1. Introduction
Stereotactic radiotherapy as a high dose per fraction treatment modality has been an emerging and quickly developing field of study for the past years. All three—stereotactic radiosurgery (SRS), stereotactic radiation therapy (SRT), and stereotactic body radiation therapy (SBR)—refer to the highly precise delivery of radiation enabled by advanced technology [
1,
2]. In our setting, the technological capability includes a Varian TrueBeam equipped with HD Multi-leaf Collimator (HD-MLC), six degree-of-freedom couch, and IDENTIFY surface guided system (SGRT) working in conjunction with a GE Discovery Computed Tomography (CT), equipped with Varian Respiratory Gating for Scanners (RGSC) utilized as a simulator. This allows us to simulate and treat both SRS and SBRT cases within our clinic while utilizing advanced scanning techniques such as 4D-CT, Deep Inspiration Breath Hold (DIBH), and End Expiration Breath Hold (EEBH) while utilizing gating and intra-fraction motion management during treatment [
3].
In order to safely standardize and improve our treatment planning processes, we evaluated the plan quality metrics first introduced by Paddick [
4]. Paddick’s work provides a conceptual framework for gradient metrics, but a practical gap exists in their direct operational use for routine quality assurance. The specific practical need and the motivation for this study was to develop an institutional, predictive QA model based on our own standardized planning conditions. The goal was to move beyond a simple pass/fail check and establish expectable and achievable gradient values based on key plan characteristics, most notably target volume. This work is not intended to re-validate the known physical relationships but rather to provide an operational contribution. However, after further literature review [
5,
6] more studies were required to investigate the two different metrics, Gradient Index (GI) and Gradient Measure (GM), in order to decide which to use as a plan differentiator when assessing dose gradients within treatment plans. GI is defined as the ratio of the volume of 50% isodose to the volume of reference isodose, while GM is a three-dimensional evaluation factor of the distance to the 50% isodose from the 100% prescription dose [
7].
A dataset was compiled to investigate the plan quality metrics that we use clinically in our department (
Table 1) [
5,
8].
2. Materials and Methods
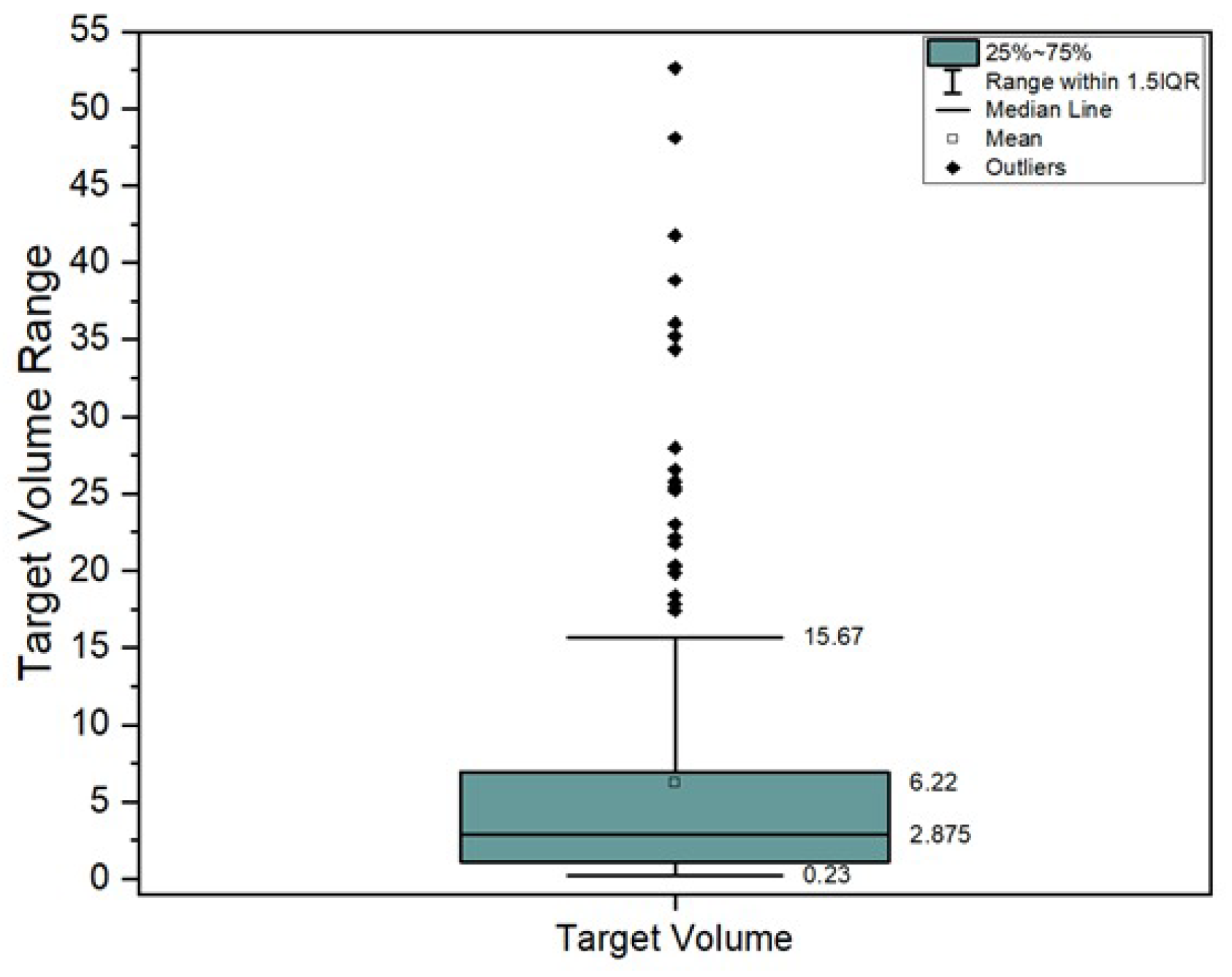
A cohort of 200 SRS-SRT individual target volumes from 104 patients were analyzed. The inclusion criteria for patient selection consisted in cerebral SRS-SRT treated lesions between 2021 and 2025 across two Neolife radiotherapy centers in Bucharest, Romania. We excluded lesions larger than 60 cc and those which did not fully complete the treatment course. Descriptive statistics can be found in
Figure 1.
The patients were simulated using a GE Discovery CT (GE HealthCare, Chicago, IL, USA) with slice thickness of 1.25 mm for all SRT patients. Encompass SRS Fibreplast system (Q-Fix, Palmyra, PA, USA) was utilized for the simulation together with a Universal Couchtop Type-S and an SRS adaptor from CQ Medical (Avondale, PA, USA). Contouring and treatment planning was performed by different personnel (physician and physicist, respectively) and was subjected to a second verification by a second physicist as per internal protocol. Since our two departments follow the same training process for all medical physicists performing the treatment plans and the same protocols for simulation, the results obtained have minimal variability. The Gross Tumor Volume (GTV) contours were defined on a post-contrast T1-weighted high resolution Magnetic Resonance Imaging (MRI) and copied over to the rigid co-registered planning CT. The Planning Target Volumes (PTV’s) were defined as a 1–3 mm isotropic expansion of the GTVs. The dose distribution was calculated using both Anisotropic Analytical Algorithm (AAA) and Acuros XB dose calculation Algorithms version 16.1, with dose grid size of 1.25 mm, divided by the two centers. Patients were treated using Varian TrueBeam (Varian Medical Systems, Inc., Palo Alto, CA, USA) with HD-MLC.
Using the gathered data, two linear regression models with GI and GM as the response variables were generated in order to ascertain the relevant regressors and coefficients that may help explain their variability.
Table 2 contains the currently recorded variables in our dataset [
9].
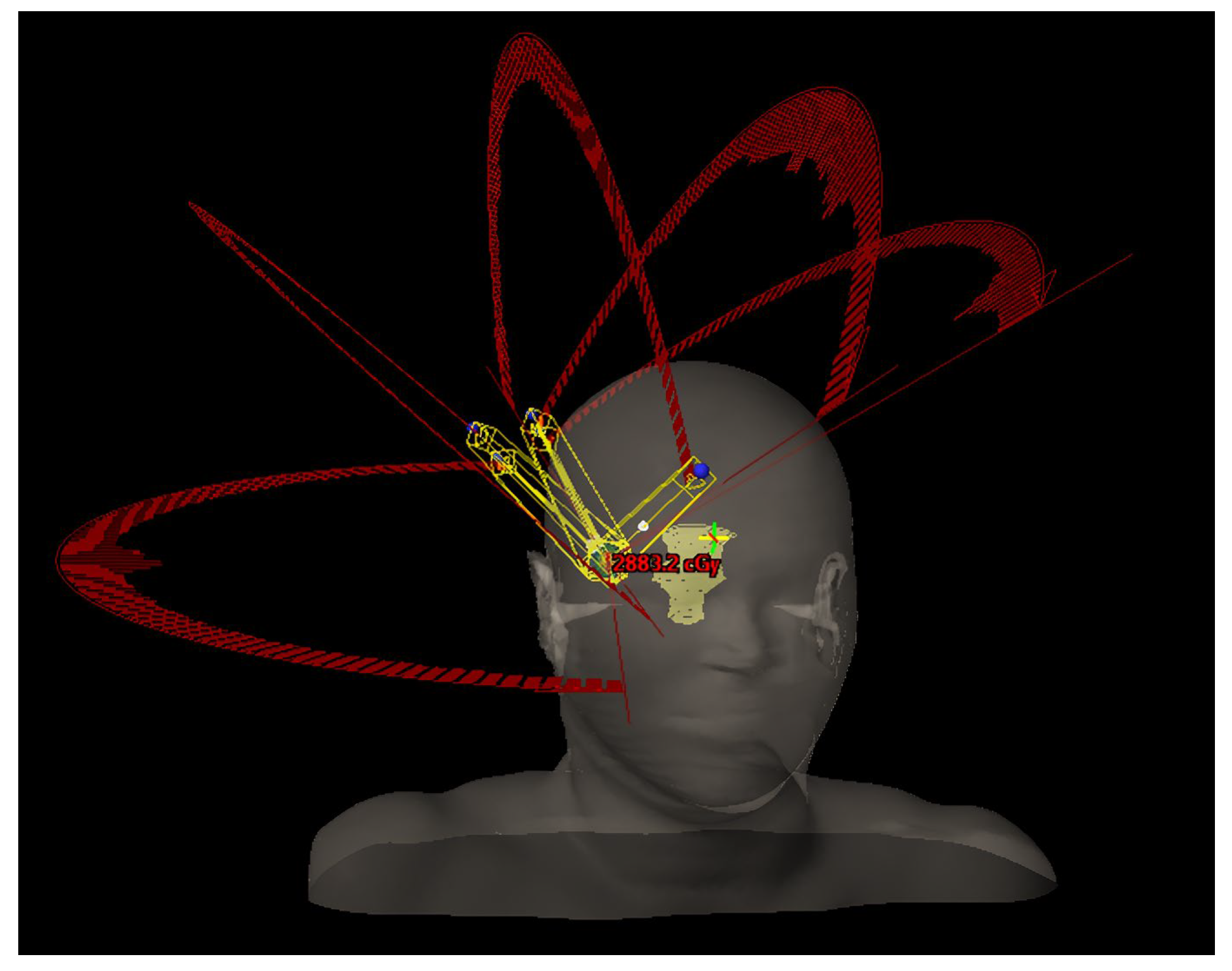
Regarding the geometrical arrangement of treatment arcs, the commonly approached technique is the following: set a partial arc or a full arc at 0° (±5°) couch angle, depending if the target is centered or not; set a partial arc at 90° or 270° (±5°), depending on the position of the target (for left hemisphere targets the chosen couch angle is 90° to prevent imager–couch collisions, and vice versa); set a minimum of 2 partial arcs, equally spaced, in the available range between the primary arcs (add extra arcs or double the existing arcs if the dose per fraction is increased over 15–20 Gy and space accordingly); adjust the length of the arcs in order to avoid direct beam entry through the eyes/ lenses; adjust the arc lengths (for targets in the posterior brain) to minimize low-dose exposure to healthy tissue [
2]. In particular, if the target position and proximity to the skull are advantageous (e.g., upper part of parietal/ frontal lobe), an additional arc with the couch angle symmetric to the midline could be used. Another key consideration based on our clinical experience, adding an arc ensures the gantry speed remains above 1.5–2° per second at each control point, preventing significant mechanical variations during beam delivery. Such example of geometry is displayed in
Figure 2.
For data collection, the above variables were recorded once the plan was finalized, reviewed by a second physicist, evaluated, and approved by the physician. The first step is normalizing so that at least 98% of the target volume receives at least 98% of the prescribed dose. Data required for dose metric calculations were extracted from the Dose Volume Histogram (DVH), either directly from the BODY and PTV structures or from an auxiliary structure encompassing the entire 50% isodose [
10]. For plans with multiple lesions, only the latter approach (auxiliary structures) was used for evaluation on the plan summation, as it yields dose metrics for each individual lesion. The rest of the data were directly gathered from the plan parameters, plan structures, and so on. The only exceptions regarding the plan normalization were the targets that posed a threat of not respecting the clinical goals of the Planning Risk Volumes (PRVs). Those particular plans were normalized to the highest coverage possible while still maintaining the clinical goals agreed upon with the physician.
Besides the data directly needed for dose metrics calculation, the other method for plan quality evaluation is visual evaluation. The aim is to obtain a uniformly distributed 50% and 25% isodoses around the target and also obtain a high dose fall-off from inside the GTV to the margin of PTV, without any significant high doses in the margin from the GTV to PTV. This fall-off allows the centering of high doses in the center of the GTV structure. In order to do so, the approached method in our departments is to optimize the plan on structures like internal GTV (internal margin of 2–3 mm from the GTV), a ring structure of the GTV (initial GTV from which we subtract the internal GTV), and a ring structure of the PTV (PTV from which we subtract the GTV). One of the limiting factors that constrains the steepness of the fall-off is the dose maximum allowed by the physician.
The statistical tools employed in this study were Spearman correlation analysis, simple T-testing, Anova, and multiple linear regression. We found the most adequate tool to use, in order to describe plan quality index behavior, to be linear regression as it can be used to model the relationship between multiple variables allowing us to estimate the separate contribution of each variable while others are held constant.
For a simple linear relationship, one can use the generic model [
11] of the form:
where
is the dependent or response variable,
is the independent or predictor variable, and ε is the error term, a statistical term representing random fluctuations, measurement errors, or the effect of outside factors within the model not explained by the
variables. As the response variable is often influenced by more than one predictor, the experimenter may add additional predictors with their own regression coefficients β.
The models may be linear with respect to β, but not necessarily in the x variables; therefore, our model—and a similar one for GM—can still be classified as a linear model.
where TV is target volume, dose/fraction is the physician-elected fractionation of the SRT plan, and MT is a flag type variable that describes whether or not the target was treated alone in a plan or together with multiple targets. The models aim to predict and explain the influence of our variables, helping guide planning decisions and set realistic expectations for achievable results. Of course, this relationship is subject to change with the advent of new technologies and novel techniques.
The study was conducted using data from patients treated between 2021 and 2025. In April 2025, preliminary approval was obtained from the ethics committee. Between April and October, anonymized dosimetric data were retrospectively processed, and in October 2025, final ethical approval was granted following the completion of the study and the presentation of the results, prior to submitting the manuscript to the journal.
The authors declare that no generative AI tools were used in data analysis or manuscript writing.
3. Results
Our study focuses on testing the correlation significance and computing Spearman correlation value for GI/GM and TV. The choice was to use TV and not equivalent sphere diameter (EQSD) due to the granularity of the data, as both showed strong statistical significance (p value of 9.01 × 10−77 for EQSD and 1.38 × 10−76 for TV) with regard to correlation to gradients (Spearman correlation of −0.908 for EQSD and −0.907 for TV).
We investigated various correlations between gradient, plan geometry, and dose metrics. There is a weak or moderate correlation between them, even though a strong statistical significance was shown. However, after running t-tests and generating several regression models trying to explain why geometry did not seem to affect our model, we realized our data are biased by our planning protocols.
Particular consideration was given to the number of couch kicks or positions in order to investigate the influence of plan geometry on gradient. However, a Spearman correlation between the number of couch positions and gradient revealed an insignificant (p = 0.76), very weak correlation (−0.02). An analysis of variance (Anova) between plans with three, four, five, and six couch positions also showed no statistical significance (p = 0.34) when looking at gradient index variances, further pointing towards a geometry bias in our dataset. We also tested whether doubling the number of arcs per couch position improved gradients; however, our data are biased, as all plans were originally created with sufficient or excess arcs and couch positions to avoid affecting the gradient. To be more specific, if the gradient of the plan was not acceptable initially, the plan was modified in geometry in order to fulfill the criteria established in our department. Therefore, with our current dataset we cannot evaluate meaningful changes based on plan geometry.
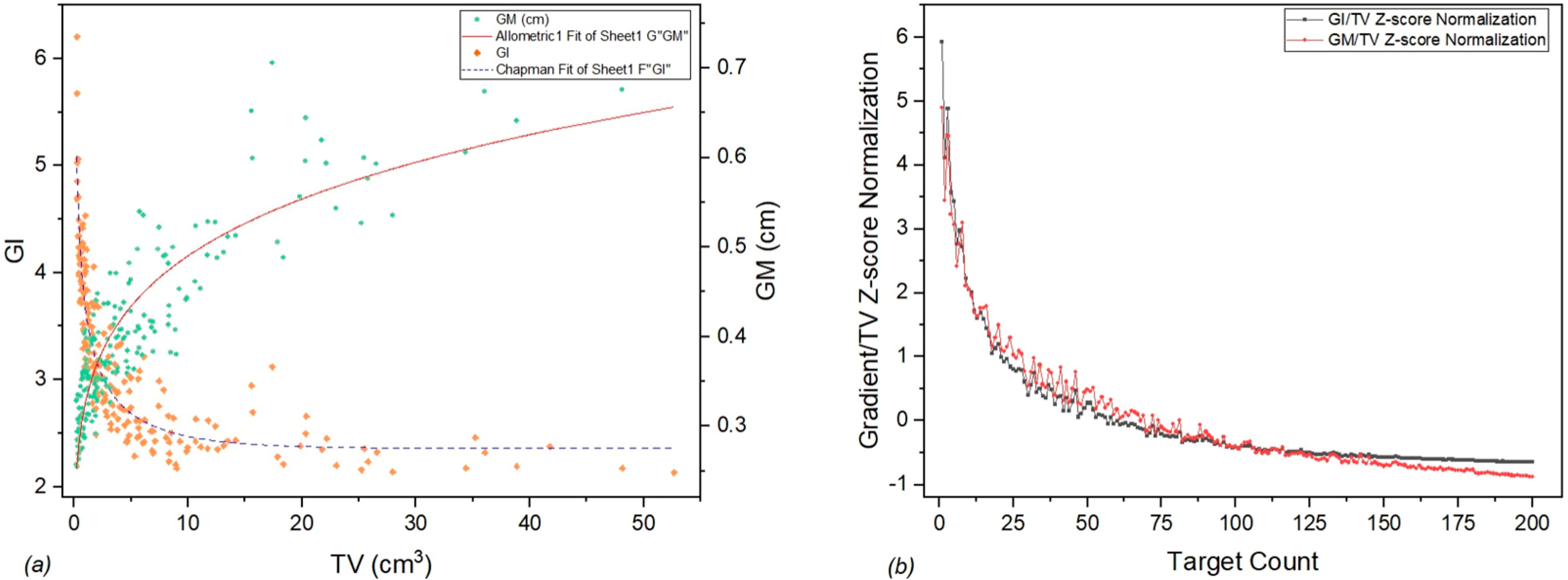
In an effort to better visualize the dependance of GI and GM depending on TV, while highlighting their differences, we have generated a graph of Standardized GI and GM using z-score normalization to account for the difference in units (
Figure 3).
As previously discussed, two linear regression models were generated for gradient index and gradient measure as summarized
Table 3 and
Table 4.
In order to account for the exponentiality of the TV regressor in an easy-to-conceptualize way, we decided to change it to a base 2 logarithm when computing the first derivative, such that we may express the variability of our response variable (GI/GM) for every TV doubling.
Rescaling to log (2) to express doubling using log change in base formula:
Rewrite predicted (fitted) GI function as:
For any given lesion, doubling its volume is predicted to change GI by
. The behavior for a large range of TV is displayed in
Table 5.
Similarly for any given lesion, doubling its volume is predicted to change GM by
. The behavior for a large range of TV is displayed in
Table 6.
4. Discussion
The linear regression models presented in this study provide a quantitative, data-driven framework for stereotactic radiotherapy plan quality assurance in our clinic. By estimating the expected GI and GM for a given target volume and configuration, planners and physicists can rapidly identify plans that deviate from institutional protocols. It can become a useful tool for standardizing treatment plans across dosimetrists and physicists and set realistic expectations for achievable dose gradients at different target sizes.
Better understanding of the relationship between plan quality metrics, such as gradient, conformity and plan geometry would also provide a useful tool for standardizing treatment planning protocols across different centers. However, this would require a treatment planning system-generated water phantom in which you can test multiple geometries while keeping a constant TV, dose prescription, anatomical features, and target position relative to plan isocenter.
Several limitations of this study should be acknowledged. The analysis was retrospective and based on a single treatment technology (Varian TrueBeam), which may limit generalizability to other systems or planning algorithms. In addition, the models were derived from plans generated within only two clinical workflows, using our own internal optimization routines and external validation was not performed.
It can be seen from
Figure 1 that target volumes larger than 20 cc are considered outliers. However, according to internal protocol, these kinds of large tumor volumes are also treated based on individual physician decisions with a larger number of fractions (e.g., five fractions). Such targets, while relatively uncommon, are still useful datapoints in determining the limitations of the protocol, technique, and technology observed in
Figure 3a as a plateau.
New technologies (such as Varian Hyperarc) and planning techniques (Rapidarc Dynamic) open new possibilities of automated plan development and delivery as well as hybrid VMAT-IMRT delivery. The impact of these emergent technologies on plan quality indexes has not been approached by this study and remains a possible future direction of research [
12].
Future research should focus on multi-institutional validation and comparison of regression parameters across planning systems and dose-calculation algorithms. This approach will be extended to other SRS/SRT non-cranial targets (lung, liver, and spine) in future research that is under development by our research team. For a more comprehensive model, future work should also focus on adapting this modeling approach to other SRS/SRT delivery systems to see if similar predictive relationships hold.
5. Conclusions
When evaluating small lesions, GI seems to be the more sensitive gradient evaluation metric for sub-cc SRT targets. Every doubling of target volume lowers GI sharply (−0.55 to −0.17). GI does exhibit an odd behavior within our model after 28 cc. We attribute this not to a true physical phenomenon, but rather to a modeling artifact resulting from the small number of cases in this upper volume range within our dataset. For lesions larger than 40 cc, the GI tends to have a constant behavior, without any significant increase nor decrease in value. The behavior of GI for larger than 20 cc volumes is influenced by the reduced number of data points in this region.
Treating multiple targets in the same plan seems to add an average of +0.19 to GI, independent of volume. Within our model, dose per fraction had no significant independent effect (p = 0.08). The model explains 85% of the variation in GI (adjusted R2 = 0.85).
When evaluating small lesions, GM seems to increase moderately (+0.024 cm to +0.07 cm). GM, according to our findings, displays a weaker sensitivity to volume than GI, making it perhaps a more useful tool when comparing GM across a wide volume spectrum of targets, though very large targets still show a penalty. Treating multiple targets in the same plan seems to add an average of +0.027 cm to GM, independent of volume. Dose per fraction had no significant effect (p = 0.1). The model explains 84% of GM variability. By verifying SRS/SRT plans using our model during the optimization process, the physics team can improve the dosimetric outcomes of the plans, with minimal time span. The generated models provide expected values of both gradient metrics for any target volume, offering a useful tool for assessing plan quality.
Author Contributions
Conceptualization, I.D., M.-Ş.B. and T.P.-B.; methodology, I.D.; software, I.D.; validation, T.P.-B., I.D. and H.-D.L.; formal analysis, I.D. and M.-Ş.B.; investigation, I.D. and T.P.-B.; resources, T.P.-B., M.-Ş.B., I.D. and H.-D.L.; data curation, I.D.; writing—original draft preparation, I.D.; writing—review and editing, M.-Ş.B., T.P.-B. and H.-D.L.; visualization, T.P.-B.; supervision, H.-D.L.; project administration, T.P.-B.; funding acquisition, H.-D.L. All authors have read and agreed to the published version of the manuscript.
Funding
Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of MNT Healthcare Europe, on 14 April 2025, according to decision 110b/14 April 2025, and confirmed on 3 October 2025, according to decision 678/3 October 2025. The present study is part of the PhD thesis of the first author.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Informed consent was requested from the patient or legal representative.
Data Availability Statement
Data are available only upon request due to ethical restrictions. The data presented in this study are available upon request from the main or corresponding author.
Acknowledgments
Authors would like to thank Neolife Radiotherapy Departments for having access to SRS/SRT plans and the physicians and physics teams for their support in contouring and planning process.
Conflicts of Interest
All authors were employed by Neolife (MNT Healthcare Europe) at the time of publication. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Statement
This article has been republished with a minor correction to the existing affiliation information. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| GI | Gradient Index |
| GM | Gradient Measure |
| SRS | Stereotactic Radiosurgery |
| SRT | Stereotactic Radiation Therapy |
| TV | Target Volume |
| MT | Multiple Targets |
| HD-MLC | HD Multi-leaf Collimator |
| SGRT | Surface Guided Radiation Therapy |
| CT | Computed Tomography |
| RGSC | Respiratory Gating for Scanners |
| DIBH | Deep Inspiration Breath Hold |
| EEBH | End Expiration Breath Hold |
| HI | Homogeneity Index |
| CI | Conformity Index |
| AAA | Anisotropic Analytical Algorithm |
| EQSD | Equivalent Sphere Diameter |
| DVH | Dose Volume Histogram |
| PTV | Planning Target Volume |
| PRV | Planning Risk Volume |
| GTV | Gross Tumor Volume |
| SBRT | Stereotactic Body Radiation Therapy |
References
- Abel, S.; Lee, S.; Ludmir, E.B.; Verma, V. Principles and Applications of Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy. Hematol. Oncol. Clin. N. Am. 2019, 33, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Vergalasova, I.; Liu, H.; Alonso-Basanta, M.; Dong, L.; Li, J.; Nie, K.; Shi, W.; Teo, B.-K.K.; Yu, Y.; Yue, N.J.; et al. Multi-Institutional Dosimetric Evaluation of Modern Day Stereotactic Radiosurgery (SRS) Treatment Options for Multiple Brain Metastases. Front. Oncol. 2019, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Baus, W.W.; Blanck, O.; Combs, S.E.; Debus, J.; Engenhart-Cabillic, R.; Gauer, T.; Grosu, A.L.; Schmitt, D.; Tanadini-Lang, S.; et al. Definition and quality requirements for stereotactic radiotherapy: Consensus statement from the DEGRO/DGMP Working Group Stereotactic Radiotherapy and Radiosurgery. Strahlenther. Onkol. 2020, 196, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Paddick, I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J. Neurosurg. 2000, 93 (Suppl. S3), 219–222. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.R.; Wessels, B.W.; Einstein, D.B.; Maciunas, R.J.; Kim, E.Y.; Kinsella, T.J. Quality of coverage: Conformity measures for stereotactic radiosurgery. J. Appl. Clin. Med. Phys. 2003, 4, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Breitman, K.; Dunscombe, P.; Spencer, D.P.; Lau, H. Evaluation of stereotactic radiosurgery conformity indices for 170 target volumes in patients with brain metastases. J. Appl. Clin. Med. Phys. 2011, 12, 3449. [Google Scholar] [CrossRef] [PubMed]
- Eclipse Photon and Electron 15.5 Reference Guide; Varian Medical Systems: Palo Alto, CA, USA, 2017; pp. 1–575.
- Patel, G.; Mandal, A.; Choudhary, S.; Mishra, R.; Shende, R. Plan evaluation indices: A journey of evolution. Rep. Pract. Oncol. Radiother. 2020, 25, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Kline, R.; Gillin, M.; Souhami, L.; Hirschfeld, A.; Dinapoli, R.; Martin, L. Radiation Therapy Oncology Group: Radiosurgery quality assurance guidelines. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 1231–1239. [Google Scholar] [CrossRef]
- Wilke, L.; Andratschke, N.; Blanck, O.; Brunner, T.B.; Combs, S.E.; Grosu, A.-L.; Moustakis, C.; Schmitt, D.; Baus, W.W.; Guckenberger, M. ICRU report 91 on prescribing, recording, and reporting of stereotactic treatments with small photon beams: Statement from the DEGRO/DGMP working group stereotactic radiotherapy and radiosurgery. ICRU-Bericht 91 über die Verschreibung, Aufzeichnung und Dokumentation von stereotaktischen Behandlungen mit kleinen Photonenfeldern: Stellungnahme der DEGRO/DGMP-Arbeitsgruppe Stereotaktische Strahlentherapie und Radiochirurgie. Strahlenther. Onkol. 2019, 195, 193–198. [Google Scholar] [CrossRef]
- Rencher Alvin, C.; Bruce Schaalje, G. Linear Models in Statistics; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Desai, D.D.; Cordrey, I.L.; Johnson, E.L.; Oldland, T.A. AFI manual planning versus HyperArc auto-planning: A head-to-head comparison of SRS plan quality. J. Appl. Clin. Med. Phys. 2024, 25, e14503. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).