Clinical Experience with URO17® in the Diagnosis and Surveillance of Bladder Cancer
Abstract
1. Introduction
2. Materials and Methods
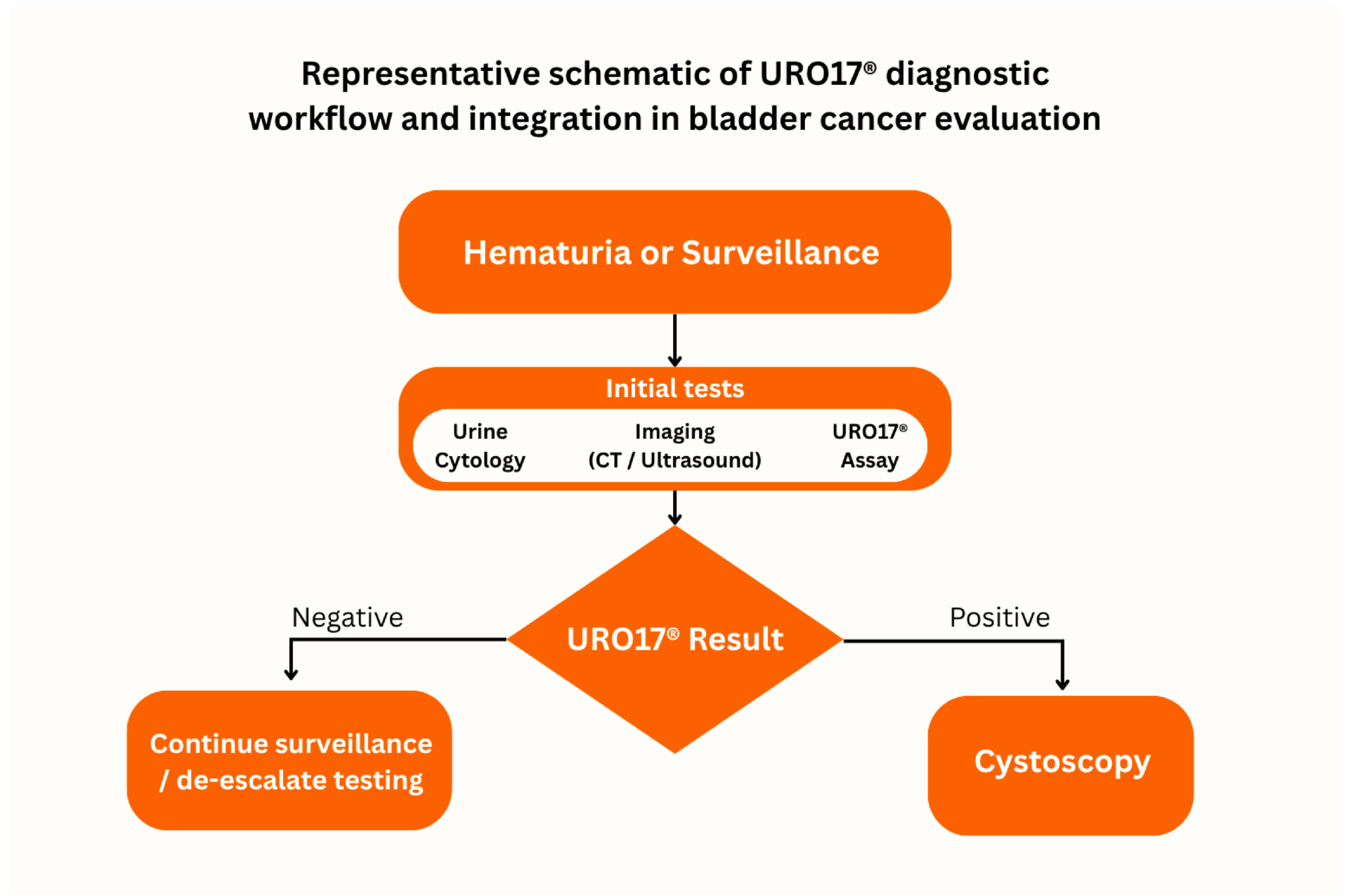
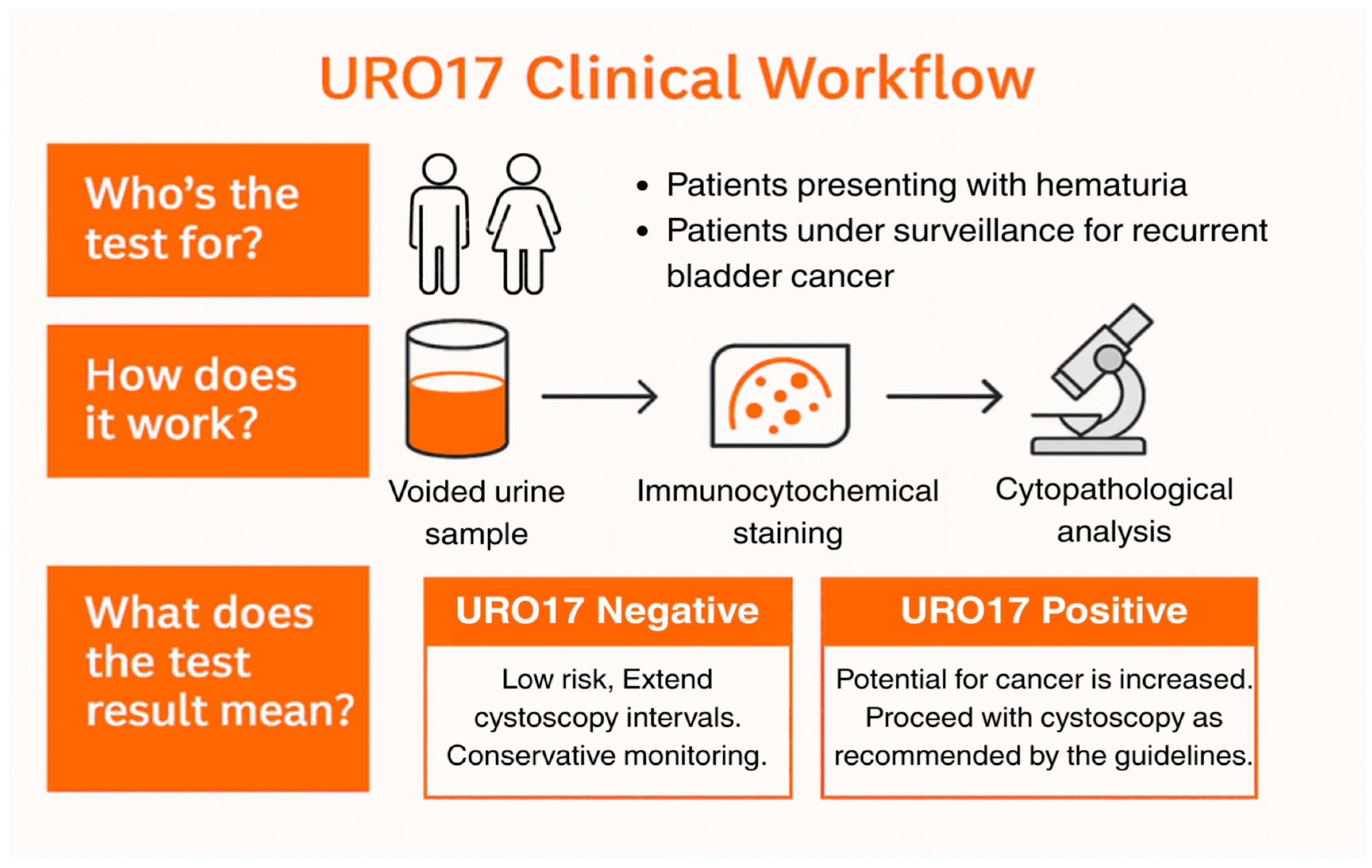
2.1. URO17® Integration in Clinical Pathways
2.2. Urine Cytology Method
2.3. URO17® Immunocytochemistry
- Negative: fewer than 5 URO17®-positive urothelial cells.
- Positive: 5 or more URO17®-positive urothelial cells.
3. Discussion
3.1. Overview of Cases and Testing Volume
3.2. Individual Case Summaries
- Case 1: Negative URO17®
- Case 2: Negative URO17®
- Case 3: Negative URO17®
- Case 4: Positive URO17®
- Case 5: Positive URO17®
- Case 6: Positive URO17®
- Case 7: Positive URO17®
3.3. Interpretation of Case Findings
3.4. Broader Clinical Context
3.5. Limitations and Future Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- American Cancer Society. Key Statistics for Bladder Cancer. American Cancer Society 2025. Available online: https://www.cancer.org/cancer/types/bladder-cancer/about/key-statistics.html (accessed on 23 September 2025).
- UpToDate. Bladder Cancer Treatment: Non-Muscle Invasive (Superficial) Cancer—Beyond the Basics. UpToDate 2024. Available online: https://www.uptodate.com/contents/bladder-cancer-treatment-non-muscle-invasive-superficial-cancer-beyond-the-basics (accessed on 23 September 2025).
- Chou, R.; Gore, J.L.; Buckley, D.; Fu, R.; Gustafson, K.; Griffin, J.C.; Selph, S. Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2015, 163, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Devlies, W.; de Jong, J.J.; Hofmann, F.; Bruins, H.M.; Zuiverloon, T.C.; Smith, E.J.; Omar, M.I. The Diagnostic Accuracy of Cystoscopy for Detecting Bladder Cancer in Adults Presenting with Haematuria: A Systematic Review from the European Association of Urology Guidelines Office. Eur. Urol. Focus 2024, 10, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Cid, A.; Cucci, J.; Kunkel, B.; Defeis, L.; Matthews, M. Acu-URO17 Is a Highly Sensitive and Specific Bladder Cancer Biomarker. BJU Compass 2024, 5, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Barocas, D.A.; Lotan, Y.; Matulewicz, R.S.; Raman, J.D.; Westerman, M.E.; Kirkby, E.; Souter, L. Updates to Microhematuria: AUA/SUFU Guideline (2025). J. Urol. 2025, 213, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, N.; Hampson, A.; Agarwal, S.; Swamy, R.; Chilvers, M.; Hampson, A.; Kim, N. The Role of URO17™ Biomarker to Enhance Diagnosis of Urothelial Cancer in New Hematuria Patients—First European Data. BJU Compass 2020, 2, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Porten, S.P.; Wang, E.Y.; Vohra, P.; Carroll, P.R.; Jahanfard, S.; Kim, N.W. Evaluation of URO17® to Improve Non-Invasive Detection of Bladder Cancer. Urol. Oncol. 2024, 42, 76.e21–76.e28. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Rabinowitz, J.; Hilbert, R.; Ghose, A.; Agarwal, S.; Swamy, R.; Vasdev, N. The Role of URO17® in Diagnosis and Follow-Up of Bladder Cancer Patients. BMC Urol. 2024, 24, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Mockler, D.C.; Roa-Peña, L.; Szygalowicz, A.; Kim, N.W.; Jahanfard, S.; Shroyer, K.R. Keratin 17 Is a Sensitive and Specific Biomarker of Urothelial Neoplasia. Mod. Pathol. 2019, 32, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Kim, N.W.; Wu, M.; Chan, I.; Escobar-Hoyos, L.F.; Shroyer, K.R. Keratin 17 Is a Novel Cytologic Biomarker for Urothelial Carcinoma Diagnosis. Am. J. Clin. Pathol. 2021, 156, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Smelser, W.; Kim, N.; Jahanfard, S.; Sarno, M.; Chang, S.S.; Giannico, G.A. Validation of Keratin 17 as a Tissue Biomarker in the Diagnosis of Upper Tract Urothelial Carcinoma. Hum. Pathol. 2024, 154, 105682. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, F.P.; Campetella, M.; Russo, P.; Palermo, G.; Moosavi, S.K.; Rossi, F.; Rocco, B. Prognostic Value of PLR, SIRI, PIV, SII, and NLR in Non-Muscle Invasive Bladder Cancer: Can Inflammatory Factors Influence Pathogenesis and Outcomes? Cancers 2025, 17, 2189. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Catellani, M.; Bianchi, R.; Fallara, G.; Tozzi, M.; Maggi, M.; Contieri, R. Enhanced Prognostic Value of Four-Tier Hybrid Grading System in Ta Non-Muscle-Invasive Bladder Cancer. BJU Int. 2025, 136, 882–890. [Google Scholar] [CrossRef] [PubMed]
| Case | Age/Sex | Clinical Context | URO17® Result(s) | Cytology | Cystoscopy/Imaging Findings | Outcome |
|---|---|---|---|---|---|---|
| 1 | 88/M | Hematuria; immunocompromised | Negative (1×) | Suspicious | Normal cystoscopy | No malignancy; post-procedure UTI |
| 2 | 76/F | Gross hematuria | Negative (3×) | Negative | Normal cystoscopy and ultrasound | No malignancy; managed conservatively |
| 3 | 66/F | Prior HG UC; under surveillance | Negative (5×) | Negative | No recurrence on serial cystoscopies | Continued surveillance |
| 4 | 93/M | Bladder wall thickening on CT | Positive (1×) | Atypical | Thickened wall; no overt lesion | HG T1 + CIS confirmed on biopsy |
| 5 | 49/F | Persistent microhematuria | Positive (1×) | Negative | Multifocal non-invasive papillary lesions | TURBT + MMC |
| 6 | 60/M | Persistent microhematuria | Positive (2×) | Negative | Patchy fluorescence on blue-light cystoscopy | CIS confirmed; IVT started |
| 7 | 81/M | NMIBC surveillance | Positive (1×) | Negative | Asymmetric left bladder wall thickening | HG Ta + CIS; managed per high-risk NMIBC protocol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholami, S.S.; Movassaghi, M.; Homayoun, S.; Vasdev, N. Clinical Experience with URO17® in the Diagnosis and Surveillance of Bladder Cancer. J. Clin. Med. 2025, 14, 8108. https://doi.org/10.3390/jcm14228108
Gholami SS, Movassaghi M, Homayoun S, Vasdev N. Clinical Experience with URO17® in the Diagnosis and Surveillance of Bladder Cancer. Journal of Clinical Medicine. 2025; 14(22):8108. https://doi.org/10.3390/jcm14228108
Chicago/Turabian StyleGholami, Shahram Shawn, Mehran Movassaghi, Sasha Homayoun, and Nikhil Vasdev. 2025. "Clinical Experience with URO17® in the Diagnosis and Surveillance of Bladder Cancer" Journal of Clinical Medicine 14, no. 22: 8108. https://doi.org/10.3390/jcm14228108
APA StyleGholami, S. S., Movassaghi, M., Homayoun, S., & Vasdev, N. (2025). Clinical Experience with URO17® in the Diagnosis and Surveillance of Bladder Cancer. Journal of Clinical Medicine, 14(22), 8108. https://doi.org/10.3390/jcm14228108







