Use of Calcium Modification in Percutaneous Coronary Intervention: A Comprehensive Review
Abstract
1. Introduction/Background
2. Materials and Methods
3. Pathophysiology of Calcium Deposition in Coronary Vessels
4. Atherosclerotic Development Timeline
4.1. Adaptive and Pathologic Intimal Thickening
4.2. Formation of Fibroatheroma
4.3. Plaque Rupture and Plaque Erosion
4.4. Variations in Calcified Plaque Morphology
5. Risk Factors and Prevalence
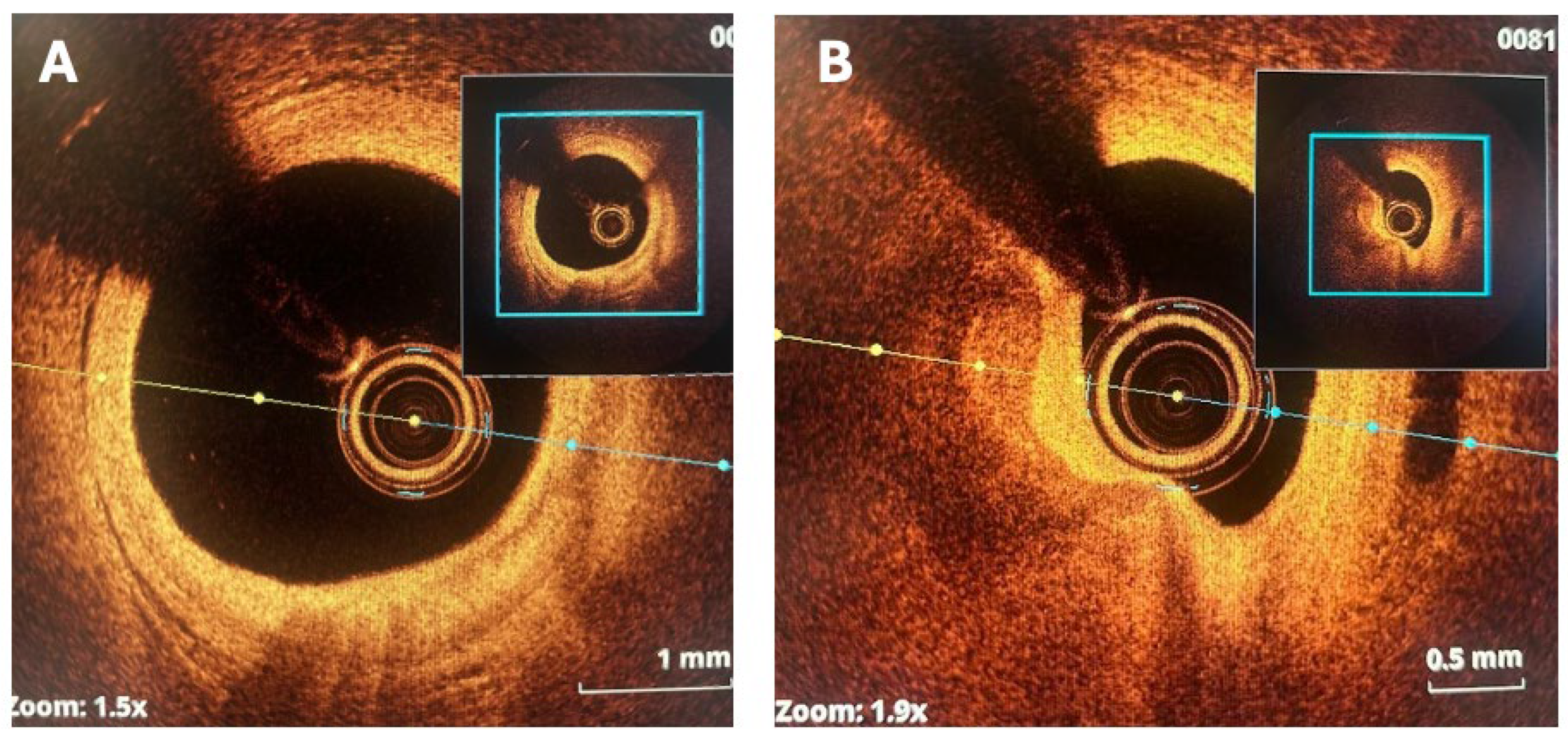
6. Role of Imaging
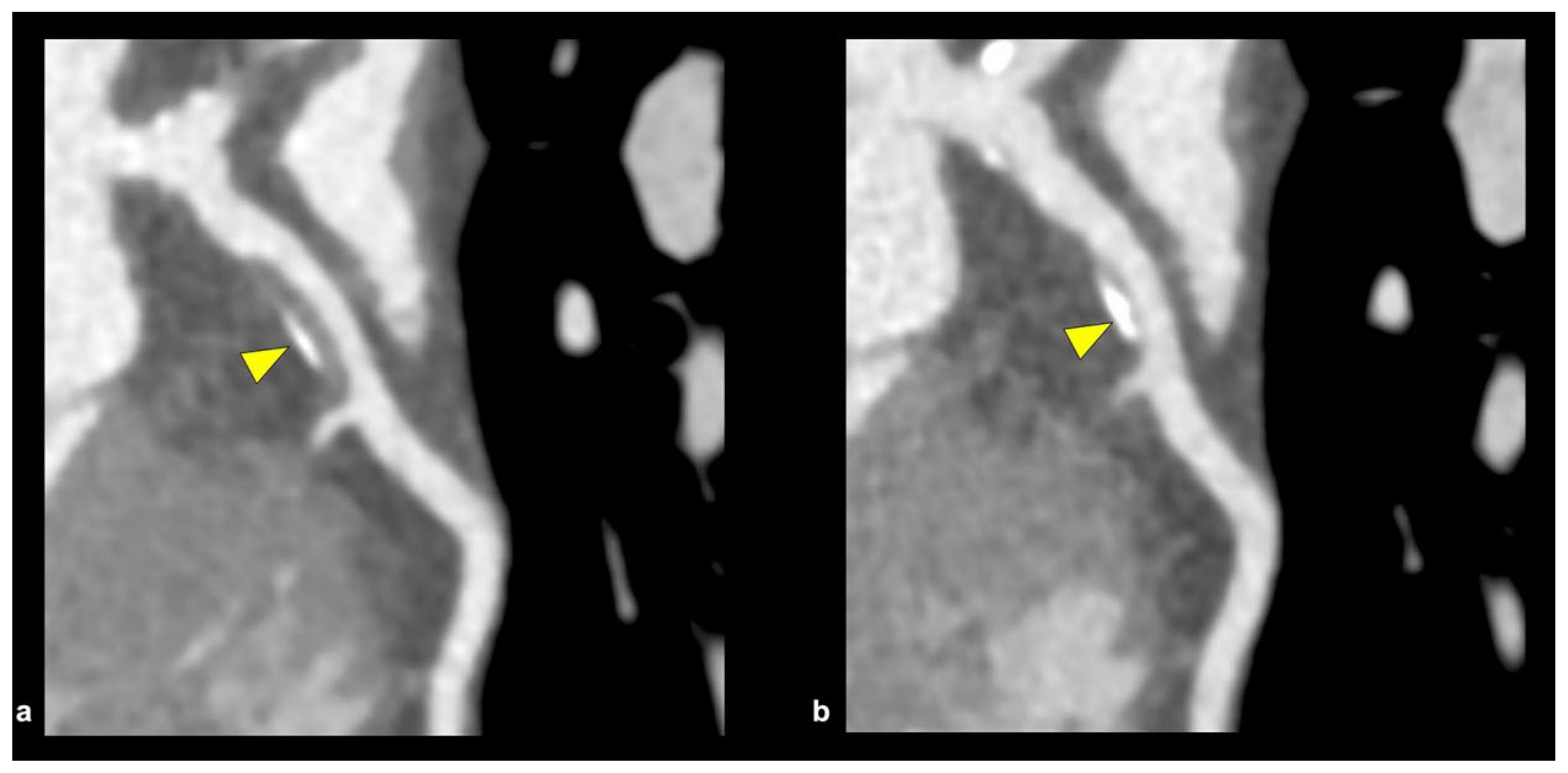
6.1. Coronary CT Angiography
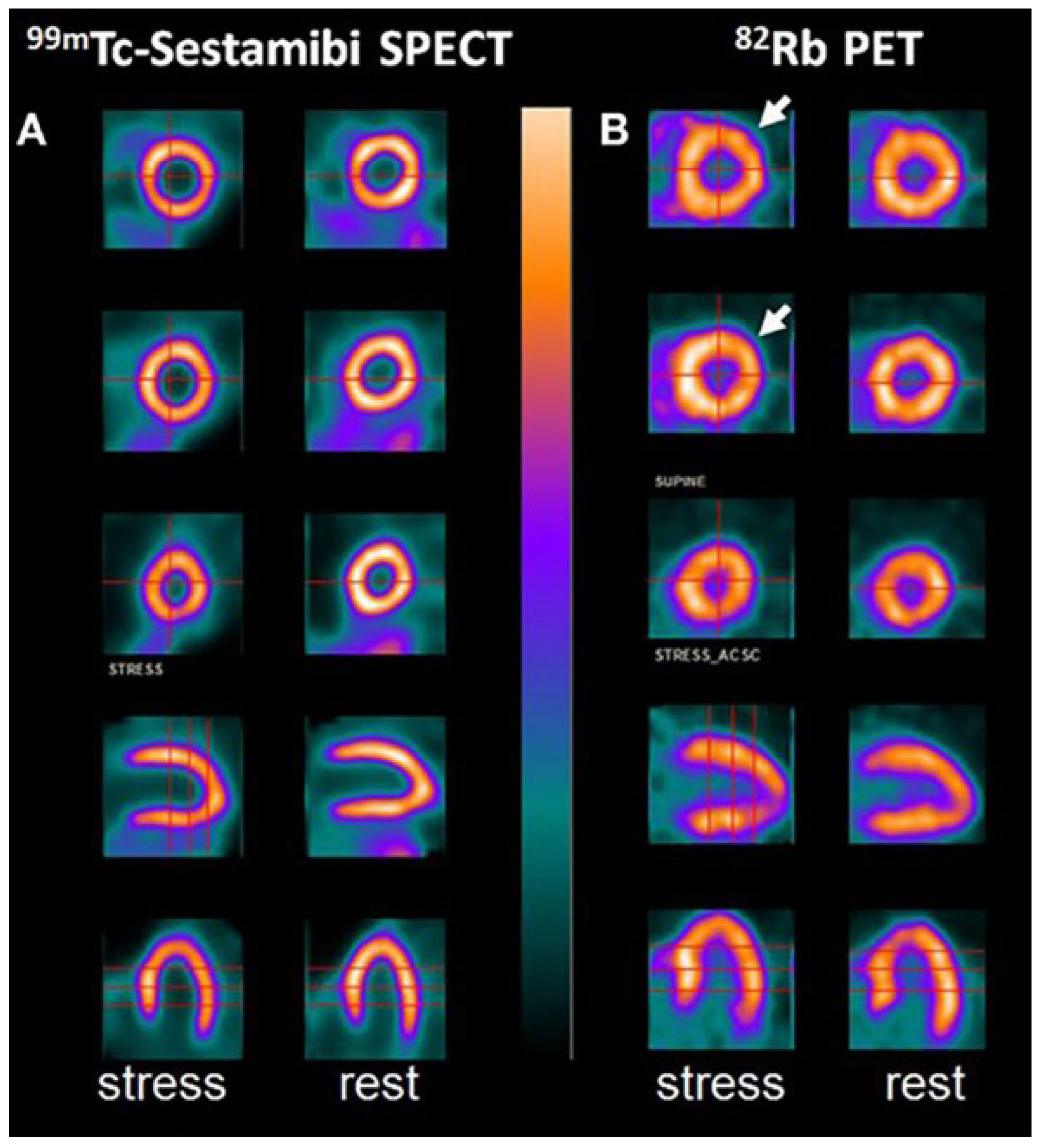
6.2. Positron Emission Tomography
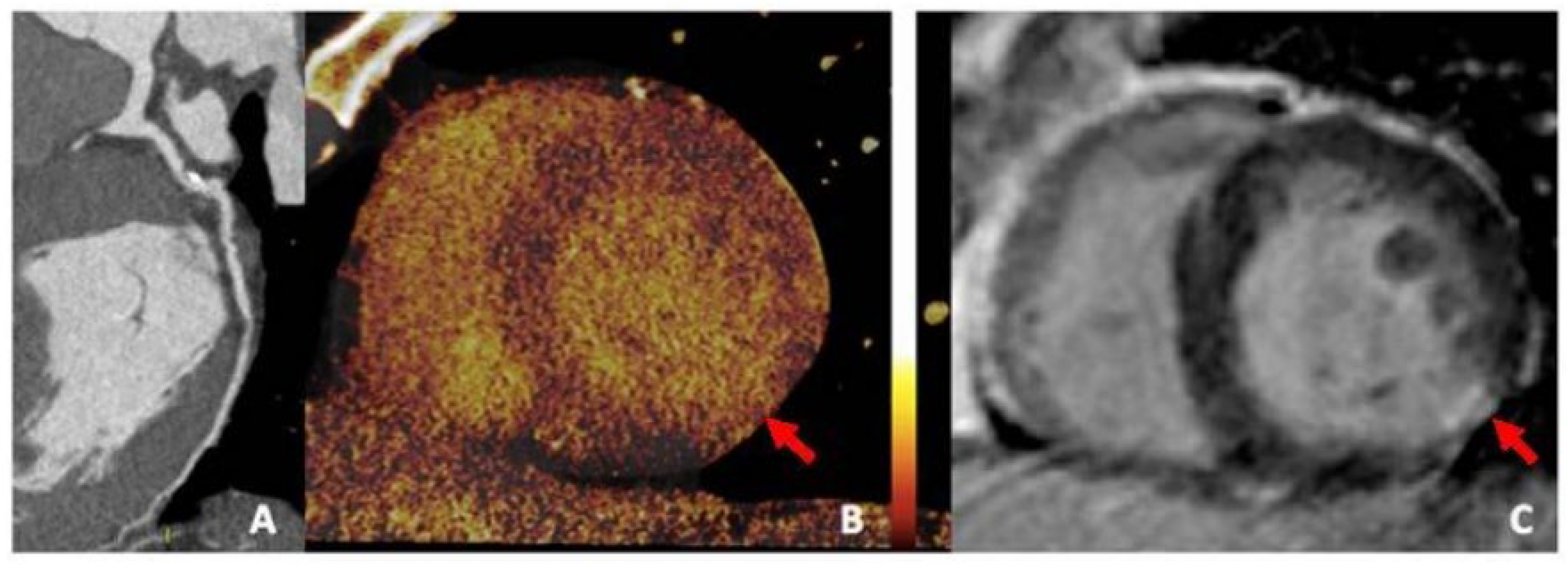
6.3. Photon-Counting CT and Dual-Energy CT
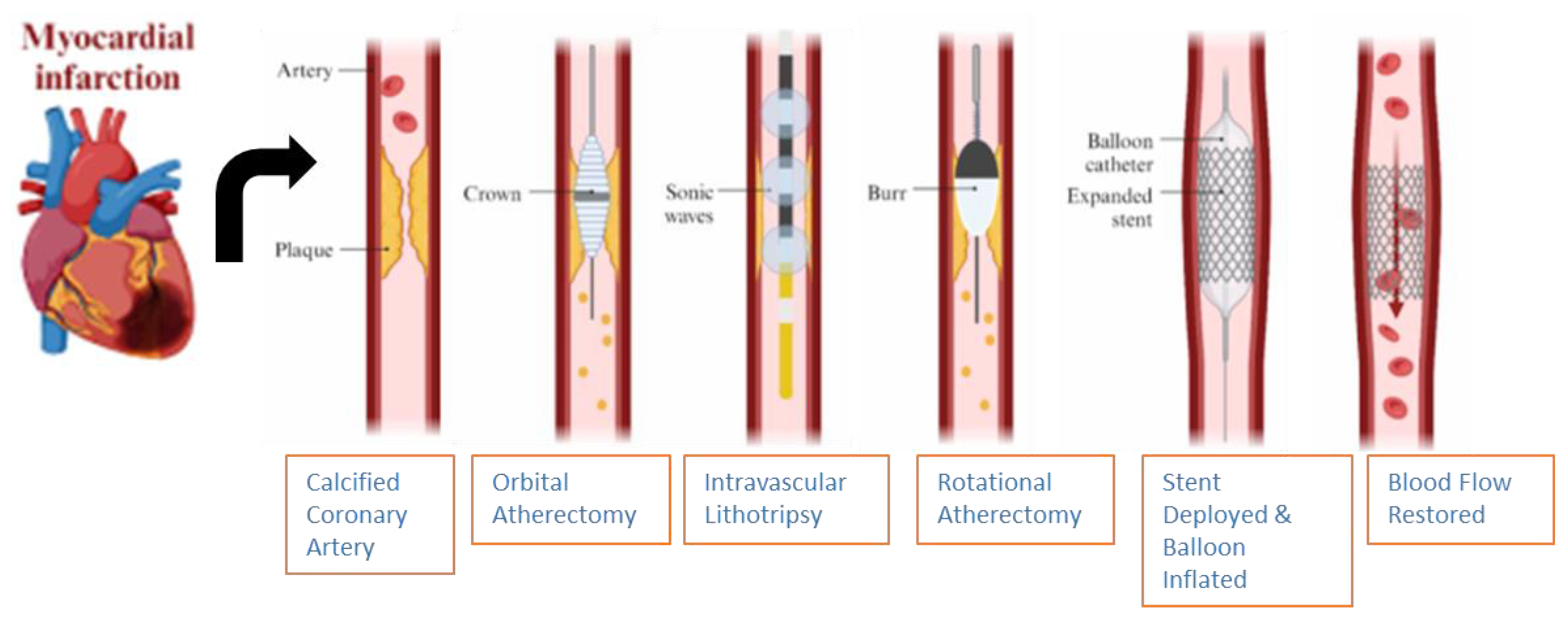
6.4. Balloon Escalation Technique
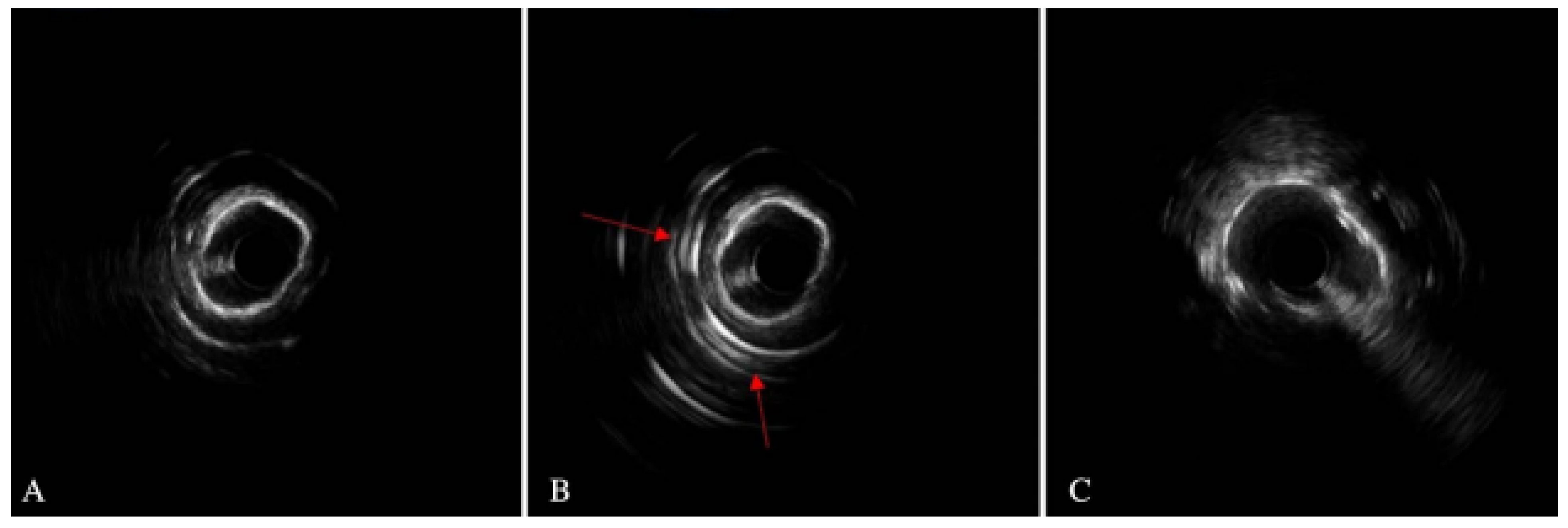
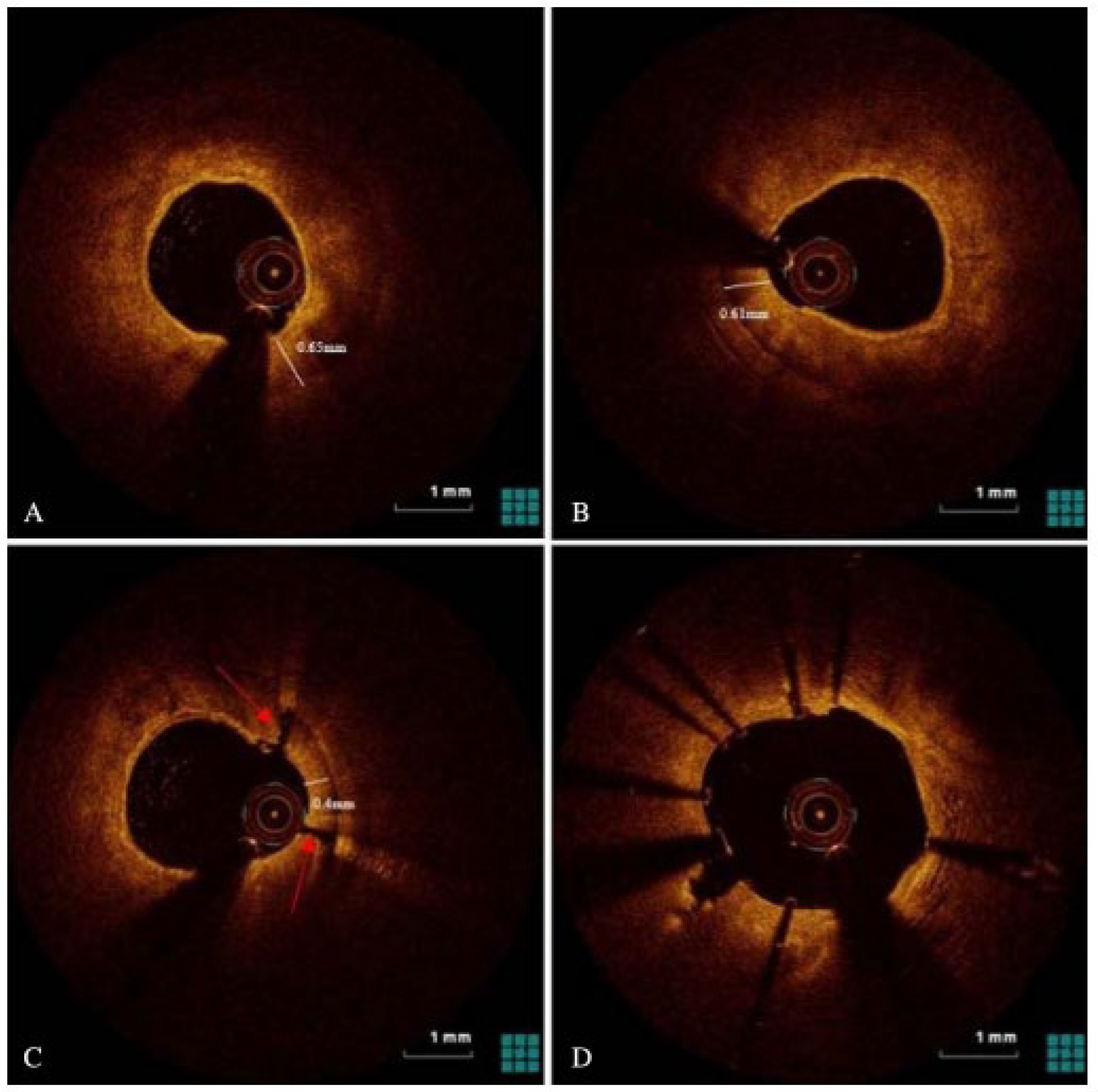
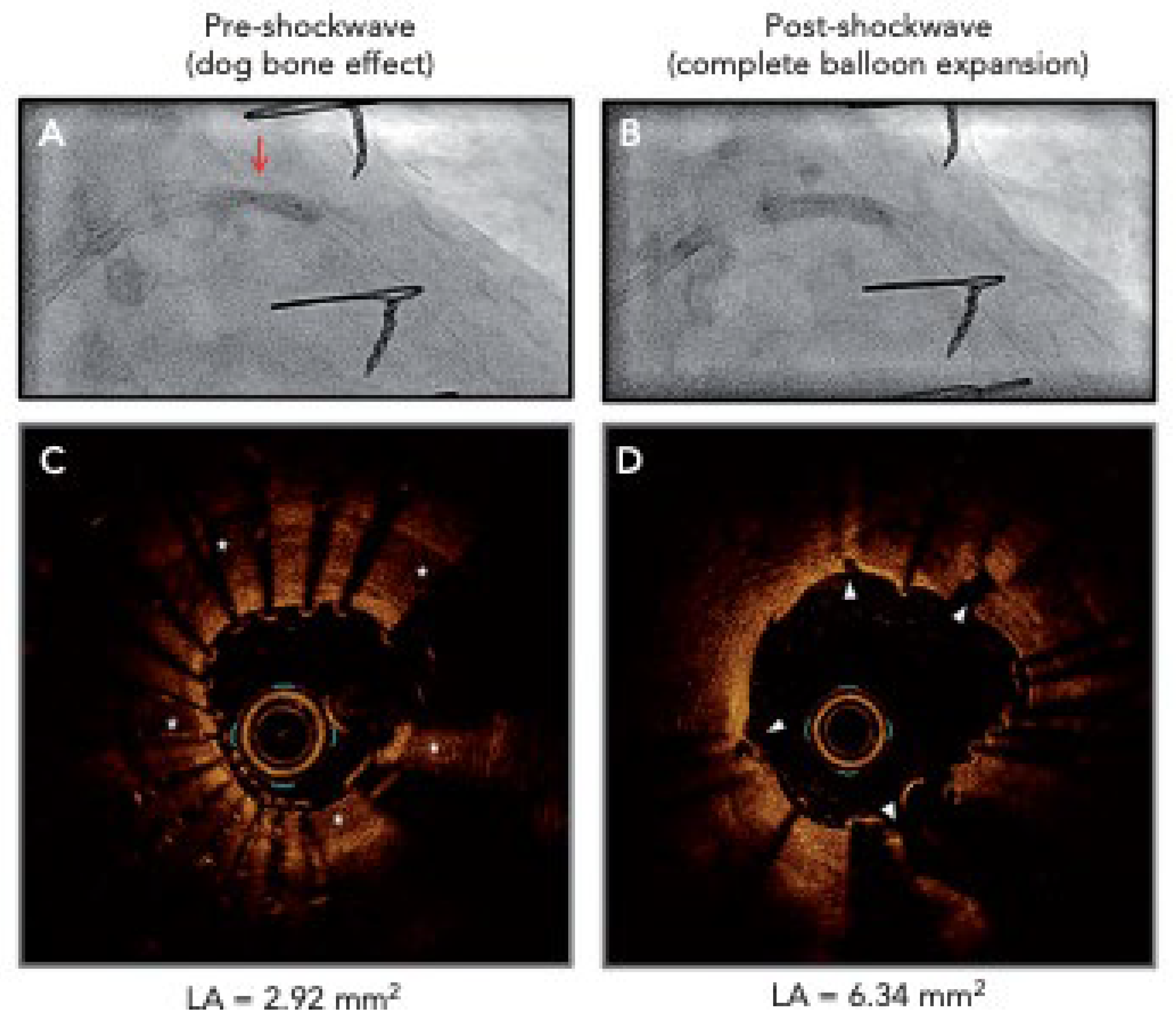
6.5. Intravascular Lithotripsy
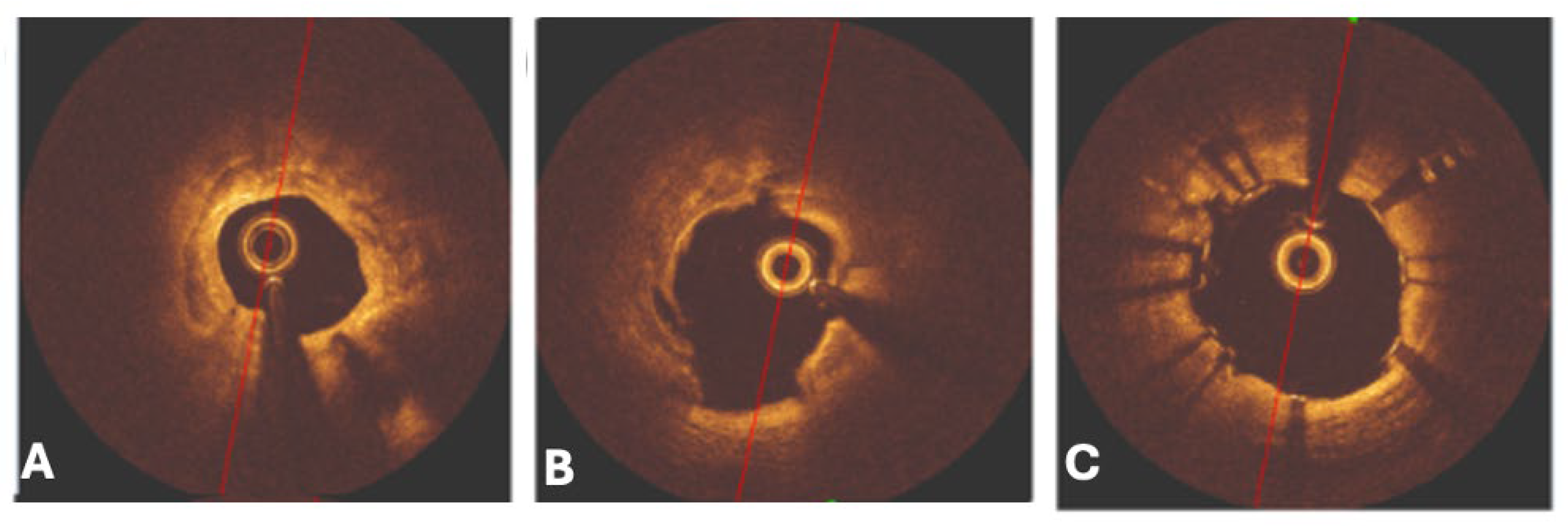
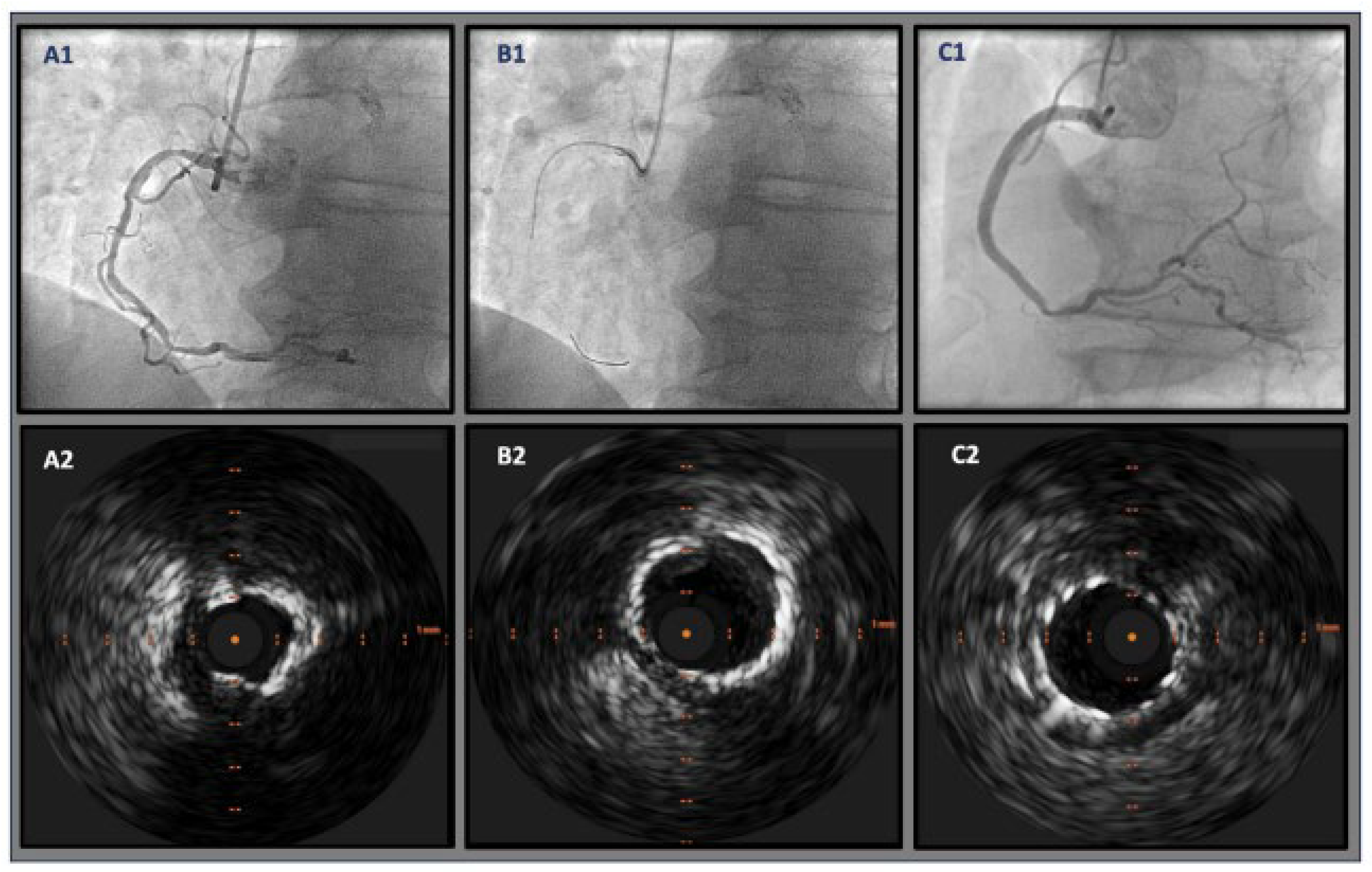
6.6. Rotational Atherectomy
6.7. Orbital Atherectomy
6.8. Laser Atherectomy
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhatt, D.L.; Lopes, R.D.; Harrington, R.A. Diagnosis and treatment of acute coronary syndromes: A review. JAMA 2022, 327, 662–675. [Google Scholar] [CrossRef]
- Panuccio, G.; Salerno, N.; De Rosa, S.; Torella, D. Timing of complete revascularization in patients with STEMI and multivessel disease: A systematic review and meta-analysis. Rev. Cardiovasc. Med. 2023, 24, 58. [Google Scholar] [CrossRef]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Williams, M.S. 2025 ACC/AHA/ACEP/NAEMSP/SCAI guideline for the management of patients with acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2025, 85, 2135–2237. [Google Scholar] [CrossRef]
- Barbato, E.; Gallinoro, E.; Abdel-Wahab, M.; Andreini, D.; Carrié, D.; Di Mario, C.; Dudek, D.; Escaned, J.; Fajadet, J.; Guagliumi, G.; et al. Management strategies for heavily calcified coronary stenoses: An EAPCI clinical consensus statement in collaboration with the EURO4C-PCR group. Eur. Heart J. 2023, 44, 4340–4356. [Google Scholar] [CrossRef]
- Gu, D.; Qu, J.; Zhang, H.; Zheng, Z. Revascularization for Coronary Artery Disease: Principle and Challenges. Adv. Exp. Med. Biol. 2020, 1177, 75–100. [Google Scholar]
- Osborne-Grinter, M.; Ali, A.; Williams, M.C. Prevalence and clinical implications of coronary artery calcium scoring on non-gated thoracic computed tomography: A systematic review and meta-analysis. Eur. Radiol. 2024, 34, 4459–4474. [Google Scholar] [CrossRef]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary artery calcification and its progression: What does it really mean? JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef]
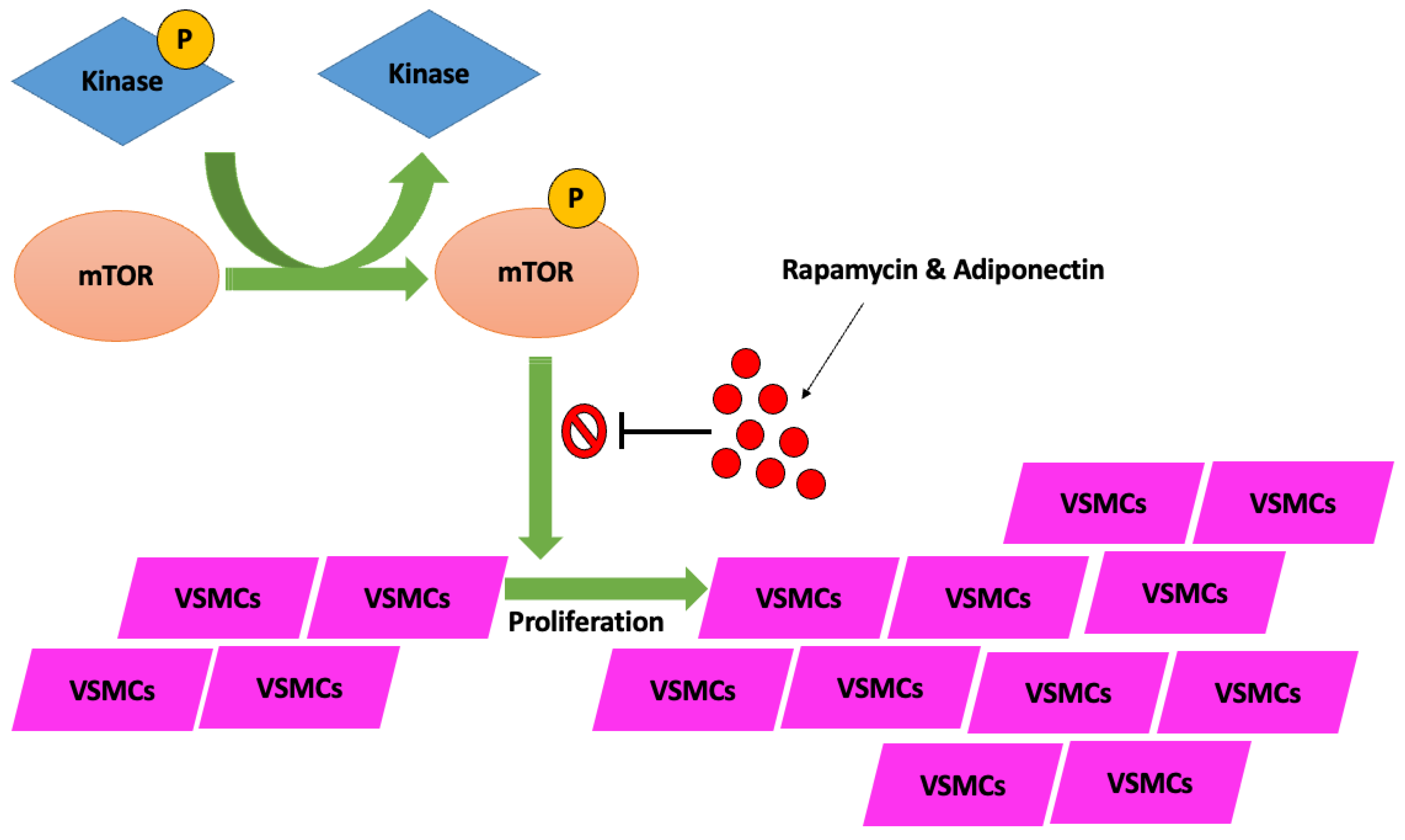
- Zhan, J.K.; Wang, Y.J.; Wang, Y.; Wang, S.; Tan, P.; Huang, W.; Liu, Y.S. The mammalian target of rapamycin signalling pathway is involved in osteoblastic differentiation of vascular smooth muscle cells. Can. J. Cardiol. 2014, 30, 568–575. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.M.; Cai, Y.; Zheng, M.F.; Sun, W.L.; Zhang, S.Y.; Kong, W.; Gu, J.; Wang, X.; Xu, M.J. Mammalian target of rapamycin signaling inhibition ameliorates vascular calcification via Klotho upregulation. Kidney Int. 2015, 88, 711–721. [Google Scholar] [CrossRef]
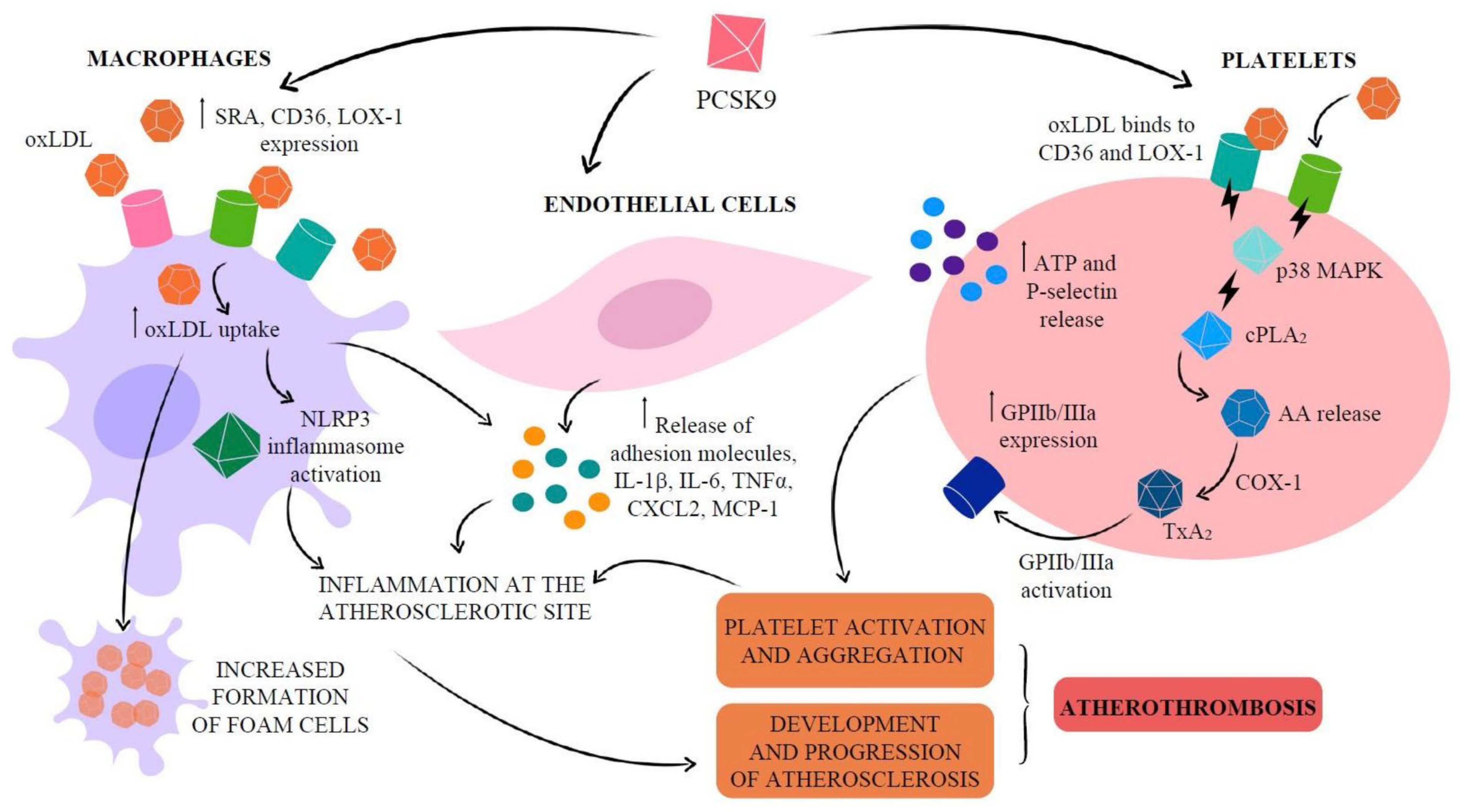
- Neels, J.G.; Leftheriotis, G.; Chinetti, G. Atherosclerosis calcification: Focus on lipoproteins. Metabolites 2023, 13, 457. [Google Scholar] [CrossRef]
- Mitsis, A.; Khattab, E.; Christodoulou, E.; Myrianthopoulos, K.; Myrianthefs, M.; Tzikas, S.; Kassimis, G. From Cells to Plaques: The Molecular Pathways of Coronary Artery Calcification and Disease. J. Clin. Med. 2024, 13, 6352. [Google Scholar] [CrossRef] [PubMed]
- Vieceli Dalla Sega, F.; Fortini, F.; Severi, P.; Rizzo, P.; Gardi, I.; Cimaglia, P.; Ferrari, R. Cardiac calcifications: Phenotypes, mechanisms, clinical and prognostic implications. Biology 2022, 11, 414. [Google Scholar] [CrossRef]
- Cabiati, M.; Vozzi, F.; Ceccherini, E.; Guiducci, L.; Persiani, E.; Gisone, I.; Del Ry, S. Exploring bone morphogenetic protein-2 and-4 mRNA expression and their receptor assessment in a dynamic in vitro model of vascular calcification. Cells 2024, 13, 2091. [Google Scholar] [CrossRef]
- Lee, G.L.; Yeh, C.C.; Wu, J.Y.; Lin, H.C.; Wang, Y.F.; Kuo, Y.Y.; Hsieh, Y.T.; Hsu, Y.J.; Kuo, C.C. TLR2 promotes vascular smooth muscle cell chondrogenic differentiation and consequent calcification via the concerted actions of osteoprotegerin suppression and IL-6–mediated RANKL induction. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 432–445. [Google Scholar] [CrossRef]
- Ren, Y.; Li, X.; Wang, S.; Pan, W.; Lv, H.; Wang, M.; Yin, D. Serum alkaline phosphatase levels are associated with coronary artery calcification patterns and plaque vulnerability. Catheter. Cardiovasc. Interv. 2021, 97, 1055–1062. [Google Scholar] [CrossRef]
- Li, M.; Han, J.; Muegge, C.; Zollinger, T.; Zhou, L.Y.; Monahan, P.; Nan, H. Age, inflammation, alkaline phosphatase, and coronary artery calcification in firefighters. BMC Cardiovasc. Disord. 2025, 25, 309. [Google Scholar] [CrossRef]
- Xu, L.; Yan, X.; Tang, Z.; Feng, B. Association between circulating oxidized OxLDL/LDL-C ratio and the severity of coronary atherosclerosis, along with other emerging biomarkers of cardiovascular disease in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2022, 191, 110040. [Google Scholar] [CrossRef]
- Wang, X.; Ren, J.; Fang, F.; Wang, E.; Li, J.; He, W.; Liu, X. Matrix vesicles from osteoblasts promote atherosclerotic calcification. Matrix Biol. 2024, 134, 79–92. [Google Scholar] [CrossRef]
- Boraldi, F.; Lofaro, F.D.; Quaglino, D. Apoptosis in the extraosseous calcification process. Cells 2021, 10, 131. [Google Scholar] [CrossRef]
- Ikari, Y.; McManus, B.M.; Kenyon, J.; Schwartz, S.M. Neonatal intima formation in the human coronary artery. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2036–2040. [Google Scholar] [CrossRef]
- Schaefer, H.E. The role of macrophages in atherosclerosis. In Disorders of the Monocyte Macrophage System: Pathophysiological and Clinical Aspects; Springer: Berlin/Heidelberg, Germany, 1981; pp. 137–142. [Google Scholar]
- Morris, M.C.; Kreutz, R.P. Coronary Calcification: Types, Morphology and Distribution. Interv. Cardiol. 2025, 20, e13. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Sato, Y.; Sakamoto, A.; Cornelissen, A.; Mori, M.; Kawakami, R.; Finn, A.V. Calcium deposition within coronary atherosclerotic lesion: Implications for plaque stability. Atherosclerosis 2020, 306, 85–95. [Google Scholar] [CrossRef]
- Hoff, H.F.; Bradley, W.A.; Heideman, C.L.; Gaubatz, J.W.; Karagas, M.D.; Gotto, A.M., Jr. Characterization of low density lipoprotein-like particle in the human aorta from grossly normal and atherosclerotic regions. Biochim. Et Biophys. Acta (BBA) Lipids Lipid Metab. 1979, 573, 361–374. [Google Scholar] [CrossRef]
- Nakashima, Y.; Fujii, H.; Sumiyoshi, S.; Wight, T.N.; Sueishi, K. Early human atherosclerosis: Accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Wight, T.N.; Sueishi, K. Early atherosclerosis in humans: Role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc. Res. 2008, 79, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Finn, A.V.; Virmani, R. Subclinical atherosclerosis: Part 1: What is it? Can it be defined at the histological level? Arterioscler. Thromb. Vasc. Biol. 2024, 44, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, Y.; Tian, M.; Deng, Q.; Qin, X.; Lu, H.; Zhang, W. Gsα regulates macrophage foam cell formation during atherosclerosis. Circ. Res. 2024, 134, e34–e51. [Google Scholar] [CrossRef]
- Nakano, M.; Yahagi, K.; Virmani, R.; Ikari, Y. Are microcalcification and hemosiderin really limitations of OCT in detection of TCFA? JACC Cardiovasc. Imaging 2016, 9, 215. [Google Scholar] [CrossRef]
- Fahed, A.C.; Jang, I.K. Plaque erosion and acute coronary syndromes: Phenotype, molecular characteristics and future directions. Nat. Rev. Cardiol. 2021, 18, 724–734. [Google Scholar] [CrossRef]
- Baaten, C.C.; Nagy, M.; Bergmeier, W.; Spronk, H.M.; van der Meijden, P.E. Platelet biology and function: Plaque erosion vs. rupture. Eur. Heart J. 2024, 45, 18–31. [Google Scholar] [CrossRef]
- Oliveri, F.; Van Oort, M.J.H.; Al Amri, I.; Bingen, B.O.; Van der Kley, F.; Jukema, J.W.; Cabezas, J.M. Coronary calcified nodules versus nonnodular coronary calcifications: A systematic review and meta-analysis. J. Cardiovasc. Med. 2024, 25, 438–449. [Google Scholar] [CrossRef]
- Alfonso, F.; Rivero, F. Superficial Calcific Sheets: A Novel Substrate for Acute Coronary Syndromes? Cardiovasc. Interv. 2019, 12, 541–544. [Google Scholar]
- Ali, Z.A.; Kereiakes, D.J.; Hill, J.M.; Saito, S.; Di Mario, C.; Honton, B.; Shlofmitz, R.A. Impact of calcium eccentricity on the safety and effectiveness of coronary intravascular lithotripsy: Pooled analysis from the disrupt CAD studies. Circ. Cardiovasc. Interv. 2023, 16, e012898. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Alaiti, A. Complications of percutaneous coronary interventions in calcified lesions: Causes, recognition, management, and how to avoid. In Debulking in Cardiovascular Interventions and Revascularization Strategies; Academic Press: Cambridge, MA, USA, 2022; pp. 311–319. [Google Scholar]
- Saleh, H.; Sharma, D.; Afify, H.; Sharma, R.; Ikram, S.; Solankhi, N. Outcomes Following Orbital Atherectomy for Coronary Calcified Nodules: A Retrospective Single-Center Experience. Catheter. Cardiovasc. Interv. 2025, 106, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Garimella, A.; Sharma, R.; Solankhi, N. TCT-373 A Single-Center Review of Outcomes of Orbital Atherectomy in Patients with Coronary Calcium Nodules. J. Am. Coll. Cardiol. 2024, 84 (Suppl. S18), B101. [Google Scholar] [CrossRef]
- Sugiyama, T.; Yamamoto, E.; Fracassi, F.; Lee, H.; Yonetsu, T.; Kakuta, T.; Jang, I.K. Calcified plaques in patients with acute coronary syndromes. Cardiovasc. Interv. 2019, 12, 531–540. [Google Scholar]
- Lei, F.; Yin, Y.; Liu, X.; Fang, C.; Jiang, S.; Xu, X.; Yu, B. Clinical outcomes of different calcified culprit plaques in patients with acute coronary syndrome. J. Clin. Med. 2022, 11, 4018. [Google Scholar] [CrossRef]
- Blaha, M.J.; DeFilippis, A.P. Multi-Ethnic Study of Atherosclerosis (MESA) JACC Focus Seminar 5/8. J. Am. Coll. Cardiol. 2021, 77, 3195–3216. [Google Scholar] [CrossRef]
- McInerney, A.; Hynes, S.O.; Gonzalo, N. Calcified Coronary Artery Disease: Pathology, Prevalence, Predictors and Impact on Outcomes. Interv. Cardiol. Rev. Res. Resour. 2025, 20, e02. [Google Scholar] [CrossRef]
- Bundy, J.D.; Chen, J.; Yang, W.; Budoff, M.; Go, A.S.; Grunwald, J.E.; CRIC Study Investigators. Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: The CRIC study. Atherosclerosis 2018, 271, 53–60. [Google Scholar] [CrossRef]
- Shikada, T.; Washio, M.; Nishizaki, A.; Kakino, T.; Ooe, K.; Ishibashi, Y.; Tashiro, H. Risk factors for coronary artery calcification in Japanese patients. J. Cardiol. 2015, 66, 36–40. [Google Scholar] [CrossRef]
- Tajima, A.; Bouisset, F.; Ohashi, H.; Sakai, K.; Mizukami, T.; Rizzini, M.L.; Collet, C. Advanced CT imaging for the assessment of calcific coronary artery disease and PCI planning. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101299. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, P.; Claessen, B.E.; Mehran, R.; Mintz, G.S.; Liu, M.; Sorrentino, S.; Stone, G.W. Coronary calcification and long-term outcomes according to drug-eluting stent generation. Cardiovasc. Interv. 2020, 13, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Najam, O.; Bhindi, R.; De Silva, K. Calcium modification techniques in complex percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2021, 14, e009870. [Google Scholar] [CrossRef] [PubMed]
- Ban, X.; Li, Z.; Duan, Y.; Xu, K.; Xiong, J.; Tu, Y. Advanced imaging modalities provide new insights into coronary artery calcification. Eur. J. Radiol. 2022, 157, 110601. [Google Scholar] [CrossRef]
- Onnis, C.; Virmani, R.; Kawai, K.; Nardi, V.; Lerman, A.; Cademartiri, F.; Saba, L. Coronary artery calcification: Current concepts and clinical implications. Circulation 2024, 149, 251–266. [Google Scholar] [CrossRef]
- Van Rosendael, A.R.; Narula, J.; Lin, F.Y.; Van Den Hoogen, I.J.; Gianni, U.; Alawamlh, O.A.H.; Shaw, L.J. Association of high-density calcified 1K plaque with risk of acute coronary syndrome. JAMA Cardiol. 2020, 5, 282–290. [Google Scholar] [CrossRef]
- Lee, S.E.; Chang, H.J.; Sung, J.M.; Park, H.B.; Heo, R.; Rizvi, A.; Min, J.K. Effects of statins on coronary atherosclerotic plaques: The Paradigm study. JACC Cardiovasc. Imaging 2018, 11, 1475–1484. [Google Scholar] [CrossRef]
- Peng, A.W.; Mirbolouk, M.; Orimoloye, O.A.; Osei, A.D.; Dardari, Z.; Dzaye, O.; Blaha, M.J. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC≥ 1000: Results from the CAC Consortium. Cardiovasc. Imaging 2020, 13 Pt 1, 83–93. [Google Scholar]
- Pontone, G.; Bertella, E.; Mushtaq, S.; Loguercio, M.; Cortinovis, S.; Baggiano, A.; Andreini, D. Coronary artery disease: Diagnostic accuracy of CT coronary angiography—A comparison of high and standard spatial resolution scanning. Radiology 2014, 271, 688–694. [Google Scholar] [CrossRef]
- Haag, N.P.; Michael, A.E.; Lennartz, S.; Panknin, C.; Niehoff, J.H.; Borggrefe, J.; Kroeger, J.R. Coronary artery calcium scoring using virtual versus true noncontrast images from photon-counting coronary CT angiography. Radiology 2024, 310, e230545. [Google Scholar] [CrossRef]
- Gupta, A.; Bera, K.; Kikano, E.; Pierce, J.D.; Gan, J.; Rajdev, M.; Gilkeson, R.C. Coronary artery calcium scoring: Current status and future directions. Radiographics 2022, 42, 947–967. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, G.; Werner, G.S.; De Rosa, S.; Torella, D.; Leistner, D.M.; Siegrist, P.T.; Abdelwahed, Y.S. Full-moon coronary calcification as detected with computed tomography angiography in chronic total occlusion percutaneous coronary intervention. Am. J. Cardiol. 2024, 222, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Doris, M.K.; Meah, M.N.; Moss, A.J.; Andrews, J.P.; Bing, R.; Gillen, R.; Adamson, P.D. Coronary 18F-fluoride uptake and progression of coronary artery calcification. Circ. Cardiovasc. Imaging 2020, 13, e011438. [Google Scholar] [CrossRef] [PubMed]
- Tzolos, E.; Dweck, M.R. 18F-sodium fluoride (18F-NaF) for imaging microcalcification activity in the cardiovascular system. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1620–1626. [Google Scholar] [CrossRef]
- Sandstedt, M.; Marsh, J.; Rajendran, K.; Gong, H.; Tao, S.; Persson, A.; McCollough, C. Improved coronary calcification quantification using photon-counting-detector CT: An ex vivo study in cadaveric specimens. Eur. Radiol. 2021, 31, 6621–6630. [Google Scholar] [CrossRef]
- VanMeter, P.D.; Marsh, J., Jr.; Rajendran, K.; Leng, S.; McCollough, C. Quantification of coronary calcification using high-resolution photon-counting-detector CT and an image domain denoising algorithm. In Medical Imaging 2022: Physics of Medical Imaging; SPIE: Bellingham, WA, USA, 2022; Volume 12031, pp. 433–438. [Google Scholar]
- Si-Mohamed, S.A.; Boccalini, S.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.A.; Douek, P.C. Coronary CT angiography with photon-counting CT: First-in-human results. Radiology 2022, 303, 303–313. [Google Scholar] [CrossRef]
- Tarkowski, P.; Czekajska-Chehab, E. Dual-energy heart CT: Beyond better angiography. J. Clin. Med. 2021, 10, 5193. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Feng, G.; Fan, T.; Jiang, H.; Wang, Z. Advances in CT techniques in vascular calcification. Front. Cardiovasc. Med. 2021, 8, 716822. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Virmani, R.; Hokama, J.Y.; Illindala, U.; Mena-Hurtado, C.; Holden, A.; Ali, Z.A. Principles of intravascular lithotripsy for calcific plaque modification. Cardiovasc. Interv. 2021, 14, 1275–1292. [Google Scholar] [CrossRef]
- Honton, B.; Monsegu, J. Best practice in intravascular lithotripsy. Interv. Cardiol. Rev. Res. Resour. 2022, 17, e02. [Google Scholar] [CrossRef] [PubMed]
- Iwańczyk, S.; Włodarczak, A.; Hiczkiewicz, J.; Faron, W.; Grygier, M.; Furtan, Ł.; Lesiak, M. Feasibility of intravascular lithotripsy for calcific coronary lesions: A multi-institutional experience. Catheter. Cardiovasc. Interv. 2021, 98, E540–E547. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Shrivastava, A.; Dugal, J.S.; Chhikara, S.; Vijayvergiya, R.; Singh, N.; Swamy, A. Coronary intravascular lithotripsy in contemporary practice: Challenges and opportunities in coronary intervention. Ther. Adv. Cardiovasc. Dis. 2024, 18, 17539447241263444. [Google Scholar] [CrossRef] [PubMed]
- Khattak, S.; Sharma, H.; Khan, S.Q. Atherectomy Techniques: Rotablation, Orbital and Laser. Interv. Cardiol. Rev. Res. Resour. 2024, 19, e21. [Google Scholar] [CrossRef]
- Florek, K.; Bartoszewska, E.; Biegała, S.; Klimek, O.; Malcharczyk, B.; Kübler, P. Rotational atherectomy, orbital atherectomy, and intravascular lithotripsy comparison for calcified coronary lesions. J. Clin. Med. 2023, 12, 7246. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Zheng, Z.; Shi, Y.; Liu, J. Comparison of intravascular lithotripsy versus rotational atherectomy for the treatment of severe coronary artery calcification. BMC Cardiovasc. Disord. 2024, 24, 311. [Google Scholar] [CrossRef]
- Mousa, M.A.; Bingen, B.O.; Al Amri, I.; Mertens, B.J.A.; Taha, S.; Tohamy, A.; Montero-Cabezas, J.M. Efficacy and safety of intravascular lithotripsy versus rotational atherectomy in balloon-crossable heavily calcified coronary lesions. Cardiovasc. Revascularization Med. 2023, 48, 1–6. [Google Scholar] [CrossRef]
- Kayani, A.M.A.; Umar, M.F.; Garcia, A.; Fretz, T.; Lemus-Zamora, R.E.; Alraies, C.; Sattar, Y. Comparative efficacy and safety of intravascular lithotripsy versus rotational atherectomy in coronary artery calcification: An updated meta-analysis. Arch. Cardiovasc. Dis. 2025; in press. [Google Scholar] [CrossRef]
- Gallinoro, E.; Monizzi, G.; Sonck, J.; Candreva, A.; Mileva, N.; Nagumo, S.; Collet, C. Physiological and angiographic outcomes of PCI in calcified lesions after rotational atherectomy or intravascular lithotripsy. Int. J. Cardiol. 2022, 352, 27–32. [Google Scholar] [CrossRef]
- Kinnaird, T.; Gallagher, S.; Sharp, A.; Protty, M.; Salim, T.; Ludman, P.; Mamas, M.A. Operator volumes and in-hospital outcomes: An analysis of 7,740 rotational atherectomy procedures from the BCIS national database. Cardiovasc. Interv. 2021, 14, 1423–1430. [Google Scholar]
- Jurado-Román, A.; Gómez-Menchero, A.; Rivero-Santana, B.; Amat-Santos, I.J.; Jiménez-Valero, S.; Caballero-Borrego, J.; Moreno, R. Rotational atherectomy, lithotripsy, or laser for calcified coronary stenosis: The ROLLER COASTR-EPIC22 trial. Cardiovasc. Interv. 2025, 18, 606–618. [Google Scholar]
- Hinton, J.; Tuffs, C.; Varma, R.; Hurwitz-Bremner, R.; Hein, A.; Kwok, C.S.; O’Kane, P. An analysis of long-term clinical outcome following the use of excimer laser coronary atherectomy in a large UK PCI center. Catheter. Cardiovasc. Interv. 2024, 104, 27–33. [Google Scholar] [CrossRef]
- Tsutsui, R.S.; Sammour, Y.; Kalra, A.; Reed, G.; Krishnaswamy, A.; Ellis, S.; Puri, R. Excimer laser atherectomy in percutaneous coronary intervention: A contemporary review. Cardiovasc. Revascularization Med. 2021, 25, 75–85. [Google Scholar] [CrossRef]






| Visual Score | Absolute CAC Score (Agatston Method) | Clinical Interpretation |
|---|---|---|
| None | 0 | Very low risk of future coronary events |
| Minimal | 1–10 | Low risk of future coronary events; low probability of MI |
| Mild | 11–100 | Medium risk of future coronary events |
| Moderate | 101–400 | Increased risk of future coronary events; consider reclassifying the individual as being at high risk |
| Severe | >400 | Increased probability of MI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramazani, N.; Abdallah, A.W.; DiCaro, M.V.; Sharma, D.; Singh, A.; Lei, K. Use of Calcium Modification in Percutaneous Coronary Intervention: A Comprehensive Review. J. Clin. Med. 2025, 14, 8130. https://doi.org/10.3390/jcm14228130
Ramazani N, Abdallah AW, DiCaro MV, Sharma D, Singh A, Lei K. Use of Calcium Modification in Percutaneous Coronary Intervention: A Comprehensive Review. Journal of Clinical Medicine. 2025; 14(22):8130. https://doi.org/10.3390/jcm14228130
Chicago/Turabian StyleRamazani, Noyan, Ala W. Abdallah, Michael V. DiCaro, Divyansh Sharma, Aditi Singh, and KaChon Lei. 2025. "Use of Calcium Modification in Percutaneous Coronary Intervention: A Comprehensive Review" Journal of Clinical Medicine 14, no. 22: 8130. https://doi.org/10.3390/jcm14228130
APA StyleRamazani, N., Abdallah, A. W., DiCaro, M. V., Sharma, D., Singh, A., & Lei, K. (2025). Use of Calcium Modification in Percutaneous Coronary Intervention: A Comprehensive Review. Journal of Clinical Medicine, 14(22), 8130. https://doi.org/10.3390/jcm14228130






