imberti, D., & Aschieri, D. (2025). Assessing Venous Congestion in Acute and Chronic Heart Failure: A Review of Splanchnic, Cardiac and Pulmonary Ultrasound: Part 1: Conventional B-Mode, Colordoppler, and Vexus Protocol. Journal of Clinical Medicine, 14(22), 8147. https://doi.org/10.3390/jcm14228147







