KELIM PSA as a Prognostic Biomarker in Castration-Resistant Prostate Cancer Treated with ARPI
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Data Collection and Variables
2.3. PSA Kinetics Modeling and KELIM Calculation
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Survival Analysis
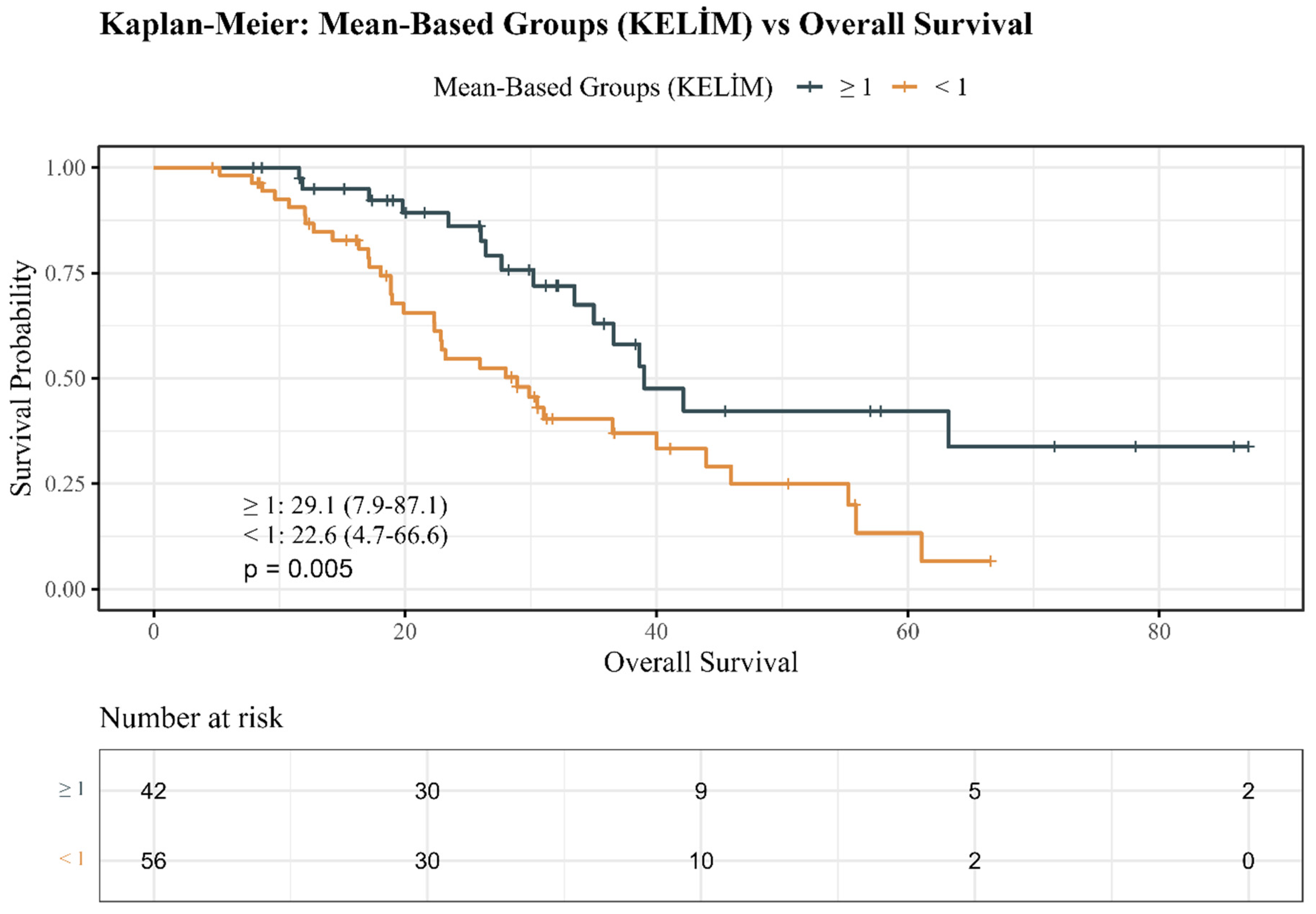
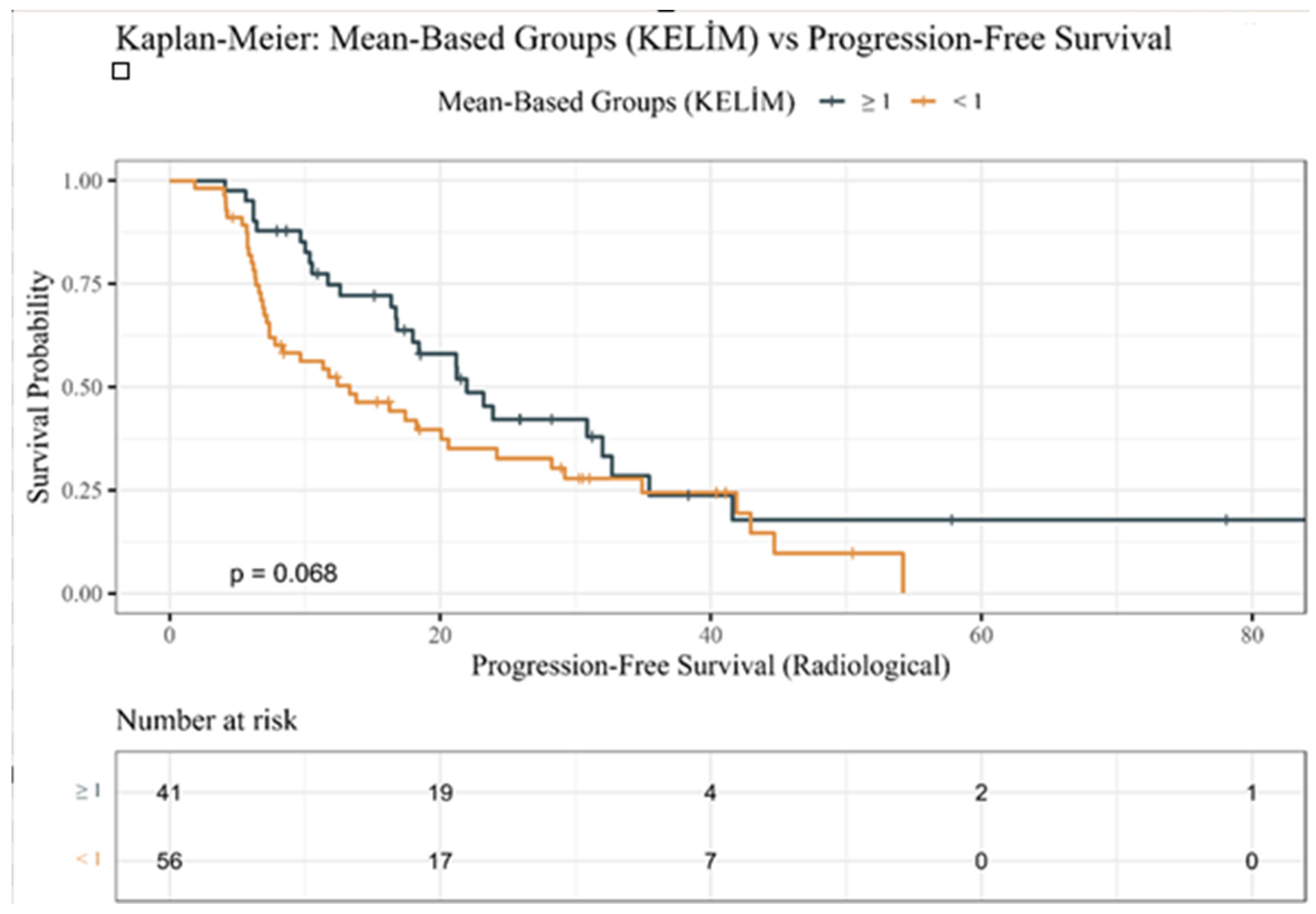
3.3. Kaplan–Meier Survival Estimates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, T.K.; Zishiri, O.T. Prostate Cancer: A Review of Genetics, Current Biomarkers and Personalised Treatments. Cancer Rep. 2024, 7, e70016. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Bailey-Whyte, M.; Minas, T.Z.; Dorsey, T.H.; Smith, C.J.; Loffredo, C.A.; Ambs, S. Systemic Inflammation Indices and Association with Prostate Cancer Survival in a Diverse Patient Cohort. Cancers 2023, 15, 1869. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.P.; Tchinde, M.J.; Sop, T.G.J.; Ndonku, S.A.; Juma, P.I. Correlation between total prostate specific antigen and histological grading of prostate cancer in Kenyan mission hospital: A five-year retrospective review. BMC Urol. 2025, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Tilling, K.; Turner, E.L.; Lane, J.A.; Simpkin, A.; Davis, M.; Donovan, J.; Hamdy, F.C.; Neal, D.E.; Martin, R.M. Investigating the prostate specific antigen, body mass index and age relationship: Is an age-BMI-adjusted PSA model clinically useful? Cancer Causes Control 2016, 27, 1465–1474. [Google Scholar] [CrossRef]
- Carrot, A.; Elaidi, R.T.; Colomban, O.; Maillet, D.; Tod, M.; You, B.; Oudard, S. Modeled Early Longitudinal PSA Kinetics Prognostic Value in Rising PSA Prostate Cancer Patients after Local Therapy Treated with ADT +/- Docetaxel. Cancers 2022, 14, 815. [Google Scholar] [CrossRef]
- Wang, Y.; Suo, J.; Wang, B.; Men, Q.; Wang, D.; Jing, H.; Li, T.; Huang, X.; Wang, C.; Luo, X.; et al. Prognostic role of prostate specific antigen kinetics in primary high volume metastatic hormonal sensitive prostate cancer treated with novel hormonal therapy agents. Sci. Rep. 2024, 14, 26712. [Google Scholar] [CrossRef] [PubMed]
- Carrot, A.; Oudard, S.; Colomban, O.; Fizazi, K.; Maillet, D.; Sartor, O.; Freyer, G.; You, B. Prognostic Value of the Modeled Prostate-Specific Antigen KELIM Confirmation in Metastatic Castration-Resistant Prostate Cancer Treated with Taxanes in FIRSTANA. JCO Clin. Cancer Inform. 2024, 8, e2300208. [Google Scholar] [CrossRef]
- Martínez-Corral, R.; De Pablos-Rodríguez, P.; Bardella-Altarriba, C.; Vera-Ballesteros, F.J.; Abella-Serra, A.; Rodríguez-Part, V.; Martínez-Corral, M.E.; Picola-Brau, N.; López-Abad, A.; Gómez-Ferrer, Á.; et al. PSA kinetics and predictors of PSA response in metastatic hormone-sensitive prostate cancer treated with androgen receptor signaling inhibitors. Urol. Oncol. 2025, 43, 527.e9–527.e15. [Google Scholar] [CrossRef]
- Oka, T.; Hatano, K.; Tani, M.; Yoshimura, A.; Horibe, Y.; Liu, Y.; Sassi, N.; Okuda, Y.; Yamamoto, A.; Uemura, T.; et al. PSA Kinetics Affect Prognosis in Patients with Castration-resistant Prostate Cancer Treated with Enzalutamide. Cancer Diagn. Progn. 2024, 4, 706–714. [Google Scholar] [CrossRef]
- Yazgan, S.C.; Sarı, A.; Bölek, H.; Yekedüz, E.; Ürün, Y. The prognostic value of elimination rate constant K score of prostate-specific antigen in metastatic castration-resistant prostate cancer patients treated with docetaxel. Prostate Int. 2025, 13, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Hata, S.; Shinohara, M.; Inoue, T.; Shibuya, T.; Ando, T.; Mimata, H.; Shin, T. MO44-1 Assessing PSA decline kinetics and Kelim as predictors of survival in docetaxel-treated prostate cancer patients. Ann. Oncol. 2024, 35, S1351. [Google Scholar] [CrossRef]
- Hakozaki, Y.; Yamada, Y.; Takeshima, Y.; Taguchi, S.; Kawai, T.; Nakamura, M.; Iwaki, T.; Teshima, T.; Kinoshita, Y.; Akiyama, Y.; et al. Low hemoglobin and PSA kinetics are prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients. Sci. Rep. 2023, 13, 2672. [Google Scholar] [CrossRef]
- Mahmoud, M.H.; Alorabi, M.O.; Ali, D.M.; Kelany, M.R.; Ismail, S.S. Importance of PSA Kinetics as a Prognostic Factor in Locally Advanced/Metastatic Prostate Cancer Patients with Prior Androgen Deprivation Therapy. QJM Int. J. Med. 2024, 117 (Suppl. S2), hcae175.607. [Google Scholar] [CrossRef]
- Desmée, S.; Mentré, F.; Veyrat-Follet, C.; Guedj, J. Nonlinear Mixed-effect Models for Prostate-specific Antigen Kinetics and Link with Survival in the Context of Metastatic Prostate Cancer: A Comparison by Simulation of Two-stage and Joint Approaches. AAPS J. 2015, 17, 691–699. [Google Scholar] [CrossRef]
- Fiala, O.; Hošek, P.; Korunkova, H.; Tkadlecova, M.; Hora, M.; Šiková, D.; Stránský, P.; Fínek, J.; Kučera, R.; Windrichová, J.; et al. Prognostic Role of Prostate-specific Antigen Isoforms and Their Early Kinetics in Patients with Metastatic Castration-resistant Prostate Cancer Receiving New Generation Androgen Receptor Targeted Agents. Vivo 2025, 39, 859–869. [Google Scholar] [CrossRef]
- Attard, G.; Borre, M.; Gurney, H.; Loriot, Y.; Andresen-Daniil, C.; Kalleda, R.; Pham, T.; Taplin, M.-E.; PLATO collaborators. Abiraterone alone or in combination with enzalutamide in metastatic castration-resistant prostate cancer with rising prostate-specific antigen during enzalutamide treatment. J. Clin. Oncol. 2018, 36, 2639–2646. [Google Scholar] [CrossRef]
- Kweldam, C.F.; Wildhagen, M.F.; Steyerberg, E.W.; Bangma, C.H.; van der Kwast, T.H.; van Leenders, G.J. Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod. Pathol. 2015, 28, 457–464. [Google Scholar] [CrossRef]
- Zhu, X.; Gou, X.; Zhou, M. Nomograms Predict Survival Advantages of Gleason Score 3+4 Over 4+3 for Prostate Cancer: A SEER-Based Study. Front. Oncol. 2019, 9, 646. [Google Scholar] [CrossRef]
- Hacioglu, M.B.; Kucukarda, A.; Gokmen, I.; Gurbuz, A.F.; Araz, M.; Kahvecioglu, F.A.; Hacibekiroglu, I.; Akdoğan, O.; Yazıcı, O.; Akkus, F.A.; et al. Prognostic Nutritional Index as a Biomarker in Metastatic Hormone-Sensitive Prostate Cancer: Impact on Survival and Treatment Optimization. Prostate 2025, 85, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.A.; Girelli, G.; Demichelis, F. Genomic Correlates to the Newly Proposed Grading Prognostic Groups for Prostate Cancer. Eur. Urol. 2016, 69, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Ozay, Z.I.; Hage Chehade, C.; Agarwal, N. On-treatment PSA kinetics as a potential biomarker: Guiding personalized treatment in metastatic hormone-sensitive prostate cancer. Med 2025, 6, 100534. [Google Scholar] [CrossRef] [PubMed]
| Overall, n (%) n = 98 | KELIM PSA ≥ 1, n (%) n = 42 | KELIM PSA < 1, n (%) n = 56 | p | |
|---|---|---|---|---|
| Age (years) * | 75.0 ± 8.6 | 74.4 ± 8.6 | 75.4 ± 8.7 | 0.559 |
| BMI (kg/m2) * | 27.5 ± 4.7 | 28.3 ± 4.6 | 27.0 ± 4.7 | 0.131 |
| BMI | 0.242 | |||
| <30 (kg/m2) | 68 (69.4) | 26 (61.9) | 42 (75.0) | |
| ≥30 (kg/m2) | 30 (30.6) | 16 (38.1) | 14 (25.0) | |
| Smoking | 0.286 | |||
| Yes | 23 (23.5) | 13 (30.9) | 10 (17.9) | |
| No | 59 (60.2) | 22 (52.4) | 37 (66.1) | |
| Unknown | 16 (16.3) | 7 (16.7) | 9 (16.1) | |
| ECOG performance score | 0.812 | |||
| 0 | 54 (55.1) | 25 (59.5) | 29 (51.8) | |
| 1 | 41 (41.8) | 16 (38.1) | 25 (44.6) | |
| 2 | 3 (3.1) | 1 (2.4) | 2 (3.6) | |
| Gleason/ISUP Score | 0.473 | |||
| 3 + 3 | 6 (6.1) | 4 (9.5) | 2 (3.6) | |
| 3 + 4 | 11 (11.2) | 4 (9.5) | 7 (12.5) | |
| 4 + 3 | 11 (11.2) | 3 (7.1) | 8 (14.3) | |
| 8 | 22 (22.6) | 8 (19.1) | 14 (25.0) | |
| 9/10 | 48 (48.9) | 23 (54.8) | 25 (44.6) | |
| Gleason/ISUP Risk Category | 0.821 | |||
| ISUP 1,2,3 | 28 (28.6) | 11 (26.2) | 17 (30.4) | |
| ISUP 4,5 | 70 (71.4) | 31 (73.8) | 39 (69.6) | |
| Previous Definitive Treatment Before Relapse | 0.236 | |||
| No treatment | 68 (69.4) | 30 (71.4) | 38 (67.9) | |
| Surgery | 8 (8.2) | 1 (2.4) | 7 (12.5) | |
| RT | 6 (6.1) | 2 (4.8) | 4 (7.1) | |
| Surgery + Salvage RT | 16 (16.3) | 9 (21.4) | 7 (12.5) | |
| Treatment in the CSPC period (sensitive) | 0.188 | |||
| ADT | 64 (65.3) | 31 (73.8) | 33 (58.9) | |
| ADT + Docetaxel | 34 (34.7) | 11 (26.2) | 23 (41.1) | |
| CRPC ARPI Series | 0.397 | |||
| 1st Line | 74 (75.5) | 34 (81.0) | 40 (71.4) | |
| 2nd Line | 24 (24.5) | 8 (19.0) | 16 (28.6) | |
| CRPC ARPI agent | 0.465 | |||
| Enzalutamide | 53 (54.1) | 25 (59.5) | 28 (50.0) | |
| Abiraterone acetate | 45 (45.9) | 17 (40.5) | 28 (50.0) | |
| Metastatic sites | ||||
| Bones | 85 (86.7) | 34 (81.0) | 51 (91.1) | 0.246 |
| Regional Lymph node | 71 (72.5) | 30 (71.4) | 41 (73.2) | >0.999 |
| Non-regional Lymph node | 50 (51.0) | 22 (52.4) | 28 (50.0) | 0.977 |
| Lung | 7 (7.1) | 2 (4.8) | 5 (8.9) | 0.695 |
| Liver | 3 (3.1) | 0 (0.0) | 3 (5.4) | 0.258 |
| De Novo/Relapse Status | 0.678 | |||
| De Novo | 69 (70.4) | 31 (73.8) | 38 (67.9) | |
| Relapse | 29 (29.6) | 11 (26.2) | 18 (32.1) | |
| Use of Bone-Modifying Agents | 68 (69.4) | 26 (61.9) | 42 (75.0) | 0.242 |
| Time to Nadir PSA level (months) * | 4.78 (−13.5–49.45) | 5.22 (0.89–49.45) | 4.14 (−13.5–21.36) | 0.071 |
| OS (months) * | 26.0 (4.7–87.1) | 29.1 (7.9–87.1) | 22.6 (4.7–66.6) | 0.050 |
| Exitus (Yes/No) | 51 (52.0)/47 (48.0) | 16 (38.1)/26 (61.9) | 35 (62.5)/21 (37.5) | 0.029 |
| Univariate | |||
|---|---|---|---|
| Characteristic | HR | 95% CI | p-Value |
| LDH level | 0.005 | ||
| ≤1.5 ULN | — | — | |
| >1.5 ULN | 2.61 | 1.34–5.08 | |
| CRPC ARPI agent | 0.204 | ||
| Enzalutamide | — | — | |
| Abiraterone acetate | 1.44 | 0.82–2.53 | |
| BMI | |||
| <30 (kg/m2) | — | — | 0.439 |
| ≥30 (kg/m2) | 1.27 | 0.69–2.33 | |
| ECOG | |||
| 0 | — | — | |
| 1 | 1.38 | 0.80–2.40 | 0.251 |
| 2 | — | 0.00–NA | 0.990 |
| Gleason/ISUP Risk Category | 0.864 | ||
| ISUP 1,2,3 | — | — | |
| ISUP 4,5 | 1.06 | 0.57–1.96 | |
| De Novo/Relapse Status | |||
| De Novo | — | — | 0.200 |
| Relapse | 0.67 | 0.36–1.24 | |
| Treatment During the CSPC Period | |||
| ADT | — | — | 0.068 |
| ADT + Docetaxel | 1.71 | 0.96–3.08 | |
| CRPC ARPI Series | 0.002 | ||
| 1st Line | — | — | |
| 2nd Line | 2.44 | 1.39–4.28 | |
| Bone metastasis | 2.74 | 0.98–7.61 | 0.054 |
| Regional Lymph node Metastasis | 0.73 | 0.41–1.28 | 0.273 |
| Non-Regional Lymph node Metastasis | 1.18 | 0.68–2.05 | 0.555 |
| Lung Metastasis | 1.67 | 0.59–4.68 | 0.332 |
| Liver Metastasis | 2.64 | 0.81–8.59 | 0.106 |
| KELIM category | 0.006 | ||
| Favorable | — | — | |
| Unfavorable | 2.30 | 1.26–4.19 | |
| Multivariate | |||
|---|---|---|---|
| Characteristic | HR | 95% CI | p-Value |
| CRPC ARPI Series | |||
| 1st Line | — | — | |
| 2nd Line | 3.19 | 1.71–5.93 | <0.001 |
| Treatment During the CSPC Period | |||
| ADT | — | — | |
| ADT + Docetaxel | 2.14 | 1.11–4.12 | 0.022 |
| KELIM category | |||
| Favorable | — | — | |
| Unfavorable | 2.09 | 1.12–3.89 | 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atalah, F.; Kuş, F.; Acarbay, A.; Karakök, A.; Alkan, O.; Nazlı, İ.; Özilice, U.; Beşiroğlu, M.; Gümüş, M. KELIM PSA as a Prognostic Biomarker in Castration-Resistant Prostate Cancer Treated with ARPI. J. Clin. Med. 2025, 14, 8114. https://doi.org/10.3390/jcm14228114
Atalah F, Kuş F, Acarbay A, Karakök A, Alkan O, Nazlı İ, Özilice U, Beşiroğlu M, Gümüş M. KELIM PSA as a Prognostic Biomarker in Castration-Resistant Prostate Cancer Treated with ARPI. Journal of Clinical Medicine. 2025; 14(22):8114. https://doi.org/10.3390/jcm14228114
Chicago/Turabian StyleAtalah, Fatih, Fatih Kuş, Aydın Acarbay, Akgün Karakök, Onur Alkan, İsmail Nazlı, Utku Özilice, Mehmet Beşiroğlu, and Mahmut Gümüş. 2025. "KELIM PSA as a Prognostic Biomarker in Castration-Resistant Prostate Cancer Treated with ARPI" Journal of Clinical Medicine 14, no. 22: 8114. https://doi.org/10.3390/jcm14228114
APA StyleAtalah, F., Kuş, F., Acarbay, A., Karakök, A., Alkan, O., Nazlı, İ., Özilice, U., Beşiroğlu, M., & Gümüş, M. (2025). KELIM PSA as a Prognostic Biomarker in Castration-Resistant Prostate Cancer Treated with ARPI. Journal of Clinical Medicine, 14(22), 8114. https://doi.org/10.3390/jcm14228114





