Prognostic Role and Therapeutic Implications of Intravascular Optical Coherence Tomography Detected Coronary Plaque Microstructures in Patients with Coronary Artery Disease
Abstract
1. Introduction
2. Prognostic Significance of Plaque Microstructures Identified by OCT Imaging
2.1. TCFA
2.2. Lipid-Rich Plaque and Lipid Burden
2.3. Healed Plaques
2.4. Macrophage Infiltration (MØI)
2.5. Cholesterol Crystals (CCs)
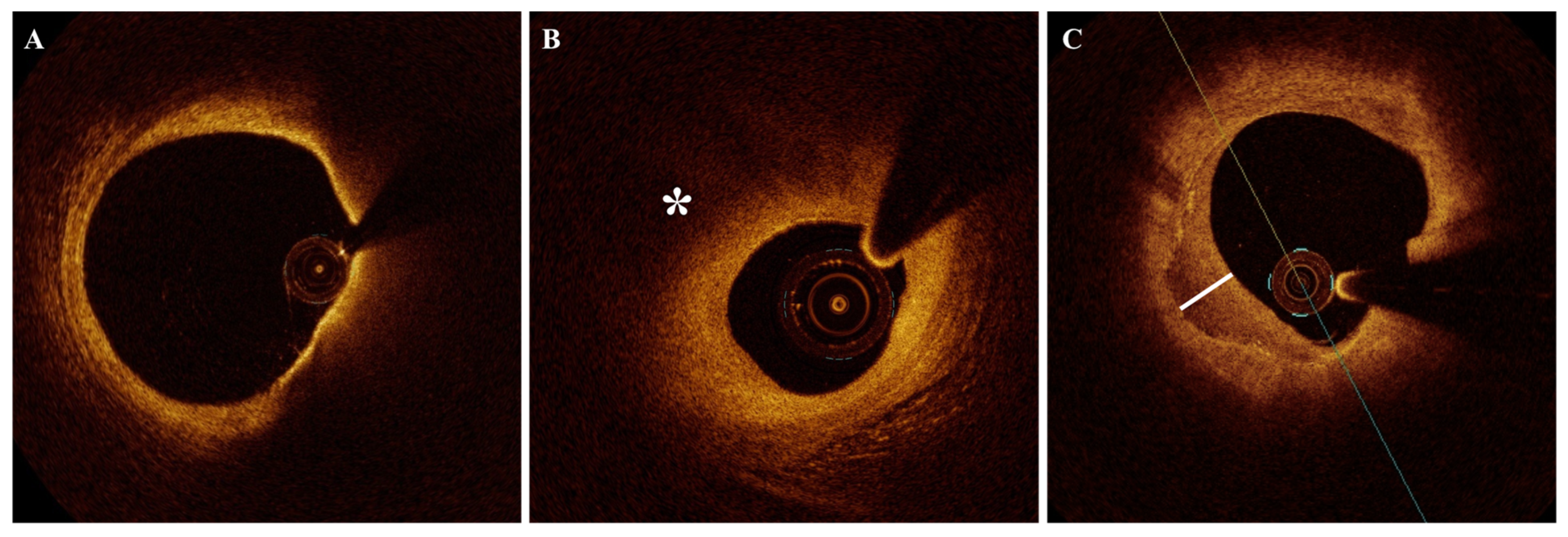
2.6. Microvessels
2.7. Calcifications
2.8. Previous Ruptures (Cavities Within the Plaque in Stable Patients)
3. Therapeutic Implications
3.1. Lipid-Lowering Therapies
3.2. Colchicine
3.3. Preventive PCI
4. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gall, E.; Pezel, T.; Lattuca, B.; Hamzi, K.; Puymirat, E.; Piliero, N.; Deney, A.; Fauvel, C.; Aboyans, V.; Schurtz, G.; et al. Profile of patients hospitalized in intensive cardiac care units in France: ADDICT-ICCU registry. Arch. Cardiovasc. Dis. 2024, 117, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Park, S.J.; Dauerman, H.L.; Uemura, S.; Kim, J.S.; Di Mario, C.; Johnson, T.W.; Guagliumi, G.; Kastrati, A.; Joner, M.; et al. Optical coherence tomography in coronary atherosclerosis assessment and intervention. Nat. Rev. Cardiol. 2022, 19, 684–703, Erratum in Nat. Rev. Cardiol. 2024, 21, 348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537, Erratum in Eur. Heart J. 2025, 46, 1565. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826, Erratum in Eur. Heart J. 2024, 45, 1145. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Guagliumi, G.; Mintz, G.S.; Costa, M.; Regar, E.; Akasaka, T.; Barlis, P.; Tearney, G.J.; Jang, I.K.; Arbustini, E.; et al. Expert review document part 2: Methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur. Heart J. 2012, 33, 2513–2520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, T.W.; Räber, L.; di Mario, C.; Bourantas, C.; Jia, H.; Mattesini, A.; Gonzalo, N.; de la Torre Hernandez, J.M.; Prati, F.; Koskinas, K.; et al. Clinical use of intracoronary imaging. Part 2: Acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2019, 40, 2566–2584. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Romagnoli, E.; Gatto, L.; La Manna, A.; Burzotta, F.; Ozaki, Y.; Marco, V.; Boi, A.; Fineschi, M.; Fabbiocchi, F.; et al. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: The CLIMA study. Eur. Heart J. 2020, 41, 383–391, Erratum in Eur. Heart J. 2020, 41, 393. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahn, J.M.; Kang, D.Y.; Yun, S.C.; Ahn, Y.K.; Kim, W.J.; Nam, C.W.; Jeong, J.O.; Chae, I.H.; Shiomi, H.; et al. Preventive percutaneous coronary intervention versus optimal medical therapy alone for the treatment of vulnerable atherosclerotic coronary plaques (PREVENT): A multicentre, open-label, randomised controlled trial. Lancet 2024, 403, 1753–1765, Erratum in Lancet 2024, 404, 1928. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47 (Suppl. 8), C13–C18. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Yasuda, S.; Noguchi, T.; Ishibashi-Ueda, H. Pathology of coronary atherosclerosis and thrombosis. Cardiovasc. Diagn. Ther. 2016, 6, 396–408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gurgoglione, F.L.; Denegri, A.; Russo, M.; Calvieri, C.; Benatti, G.; Niccoli, G. Intracoronary Imaging of Coronary Atherosclerotic Plaque: From Assessment of Pathophysiological Mechanisms to Therapeutic Implication. Int. J. Mol. Sci. 2023, 24, 5155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burke, A.P.; Farb, A.; Malcom, G.T.; Liang, Y.H.; Smialek, J.; Virmani, R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N. Engl. J. Med. 1997, 336, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Soeda, T.; Kim, H.O.; Thondapu, V.; Russo, M.; Kurihara, O.; Shinohara, H.; Minami, Y.; Higuma, T.; Lee, H.; et al. Spatial Distribution of Vulnerable Plaques: Comprehensive In Vivo Coronary Plaque Mapping. JACC Cardiovasc. Imaging 2020, 13, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, M.; Quadri, G.; Taha, S.; D’Ascenzo, F.; Montefusco, A.; Omede’, P.; Jang, I.K.; Niccoli, G.; Souteyrand, G.; Yundai, C.; et al. Prevalence and predictors of culprit plaque rupture at OCT in patients with coronary artery disease: A meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Raffel, O.C.; Tearney, G.J.; Gauthier, D.D.; Halpern, E.F.; Bouma, B.E.; Jang, I.K. Relationship between a systemic inflammatory marker, plaque inflammation, and plaque characteristics determined by intravascular optical coherence tomography. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1820–1827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aguirre, A.D.; Arbab-Zadeh, A.; Soeda, T.; Fuster, V.; Jang, I.K. Optical Coherence Tomography of Plaque Vulnerability and Rupture: JACC Focus Seminar Part 1/3. J. Am. Coll. Cardiol. 2021, 78, 1257–1265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xue, C.; Chen, Q.; Bian, L.; Yin, Z.; Xu, Z.; Zhang, H.; Zhang, Q.; Zhang, J.; Wang, C.; Du, R.; et al. The relationships between cholesterol crystals, NLRP3 inflammasome, and coronary atherosclerotic plaque vulnerability in acute coronary syndrome: An optical coherence tomography study. Front. Cardiovasc. Med. 2022, 9, 905363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, C.; Deng, C.; Xia, J.; Wang, S.; Zhang, L.; Liu, Z.; Zhang, W.; Deng, Y.; Lu, S.; Xu, G.; et al. Peri-coronary adipose tissue attenuation and its association with plaque vulnerability and clinical outcomes in coronary artery disease using combined CCTA and OCT. Sci. Rep. 2025, 15, 16520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katamine, M.; Minami, Y.; Nagata, T.; Asakura, K.; Muramatsu, Y.; Kinoshita, D.; Fujiyoshi, K.; Ako, J. High-sensitivity C-reactive protein, plaque vulnerability and adverse events in patients with stable coronary disease: An optical coherence tomography study. Int. J. Cardiol. 2025, 421, 132924. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhao, J.; Xu, X.; Chen, Y.; Sun, S.; Li, S.; Cui, L.; Wang, Y.; Li, L.; Guo, R.; et al. Long-Term Prognostic Implications of Non-Culprit Lesions in Patients Presenting with an Acute Myocardial Infarction: Is It the Angiographic Stenosis Severity or the Underlying High-Risk Morphology? Circulation 2025, 151, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Biccirè, F.G.; Fabbiocchi, F.; Gatto, L.; La Manna, A.; Ozaki, Y.; Romagnoli, E.; Marco, V.; Boi, A.; Fineschi, M.; Piedimonte, G.; et al. Long-Term Prognostic Impact of OCT-Derived High-Risk Plaque Features: Extended Follow-Up of the CLIMA Study. JACC Cardiovasc. Interv. 2025, 18, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- Fabris, E.; Berta, B.; Hommels, T.; Roleder, T.; Hermanides, R.S.; Rivero, F.; von Birgelen, C.; Escaned, J.; Camaro, C.; Kennedy, M.W.; et al. Long-term outcomes of patients with normal fractional flow reserve and thin-cap fibroatheroma. EuroIntervention 2023, 18, e1099–e1107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gallone, G.; Bellettini, M.; Gatti, M.; Tore, D.; Bruno, F.; Scudeler, L.; Cusenza, V.; Lanfranchi, A.; Angelini, A.; de Filippo, O.; et al. Coronary Plaque Characteristics Associated with Major Adverse Cardiovascular Events in Atherosclerotic Patients and Lesions: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2023, 16, 1584–1604, Erratum in JACC Cardiovasc. Imaging 2024, 17, 578. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Fang, C.; Xu, X.; Xing, L.; Sun, S.; Peng, C.; Yin, Y.; Lei, F.; Wang, Y.; Li, L.; et al. Identification of High-Risk Coronary Lesions by 3-Vessel Optical Coherence Tomography. J. Am. Coll. Cardiol. 2023, 81, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Ino, Y.; Mintz, G.S.; Shiono, Y.; Shimamura, K.; Takahata, M.; Terada, K.; Higashioka, D.; Emori, H.; Wada, T.; et al. Optical coherence tomography detection of vulnerable plaques at high risk of developing acute coronary syndrome. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Kanenawa, K.; Morofuji, T.; Nishikawa, R.; Imada, K.; Kohjitani, H.; Watanabe, H.; Tazaki, J.; Taniwaki, M.; Koga, S.; et al. Serial Optical Coherence Tomography Assessment of Coronary Atherosclerosis and Long-Term Clinical Outcomes. J. Am. Heart Assoc. 2024, 13, e034458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kedhi, E.; Berta, B.; Roleder, T.; Hermanides, R.S.; Fabris, E.; IJsselmuiden, A.J.J.; Kauer, F.; Alfonso, F.; von Birgelen, C.; Escaned, J.; et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: The COMBINE OCT-FFR trial. Eur. Heart J. 2021, 42, 4671–4679. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Fracassi, F.; Kurihara, O.; Kim, H.O.; Thondapu, V.; Araki, M.; Shinohara, H.; Sugiyama, T.; Yamamoto, E.; Lee, H.; et al. Healed Plaques in Patients With Stable Angina Pectoris. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, R.; Crea, F. Atherosclerotic Plaque Healing. N. Engl. J. Med. 2020, 383, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Yonetsu, T.; Kurihara, O.; Nakajima, A.; Lee, H.; Soeda, T.; Minami, Y.; McNulty, I.; Uemura, S.; Kakuta, T.; et al. Predictors of Rapid Plaque Progression: An Optical Coherence Tomography Study. JACC Cardiovasc. Imaging 2021, 14, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, J.; He, L.; Zhang, D.; Yi, B.; Zuo, Y.; Qin, Y.; Zhao, C.; Weng, Z.; Sun, Y.; et al. Predictors and Mechanisms of Nonculprit Plaque Progression in Patients With Acute Coronary Syndromes: An In-Vivo Serial Optical Coherence Tomography Study. Am. J. Cardiol. 2025, 249, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Kim, H.O.; Kurihara, O.; Araki, M.; Shinohara, H.; Thondapu, V.; Yonetsu, T.; Soeda, T.; Minami, Y.; Higuma, T.; et al. Characteristics of non-culprit plaques in acute coronary syndrome patients with layered culprit plaque. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Sibbald, M.; Pinilla-Echeverri, N.; Alameer, M.; Chavarria, J.; Dutra, G.; Sheth, T. Using Optical Coherence Tomography to Identify Lipid and Its Impact on Interventions and Clinical Events—A Scoping Review. Circ. J. 2021, 85, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Yonetsu, T.; Kim, S.J.; Xing, L.; Lee, H.; McNulty, I.; Yeh, R.W.; Sakhuja, R.; Zhang, S.; Uemura, S.; et al. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non-acute coronary syndromes: A 3-vessel optical coherence tomography study. Circ. Cardiovasc. Imaging 2012, 5, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, L.; Agozzino, M.; Marco, V.; Ricciardi, A.; Concardi, M.; Romagnoli, E.; Gatto, L.; Calogero, G.; Tavazzi, L.; Arbustini, E.; et al. Identification and quantification of macrophage presence in coronary atherosclerotic plaques by optical coherence tomography. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Araki, M.; Kurihara, O.; Minami, Y.; Soeda, T.; Yonetsu, T.; Higuma, T.; Kakuta, T.; McNulty, I.; Lee, H.; et al. Predictors for Rapid Progression of Coronary Calcification: An Optical Coherence Tomography Study. J. Am. Heart Assoc. 2021, 10, e019235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xing, L.; Higuma, T.; Wang, Z.; Aguirre, A.D.; Mizuno, K.; Takano, M.; Dauerman, H.L.; Park, S.J.; Jang, Y.; Kim, C.J.; et al. Clinical Significance of Lipid-Rich Plaque Detected by Optical Coherence Tomography: A 4-Year Follow-Up Study. J. Am. Coll. Cardiol. 2017, 69, 2502–2513. [Google Scholar] [CrossRef] [PubMed]
- Biccirè, F.G.; Budassi, S.; Ozaki, Y.; Boi, A.; Romagnoli, E.; Di Pietro, R.; Bourantas, C.V.; Marco, V.; Paoletti, G.; Debelak, C.; et al. Optical coherence tomography-derived lipid core burden index and clinical outcomes: Results from the CLIMA registry. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Fabris, E.; Berta, B.; Roleder, T.; Hermanides, R.S.; IJsselmuiden, A.J.J.; Kauer, F.; Alfonso, F.; von Birgelen, C.; Escaned, J.; Camaro, C.; et al. Thin-Cap Fibroatheroma Rather than Any Lipid Plaques Increases the Risk of Cardiovascular Events in Diabetic Patients: Insights From the COMBINE OCT-FFR Trial. Circ. Cardiovasc. Interv. 2022, 15, e011728. [Google Scholar] [CrossRef] [PubMed]
- Shimokado, A.; Matsuo, Y.; Kubo, T.; Nishiguchi, T.; Taruya, A.; Teraguchi, I.; Shiono, Y.; Orii, M.; Tanimoto, T.; Yamano, T.; et al. In vivo optical coherence tomography imaging and histopathology of healed coronary plaques. Atherosclerosis 2018, 275, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Rinaldi, R.; Camilli, M.; Bonanni, A.; Caffè, A.; Basile, M.; Salzillo, C.; Colucci, M.; Torre, I.; Sanna, T.; et al. Air pollution and plaque healing in acute coronary syndromes. Eur. Heart J. 2023, 44, 2403–2405. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.P.; Kolodgie, F.D.; Farb, A.; Weber, D.K.; Malcom, G.T.; Smialek, J.; Virmani, R. Healed plaque ruptures and sudden coronary death: Evidence that subclinical rupture has a role in plaque progression. Circulation 2001, 103, 934–940. [Google Scholar] [CrossRef]
- Mann, J.; Davies, M.J. Mechanisms of progression in native coronary artery disease: Role of healed plaque disruption. Heart 1999, 82, 265–268. [Google Scholar] [CrossRef]
- Yamamoto, M.H.; Yamashita, K.; Matsumura, M.; Fujino, A.; Ishida, M.; Ebara, S.; Okabe, T.; Saito, S.; Hoshimoto, K.; Amemiya, K.; et al. Serial 3-vessel optical coherence tomography and intravascular ultrasound analysis of changing morphologies associated with lesion progression in patients with stable angina pectoris. Circ. Cardiovasc. Imaging 2017, 10, e006347. [Google Scholar] [CrossRef]
- Araki, M.; Yonetsu, T.; Russo, M.; Kurihara, O.; Kim, H.O.; Shinohara, H.; Thondapu, V.; Soeda, T.; Minami, Y.; Higuma, T.; et al. Predictors for layered coronary plaques: An optical coherence tomography study. J. Thromb. Thrombolysis 2020, 50, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, O.; Russo, M.; Kim, H.O.; Araki, M.; Shinohara, H.; Lee, H.; Takano, M.; Mizuno, K.; Jang, I.K. Clinical significance of healed plaque detected by optical coherence tomography: A 2-year follow-up study. J. Thromb. Thrombolysis 2020, 50, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; He, L.; Zhang, D.; Zeng, M.; Zhao, C.; Meng, W.; Qin, Y.; Weng, Z.; Xu, Y.; Liu, M.; et al. Non-culprit plaque healing on serial OCT imaging and future outcome in patients with acute coronary syndromes. Atherosclerosis 2025, 401, 119092. [Google Scholar] [CrossRef] [PubMed]
- Usui, E.; Mintz, G.S.; Lee, T.; Matsumura, M.; Zhang, Y.; Hada, M.; Yamaguchi, M.; Hoshino, M.; Kanaji, Y.; Sugiyama, T.; et al. Prognostic impact of healed coronary plaque in non-culprit lesions assessed by optical coherence tomography. Atherosclerosis 2020, 309, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Del Val, D.; Berta, B.; Roleder, T.; Malinowski, K.; Bastante, T.; Hermanides, R.S.; Wojakowski, W.; Fabris, E.; Cuesta, J.; De Luca, G.; et al. Vulnerable plaque features and adverse events in patients with diabetes mellitus: A post hoc analysis of the COMBINE OCT-FFR trial. EuroIntervention 2024, 20, e707–e717. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Isshiki, A.; Shimizu, M.; Fujii, H.; Suzuki, M. Clinical Significance of Coronary Healed Plaques in Stable Angina Pectoris Patients Undergoing Percutaneous Coronary Intervention. Circ. J. 2023, 87, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Fracassi, F.; Crea, F.; Sugiyama, T.; Yamamoto, E.; Uemura, S.; Vergallo, R.; Porto, I.; Lee, H.; Fujimoto, J.; Fuster, V.; et al. Healed Culprit Plaques in Patients with Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2019, 73, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Fang, C.; Jiang, S.; Wang, J.; Wang, Y.; Guo, J.; Lei, F.; Sun, S.; Pei, X.; Jia, R.; et al. In vivo evidence of atherosclerotic plaque erosion and healing in patients with acute coronary syndrome using serial optical coherence tomography imaging. Am. Heart J. 2022, 243, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Fang, C.; Zhang, S.; Li, L.; Wang, Y.; Xing, L.; Yu, H.; Jiang, S.; Yin, Y.; Wang, J.; et al. Frequency, Predictors, Distribution, and Morphological Characteristics of Layered Culprit and Nonculprit Plaques of Patients with Acute Myocardial Infarction: In Vivo 3-Vessel Optical Coherence Tomography Study. Circ. Cardiovasc. Interv. 2020, 13, e009125. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, S.; Wu, J.; Yu, H.; Pan, W.; Qin, Y.; He, L.; Li, L.; Hou, J.; Zhang, S.; et al. Characteristics and significance of healed plaques in patients with acute coronary syndrome and stable angina: An in vivo OCT and IVUS study. EuroIntervention 2019, 15, e771–e778. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, R.; Porto, I.; D’Amario, D.; Annibali, G.; Galli, M.; Benenati, S.; Bendandi, F.; Migliaro, S.; Fracassi, F.; Aurigemma, C.; et al. Coronary Atherosclerotic Phenotype and Plaque Healing in Patients with Recurrent Acute Coronary Syndromes Compared with Patients with Long-term Clinical Stability: An In Vivo Optical Coherence Tomography Study. JAMA Cardiol. 2019, 4, 321–329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurihara, O.; Shinohara, H.; Kim, H.O.; Russo, M.; Araki, M.; Nakajima, A.; Lee, H.; Takano, M.; Mizuno, K.; Komuro, I.; et al. Comparison of post-stent optical coherence tomography findings: Layered versus non-layered culprit lesions. Catheter. Cardiovasc. Interv. 2021, 97, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Yabushita, H.; Houser, S.L.; Aretz, H.T.; Jang, I.K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Halpern, E.F.; Bouma, B.E. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 2003, 107, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Phipps, J.E.; Vela, D.; Hoyt, T.; Halaney, D.L.; Mancuso, J.J.; Buja, L.M.; Asmis, R.; Milner, T.E.; Feldman, M.D. Macrophages and intravascular OCT bright spots: A quantitative study. JACC Cardiovasc. Imaging 2015, 8, 63–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minami, Y.; Phipps, J.E.; Hoyt, T.; Milner, T.E.; Ong, D.S.; Soeda, T.; Vergallo, R.; Feldman, M.D.; Jang, I.K. Clinical utility of quantitative bright spots analysis in patients with acute coronary syndrome: An optical coherence tomography study. Int. J. Cardiovasc. Imaging 2015, 31, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, F.; Hu, Y.; Wang, L.; Li, X.; Cong, H.; Zhang, J. The relationships between inflammatory biomarkers, plaque characteristics, and macrophage clusters in coronary plaque: A quantitative assessment of macrophages based on optical coherence tomography. Front. Cardiovasc. Med. 2025, 12, 1625239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rico-Jimenez, J.J.; Campos-Delgado, D.U.; Buja, L.M.; Vela, D.; Jo, J.A. Intravascular optical coherence tomography method for automated detection of macrophage infiltration within atherosclerotic coronary plaques. Atherosclerosis 2019, 290, 94–102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacNeill, B.D.; Jang, I.K.; Bouma, B.E.; Iftimia, N.; Takano, M.; Yabushita, H.; Shishkov, M.; Kauffman, C.R.; Houser, S.L.; Aretz, H.T.; et al. Focal and multi-focal plaque macrophage distributions in patients with acute and stable presentations of coronary artery disease. J. Am. Coll. Cardiol. 2004, 44, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Scalone, G.; Niccoli, G.; Refaat, H.; Vergallo, R.; Porto, I.; Leone, A.M.; Burzotta, F.; D’Amario, D.; Liuzzo, G.; Fracassi, F.; et al. Not all plaque ruptures are born equal: An optical coherence tomography study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Yuki, H.; Sugiyama, T.; Suzuki, K.; Kinoshita, D.; Niida, T.; Nakajima, A.; Araki, M.; Dey, D.; Lee, H.; McNulty, I.; et al. Coronary Inflammation and Plaque Vulnerability: A Coronary Computed Tomography and Optical Coherence Tomography Study. Circ. Cardiovasc. Imaging 2023, 16, e014959. [Google Scholar] [CrossRef] [PubMed]
- Gatto, L.; Alfonso, F.; Paoletti, G.; Burzotta, F.; La Manna, A.; Budassi, S.; Biccirè, F.G.; Fineschi, M.; Marco, V.; Fabbiocchi, F.; et al. Relationship betweeen the amount and location of macrophages and clinical outcome: Subanalysis of the CLIMA-study. Int. J. Cardiol. 2022, 346, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, M.; Souteyrand, G.; Niccoli, G.; Mancone, M.; Sardella, G.; Tamburino, C.; Templin, C.; Gili, S.; Boccuzzi, G.G.; D’Ascenzo, F. Clinical impact of optical coherence tomography findings on culprit plaque in acute coronary syndrome: The OCT-FORMIDABLE study registry. Catheter. Cardiovasc. Interv. 2018, 92, E486–E492. [Google Scholar] [CrossRef] [PubMed]
- Burgmaier, M.; Milzi, A.; Dettori, R.; Burgmaier, K.; Hellmich, M.; Almalla, M.; Marx, N.; Reith, S. Colocalization of plaque macrophages and calcification in coronary plaques as detected by optical coherence tomography predicts cardiovascular outcome. Cardiol. J. 2020, 27, 303–306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fracassi, F.; Niccoli, G.; Vetrugno, V.; Russo, M.; Rettura, F.; Vergni, F.; Scalone, G.; Montone, R.A.; Vergallo, R.; D’Amario, D.; et al. Optical coherence tomography and C-reactive protein in risk stratification of acute coronary syndromes. Int. J. Cardiol. 2019, 286, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Vetrugno, V.; Camilli, M.; Russo, M.; Fracassi, F.; Khan, S.Q.; Doshi, S.N.; Townend, J.N.; Ludman, P.F.; Trani, C.; et al. Macrophage infiltrates in coronary plaque erosion and cardiovascular outcome in patients with acute coronary syndrome. Atherosclerosis 2020, 311, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Jang, I.K. Cholesterol crystals in atherosclerotic plaques: A future target to reduce the risk of plaque rupture? Int. J. Cardiol. 2022, 365, 30–31. [Google Scholar] [CrossRef] [PubMed]
- Abela, G.S.; Aziz, K.; Vedre, A.; Pathak, D.R.; Talbott, J.D.; Dejong, J. Effect of cholesterol crystals on plaques and intima in arteries of patients with acute coronary and cerebrovascular syndromes. Am. J. Cardiol. 2009, 103, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Fiolet, A.; Abela, G.S. Viewing atherosclerosis through a crystal lens: How the evolving structure of cholesterol crystals in atherosclerotic plaque alters its stability. J. Clin. Lipidol. 2020, 14, 619–630. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Sato, Y.; Torii, S.; Sakamoto, A.; Cornelissen, A.; Bhoite, R.R.; Kuntz, S.; Guo, L.; Paek, K.H.; Fernandez, R.; et al. Detection of cholesterol crystals by optical coherence tomography. EuroIntervention 2020, 16, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Tanaka, A.; Taruya, A.; Kashiwagi, M.; Nishiguchi, T.; Ozaki, Y.; Matsuo, Y.; Kitabata, H.; Kubo, T.; Shimada, E.; et al. Feasibility and Clinical Significance of In Vivo Cholesterol Crystal Detection Using Optical Coherence Tomography. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Liu, Z.; Li, C.; Xu, G.; Zhang, R.; Bai, Z.; Hu, X.; Xia, Q.; Pan, L.; Wang, S.; et al. Predictive models for cholesterol crystals and plaque vulnerability in acute myocardial infarction: Insights from an optical coherence tomography study. Int. J. Cardiol. 2025, 418, 132610. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Cao, M.; Xi, X.; Zhang, Y.; Wang, Z.; Zhao, S.; Tian, Y.; Xu, Q.; Yu, H.; Tian, J.; et al. Cholesterol crystals in non-culprit plaques of STEMI patients: A 3-vessel OCT study. Int. J. Cardiol. 2022, 364, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Ehara, S.; Hasegawa, T.; Matsumoto, K.; Yoshikawa, J.; Shimada, K. Cholesterol crystal as a new feature of coronary vulnerable plaques: An optical coherence tomography study. J. Cardiol. 2017, 69, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, K.; Minami, Y.; Ishida, K.; Kato, A.; Katsura, A.; Muramatsu, Y.; Sato, T.; Kakizaki, R.; Nemoto, T.; Hashimoto, T.; et al. Incidence, factors, and clinical significance of cholesterol crystals in coronary plaque: An optical coherence tomography study. Atherosclerosis 2019, 283, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Nelles, G.; Abdelwahed, Y.S.; Seppelt, C.; Meteva, D.; Stähli, B.E.; Rai, H.; Seegers, L.M.; Sieronski, L.; Musfeldt, J.; Gerhardt, T.; et al. Cholesterol crystals at the culprit lesion in patients with acute coronary syndrome are associated with worse cardiovascular outcomes at two years follow up—Results from the translational OPTICO-ACS study program. Int. J. Cardiol. 2024, 399, 131665. [Google Scholar] [CrossRef] [PubMed]
- Usui, E.; Matsumura, M.; Mintz, G.S.; Zhou, Z.; Hada, M.; Yamaguchi, M.; Hoshino, M.; Kanaji, Y.; Sugiyama, T.; Murai, T.; et al. Clinical outcomes of low-intensity area without attenuation and cholesterol crystals in non-culprit lesions assessed by optical coherence tomography. Atherosclerosis 2021, 332, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.R.; Purushothaman, K.R.; Fuster, V.; Echeverri, D.; Truszczynska, H.; Sharma, S.K.; Badimon, J.J.; O’Connor, W.N. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: Implications for plaque vulnerability. Circulation 2004, 110, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Kume, T.; Okura, H.; Yamada, R.; Koyama, T.; Fukuhara, K.; Kawamura, A.; Imai, K.; Neishi, Y.; Uemura, S. Detection of Plaque Neovascularization by Optical Coherence Tomography: Ex Vivo Feasibility Study and In Vivo Observation in Patients with Angina Pectoris. J. Invasive Cardiol. 2016, 28, 17–22. [Google Scholar] [PubMed]
- Aoki, T.; Rodriguez-Porcel, M.; Matsuo, Y.; Cassar, A.; Kwon, T.G.; Franchi, F.; Gulati, R.; Kushwaha, S.S.; Lennon, R.J.; Lerman, L.O.; et al. Evaluation of coronary adventitial vasa vasorum using 3D optical coherence tomography–animal and human studies. Atherosclerosis 2015, 239, 203–208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kitabata, H.; Tanaka, A.; Kubo, T.; Takarada, S.; Kashiwagi, M.; Tsujioka, H.; Ikejima, H.; Kuroi, A.; Kataiwa, H.; Ishibashi, K.; et al. Relation of microchannel structure identified by optical coherence tomography to plaque vulnerability in patients with coronary artery disease. Am. J. Cardiol. 2010, 105, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Koizumi, M.; Okubo, R.; Yabe, T.; Watanabe, I.; Saito, D.; Toda, M.; Ikeda, T. Comparison of Coronary Intimal Plaques by Optical Coherence Tomography in Arteries With Versus Without Internal Running Vasa Vasorum. Am. J. Cardiol. 2017, 119, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Uemura, S.; Ishigami, K.; Soeda, T.; Okayama, S.; Sung, J.H.; Nakagawa, H.; Somekawa, S.; Takeda, Y.; Kawata, H.; Horii, M.; et al. Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur. Heart J. 2012, 33, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Takeshige, R.; Otake, H.; Kawamori, H.; Toba, T.; Nagano, Y.; Tsukiyama, Y.; Yanaka, K.I.; Yamamoto, H.; Nagasawa, A.; Onishi, H.; et al. Progression from normal vessel wall to atherosclerotic plaque: Lessons from an optical coherence tomography study with follow-up of over 5 years. Heart Vessel. 2022, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Suwa, K.; Kawakami, R.; Finn, A.V.; Maekawa, Y.; Virmani, R.; Finn, A.V. Significance of Intra-plaque Hemorrhage for the Development of High-Risk Vulnerable Plaque: Current Understanding from Basic to Clinical Points of View. Int. J. Mol. Sci. 2023, 24, 13298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, T.; Lin, L.; Chen, M.; Zhang, J.J.; Ye, F.; Pan, T.; Huang, X.; Chen, S.L. Coronary Artery Intraplaque Microvessels by Optical Coherence Tomography Correlate with Vulnerable Plaque and Predict Clinical Outcomes in Patients with Ischemic Angina. JACC Cardiovasc. Interv. 2018, 11, 1421–1422. [Google Scholar] [CrossRef] [PubMed]
- Waring, O.J.; Skenteris, N.T.; Biessen, E.A.L.; Donners, M.M.P.C. Two-faced Janus: The dual role of macrophages in atherosclerotic calcification. Cardiovasc. Res. 2022, 118, 2768–2777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yabushita, H.; Bouma, B.E.; Houser, S.L.; Aretz, H.T.; Jang, I.K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Kang, D.H.; Halpern, E.F.; et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 2002, 106, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, F.; Hu, Y.; Wang, L.; Li, X.; Cong, H.; Zhang, J. Relationship between coronary artery calcification and plaque vulnerability, a qualitative and quantitative optical coherence tomography study. Int. J. Cardiol. 2025, 440, 133707. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, D.; Kinoshita, D.; Suzuki, K.; Niida, T.; Yuki, H.; McNulty, I.; Lee, H.; Otake, H.; Shite, J.; Ferencik, M.; et al. Relationship Between Calcified Plaque Burden, Vascular Inflammation, and Plaque Vulnerability in Patients with Coronary Atherosclerosis. JACC Cardiovasc. Imaging 2024, 17, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Yamamoto, E.; Fracassi, F.; Lee, H.; Yonetsu, T.; Kakuta, T.; Soeda, T.; Saito, Y.; Yan, B.P.; Kurihara, O.; et al. Calcified Plaques in Patients With Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2019, 12, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, A.; Servadei, F.; Zoccai, G.B.; Giacobbi, E.; Anemona, L.; Bonanno, E.; Casella, S. Coronary calcification identifies the vulnerable patient rather than the vulnerable Plaque. Atherosclerosis 2013, 229, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Grandhi, G.R.; Mszar, R.; Cainzos-Achirica, M.; Rajan, T.; Latif, M.A.; Bittencourt, M.S.; Shaw, L.J.; Batlle, J.C.; Blankstein, R.; Blaha, M.J.; et al. Coronary Calcium to Rule Out Obstructive Coronary Artery Disease in Patients with Acute Chest Pain. JACC Cardiovasc. Imaging 2022, 15, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, D.; Kinoshita, D.; Suzuki, K.; Niida, T.; Yuki, H.; McNulty, I.; Lee, H.; Otake, H.; Shite, J.; Ferencik, M.; et al. Coronary spotty calcification, compared with macro calcification, is associated with a higher level of vascular inflammation and plaque vulnerability in patients with stable angina. Atherosclerosis 2025, 405, 119237. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, M.; Hasegawa, T.; Ehara, S.; Matsumoto, K.; Mizutani, K.; Iguchi, T.; Ishii, H.; Nakagawa, M.; Shimada, K.; Yoshiyama, M. New insights into spotty calcification and plaque rupture in acute coronary syndrome: An optical coherence tomography study. Heart Vessel. 2016, 31, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Puri, R.; Hammadah, M.; Duggal, B.; Uno, K.; Kapadia, S.R.; Tuzcu, E.M.; Nissen, S.E.; Nicholls, S.J. Spotty calcification and plaque vulnerability in vivo: Frequency-domain optical coherence tomography analysis. Cardiovasc. Diagn. Ther. 2014, 4, 460–469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, X.; Gao, J.; Lv, Q.; Cai, H.; Wang, F.; Ye, R.; Liu, X. Calcification in Atherosclerotic Plaque Vulnerability: Friend or Foe? Front. Physiol. 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kinoshita, D.; Suzuki, K.; Yuki, H.; Niida, T.; Fujimoto, D.; Minami, Y.; Dey, D.; Lee, H.; McNulty, I.; Ako, J.; et al. Coronary plaque phenotype associated with positive remodeling. J. Cardiovasc. Comput. Tomogr. 2024, 18, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, T.; Tan, J.; Chen, Y.; Xu, X.; Guo, Y.; Jin, C.; Xiu, L.; Zhao, R.; Sun, S.; et al. Pancoronary plaque characteristics in STEMI patients with rapid plaque progression: An optical coherence tomography study. Int. J. Cardiol. 2024, 400, 131821. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Zimarino, M. Coronary calcifications are the growth rings of coronary atherosclerotic plaque. Int. J. Cardiol. 2025, 442, 133902. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Mintz, G.S.; Matsumura, M.; Zhang, W.; Cao, Y.; Usui, E.; Kanaji, Y.; Murai, T.; Yonetsu, T.; Kakuta, T.; et al. Prevalence, Predictors, and Clinical Presentation of a Calcified Nodule as Assessed by Optical Coherence Tomography. JACC Cardiovasc. Imaging 2017, 10, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Kakuta, T.; Hoshino, M.; Hada, M.; Yonetsu, T.; Usui, E.; Hanyu, Y.; Nagamine, T.; Nogami, K.; Ueno, H.; et al. Predictors of Optical Coherence Tomography-Defined Calcified Nodules in Patients with Acute Coronary Syndrome—A Substudy From the TACTICS Registry. Circ. J. 2024, 88, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Nelles, G.; Abdelwahed, Y.S.; Alyaqoob, A.; Seppelt, C.; Stähli, B.E.; Meteva, D.; Kränkel, N.; Haghikia, A.; Skurk, C.; Dreger, H.; et al. Spotty calcium deposits within acute coronary syndrome (ACS)-causing culprit lesions impact inflammatory vessel-wall interactions and are associated with higher cardiovascular event rates at one year follow-up: Results from the prospective translational OPTICO-ACS study program. Atherosclerosis 2023, 385, 117284. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Gatto, L.; Fabbiocchi, F.; Vergallo, R.; Paoletti, G.; Ruscica, G.; Marco, V.; Romagnoli, E.; Boi, A.; Fineschi, M.; et al. Clinical outcomes of calcified nodules detected by optical coherence tomography: A sub-analysis of the CLIMA study. EuroIntervention 2020, 16, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Yokomine, T.; Kajiya, T.; Takei, T.; Kitazono, K.; Ninomiya, T.; Inoue, T.; Takaoka, J.; Atsuchi, Y.; Atsuchi, N.; Ohishi, M. Impact of Calcified Nodules on Clinical Outcomes in Hemodialysis Patients Undergoing Percutaneous Coronary Intervention. Am. J. Cardiol. 2025, 245, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Yin, Y.; Liu, X.; Fang, C.; Jiang, S.; Xu, X.; Sun, S.; Pei, X.; Jia, R.; Tang, C.; et al. Clinical Outcomes of Different Calcified Culprit Plaques in Patients with Acute Coronary Syndrome. J. Clin. Med. 2022, 11, 4018. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okamura, A.; Okura, H.; Iwai, S.; Sakagami, A.; Kamon, D.; Hashimoto, Y.; Ueda, T.; Soeda, T.; Watanabe, M.; Saito, Y. Incidence and prognostic impact of the calcified nodule in coronary artery disease patients with end-stage renal disease on dialysis. Heart Vessel. 2022, 37, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Sugizaki, Y.; Matsumura, M.; Chen, Y.; Tsukui, T.; Shlofmitz, E.; Thomas, S.V.; Malik, S.; Dakroub, A.; Singh, M.; Shin, D.; et al. Natural history of a newly developed calcified nodule: Incidence, predictors, and clinical outcomes. EuroIntervention 2024, 20, e1330–e1339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rai, H.; Harzer, F.; Otsuka, T.; Abdelwahed, Y.S.; Antuña, P.; Blachutzik, F.; Koppara, T.; Räber, L.; Leistner, D.M.; Alfonso, F.; et al. Stent Optimization Using Optical Coherence Tomography and Its Prognostic Implications After Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2022, 11, e023493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagata, T.; Minami, Y.; Katsura, A.; Asakura, K.; Katamine, M.; Muramatsu, Y.; Fujiyoshi, K.; Kinoshita, D.; Ako, J. Optical coherence tomography factors for adverse events in patients with severe coronary calcification. Int. J. Cardiol. 2023, 376, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kawamori, H.; Toba, T.; Kakizaki, S.; Nakamura, K.; Fujimoto, D.; Sasaki, S.; Fujii, H.; Hamana, T.; Osumi, Y.; et al. Clinical impact of optical coherence tomography findings after drug-coated balloon treatment for patients with acute coronary syndromes. Int. J. Cardiol. 2023, 387, 131149. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Koide, M.; Takamatsu, K.; Sugimoto, H.; Takeda, Y.; Akabame, S.; Seki, T.; Zen, K.; Matoba, S. Clinical Outcomes of Percutaneous Coronary Intervention Using Drug-Coated Balloons for De Novo Coronary Lesions with Eruptive Calcified Nodules as Detected by Optical Coherence Tomography. Circ. J. 2025, 89, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Lu, W.; Han, Z.; Pan, S.; Li, X.; Wang, X.; Pan, L.; Wang, X.; Zheng, X.; Li, R.; et al. Long-term outcomes of drug-coated balloon treatment of calcified coronary artery lesions: A multicenter, retrospective, propensity matching study. Front. Cardiovasc. Med. 2023, 10, 1122290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davies, M.J.; Bland, J.M.; Hangartner, J.R.; Angelini, A.; Thomas, A.C. Factors influencing the presence or absence of acute coronary artery thrombi in sudden ischaemic death. Eur. Heart J. 1989, 10, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, R.; Uemura, S.; Soeda, T.; Minami, Y.; Cho, J.M.; Ong, D.S.; Aguirre, A.D.; Gao, L.; Biasucci, L.M.; Crea, F.; et al. Prevalence and Predictors of Multiple Coronary Plaque Ruptures: In Vivo 3-Vessel Optical Coherence Tomography Imaging Study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Volleberg, R.H.J.A.; Rroku, A.; Mol, J.Q.; Hermanides, R.S.; van Leeuwen, M.; Berta, B.; Meuwissen, M.; Alfonso, F.; Wojakowski, W.; Belkacemi, A.; et al. FFR-Negative Nonculprit High-Risk Plaques and Clinical Outcomes in High-Risk Populations: An Individual Patient-Data Pooled Analysis From COMBINE (OCT-FFR) and PECTUS-obs. Circ. Cardiovasc. Interv. 2025, 18, e014667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kataoka, Y.; Hammadah, M.; Puri, R.; Duggal, B.; Uno, K.; Kapadia, S.R.; Murat Tuzcu, E.; Nissen, S.E.; Nicholls, S.J. Plaque microstructures in patients with coronary artery disease who achieved very low low-density lipoprotein cholesterol levels. Atherosclerosis 2015, 242, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hou, J.; Wan, J.; Yang, Y.; Wang, D.; Liang, D.; Wang, X.; Zhou, P.; Wang, P. Effect of Intensive Lipid-Lowering Therapy on Coronary Plaque Stabilization Derived from Optical Coherence Tomography: A Meta-analysis and Meta-regression. Cardiovasc. Drugs Ther. 2025, 39, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Yokoyama, T.; Miyauchi, K.; Shimada, K.; Kurata, T.; Sato, H.; Daida, H. Early statin treatment in patients with acute coronary syndrome: Demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: The ESTABLISH Study. Circulation 2004, 110, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, H.; Han, Y.; Yuan, X.; Jiang, M.; Wang, W.; Gao, L. Plaque Stabilization and Regression, from Mechanisms to Surveillance and Clinical Strategies. Rev. Cardiovasc. Med. 2024, 25, 459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takagi, T.; Yoshida, K.; Akasaka, T.; Hozumi, T.; Morioka, S.; Yoshikawa, J. Intravascular ultrasound analysis of reduction in progression of coronary narrowing by treatment with pravastatin. Am. J. Cardiol. 1997, 79, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.P.; Lum, M.; Nerleker, N.; Nicholls, S.J.; Layland, J. Coronary Atherosclerotic Plaque Regression: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Hiro, T.; Yamagishi, M.; Daida, H.; Hirayama, A.; Saito, S.; Yamaguchi, T.; Matsuzaki, M.; COSMOS Investigators. Effect of rosuvastatin on coronary atheroma in stable coronary artery disease: Multicenter coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Circ. J. 2009, 73, 2110–2117. [Google Scholar] [CrossRef] [PubMed]
- Räber, L.; Taniwaki, M.; Zaugg, S.; Kelbæk, H.; Roffi, M.; Holmvang, L.; Noble, S.; Pedrazzini, G.; Moschovitis, A.; Lüscher, T.F.; et al. Effect of high-intensity statin therapy on atherosclerosis in non-infarct-related coronary arteries (IBIS-4): A serial intravascular ultrasonography study. Eur. Heart J. 2015, 36, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Tuzcu, E.M.; Schoenhagen, P.; Brown, B.G.; Ganz, P.; Vogel, R.A.; Crowe, T.; Howard, G.; Cooper, C.J.; Brodie, B.; et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: A randomized controlled trial. JAMA 2004, 291, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Ballantyne, C.M.; Barter, P.J.; Chapman, M.J.; Erbel, R.M.; Libby, P.; Raichlen, J.S.; Uno, K.; Borgman, M.; Wolski, K.; et al. Effect of two intensive statin regimens on progression of coronary disease. N. Engl. J. Med. 2011, 365, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Hiro, T.; Kimura, T.; Morimoto, T.; Miyauchi, K.; Nakagawa, Y.; Yamagishi, M.; Ozaki, Y.; Kimura, K.; Saito, S.; Yamaguchi, T.; et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: A multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J. Am. Coll. Cardiol. 2009, 54, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Kini, A.S.; Baber, U.; Kovacic, J.C.; Limaye, A.; Ali, Z.A.; Sweeny, J.; Maehara, A.; Mehran, R.; Dangas, G.; Mintz, G.S.; et al. Changes in plaque lipid content after short-term intensive versus standard statin therapy: The YELLOW trial (reduction in yellow plaque by aggressive lipid-lowering therapy). J. Am. Coll. Cardiol. 2013, 62, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Komukai, K.; Kubo, T.; Kitabata, H.; Matsuo, Y.; Ozaki, Y.; Takarada, S.; Okumoto, Y.; Shiono, Y.; Orii, M.; Shimamura, K.; et al. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: The EASY-FIT study. J. Am. Coll. Cardiol. 2014, 64, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Arsanjani, R.; Sung, J.M.; Heo, R.; Lee, B.K.; Lin, F.Y.; Hadamitzky, M.; Kim, Y.J.; Conte, E.; Andreini, D.; et al. Impact of statins based on high-risk plaque features on coronary plaque progression in mild stenosis lesions: Results from the PARADIGM study. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, D.; Suzuki, K.; Usui, E.; Hada, M.; Yuki, H.; Niida, T.; Minami, Y.; Lee, H.; McNulty, I.; Ako, J.; et al. High-Risk Plaques on Coronary Computed Tomography Angiography: Correlation with Optical Coherence Tomography. JACC Cardiovasc. Imaging 2024, 17, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Shen, Y.; Li, Y.; Wang, X. Meta-analysis and trial sequential analysis of ezetimibe for coronary atherosclerotic plaque compositions. Front. Pharmacol. 2023, 14, 1166762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.J.; Xu, M.; Duan, J.Q.; Wang, D.J.; Han, S.L. Effect of ezetimibe-statin combination therapy vs. statin monotherapy on coronary atheroma phenotype and lumen stenosis in patients with coronary artery disease: A meta-analysis and trial sequential analysis. Front. Pharmacol. 2024, 15, 1343582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hibi, K.; Sonoda, S.; Kawasaki, M.; Otsuji, Y.; Murohara, T.; Ishii, H.; Sato, K.; Koshida, R.; Ozaki, Y.; Sata, M.; et al. Effects of Ezetimibe-Statin Combination Therapy on Coronary Atherosclerosis in Acute Coronary Syndrome. Circ. J. 2018, 82, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Habara, M.; Nasu, K.; Terashima, M.; Ko, E.; Yokota, D.; Ito, T.; Kurita, T.; Teramoto, T.; Kimura, M.; Kinoshita, Y.; et al. Impact on optical coherence tomographic coronary findings of fluvastatin alone versus fluvastatin + ezetimibe. Am. J. Cardiol. 2014, 113, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Hougaard, M.; Hansen, H.S.; Thayssen, P.; Maehara, A.; Antonsen, L.; Junker, A.; Mintz, G.S.; Jensen, L.O. Influence of Ezetimibe on Plaque Morphology in Patients with ST Elevation Myocardial Infarction Assessed by Optical Coherence Tomography: An OCTIVUS Sub-Study. Cardiovasc. Revasc. Med. 2020, 21, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016, 316, 2373–2384. [Google Scholar] [CrossRef] [PubMed]
- Ako, J.; Hibi, K.; Tsujita, K.; Hiro, T.; Morino, Y.; Kozuma, K.; Shinke, T.; Otake, H.; Uno, K.; Louie, M.J.; et al. Effect of Alirocumab on Coronary Atheroma Volume in Japanese Patients With Acute Coronary Syndrome—The ODYSSEY J-IVUS Trial. Circ. J. 2019, 83, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Kataoka, Y.; Nissen, S.E.; Prati, F.; Windecker, S.; Puri, R.; Hucko, T.; Aradi, D.; Herrman, J.R.; Hermanides, R.S.; et al. Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients with Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Revaiah, P.C.; Serruys, P.W.; Onuma, Y.; Andreini, D.; Budoff, M.J.; Sharif, F.; Chernofsky, A.; Vikarunnessa, S.; Wiethoff, A.J.; Yates, D.; et al. Design and rationale of a randomized clinical trial assessing the effect of inclisiran on atherosclerotic plaque in individuals without previous cardiovascular event and without flow-limiting lesions identified in an in-hospital screening: The VICTORION-PLAQUE primary prevention trial. Am. Heart J. 2026, 291, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Guarnieri, C.; Ussia, G.P.; Zimarino, M.; Traini, A.M.; Parlangeli, R.; Vaona, I.; Branzi, A.; Magnani, B. Limitation of myocardial infarct size by nicorandil after sustained ischemia in pigs. J. Cardiovasc. Pharmacol. 1995, 26, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Camera, M.; Brambilla, M.; Sarto, G.; Spadafora, L.; Bernardi, M.; Iaconelli, A.; D’Amario, D.; Biondi-Zoccai, G.; Celia, A.I.; et al. Combining Colchicine and Antiplatelet Therapy to Tackle Atherothrombosis: A Paradigm in Transition? Int. J. Mol. Sci. 2025, 26, 1136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Andreis, A.; Imazio, M.; Piroli, F.; Avondo, S.; Casula, M.; Paneva, E.; De Ferrari, G.M. Efficacy and safety of colchicine for the prevention of major cardiovascular and cerebrovascular events in patients with coronary artery disease: A systematic review and meta-analysis on 12,869 patients. Eur. J. Prev. Cardiol. 2022, 28, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yang, Y.; Dong, S.L.; Zhao, C.; Yang, F.; Yuan, Y.F.; Liao, Y.H.; He, S.L.; Liu, K.; Wei, F.; et al. Effect of Colchicine on Coronary Plaque Stability in Acute Coronary Syndrome as Assessed by Optical Coherence Tomography: The COLOCT Randomized Clinical Trial. Circulation 2024, 150, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Psaltis, P.J.; Nguyen, M.T.; Singh, K.; Sinhal, A.; Wong, D.T.L.; Alcock, R.; Rajendran, S.; Dautov, R.; Barlis, P.; Patel, S.; et al. Optical coherence tomography assessment of the impact of colchicine on non-culprit coronary plaque composition after myocardial infarction. Cardiovasc. Res. 2025, 121, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Wykrzykowska, J.J.; Diletti, R.; Gutierrez-Chico, J.L.; van Geuns, R.J.; van der Giessen, W.J.; Ramcharitar, S.; Duckers, H.E.; Schultz, C.; de Feyter, P.; van der Ent, M.; et al. Plaque sealing and passivation with a mechanical self-expanding low outward force nitinol vShield device for the treatment of IVUS and OCT-derived thin cap fibroatheromas (TCFAs) in native coronary arteries: Report of the pilot study vShield Evaluated at Cardiac hospital in Rotterdam for Investigation and Treatment of TCFA (SECRITT). EuroIntervention 2012, 8, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Ali, Z.A.; Held, C.; Matsumura, M.; Kjøller-Hansen, L.; Bøtker, H.E.; Maeng, M.; Engstrøm, T.; Wiseth, R.; et al. Percutaneous Coronary Intervention for Vulnerable Coronary Atherosclerotic Plaque. J. Am. Coll. Cardiol. 2020, 76, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Zimarino, M.; Renda, G.; De Caterina, R. Optimal duration of antiplatelet therapy in recipients of coronary drug-eluting stents. Drugs 2005, 65, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Zimarino, M.; Angiolillo, D.J.; Dangas, G.; Capodanno, D.; Barbato, E.; Hahn, J.Y.; Giustino, G.; Watanabe, H.; Costa, F.; Cuisset, T.; et al. Antithrombotic therapy after percutaneous coronary intervention of bifurcation lesions. EuroIntervention 2021, 17, 59–66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, M.; Bacigalupi, E.; Radico, F.; Scorpiglione, L.; Gurgoglione, F.L.; Russo, A.; Vigna, C.; Galli, M.; Benenati, S.; Vergallo, R.; et al. Prognostic Role and Therapeutic Implications of Intravascular Optical Coherence Tomography Detected Coronary Plaque Microstructures in Patients with Coronary Artery Disease. J. Clin. Med. 2025, 14, 8132. https://doi.org/10.3390/jcm14228132
Russo M, Bacigalupi E, Radico F, Scorpiglione L, Gurgoglione FL, Russo A, Vigna C, Galli M, Benenati S, Vergallo R, et al. Prognostic Role and Therapeutic Implications of Intravascular Optical Coherence Tomography Detected Coronary Plaque Microstructures in Patients with Coronary Artery Disease. Journal of Clinical Medicine. 2025; 14(22):8132. https://doi.org/10.3390/jcm14228132
Chicago/Turabian StyleRusso, Michele, Elena Bacigalupi, Francesco Radico, Luca Scorpiglione, Filippo Luca Gurgoglione, Alessandro Russo, Carlo Vigna, Mattia Galli, Stefano Benenati, Rocco Vergallo, and et al. 2025. "Prognostic Role and Therapeutic Implications of Intravascular Optical Coherence Tomography Detected Coronary Plaque Microstructures in Patients with Coronary Artery Disease" Journal of Clinical Medicine 14, no. 22: 8132. https://doi.org/10.3390/jcm14228132
APA StyleRusso, M., Bacigalupi, E., Radico, F., Scorpiglione, L., Gurgoglione, F. L., Russo, A., Vigna, C., Galli, M., Benenati, S., Vergallo, R., Montone, R. A., Benedetto, U., Niccoli, G., Prati, F., & Zimarino, M. (2025). Prognostic Role and Therapeutic Implications of Intravascular Optical Coherence Tomography Detected Coronary Plaque Microstructures in Patients with Coronary Artery Disease. Journal of Clinical Medicine, 14(22), 8132. https://doi.org/10.3390/jcm14228132







