Vacuum-Assisted Wound Therapy in Bothrops Snakebite Injuries: A Case Series from the Brazilian Amazon
Abstract
1. Introduction
2. Case Series Presentation
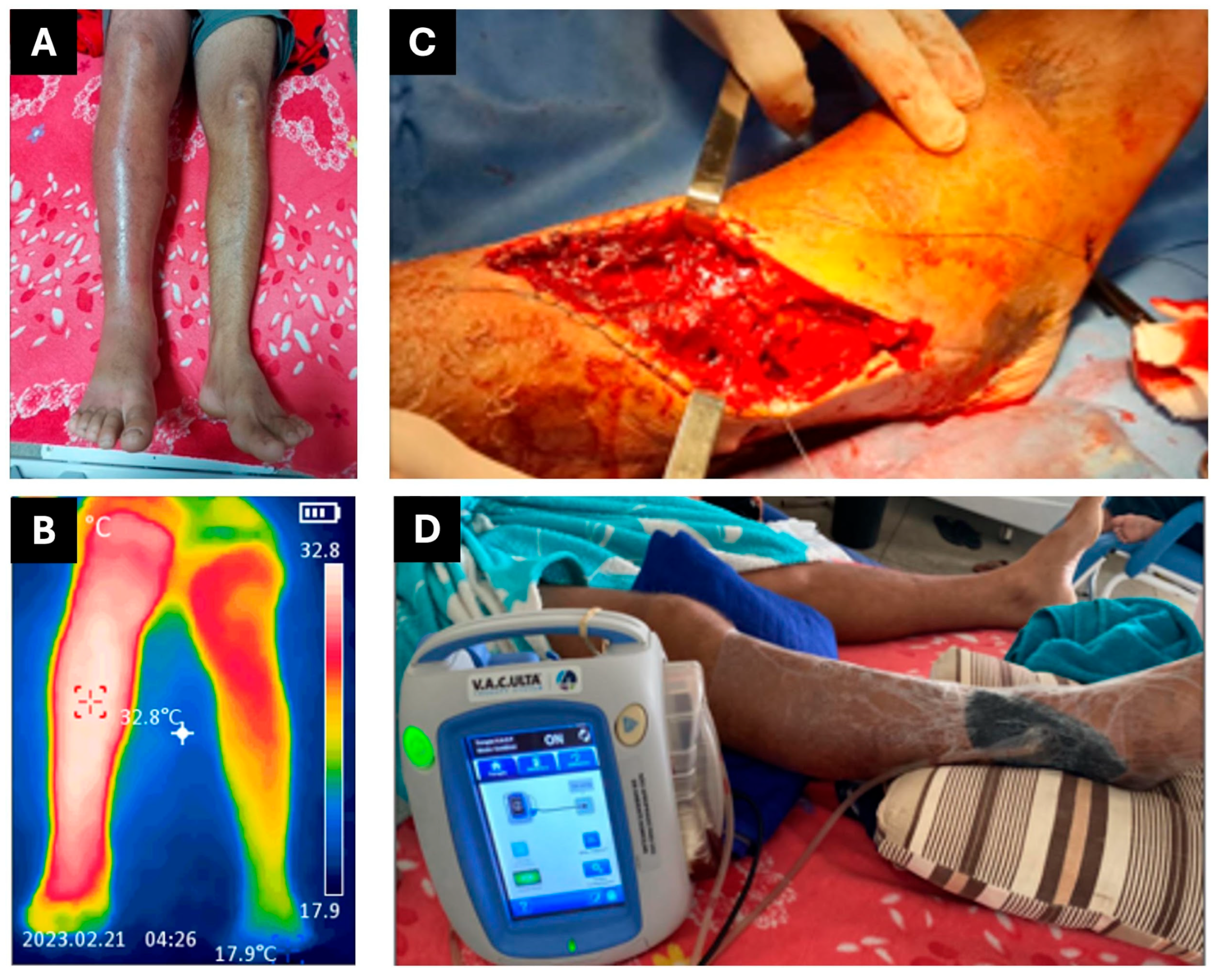
2.1. CASE REPORT 1
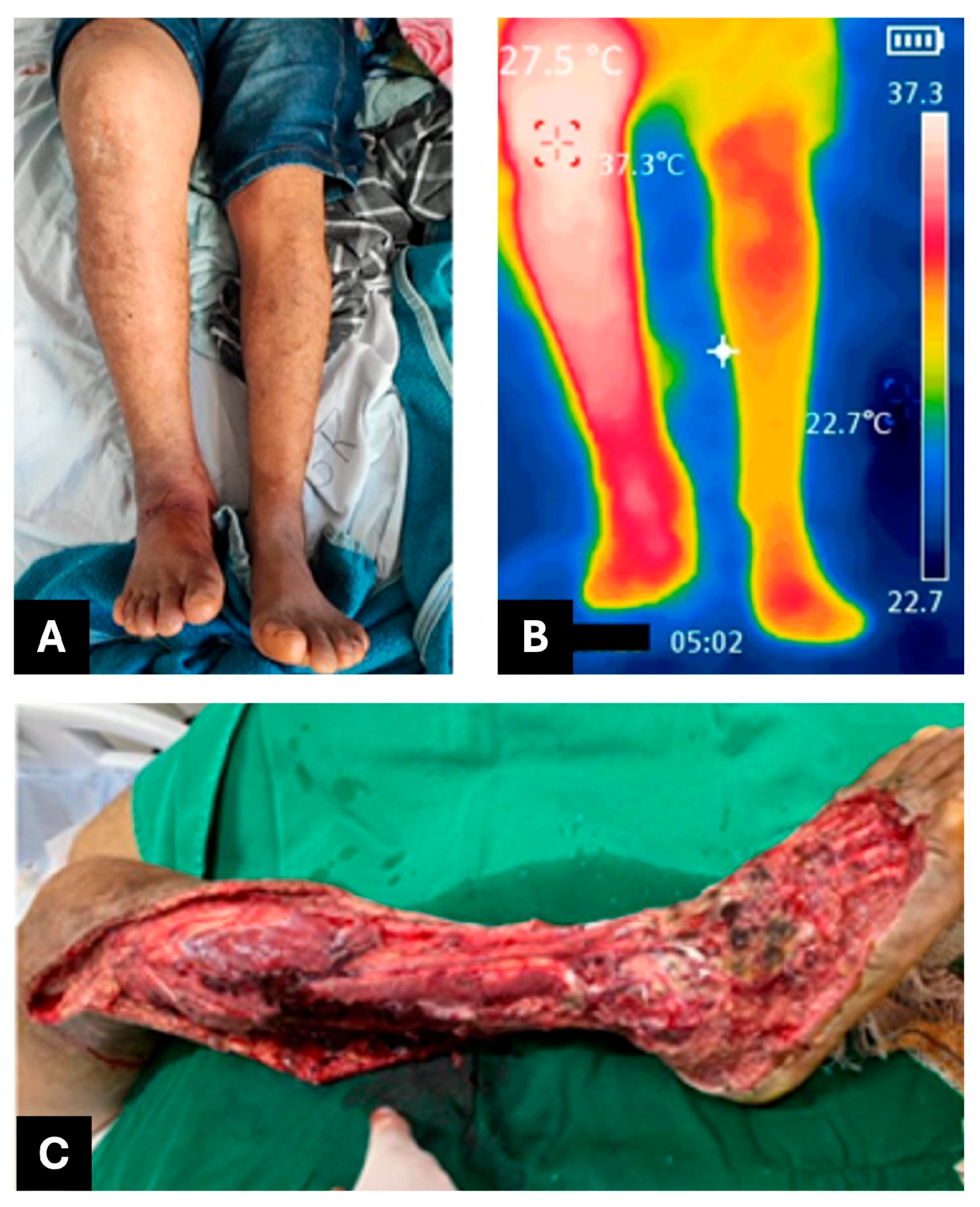
2.2. CASE REPORT 2
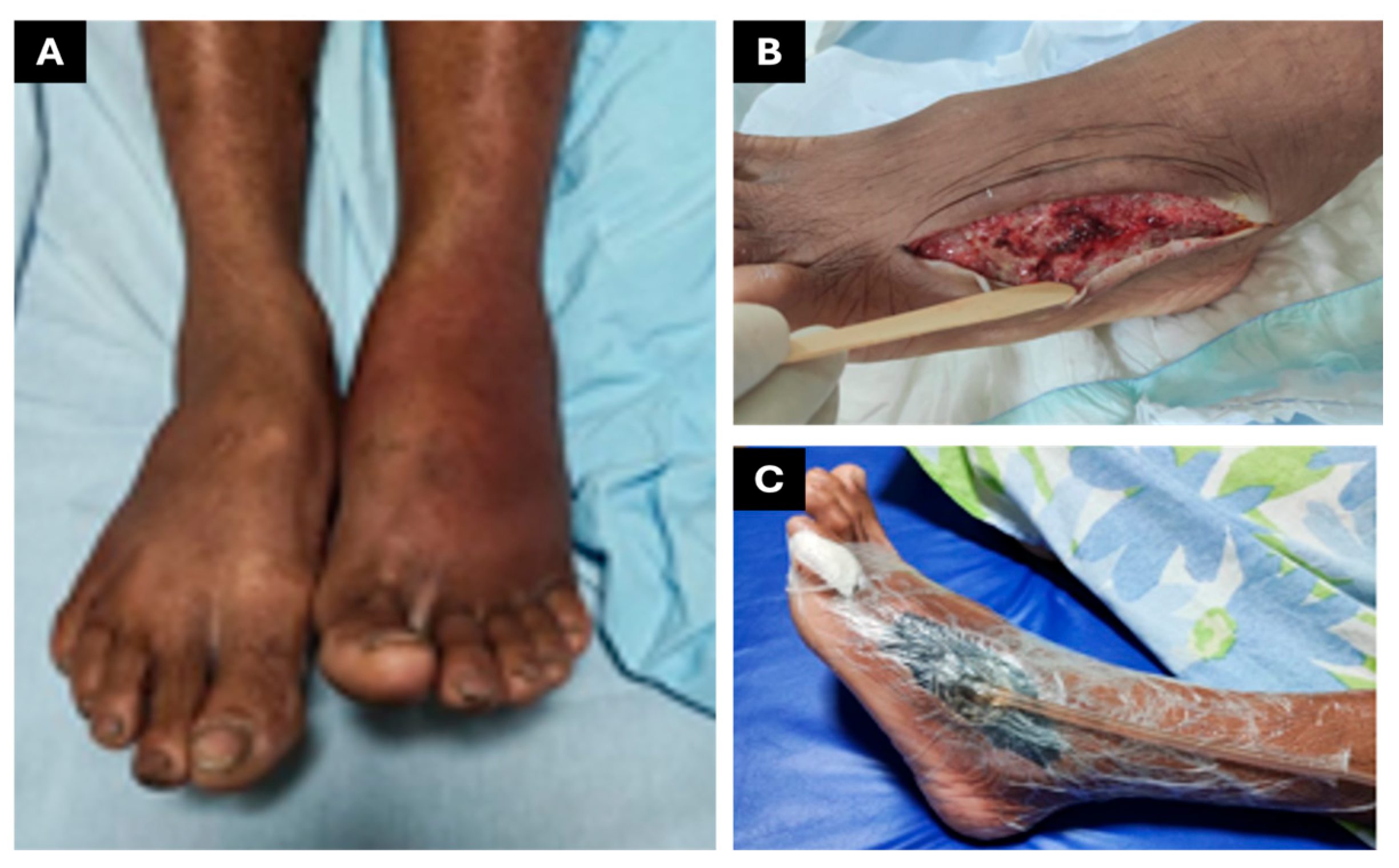
2.3. CASE REPORT 3
2.4. CASE REPORT 4
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Snakebite Envenoming—A Strategy for Prevention and Control. Available online: https://www.who.int/publications/i/item/9789241515641 (accessed on 2 June 2025).
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite Envenoming. Nat. Rev. Dis. Primers 2017, 3, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef]
- Williams, D.; Gutiérrez, J.M.; Harrison, R.; Warrell, D.A.; White, J.; Winkel, K.D.; Gopalakrishnakone, P. The Global Snake Bite Initiative: An Antidote for Snake Bite. Lancet 2010, 375, 89–91. [Google Scholar] [CrossRef]
- Acidentes por Animais Peçonhentos. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/a/animais-peconhentos/animais-peconhentos (accessed on 2 June 2025).
- Natural History of Snakes in Forests of the Manaus Region, Central Amazonia, Brazil. Available online: https://www.researchgate.net/publication/236897126_Natural_history_of_snakes_in_Forests_of_the_Manaus_Region_Central_Amazonia_Brazil (accessed on 2 June 2025).
- Bernarde, P.S.; Gomes, J.d.O. Serpentes peçonhentas e ofidismo em Cruzeiro do Sul, Alto Juruá, Estado do Acre, Brasil. Acta Amaz. 2012, 42, 65–72. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C. Hemorrhage Caused by Snake Venom Metalloproteinases: A Journey of Discovery and Understanding. Toxins 2016, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Snake Bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Anz, A.W.; Schweppe, M.; Halvorson, J.; Bushnell, B.; Sternberg, M.; Andrew Koman, L. Management of Venomous Snakebite Injury to the Extremities. J. Am. Acad. Orthop. Surg. 2010, 18, 749–759. [Google Scholar] [CrossRef]
- de Farias, A.S.; Cristino, J.S.; da Costa Arévalo, M.; Carneiro Junior, A.; Gomes Filho, M.R.; Ambrosio, S.A.; Nickenig Vissoci, J.; Wen, F.H.; Azevedo Machado, V.; Sachett, J.; et al. Children Growing Up with Severe Disabilities as a Result of Snakebite Envenomations in Indigenous Villages of the Brazilian Amazon: Three Cases and Narratives. Toxins 2023, 15, 352. [Google Scholar] [CrossRef]
- Gabriel, A.; Heinrich, C.; Shores, J.; Cho, D.; Baqai, W.; Moores, D.; Miles, D.; Gupta, S. Outcomes of Vacuum-Assisted Closure for the Treatment of Wounds in a Paediatric Population: Case Series of 58 Patients. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 1428–1436. [Google Scholar] [CrossRef]
- Albuquerque Barbosa, F.B.; de Raad, R.S.; Santos Ibiapina, H.N.; Freire Dos Reis, M.; Neves, J.C.F.; Andrade, R.V.; Nascimento, T.P.; Valle, F.F.; Casewell, N.R.; Sachett, J.; et al. Dermatopathological Findings of Bothrops Atrox Snakebites: A Case Series in the Brazilian Amazon. PLoS Negl. Trop. Dis. 2024, 18, e0012704. [Google Scholar] [CrossRef]
- Juckett, G.; Hancox, J.G. Venomous Snakebites in the United States: Management Review and Update. Am. Fam. Physician 2002, 65, 1367–1374. [Google Scholar] [PubMed]
- Lima, R.V.K.S.; Coltro, P.S.; Farina, J.A. Terapia por pressão negativa no tratamento de feridas complexas. Rev. Col. Bras. Cir. 2017, 44, 81–93. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Tuma, P.; Carvalho, V.F.; Kamamoto, F. Complex Wounds. Clinics 2006, 61, 571–578. [Google Scholar] [CrossRef]
- Shoufani, A.; Samuelov, R. Vacuum assisted closure—A new method for wound control and treatment. Harefuah 2003, 142, 837–877. [Google Scholar]
- Kim, K.J.; Min, J.H.; Yoo, I.; Kim, S.W.; Lee, J.; Ryu, S.; You, Y.H.; Park, J.S.; Jeong, W.J.; Cho, Y.C.; et al. Negative pressure wound therapy for skin necrosis prevention after snakebite in the emergency department: A retrospective cohort study. Medicine 2021, 100, e24290. [Google Scholar] [CrossRef]
- Schlatterer, D.R.; Hirschfeld, A.G.; Webb, L.X. Negative Pressure Wound Therapy in Grade IIIB Tibial Fractures: Fewer Infections and Fewer Flap Procedures? Clin. Orthop. Relat. Res. 2015, 473, 1802–1811. [Google Scholar] [CrossRef]
- Cheng, H.-T.; Hsu, Y.-C.; Wu, C.-I. Risk of Infection with Delayed Wound Coverage by Using Negative-Pressure Wound Therapy in Gustilo Grade IIIB/IIIC Open Tibial Fracture: An Evidence-Based Review. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.K.; Alcantara, S.; Goss, S.; Lantis, J.C. Cost Analysis of Negative-Pressure Wound Therapy with Instillation for Wound Bed Preparation Preceding Split-Thickness Skin Grafts for Massive (>100 cm2) Chronic Venous Leg Ulcers. J. Vasc. Surg. 2015, 61, 995–999. [Google Scholar] [CrossRef]
- Ferreira, M.C.; de Carvalho, V.F.; Kamamoto, F.; Tuma Junior, P.; Paggiaro, A.O. Terapia por pressão negativa (vácuo) no preparo do leito da ferida em pacientes diabéticos: Série de casos. Sao Paulo Med. J. 2009, 127, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Tiong, W.H.C.; Ismael, T.; McCann, J. Post-Traumatic and Post-Surgical Absidia Corymbifera Infection in a Young, Healthy Man. J. Plast. Reconstr. Aesthetic Surg. 2006, 59, 1367–1371. [Google Scholar] [CrossRef]
- Cheatham, M.L.; Demetriades, D.; Fabian, T.C.; Kaplan, M.J.; Miles, W.S.; Schreiber, M.A.; Holcomb, J.B.; Bochicchio, G.; Sarani, B.; Rotondo, M.F. Prospective Study Examining Clinical Outcomes Associated with a Negative Pressure Wound Therapy System and Barker’s Vacuum Packing Technique. World J. Surg. 2013, 37, 2018–2030. [Google Scholar] [CrossRef]
- Kirkpatrick, A.W.; Roberts, D.J.; Faris, P.D.; Ball, C.G.; Kubes, P.; Tiruta, C.; Xiao, Z.; Holodinsky, J.K.; McBeth, P.B.; Doig, C.J.; et al. Active Negative Pressure Peritoneal Therapy After Abbreviated Laparotomy: The Intraperitoneal Vacuum Randomized Controlled Trial. Ann. Surg. 2015, 262, 38–46. [Google Scholar] [CrossRef]
- Argenta, L.C.; Morykwas, M.J. Vacuum-Assisted Closure: A New Method for Wound Control and Treatment: Clinical Experience. Ann. Plast. Surg. 1997, 38, 563–576; discussion 577. [Google Scholar] [CrossRef]
- Wang, G.; Xu, H.; Xu, G.; Zhang, H.; Li, Z.; Liu, D. Clinical Outcomes of Negative Pressure Wound Therapy with Instillation vs Standard Negative Pressure Wound Therapy for Wounds: A Meta-Analysis of Randomised Controlled Trials. Int. Wound J. 2023, 20, 1739–1749. [Google Scholar] [CrossRef]
- Norman, G.; Shi, C.; Goh, E.L.; Murphy, E.M.; Reid, A.; Chiverton, L.; Stankiewicz, M.; Dumville, J.C. Negative Pressure Wound Therapy for Surgical Wounds Healing by Primary Closure. Cochrane Database Syst. Rev. 2022, 2022, CD009261. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Liu, Z.; Norman, G.; Dumville, J.C.; Chiverton, L.; Scuffham, P.; Stankiewicz, M.; Chaboyer, W.P. Negative Pressure Wound Therapy for Surgical Wounds Healing by Primary Closure. Cochrane Database Syst. Rev. 2019, 3, CD009261. [Google Scholar] [CrossRef]
- Campitiello, F.; Mancone, M.; Corte, A.D.; Guerniero, R.; Canonico, S. Expanded Negative Pressure Wound Therapy in Healing Diabetic Foot Ulcers: A Prospective Randomised Study. J. Wound Care 2021, 30, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Irgit, K.; Strohecker, K.A.; Matzko, M.E.; Wingert, N.C.; DeSantis, J.G.; Smith, W.R. Delayed Flap Reconstruction with Vacuum-Assisted Closure Management of the Open IIIB Tibial Fracture. J. Trauma 2011, 71, 1705–1708. [Google Scholar] [CrossRef]
- Mouës, C.M.; Vos, M.C.; van den Bemd, G.-J.C.M.; Stijnen, T.; Hovius, S.E.R. Bacterial Load in Relation to Vacuum-Assisted Closure Wound Therapy: A Prospective Randomized Trial. Wound Repair. Regen. 2004, 12, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Banasiewicz, T.; Borejsza-Wysocki, M.; Meissner, W.; Malinger, S.; Szmeja, J.; Kościński, T.; Ratajczak, A.; Drews, M. Vacuum-Assisted Closure Therapy in Patients with Large Postoperative Wounds Complicated by Multiple Fistulas. Wideochir. Inne Tech. Maloinwazyjne 2011, 6, 155–163. [Google Scholar] [CrossRef]
- Widigdo, D.A.M.; Sofro, Z.M.; Pangastuti, H.S.; Dachlan, I. The Efficacy of Negative Pressure Wound Therapy (NPWT) on Healing of Diabetic Foot Ulcers: A Literature Review. Curr. Diabetes Rev. 2024, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Plikaitis, C.M.; Molnar, J.A. Subatmospheric Pressure Wound Therapy and the Vacuum-Assisted Closure Device: Basic Science and Current Clinical Successes. Expert Rev. Med. Devices 2006, 3, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dumville, J.C.; Hinchliffe, R.J.; Cullum, N.; Game, F.; Stubbs, N.; Sweeting, M.; Peinemann, F. Negative Pressure Wound Therapy for Treating Foot Wounds in People with Diabetes Mellitus. Cochrane Database Syst. Rev. 2018, 10, CD010318. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, C.; Davis, L.; Henderson, V.; Searle, R. The Economic Benefits of Negative Pressure Wound Therapy in Community-Based Wound Care in the NHS. Int. Wound J. 2012, 9, 544–552. [Google Scholar] [CrossRef]


| Analytes * | 16/02 00:07 | 16/02 09:46 | 16/02 20:03 | 26/02 | 01/03 | 03/03 Before VAWT Therapy | 09/03 During VAWT Therapy | Reference Range ** |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin 1 | 15.6 | 15.9 | 15 | 12.3 | 11.3 | 10.8 | 9.4 | 13.5–18.0 g/dL |
| Leucocytes 1 | 26,500 | 21,400 | 21,500 | 18,650 | 17,590 | 16,360 | 11,200 | 4000–10,000 cells/µL |
| Neutrophils 1 | 91% | 82% | 79% | 76.3% | 76.3% | 74.2% | 64.2% | 50–70% |
| Platelets 1,*** | 283,000 | 303,000 | 239,000 | 684,000 | 776,000 | 748,000 | 500,000 | 150,000–400,000/µL |
| PT 2 | Uncoagulable | - | 14.5 | 13.2 | 13.7 | 13.0 | - | 10–14 s |
| aPTT | 48.9 | - | 31.8 | 25.4 | 29.3 | 29.6 | - | 25–39 s |
| Urea 3 | 31 | 30 | 30 | 23.64 | 27.79 | 27.09 | 17.63 | 16–40 mg/dL |
| Creatinine 4 | 1.0 | 0.7 | 1.0 | 0.84 | 0.97 | 0.85 | 0.94 | 0.7–1.4 mg/dL |
| ALT 3 | - | - | 54 | 51.73 | 42.35 | 30.11 | 17.95 | 5–48 U/mL |
| AST 5 | - | - | 87 | 34.42 | 31.31 | 24.08 | 15.54 | 5–48 U/mL |
| CRP 6 | - | - | - | 78.67 | 66.96 | 98.5 | 14.82 | 0.0–8.0 mg/L |
| Analytes * | 27/04 | 30/04 | 06/05 | 13/05 | 21/05 | 28/05 | 16/06 | 23/06 | Reference Range ** |
|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin 1 | 14.4 | 11.3 | 6.5 | 9.5 | 9.6 | 9.6 | 8.6 | 9.8 | 13.5–18.0 g/dL |
| Leucocytes 1 | 22,900 | 16,980 | 18,610 | 11,460 | 8140 | 4870 | 9200 | 5970 | 4000–10,000 cells/µL |
| Neutrophils 1 | 86.5% | 81.9% | 76.6% | 69.4% | 60% | 25% | 62.2% | 41.8% | 50–70% |
| Platelets 1,*** | 383,000 | 89,000 | 785,000 | 1019,000 | 599,000 | 571,000 | 449,000 | 479,000 | 150,000–400,000/µL |
| PT 2 | - | 23.9 | 12.4 | 12.1 | 13.0 | 12.9 | - | - | 10–14 s |
| aPTT | - | 47.1 | 28.7 | 30.0 | 31.7 | 36.3 | - | - | 25–39 s |
| Urea 3 | 32.56 | 20.81 | 19.51 | 17.45 | 18.53 | 13.8 | 17 | 24 | 16–40 mg/dL |
| Creatinine 4 | 0.8 | 0.96 | 0.78 | 0.81 | 0.71 | 0.87 | 0.8 | 0.8 | 0.7–1.4 mg/dL |
| ALT 3 | 37.68 | 25.94 | - | 76.81 | 45.49 | 42.8 | 39 | 54 | 5–48 U/mL |
| AST 5 | 39.42 | 46.66 | - | 32.85 | 19.76 | 22.69 | 18 | 30 | 5–48 U/mL |
| CRP 6 | 2.67 | 156.83 | 79.31 | 2.77 | 3.88 | 67 | 1.7 | 0.75 | 0.0–8.0 mg/L |
| Analytes * | 24/05 | 27/05 | 03/06 | 09/06 | 16/06 | 23/06 | 11/07 | 17/07 | Reference Range ** |
|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin 1 | 14.9 | 12.4 | 11 | 10.6 | 10.9 | 10.9 | 11.8 | 11.0 | 13.5–18.0 g/dL |
| Leucocytes 1 | 23,980 | 12,290 | 5380 | 6230 | 4620 | 3200 | 5200 | 5270 | 4000–10,000 cells/µL |
| Neutrophils 1 | 94% | 81% | 69.2% | 68% | 52.6% | 41.5% | 49% | 57.3% | 50–70% |
| Platelets 1,*** | 192,000 | 227,000 | 418,000 | 493,000 | 309,000 | 216,000 | 284,000 | 208,000 | 150,000–400,000/µL |
| PT 2 | 14.2 | 15.3 | 14.3 | 13.7 | 14 | - | - | - | 10–14 s |
| aPTT | 35.4 | 32.6 | 32.5 | 33.3 | 32.9 | - | - | - | 25–39 s |
| Urea 3 | 91.22 | - | 26.9 | 26 | 17 | 9.4 | 14 | 21.63 | 16–40 mg/dL |
| Creatinine 4 | 1.98 | 0.74 | 0.77 | 0.92 | 0.97 | 0.67 | 0.7 | 0.59 | 0.7–1.4 mg/dL |
| ALT 3 | 33.18 | - | 41.57 | 33.2 | 24 | 29 | 14 | 14.9 | 5–48 U/mL |
| AST 5 | 37 | 15.65 | 32.47 | 33.1 | 22 | 25 | 15 | 11.89 | 5–48 U/mL |
| CRP 6 | 165 | 139 | 58.4 | 50 | 9.69 | 4 | 1.52 | 1.06 | 0.0–8.0 mg/L |
| Analytes * | 11/05 | 12/05 | 14/05 | 20/05 | Reference Range ** |
|---|---|---|---|---|---|
| Hemoglobin 1 | 15.3 | 16.1 | 15.7 | 15.7 | 13.5–18.0 g/dL |
| Leucocytes 1 | 9410 | 14,740 | 14,960 | 7890 | 4000–10,000 cells/µL |
| Neutrophils 1 | 67.5% | 76.8% | 80.3% | 58.3% | 50–70% |
| Platelets 1,*** | 192,000 | 227,000 | 418,000 | 493,000 | 150,000–400,000/µL |
| PT 2 | 14.9 | 13.8 | 12.8 | 14.2 | 10–14 s |
| aPTT | 31.2 | 27.6 | 29.8 | 30.7 | 25–39 s |
| Urea 3 | 33.66 | 26.07 | 16.91 | 35.18 | 16–40 mg/dL |
| Creatinine 4 | 0.87 | 1.19 | 1.01 | 1.14 | 0.7–1.4 mg/dL |
| ALT 3 | 27.62 | 34.09 | 37.62 | 38.54 | 5–48 U/mL |
| AST 5 | 29.51 | 58.95 | 30.44 | 23.41 | 5–48 U/mL |
| CRP 6 | 0.47 | 59.27 | 157.09 | 25.79 | 0.0–8.0 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonell, R.C.C.; Garcês-Filho, A.Q.; Galan, L.E.B.; Romanazzi, M.; Malachias-Pires, G.M.; Almeida, J.R.; Nattrodt, J.J.M.; Carbonell, J.d.L.B.; Cerni, F.A.; Vaiyapuri, S.; et al. Vacuum-Assisted Wound Therapy in Bothrops Snakebite Injuries: A Case Series from the Brazilian Amazon. J. Clin. Med. 2025, 14, 8129. https://doi.org/10.3390/jcm14228129
Carbonell RCC, Garcês-Filho AQ, Galan LEB, Romanazzi M, Malachias-Pires GM, Almeida JR, Nattrodt JJM, Carbonell JdLB, Cerni FA, Vaiyapuri S, et al. Vacuum-Assisted Wound Therapy in Bothrops Snakebite Injuries: A Case Series from the Brazilian Amazon. Journal of Clinical Medicine. 2025; 14(22):8129. https://doi.org/10.3390/jcm14228129
Chicago/Turabian StyleCarbonell, Roberto C. C., Allan Q. Garcês-Filho, Luis E. B. Galan, Marcela Romanazzi, Geovanna M. Malachias-Pires, José R. Almeida, Jânio J. M. Nattrodt, Joquebede de L. B. Carbonell, Felipe A. Cerni, Sakthivel Vaiyapuri, and et al. 2025. "Vacuum-Assisted Wound Therapy in Bothrops Snakebite Injuries: A Case Series from the Brazilian Amazon" Journal of Clinical Medicine 14, no. 22: 8129. https://doi.org/10.3390/jcm14228129
APA StyleCarbonell, R. C. C., Garcês-Filho, A. Q., Galan, L. E. B., Romanazzi, M., Malachias-Pires, G. M., Almeida, J. R., Nattrodt, J. J. M., Carbonell, J. d. L. B., Cerni, F. A., Vaiyapuri, S., & Pucca, M. B. (2025). Vacuum-Assisted Wound Therapy in Bothrops Snakebite Injuries: A Case Series from the Brazilian Amazon. Journal of Clinical Medicine, 14(22), 8129. https://doi.org/10.3390/jcm14228129









