Disease-Modifying Treatment Options in Very Early Onset Multiple Sclerosis—What Choices Are There for Onset Under 5 Years of Age? A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Data Analysis
2.3. Statistical Analysis
3. Results
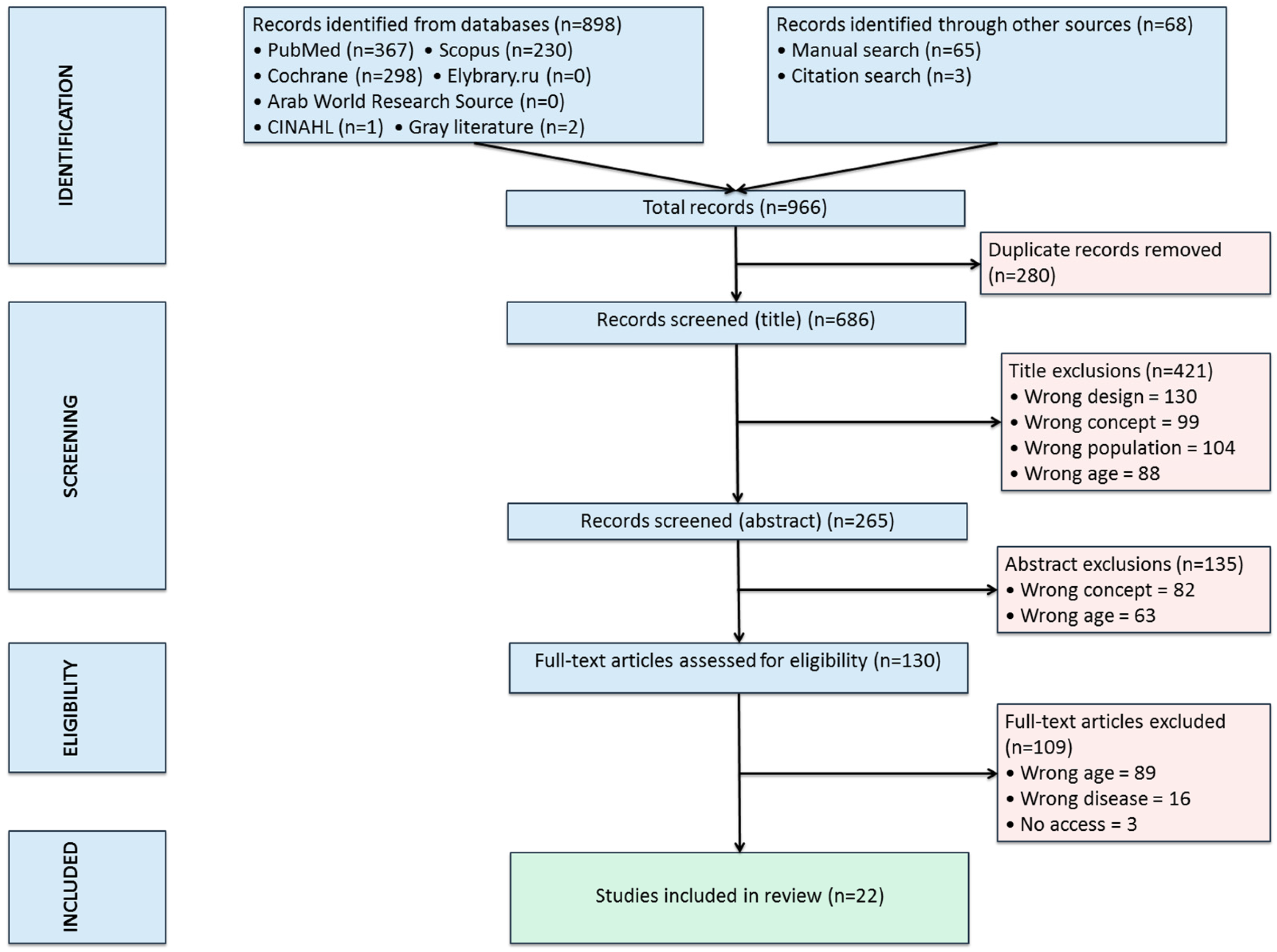
3.1. Literature Search
3.2. Data Analysis
3.2.1. Demographic and Clinical Data
3.2.2. Paraclinical Data
- Imaging: MRI was performed in 98 cases. Supratentorial lesions predominated (57.4%), most commonly periventricular (59.1%); infratentorial lesions were primarily located in the brainstem (32.7% of all cases) (Table 3). Cerebellar lesions were observed in 10 cases (10.2% of all cases) and spinal lesions in 12 cases (10.2% of all cases). Six patients underwent computer tomography (CT) examination (three had only a CT-scan), of which five revealed hypointense lesions. Two of the patients had a normal CT but abnormal IRM at the follow-up. The other one with normal CT had MS characteristic lesions that were confirmed by autopsy.
3.2.3. Cereb
- Cerebrospinal fluid (CSF) analysis performed in 99 cases showed pleocytosis (45 out of 999 cases/45.5%), hyper-proteinorrachia (27 out of 99 cases/27.3%), positive oligoclonal bands (32 out of 99 cases/32.3%), elevated IgG index (5 of 9 tested/55.5%) and anti-myelin basic protein antibodies (3 out of 3 cases/100%).
- Visual evoked potentials (VEPs) were abnormal in 10 out of 30 patients (33,3%). Autopsies were performed on two deceased patients, revealing multiple small sclerotic lesions, some with cystic components, distributed within the white matter, predominantly supratentorial periventricular.
3.2.4. Treatment
- Regarding treatment, 43 of all treated patients (95.6%) received steroids for at least one relapse (Table 4). In most cases, high-dose intravenous methylprednisolone was used, with or without subsequent tapering with oral prednisone.
- Three additional patients received intravenous immunoglobulin (IVIG) and steroids.
- Disease-modifying therapies were initiated in 11 cases (24.4% of all treated patients), of which 6 received low-efficacy agents (4 interferon, 1 Dimethyl fumarate, and 1 Glatiramer acetate) and 2 received high-efficacy agents (1 Natalizumab and 1 Rituximab). Azathioprine was administered in three cases.
3.2.5. Outcomes
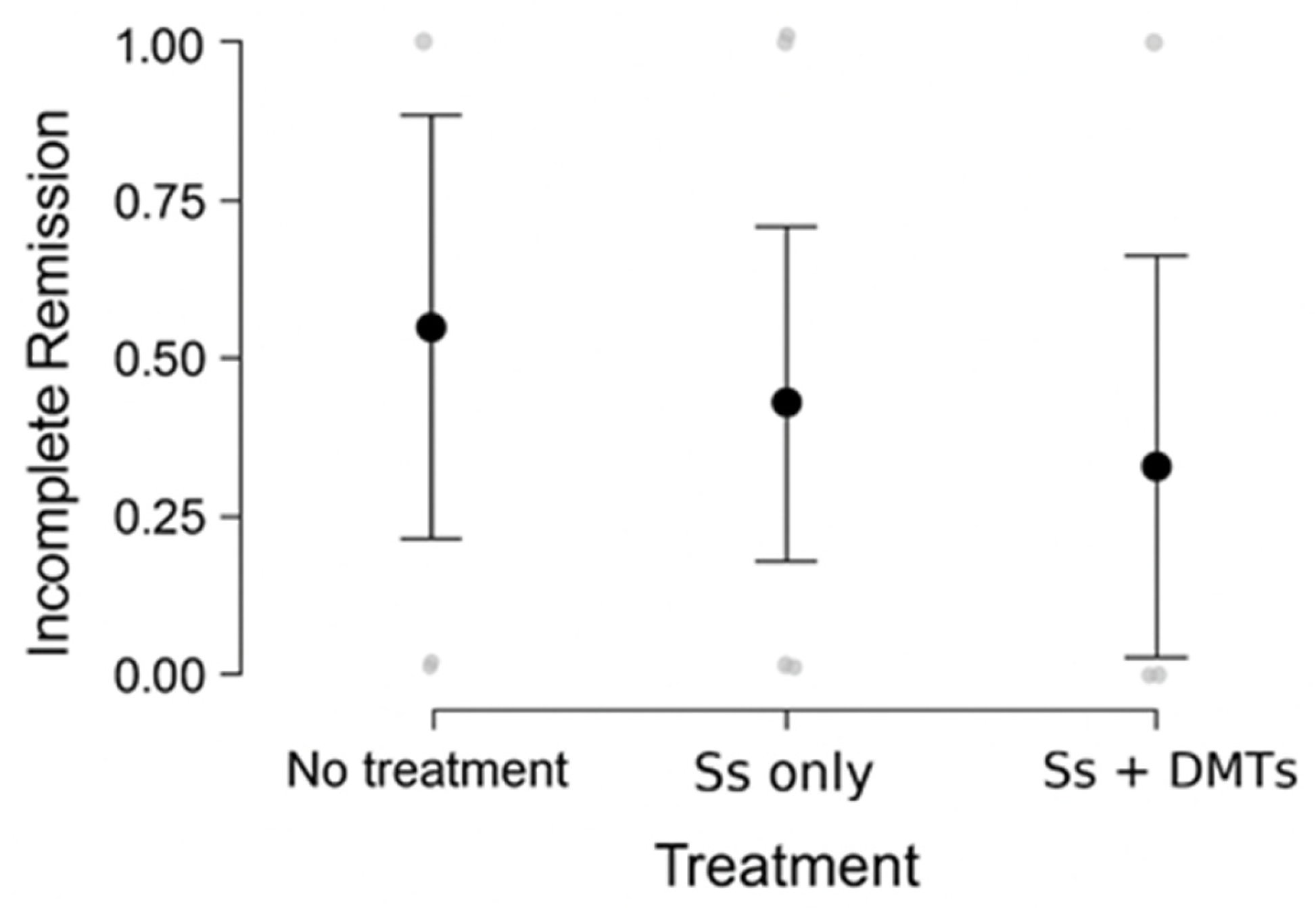
- Complete remission was documented in 88% of cases.
- Poor outcomes were observed in 12 cases, characterized by multiple relapses with incomplete recovery, progressive course, or lack of remission. Two deaths occurred, both in patients from the pre-2001 cohort who had not received treatment. Among patients with incomplete remission, 8 were treated with steroids, and 1 with steroids and Dimethyl fumarate.
- Escalation to higher efficacy therapies (Rituximab, Natalizumab, and Azathioprine) occurred in three cases, with adequate disease control achieved in the first two cases.
3.2.6. Statistical Analysis of Treatment Outcomes
3.3. Clinical Vignette
3.3.1. Workup
3.3.2. Evolution Under Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADEM | Acute disseminated encephalomyelitis |
| ANAs | Anti-nuclear antibodies |
| Anti-dsDNA | Anti-double-stranded DNA antibody |
| anti-Sm | Anti-Smith antibody |
| AQP4 | Serum aquaporin-4 autoantibodies |
| CI | Confidence interval |
| CSF | Cerebrospinal fluid |
| CT | Computer tomography |
| DIS | Dissemination in time |
| DIT | Dissemination in space |
| DMT | Disease-modifying therapy |
| EBV | Epstein–Barr virus |
| EDSS | Expanded Disability Status Scale |
| GenAI | Generative artificial intelligence |
| HBV | Hepatitis B virus |
| HIV | Human immunodeficiency virus |
| IVIG | Intravenous immunoglobulins |
| JCV | John Cunningham virus |
| LP | Lumbar puncture |
| MOG | Myelin oligodendrocyte glycoprotein |
| MOGAD | Myelin oligodendrocyte glycoprotein-associated disease |
| MRI | Magnetic resonance imaging |
| MS | Multiple sclerosis |
| NMO | Neuromyelitis optica |
| NMOSD | Neuromyelitis optic spectrum disorder |
| OCBs | Oligoclonal bands |
| POMS | Pediatric-onset multiple sclerosis |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RRMS | Relapsing remitting multiple sclerosis |
| Ss | Steroids |
| SD | Standard deviation |
| VEPs | Visual evoked potentials |
References
- Kauth, F.; Bertolini, A.; Wendel, E.-M.; Koukou, G.; El Naggar, I.; Chung, J.; Baumann, M.; Schödl, C.; Lechner, C.; Bigi, S.; et al. Characterization of children with early onset pediatric multiple sclerosis. Eur. J. Paediatr. Neurol. 2025, 54, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Glanz, B.; Jaffin, S.; Healy, B. Demographics of pediatric-onset multiple sclerosis in an MS center population from the Northeastern United States. Mult. Scler. 2009, 15, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Ness, J.M.; Chabas, D.; Sadovnick, A.D.; Pohl, D.; Banwell, B.; Weinstock-Guttman, B.; International Pediatric MS Study Group. Clinical features of children and adolescents with multiple sclerosis. Neurology 2007, 68 (Suppl. 2), S37–S45. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, S.; Banwell, B. Pediatric multiple sclerosis. Neurologist 2010, 16, 92–105. [Google Scholar] [CrossRef]
- Arkar, U.; Vipotnik Vesnaver, T.; Osredkar, D.; Perković Benedik, M.; Bizjak, N. Multiple sclerosis in a 4-year-old boy: A case report and literature review. Front. Neurol. 2024, 15, 1359938. [Google Scholar] [CrossRef]
- Mavridi, A.; Bompou, M.E.; Redmond, A.; Archontakis-Barakakis, P.; Vavougios, G.D.; Mitsikostas, D.D.; Mavridis, T. Current and emerging treatment options in pediatric onset multiple sclerosis. Sclerosis 2024, 2, 88–107. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Poser, C.M.; Paty, D.W.; Scheinberg, L.; McDonald, W.I.; Davis, F.A.; Ebers, G.C.; Johnson, K.P.; Sibley, W.A.; Silberberg, D.H.; Tourtellotte, W.W. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann. Neurol. 1983, 13, 227–231. [Google Scholar] [CrossRef]
- Miller, D.H.; Weinshenker, B.G.; Filippi, M.; Banwell, B.L.; Cohen, J.A.; Freedman, M.S.; Galetta, S.L.; Hutchinson, M.; Johnson, R.T.; Kappos, L.; et al. Differential diagnosis of suspected multiple sclerosis: A consensus approach. Mult. Scler. 2008, 14, 1157–1174. [Google Scholar] [CrossRef]
- de Mol, C.L.; Wong, Y.; van Pelt, E.D.; Wokke, B.; Siepman, T.; Neuteboom, R.F.; Hamann, D.; Hintzen, R.Q. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult. Scler. 2020, 26, 806–814. [Google Scholar] [CrossRef]
- Carvalho, I.V.; Dos Santos, C.S.; Amaral, J.; Ribeiro, J.A.; Pereira, C.; Pais, R.P.; Palavra, F. Multiple sclerosis under the age of ten: The challenge of a rare diagnosis in a special population— a case series. Front. Neurosci. 2023, 17, 1297171. [Google Scholar] [CrossRef]
- Cole, G.F.; Auchterlonie, L.A.; Best, P.V. Very early onset multiple sclerosis. Dev. Med. Child Neurol. 1995, 37, 667–672. [Google Scholar] [CrossRef]
- Calcii, C.; Aminov, D.; Sprincean, M.; Marga, S.; Anton-Paduraru, D.-T.; Revenco, N.; Groppa, S.; Palii, I.; Pirtu, L.; Hadjiu, S. Very rare incident of pediatric onset multiple sclerosis complicated with status epilepticus: A case report. Austin Neurol. Neurosci. 2023, 6, 1028. [Google Scholar]
- Gaccon, L. A case of childhood multiple sclerosis. Br. Ir. Orthopt. J. 2006, 3, 44–46. [Google Scholar] [CrossRef]
- Gargouri, L.; Safi, F.; Fourati, H.; Kmiha, S.; Turki, F.; Zidi, F.; Mnif, Z.; Mahfoudh, A. Early-onset of multiple sclerosis in a 5-year-old girl. Arch. Pediatr. 2014, 21, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Guilhoto, L.M.d.F.F.; Osório, C.A.M.; Machado, L.R.; de Castro, C.P.; Manreza, M.L.G.; Callegaro, D.; Kok, F.; Diament, A. Pediatric multiple sclerosis: Report of 14 cases. Brain Dev. 1995, 17, 9–12. [Google Scholar] [CrossRef]
- Hauser, S.L.; Reinherz, E.L.; Hoban, C.J.; Schlossman, S.F.; Weiner, H.L. Immunoregulatory T-cells and lymphocytotoxic antibodies in active multiple sclerosis: Weekly analysis over a six-month period. Ann. Neurol. 1983, 13, 418–425. [Google Scholar] [CrossRef]
- Hwang, J.S.; Kim, S.J.; Yu, Y.S.; Chung, H. Clinical characteristics of multiple sclerosis and associated optic neuritis in Korean children. J. AAPOS 2007, 11, 559–563. [Google Scholar] [CrossRef]
- Maeda, Y.; Kitamoto, I.; Kurokawa, T.; Ueda, K.; Hasuo, K.; Fujioka, K. Infantile multiple sclerosis with extensive white matter lesions. Pediatr. Neurol. 1989, 5, 317–319. [Google Scholar] [CrossRef]
- Mikaeloff, Y.; Caridade, G.; Assi, S.; Suissa, S.; Tardieu, M. Prognostic factors for early severity in a childhood multiple sclerosis cohort. Pediatrics 2006, 118, 1133–1139. [Google Scholar] [CrossRef]
- Pérez-Fernández, V.; Martínez-Salcedo, E.; Climent-Oltra, V.; Domingo-Jiménez, R.; Casas-Fernández, C. Esclerosis múltiple: A propósito de un caso de inicio muy precoz. Rev. Neurol. 1999, 28, 488–491. [Google Scholar] [CrossRef]
- Rai, B.; Riazat, M.I.; Sharif, F. P652: An unusual case of recurring demyelinating neurological disorder in a 3-year-old. Arch. Dis. Child. 2019, 104, A410. [Google Scholar]
- Rodríguez Núñez, A.; Redondo, J.L.; Cabadas, R.; Sánchez, J.M.C.; Gago, M.C. Esclerosis múltiple en la edad preescolar: Aportación diagnóstica de la resonancia magnética. An. Esp. Pediatr. 1992, 37, 405–407. [Google Scholar] [PubMed]
- Ruggieri, M.; Polizzi, A.; Pavone, L.; Grimaldi, L.M.E. Multiple sclerosis in children under 6 years of age. Neurology 1999, 53, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Rushdi, R.; Shalaby, N. Pediatric multiple sclerosis: Unveiling the trajectory of a toddler-onset case. Neuroimmunol. Rep. 2024, 6, 100222. [Google Scholar] [CrossRef]
- Saijo, N.; Abe, Y.; Oikawa, Y.; Okubo, Y.; Endo, W.; Numata-Uematsu, Y.; Takahashi, T.; Uematsu, M. Successful treatment with Dimethyl fumarate in a child with relapsing–remitting multiple sclerosis. Brain Dev. 2022, 44, 353–356. [Google Scholar] [CrossRef]
- Sánchez-Calderón, M.; De Santos, T.; Martín, S.; Angulo, T.; Careaga, J.; Campos-Castelló, J. Esclerosis múltiple en la infancia: Nuestra experiencia y revisión de la literatura. Rev. Neurol. 1998, 27, 237–241. [Google Scholar] [CrossRef]
- Sawant, T.A.; Lynch, B.; Sharif, F. A child with early onset multiple sclerosis. BMJ Paediatr. Open 2024, 8 (Suppl. S1), A115. [Google Scholar]
- Shaw, C.-M.; Alvord, E.C. Multiple sclerosis beginning in infancy. J. Child Neurol. 1987, 2, 252–256. [Google Scholar] [CrossRef]
- Sivaraman, I.; Moodley, M. Multiple sclerosis in the very young: A case report and review of the literature. Neurodegener. Dis. Manag. 2016, 6, 31–36. [Google Scholar] [CrossRef]
- Sotgiu, S.; Nieddu, A.; Pruna, D.; Madrau, A.; Zarbo, I.R.; Carta, A. On a 5-year-old girl with multiple sclerosis treated with Natalizumab. Neurol. Sci. 2023, 44, 2963–2965. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Huppke, P.; Huppke, B.; Ellenberger, D.; Rostasy, K.; Hummel, H.; Stark, W.; Brück, W.; Gärtner, J. Therapy of highly active pediatric multiple sclerosis. Mult. Scler. 2019, 25, 72–80. [Google Scholar] [CrossRef] [PubMed]
- McElroy, J.P.; Krupp, L.B.; Johnson, B.A.; McCauley, J.L.; Qi, Z.; Caillier, S.J.; Gourraud, P.A.; Yu, J.; Nathanson, L.; Belman, A.L.; et al. Copy number variation in pediatric multiple sclerosis. Mult. Scler. 2013, 19, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Chou, I.J.; Wang, H.S.; Whitehouse, W.P.; Constantinescu, C.S. Paediatric multiple sclerosis: Update on diagnostic criteria, imaging, histopathology and treatment choices. Curr. Neurol. Neurosci. Rep. 2016, 16, 68. [Google Scholar] [CrossRef]
- Fadda, G.; Waters, P.; Woodhall, M.; Brown, R.A.; O’Mahony, J.; Castro, D.A.; Longoni, G.; Yeh, E.A.; Marrie, R.A.; Arnold, D.L.; et al. Serum MOG-IgG in children meeting multiple sclerosis diagnostic criteria. Mult. Scler. 2022, 28, 1697–1709. [Google Scholar] [CrossRef]
- Vergani, M.I.; Reimão, R.; Silva, A.M.; Muskat, M.; Espósito, S.; Diament, A. Multiple sclerosis with early childhood onset. A case report. Arq. Neuropsiquiatr. 1988, 46, 195–197. [Google Scholar] [CrossRef]
- Asai, K.; Inagaki, M.; Maegaki, Y.; Yamamoto, T.; Suzaki, I.; Ohta, S. An early-onset case of multiple sclerosis with thalamic lesions on MRI. Acta Paediatr. Jpn. 1994, 36, 431–434. [Google Scholar] [CrossRef]
- Murakami, Y.; Matsuishi, T.; Shimizu, T.; Yamashita, Y.; Nagamitsu, S.; Kojima, K.; Kato, H.; Tabira, T. Baló’s concentric sclerosis in a 4-year-old Japanese infant. Brain Dev. 1998, 20, 250–252. [Google Scholar] [CrossRef]
- Bejar, J.M.; Ziegler, D.K. Onset of multiple sclerosis in a 24-month-old child. Arch. Neurol. 1984, 41, 881–882. [Google Scholar] [CrossRef]
- Brandt, S.; Gyldensted, C.; Offner, H.; Melchior, J.C. Multiple sclerosis with onset in a two-year old boy. Neuropediatrics 1981, 12, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, F.; Bauer, H.J.; Christen, H.J.; Kruse, B.; Bruhn, H.; Frahm, J. Multiple sclerosis in childhood: Report of 15 cases. Brain Dev. 1991, 13, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.J.; Hanefeld, F.; Christen, H.J. Multiple sclerosis in early childhood. Lancet 1990, 336, 1190. [Google Scholar] [CrossRef] [PubMed]
- Boutin, B.; Esquivel, E.; Mayer, M.; Chaumet, S.; Ponsot, G.; Arthuis, M. Multiple sclerosis in children: Report of clinical and paraclinical features of 19 cases. Neuropediatrics 1988, 19, 118–123. [Google Scholar] [CrossRef]
- Bye, A.M.E.; Kendall, B.; Wilson, J. Multiple sclerosis in childhood: A new look. Dev. Med. Child Neurol. 1985, 27, 215–222. [Google Scholar] [CrossRef]
- Haas, G.; Schroth, G.; Krägeloh-Mann, I.; Buchwald-Saal, M. Magnetic resonance imaging of the brain of children with multiple sclerosis. Dev. Med. Child Neurol. 1987, 29, 586–591. [Google Scholar] [CrossRef]
- Corbali, O.; Chitnis, T. Pathophysiology of myelin oligodendrocyte glycoprotein antibody disease. Front. Neurol. 2023, 14, 1137998. [Google Scholar] [CrossRef]
- O’Connor, K.C.; McLaughlin, K.A.; De Jager, P.L.; Chitnis, T.; Bettelli, E.; Xu, C.; Robinson, W.H.; Cherry, S.V.; Bar-Or, A.; Banwell, B.; et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat. Med. 2007, 13, 211–217. [Google Scholar] [CrossRef]
- Banwell, B.; Bennett, J.L.; Marignier, R.; Kim, H.J.; Brilot, F.; Flanagan, E.P.; Ramanathan, S.; Waters, P.; Tenembaum, S.; Graves, J.S.; et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. 2023, 22, 268–282. [Google Scholar] [CrossRef]
- Krupp, L.B.; Tardieu, M.; Amato, M.P.; Banwell, B.; Chitnis, T.; Dale, R.C.; Ghezzi, A.; Hintzen, R.; Kornberg, A.; Pohl, D.; et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: Revisions to the 2007 definitions. Mult. Scler. 2013, 19, 1261–1267. [Google Scholar] [CrossRef]
- Dică, A.D.; Craiu, D.; Linca, F.I.; Budișteanu, M.; Iliescu, C.; Sandu, C.; Pomeran, C.; Bârcă, D.; Butoianu, N.; Burloiu, C.; et al. Age-onset-related particularities of pediatric MS—Understanding the spectrum: A tertiary center experience. Diseases 2025, 13, 193. [Google Scholar] [CrossRef]
- Renoux, C.; Vukusic, S.; Mikaeloff, Y.; Edan, G.; Clanet, M.; Dubois, B.; Debouverie, M.; Brochet, B.; Lebrun-Frenay, C.; Pelletier, J.; et al. Natural history of multiple sclerosis with childhood onset. N. Engl. J. Med. 2007, 356, 2603–2613. [Google Scholar] [CrossRef]
- Neuß, F.; von Podewils, F.; Wang, Z.I.; Süße, M.; Zettl, U.K.; Grothe, M. Epileptic seizures in multiple sclerosis: Prevalence, competing causes and diagnostic accuracy. J. Neurol. 2021, 268, 1721–1727. [Google Scholar] [CrossRef]
- Baroncini, D.; Simone, M.; Iaffaldano, P.; Brescia Morra, V.; Lanzillo, R.; Filippi, M.; Romeo, M.; Patti, F.; Chisari, C.G.; Cocco, E.; et al. Risk of persistent disability in patients with pediatric-onset multiple sclerosis. JAMA Neurol. 2021, 78, 726–735. [Google Scholar] [CrossRef]
- Baroncini, D.; Ghezzi, A.; Guaschino, C.; Moiola, L.; Filippi, M.; Ianniello, A.; Pozzilli, C.; Lanzillo, R.; Brescia-Morra, V.; Margoni, M.; et al. Long-term follow-up (up to 11 years) of an Italian pediatric MS cohort treated with Natalizumab: A multicenter, observational study. Neurol. Sci. 2022, 43, 6415–6423. [Google Scholar] [CrossRef] [PubMed]
- Menascu, S.; Fattal-Valevski, A.; Vaknin-Dembinsky, A.; Milo, R.; Geva, K.; Magalashvili, D.; Dolev, M.; Flecther, S.; Kalron, A.; Miron, S.; et al. Effect of Natalizumab treatment on the rate of No Evidence of Disease Activity in young adults with multiple sclerosis in relation to pubertal stage. J. Neurol. Sci. 2022, 432, 120074. [Google Scholar] [CrossRef] [PubMed]
- Tysabri, INN: Natalizumab. Annex I, Summary Of Product Characteristics. Available online: https://ec.europa.eu/health/documents/community-register/2016/20160425134515/anx_134515_en.pdf (accessed on 16 September 2025).
- Simone, I.L.; Carrara, D.; Tortorella, C.; Liguori, M.; Lepore, V.; Pellegrini, F.; Bellacosa, A.; Ceccarelli, A.; Pavone, I.; Livrea, P. Course and prognosis in early-onset MS: Comparison with adult-onset forms. Neurology 2002, 59, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- JASP (Version 095.1). Available online: https://jasp-stats.org/download (accessed on 4 September 2025).
- Generative Artificial Intelligence (GenAI). Available online: https://chat.chatbot.app/gpt5?gad_campaignid=23089952391 (accessed on 16 September 2025).


| Features | |
|---|---|
| Sex | |
| Male | 42 |
| Female | 59 |
| F:M ratio | 1.47:1 |
| Age at onset (mo.) mean ± SD (range) | 36 ± 13.29 (10–60) |
| Mean total number of attacks | 4.1 |
| Mean number of attacks in the first 2 years | 2.3 |
| Follow-up time (mo.) mean (range) | 26.4 (10–48) |
| Features | Onset (% of Cases) | Evolution (% of Cases) |
|---|---|---|
| Symptoms | ||
| Ataxia | 57.4% | 42.9.% |
| Pyramidal | 41.4% | 47.5% |
| Fever ± lethargy/altered consciousness | 17.2% | 4.9% |
| Ophthalmoplegia | 10.3% | 3.9% |
| Optic neuritis | 6.9% | 21.7% |
| Seizures | 4.3% | 22.7% |
| Neurogenic bladder | 3.9% | 3.9% |
| Other cranial nerve palsies | 3.4% | 19.8% |
| Features | % of All Cases | % of Tested Cases |
|---|---|---|
| MRI | ||
| Supratentorial lesions | 57.4% | 59.1% |
| Periventricular | 57.4% | 59.1% |
| Cortical/subcortical | 24.7% | 25.5% |
| Infratentorial lesions | 36.6% | 37.7% |
| Brainstem | 31.7% | 32.7% |
| Spinal cord | 11.9% | 12.2% |
| Cerebellum | 9.9% | 10.2% |
| CSF | ||
| Normal CSF | 11.9% | 12.1% |
| Oligoclonal bands (positive) | 31.7% | 32.3% |
| Pleocytosis | 44.5% | 45.5% |
| Elevated protein | 26.7% | 27.3% |
| VEPs | ||
| Abnormal VEPs | 9.9% | 33.3% |
| Normal VEPs | 19.8% | 66.7% |
| Features | Number | % of All Cases | % of Treated Cases |
|---|---|---|---|
| Steroid therapy | 44 | 43.5% | 80% |
| Methylprednisolone | 26 | 25.7% | 47.3% |
| Prednisone/prednisolone | 17 | 16.8% | 30.9% |
| Other steroids | 3 | 2.9% | 3.7% |
| Intravenous immunoglobulin | 3 | 2.9% | 3.7% |
| Disease-modifying therapy | 11 | 10.9% | 20.0% |
| Interferons | 4 | 4.0% | 7.3% |
| Azathioprine | 3 | 2.9% | 3.7% |
| Dimethyl fumarate | 1 | 1.0% | 1.8% |
| Glatiramer acetate | 1 | 1.0% | 1.8% |
| Natalizumab | 1 | 1.0% | 1.8% |
| Rituximab | 1 | 1.0% | 1.8% |
| Episode | Age | Symptoms | Episode Duration | Type of Remission | Residual Symptoms | Free Interval After Episode | Treatment in Episode | DMT | Observations |
|---|---|---|---|---|---|---|---|---|---|
| I | 2 y 4 m | Severe truncal ataxia Irritability | 5 w | Complete | - | 30 d | ACTH 0.5 mg/day—14 d Prednisone 0.5 mg/kg/day—7 d | - | |
| II | 2 y 6 m | Severe ataxia (R > L CS) Irritability | 5 w | Complete | - | 30 d | Dexamethasone 8 mg/day—3 d ACTH 1/3 mg/day—14 d | - | |
| III | 2 y 8 m | Severe ataxia (CS) L > R PS Irritability Saccadic speech (CS) | 3 w | Incomplete | L pyramidal Mild ataxia | 30 d | Methylprednisolone i.v. 30 mg/kg/day—6 d Tapered with Medrol IVIG 2 g/kg (in 6 d) | - | Diagnosis = RRMS |
| IV | 2 y 10 m | Severe ataxia (CS) L > R PS Irritability | 1 w | Incomplete | L pyramidal Mild ataxia | 30 d | Methylprednisolone i.v. 30 mg/kg/day—5 d Tapered with Medrol | IFN beta-1a * | |
| V | 2 y 11 m | Severe ataxia (CS) L > R PS Irritability | 1 w | Incomplete | L pyramidal Mild ataxia | 60 d | Methylprednisolone i.v. 30 mg/kg/day—5 d Tapered with Medrol | ||
| VI | 3 y 1 m | Irritability, mild ataxia | 2 d | Incomplete | Mild L pyramidal | 60 d | - | ||
| VII | 3 y 3 m | Irritability, mild ataxia | 2 d | Incomplete | Mild L pyramidal | 10 m | - | ||
| VIII | 4 y 1 m | Slight left intentional tremor (CS) Mild left hemiparesis (PS) | Not known | Incomplete | Mild L pyramidal | 13 m | Methylprednisolone i.v. 30 mg/kg/day—5 d Tapered with Medrol | IVIG 2 g/kg/administration—monthly | |
| IX | 5 y 2 m | Mild left hemiparesis | 1 w | Incomplete | Mild L pyramidal | 11 m | Methylprednisolone i.v. 30 mg/kg/day—5 d Tapered with Medrol | ||
| X | 6 y 1 m | Mild left hemiparesis | 1 w | Incomplete | Mild L pyramidal | 12 m | Methylprednisolone i.v. 30 mg/kg/day—5 d Tapered with Medrol | ||
| XI | 7 y 1 m | Mild left hemiparesis | 1 w | Incomplete | Mild L pyramidal | 9 y–present | Methylprednisolone i.v. 30 mg/kg/day—5 d Tapered with Medrol | Natalizumab 300 mg/dose every 28 d | No relapses since initiation |
| Differential Diagnosis | Observations | Investigation |
|---|---|---|
| NMO, recurrent | - No optic neuritis -Spinal cord involvement less than 3 spinal segments | AQP4 negative Oligoclonal bands negative |
| ADEM, recurrent | -No encephalopathy (except behavioral symptoms at first episode) Remission of initial symptoms followed by new symptoms after interval of 1 month (MS more probable), Miller et al. [29] | MRI—DIT, DIS, lesions typical for MS |
| Infectious diseases, including Borreliosis, HIV, HBV, EBV, cysticercosis | No fever No other organ involvement | LP—CSF and blood serology negative |
| Autoimmune disorders | No other organ involvement | Blood serology negative (ANA, anti-dsDNA antibodies, anti-Sm antibodies, serum complement) |
| Mitochondrial disorder | - No other organ involvement - Clinical evolution typical for MS - Treatment—efficacious | Lactic acid negative—blood and CSF; MRI—lesions typical for MS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craiu, D.; Dica, A.D.; Pomeran, C.; Pescaru, G.; Menascu, S.; Simu, M. Disease-Modifying Treatment Options in Very Early Onset Multiple Sclerosis—What Choices Are There for Onset Under 5 Years of Age? A Systematic Review. J. Clin. Med. 2025, 14, 8133. https://doi.org/10.3390/jcm14228133
Craiu D, Dica AD, Pomeran C, Pescaru G, Menascu S, Simu M. Disease-Modifying Treatment Options in Very Early Onset Multiple Sclerosis—What Choices Are There for Onset Under 5 Years of Age? A Systematic Review. Journal of Clinical Medicine. 2025; 14(22):8133. https://doi.org/10.3390/jcm14228133
Chicago/Turabian StyleCraiu, Dana, Alice Denisa Dica, Cristina Pomeran, George Pescaru, Shay Menascu, and Mihaela Simu. 2025. "Disease-Modifying Treatment Options in Very Early Onset Multiple Sclerosis—What Choices Are There for Onset Under 5 Years of Age? A Systematic Review" Journal of Clinical Medicine 14, no. 22: 8133. https://doi.org/10.3390/jcm14228133
APA StyleCraiu, D., Dica, A. D., Pomeran, C., Pescaru, G., Menascu, S., & Simu, M. (2025). Disease-Modifying Treatment Options in Very Early Onset Multiple Sclerosis—What Choices Are There for Onset Under 5 Years of Age? A Systematic Review. Journal of Clinical Medicine, 14(22), 8133. https://doi.org/10.3390/jcm14228133





