Impact of Metabolic Dysfunction-Associated Steatotic Liver Disease on Fatigue and Pruritus in Primary Sclerosing Cholangitis: A U.S. Single-Center Study
Abstract
1. Introduction
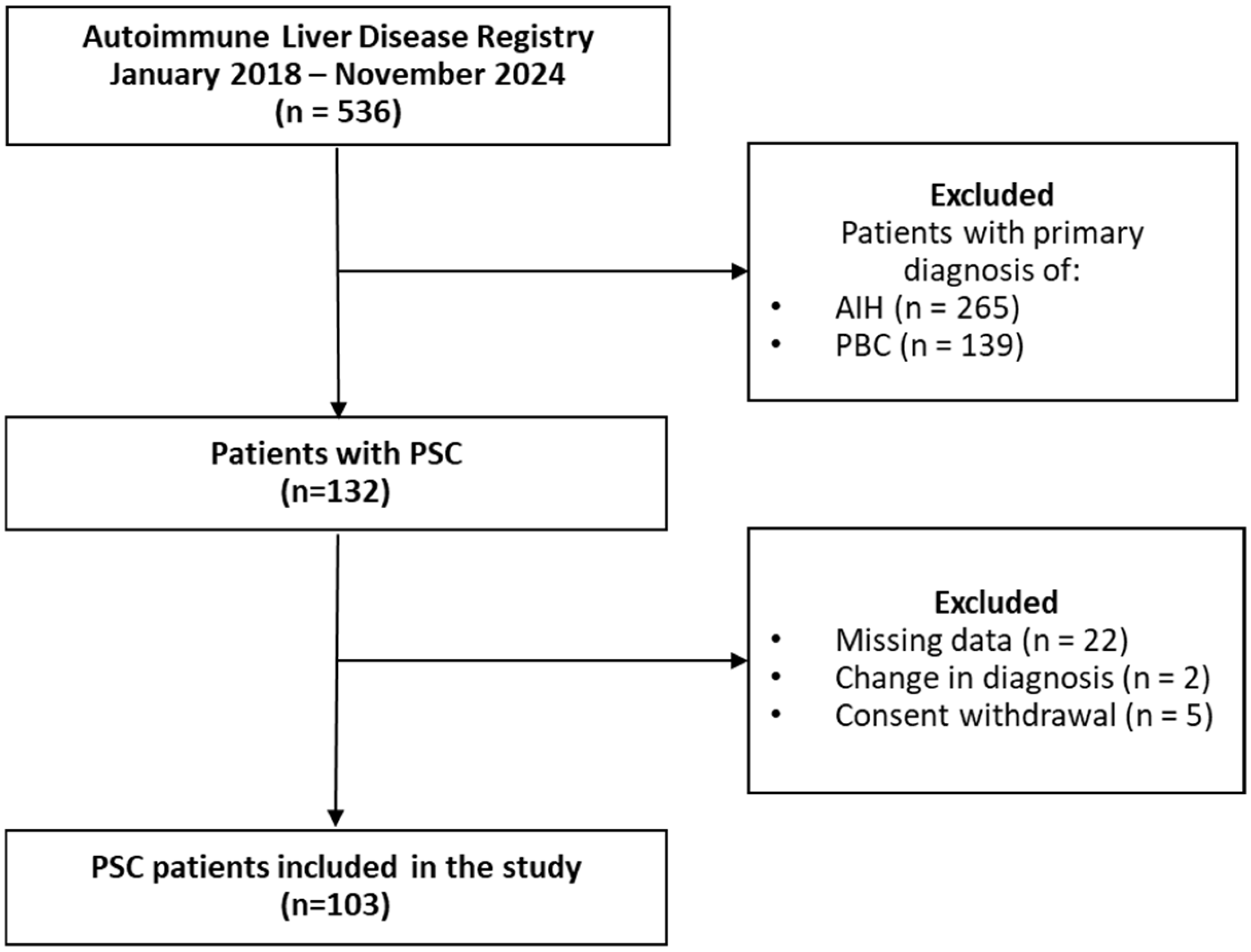
2. Materials and Methods
2.1. Study Population
2.2. Study Outcome, Variables, and Definitions
2.3. Statistical Analysis
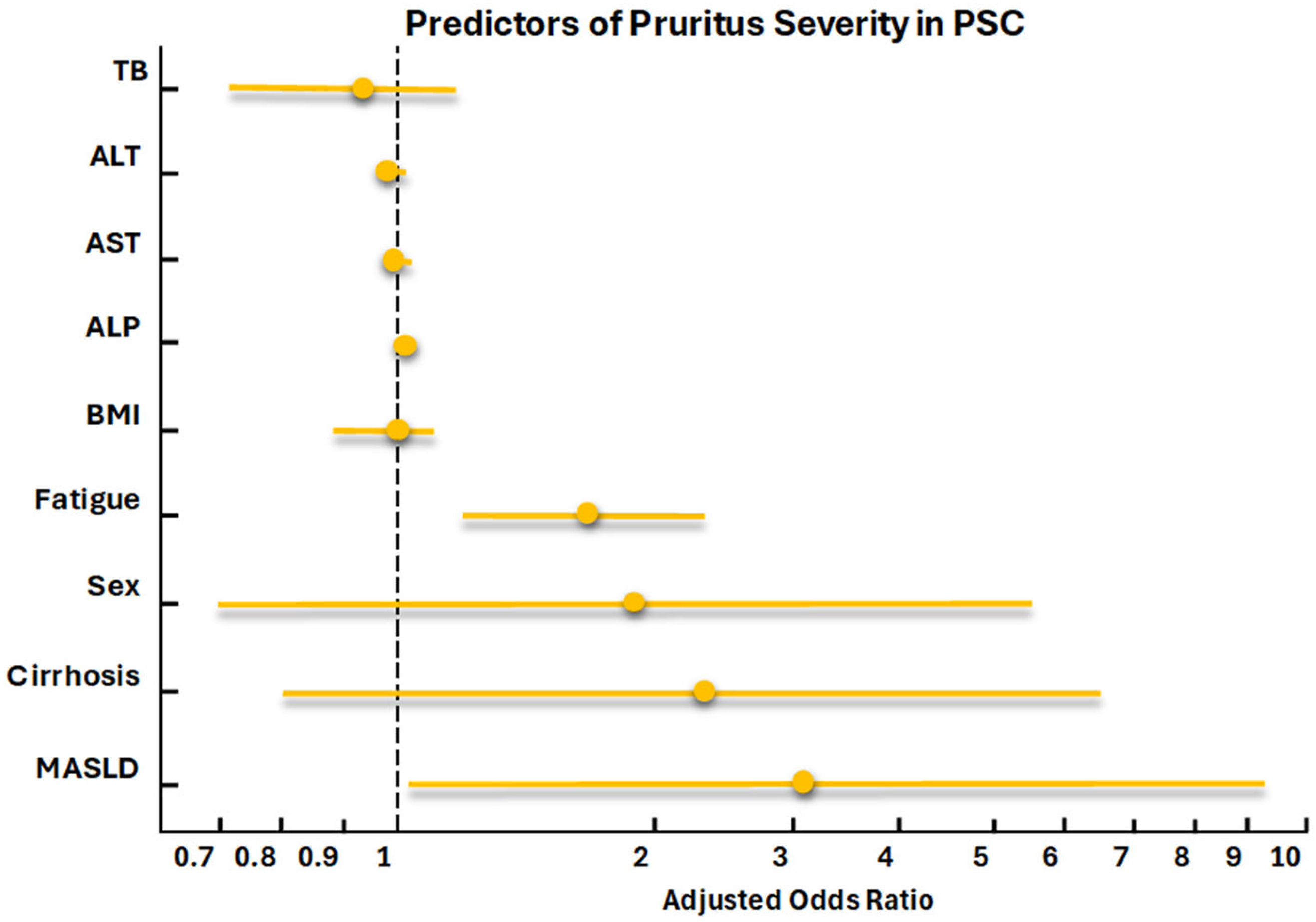
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AASLD | American Association for the Study of Liver Diseases |
| AIH | Autoimmune hepatitis |
| ALB | Albumin |
| ALD | Alcohol-related liver disease |
| ALP | Alkaline phosphatase |
| ALT | Alanine transaminase |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| CLDQ | Chronic Liver Disease Questionnaire |
| DM | Diabetes mellitus |
| GPAM | Glycerol-3-phosphate acyltransferase, mitochondrial |
| IBD | Inflammatory bowel disease |
| INR | International normalized ratio |
| IQR | Interquartile ranges |
| ldPSC | Large duct involvement |
| LPA | Lysophosphatidic acid |
| LT | Liver transplantation |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MetALD | Metabolic and alcohol-associated liver disease |
| miRNAs | MicroRNAs |
| MRI | Magnetic resonance imaging |
| PBC | Primary biliary cholangitis |
| PCC | Patient-centered care |
| PL | Platelets |
| PROs | Patient-reported outcome |
| PSC | Primary sclerosing cholangitis |
| PT | Prothrombin time |
| REDCap | Research Electronic Data Capture |
| sdPSC | Small duct PSC |
| TB | Total bilirubin |
| UC | Ulcerative Colitis |
| VCTE | Vibration-Controlled Transient Elastography |
| WBC | White blood cell count |
References
- Díaz, L.A.; Lazarus, J.V.; Fuentes-López, E.; Idalsoaga, F.; Ayares, G.; Desaleng, H.; Danpanichkul, P.; Cotter, T.G.; Dunn, W.; Barrera, F.; et al. Disparities in steatosis prevalence in the United States by race or ethnicity according to the 2023 criteria. Commun. Med. 2024, 4, 219. [Google Scholar] [CrossRef]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Zhang, X.; Huang, J.; Chen, H.; Tang, H. Prevalence of steatotic liver disease and associated fibrosis in the United States: Results from NHANES 2017–March 2020. J. Hepatol. 2024, 80, e70–e71. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, Y.; Zhu, Z.; Li, Y.; Li, X.; Li, Y.; Shen, H.; Wu, W.; Liu, Y.; Han, C. Changes in the global, regional, and national burdens of NAFLD from 1990 to 2019: A systematic analysis of the Global Burden of Disease Study 2019. Front. Nutr. 2022, 9, 1047129. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. 2025, 31, S32–S50. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Bowlus, C.L.; Yimam, K.K.; Razavi, H.; Estes, C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: A systematic review of population-based studies. Clin. Gastroenterol. Hepatol. 2022, 20, 1687–1700.e4. [Google Scholar] [CrossRef]
- Bambha, K.; Kim, W.R.; Talwalkar, J.; Torgerson, H.; Benson, J.T.; Therneau, T.M.; Loftus, E.V.; Yawn, B.P.; Dickson, E.; Melton, L. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology 2003, 125, 1364–1369. [Google Scholar] [CrossRef]
- Doycheva, I.; Cox, K.; Haseeb, A.; Adler, D.G. Prevalence and relevance of nonalcoholic fatty liver disease in patients with primary sclerosing cholangitis. Dig. Dis. Sci. 2014, 59, 1645–1646. [Google Scholar] [CrossRef]
- Danielsson, O.; Vesterinen, T.; Arola, J.; Åberg, F.; Nissinen, M.J. Coexistence of metabolic-associated fatty liver disease and autoimmune or toxic liver disease. Eur. J. Gastroenterol. Hepatol. 2024, 36, 961–969. [Google Scholar] [CrossRef]
- Kimbell, B.; Boyd, K.; Kendall, M.; Iredale, J.; Murray, S.A. Managing uncertainty in advanced liver disease: A qualitative, multiperspective, serial interview study. BMJ Open 2015, 5, e009241. [Google Scholar] [CrossRef]
- Loesken, C.; Maehder, K.; Buck, L.; Hartl, J.; Löwe, B.; Schramm, C.; Toussaint, A. Understanding illness experiences of patients with primary sclerosing cholangitis: A qualitative analysis within the SOMA.LIV study. BMC Gastroenterol. 2023, 23, 12. [Google Scholar] [CrossRef]
- Swain, M.G.; Pettersson, B.; Meyers, O.; Venerus, M.; Oscarsson, J. A qualitative patient interview study to understand the experience of patients with nonalcoholic steatohepatitis. Hepatol. Commun. 2023, 7, e0036. [Google Scholar] [CrossRef]
- van Munster, K.N.; Dijkgraaf, M.G.W.; Oude Elferink, R.P.J.; Beuers, U.; Ponsioen, C.Y. Symptom patterns in the daily life of PSC patients. Liver Int. 2022, 42, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Keklikkiran, C.; Racila, A.; Stepanova, M.; Younossi, Z.M. Pruritus and fatigue in patients with metabolic dysfunction–associated steatotic liver disease: A study of Turkish patients from the Global NASH/MASH Registry. Clin. Transl. Gastroenterol. 2025, 16, e00844. [Google Scholar] [CrossRef] [PubMed]
- Royeck, S.; Mess, C.; Weigel, A.; Löwe, B.; Toussaint, A.; Schramm, C.; Mora, M.S.; Huber, T.B.; Zeidler, C.; Witte, F.; et al. Comparative analysis of patient-reported outcomes in chronic pruritus: Patients with chronic liver or kidney diseases exhibit higher psychological distress than those with atopic dermatitis. Dermatol. Ther. 2025, 15, 3361–3375. [Google Scholar] [CrossRef] [PubMed]
- Kanagalingam, G.; Allen, J.; Chin, G.H.; Lee, H.M. Palliative care and chronic liver disease: Barriers to care, health disparities and the role of health literacy. Ann. Palliat. Med. 2025, 14, 353–368. [Google Scholar] [CrossRef]
- Hansen, L.; Chang, M.F.; Hiatt, S.; Dieckmann, N.F.; Lee, C.S. Distinct longitudinal trajectories of symptom burden predict clinical outcomes in end-stage liver disease. Clin. Transl. Gastroenterol. 2024, 15, e00728. [Google Scholar] [CrossRef]
- Kim, H.P.; Lieber, S.R.; Rogers, M.E.; Moon, A.M.; Loiselle, M.; Walker, J.; Assis, D.N.; Safer, R.; Gomel, R.; Evon, D.M. A systematic review of patient-reported outcomes in primary biliary cholangitis and primary sclerosing cholangitis. Hepatol. Commun. 2020, 4, 1502–1515. [Google Scholar] [CrossRef]
- Bowlus, C.L.; Arrivé, L.; Bergquist, A.; Deneau, M.; Forman, L.; Ilyas, S.I.; Lunsford, K.E.; Martinez, M.; Sapisochin, G.; Shroff, R.; et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2023, 77, 659–702. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Hernández-Pérez, M.; Riado, D.; Pena, E.; Méndez, C.; Pinedo, F.; Ramos, P.; Castillo, P.; Romero, M.; Fernández-Rodríguez, C.; Olveira, A. The overlap with metabolic dysfunction-associated steatotic liver disease negatively affects outcomes of primary biliary cholangitis. Aliment. Pharmacol. Ther. 2024, 60, 613–619. [Google Scholar] [CrossRef]
- Mehta, T.I.; Weissman, S.; Fung, B.M.; Sotiriadis, J.; Lindor, K.D.; Tabibian, J.H. Global incidence, prevalence and features of primary sclerosing cholangitis: A systematic review and meta-analysis. Liver Int. 2021, 41, 2418–2426. [Google Scholar] [CrossRef]
- Sohal, A.; Kayani, S.; Kowdley, K.V. Primary Sclerosing Cholangitis: Epidemiology, Diagnosis, and Presentation. Clin. Liver Dis. 2024, 28, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Takakura, W.R.; Tabibian, J.H.; Bowlus, C.L. The evolution of natural history of primary sclerosing cholangitis. Curr. Opin. Gastroenterol. 2017, 33, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Vander Does, A.; Levy, C.; Yosipovitch, G. Cholestatic Itch: Our Current Understanding of Pathophysiology and Treatments. Am. J. Clin. Dermatol. 2022, 23, 647–659. [Google Scholar] [CrossRef] [PubMed]
- He, L.; She, X.; Guo, L.; Gao, M.; Wang, S.; Lu, Z.; Guo, H.; Li, R.; Nie, Y.; Xing, J.; et al. Hepatic AKAP1 deficiency exacerbates diet-induced MASLD by enhancing GPAT1-mediated lysophosphatidic acid synthesis. Nat. Commun. 2025, 16, 4286. [Google Scholar] [CrossRef]
- Patel, S.P.; Khanna, R.; Choi, J.; Williams, K.A.; Roh, Y.S.; Hong, M.S.; Sutaria, N.H.; Pritchard, T.; Kwatra, M.M.; Kwatra, S.G. Sleep disturbance in adults with chronic pruritic dermatoses is associated with increased C-reactive protein levels. J. Am. Acad. Dermatol. 2021, 84, 265–272. [Google Scholar] [CrossRef]
- Weismüller, T.J.; Trivedi, P.J.; Bergquist, A.; Imam, M.; Lenzen, H.; Ponsioen, C.Y.; Holm, K.; Gotthardt, D.; Färkkilä, M.A.; Marschall, H.-U.; et al. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology 2017, 152, 1975–1984.e8. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Kline, K.T.; Stevenson, H.L.; Khan, K.; Parupudi, S. Small duct primary sclerosing cholangitis: A discrete variant or a bridge to large duct disease, a practical review. World J. Hepatol. 2022, 14, 495–503. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hurley, E.H.; Park, Y.; Ko, S. Primary sclerosing cholangitis (PSC) and inflammatory bowel disease (IBD): A condition exemplifying the crosstalk of the gut-liver axis. Exp. Mol. Med. 2023, 55, 1380–1387. [Google Scholar] [CrossRef]
- Popa, M.L.; Ichim, C.; Anderco, P.; Todor, S.B.; Pop-Lodromanean, D. MicroRNAs in the Diagnosis of Digestive Diseases: A Comprehensive Review. J. Clin. Med. 2025, 14, 2054. [Google Scholar] [CrossRef]
- Hasegawa, S.; Yoneda, M.; Kurita, Y.; Nogami, A.; Honda, Y.; Hosono, K.; Nakajima, A. Cholestatic Liver Disease: Current Treatment Strategies and New Therapeutic Agents. Drugs 2021, 81, 1181–1192. [Google Scholar] [CrossRef]
- Björnsson, E.S.; Kalaitzakis, E. Recent advances in the treatment of primary sclerosing cholangitis. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 413–425. [Google Scholar] [CrossRef]

| Variable | PSC Only (n = 69) | PSC/MASLD (n = 34) | p Value |
|---|---|---|---|
| Age, (IQR) | 46 (35–60) | 55 (47–66) | <0.006 * |
| Sex, n (%) | 0.835 | ||
| Female | 34 (49) | 18 (53) | |
| Male | 35 (51) | 16 (47) | |
| Race/ethnicity, n (%) | 0.203 | ||
| White Caucasian | 57 (83) | 26 (76) | |
| Black | 7 (10) | 2 (6) | |
| Hispanic | 1 (1) | 4 (12) | |
| Asian | 2 (2) | 1 (3) | |
| BMI, (IQR) | 25 (23–28) | 30 (26–34) | <0.001 * |
| Bile Duct Involvement, n (%) | 0.002 * | ||
| Small duct disease | 7 (12) | 16 (48) | |
| Large duct disease | 30 (51) | 10 (30) | |
| Dominant stricture | 17 (29) | 5 (15) | |
| Unknown | 5 (8) | 2 (6) | |
| IBD, n (%) | 0.219 | ||
| Ulcerative colitis | 36 (52) | 13 (38) | |
| Crohn’s disease | 9 (13) | 3 (9) | |
| Cirrhosis, n (%) | 23 (34) | 7 (21) | 0.249 |
| Cholangiocarcinoma, n (%) | 1 (1.4) | 0 | 1.000 |
| Liver Transplant, n (%) | 12 (18) | 1 (3) | 0.055 |
| Hypertension, n (%) | 10 (14) | 13 (38) | 0.011 * |
| DM, n (%) | 1 (1) | 5 (15) | 0.014 * |
| Laboratory values | |||
| ALP, (IQR) | 108 (86–250) | 125 (92–233) | 0.680 |
| ALT, (IQR) | 28 (16–59) | 31 (22–49) | 0.667 |
| AST, (IQR) | 29 (20–52) | 28 (22–40) | 0.735 |
| Total Cholesterol, (IQR) | 189 (169–215) | 197 (142–231) | 0.970 |
| LDL, (IQR) | 101 (74–132) | 97 (74–133) | 0.801 |
| HDL, (IQR) | 65 (46–76) | 58 (50–83) | 0.902 |
| Triglycerides | 97 (65–134) | 94 (61–163) | 0.924 |
| Total Bilirubin, (IQR) | 0.6 (0.4–1.1) | 0.5 (0.4–0.6) | 0.129 |
| Albumin, (IQR) | 4.4 (4–4.6) | 4.4 (4–4.6) | 0.845 |
| WBC–Leukocytes (IQR) | 6.2 (4.6–8.1) | 7.5 (5.4–8.6) | 0.037 * |
| Platelets, (IQR) | 208 (163–286) | 268 (220–348) | 0.011 * |
| INR, (IQR) | 1.1 (1–1.2) | 1.1 (1–1.1) | 0.292 |
| Creatinine, (IQR) | 0.8 (0.7–0.9) | 0.8 (0.7–1) | 0.584 |
| Patient-Reported Outcome | |||
| Fatigue Intensity, n (%) | 0.566 | ||
| None of the time | 11 (16) | 3 (9) | |
| Hardly any of the time | 13 (19) | 9 (26) | |
| A little of the time | 13 (19) | 9 (26) | |
| Some of the time | 12 (17) | 7 (21) | |
| A good bit of the time | 5 (7) | 3 (9) | |
| Most of the time | 9 (13) | 1 (3) | |
| All of the time | 6 (9) | 2 (6) | |
| Pruritus, n (%) | 0.390 | ||
| Not present | 46 (66) | 19 (55) | |
| Mild | 14 (20) | 8 (23) | |
| Moderate | 7 (10) | 7 (20) | |
| Unbearable | 2 (3) | 0 (0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Amaris, N.; Marenco-Flores, A.; Lara-Romero, C.; Barba, R.; Rubio-Cruz, D.; Parraga, X.; Goyes, D.; Medina-Morales, J.E.; Sierra, L.; Romero-Gomez, M.; et al. Impact of Metabolic Dysfunction-Associated Steatotic Liver Disease on Fatigue and Pruritus in Primary Sclerosing Cholangitis: A U.S. Single-Center Study. J. Clin. Med. 2025, 14, 8083. https://doi.org/10.3390/jcm14228083
Rojas-Amaris N, Marenco-Flores A, Lara-Romero C, Barba R, Rubio-Cruz D, Parraga X, Goyes D, Medina-Morales JE, Sierra L, Romero-Gomez M, et al. Impact of Metabolic Dysfunction-Associated Steatotic Liver Disease on Fatigue and Pruritus in Primary Sclerosing Cholangitis: A U.S. Single-Center Study. Journal of Clinical Medicine. 2025; 14(22):8083. https://doi.org/10.3390/jcm14228083
Chicago/Turabian StyleRojas-Amaris, Natalia, Ana Marenco-Flores, Carmen Lara-Romero, Romelia Barba, Denisse Rubio-Cruz, Ximena Parraga, Daniela Goyes, John Esli Medina-Morales, Leandro Sierra, Manuel Romero-Gomez, and et al. 2025. "Impact of Metabolic Dysfunction-Associated Steatotic Liver Disease on Fatigue and Pruritus in Primary Sclerosing Cholangitis: A U.S. Single-Center Study" Journal of Clinical Medicine 14, no. 22: 8083. https://doi.org/10.3390/jcm14228083
APA StyleRojas-Amaris, N., Marenco-Flores, A., Lara-Romero, C., Barba, R., Rubio-Cruz, D., Parraga, X., Goyes, D., Medina-Morales, J. E., Sierra, L., Romero-Gomez, M., Lai, M., Saberi, B., Patwardhan, V., & Bonder, A. (2025). Impact of Metabolic Dysfunction-Associated Steatotic Liver Disease on Fatigue and Pruritus in Primary Sclerosing Cholangitis: A U.S. Single-Center Study. Journal of Clinical Medicine, 14(22), 8083. https://doi.org/10.3390/jcm14228083






