Adequacy of Anesthesia Guidance Combined with Peribulbar Blocks Shows Potential Benefit in High-Risk PONV Patients Undergoing Vitreoretinal Surgeries
Abstract
1. Introduction
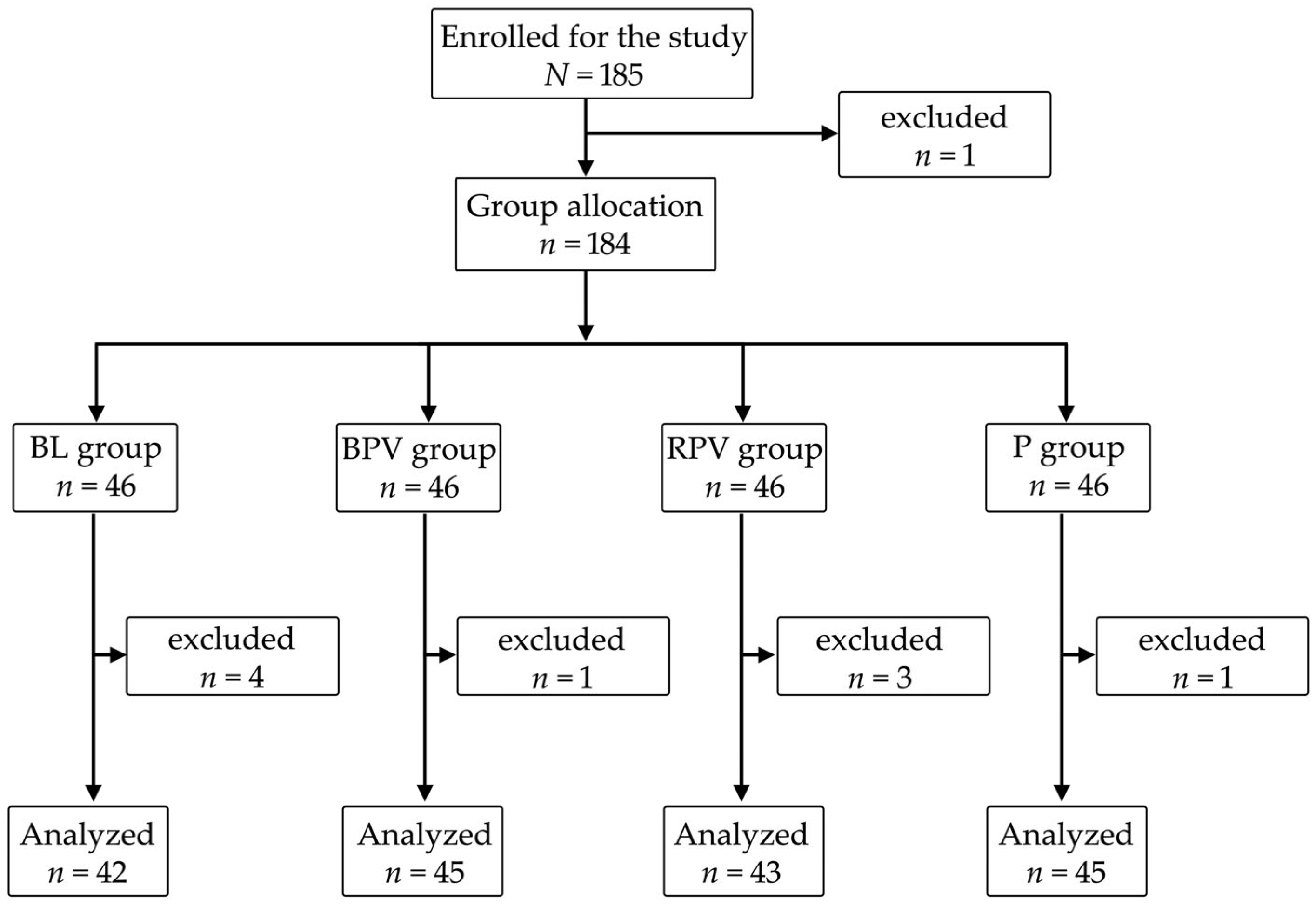
2. Materials and Methods
2.1. Anesthesia Technique
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AoA | Adequacy of Anesthesia |
| BL | Bupivacaine/lidocaine |
| BPV | Bupivacaine |
| COX-3 | Cyclooxygenase-3 |
| DM | Diabetes mellitus |
| FNT | Fentanyl |
| GA | General anesthesia |
| IPPP | Inappropriate postoperative pain perception |
| MAP | Mean arterial pressure |
| NPRS | Numeric pain rating scale |
| OCR | Oculocardiac reflex |
| OER | Oculoemetic reflex |
| P | Paracetamol |
| PA | Preventive analgesia |
| PACU | Post-anesthesia care unit |
| PBB | Peribulbar block |
| PONV | Postoperative nausea and vomiting |
| RE | Response entropy |
| RPV | Ropivacaine |
| SE | State entropy |
| SPI | Surgical pleth index |
Appendix A. Detailed Anesthesia Protocol
Appendix A.1. Stage 1
Appendix A.2. Stage 2
Appendix A.3. Stage 3—Intraoperatively
Appendix A.4. Stage 4—Postoperatively
References
- Weibel, S.; Rücker, G.; Eberhart, L.H.; Pace, N.L.; Hartl, H.M.; Jordan, O.L.; Mayer, D.; Riemer, M.; Schaefer, M.S.; Raj, D.; et al. Drugs for Preventing Postoperative Nausea and Vomiting in Adults after General Anaesthesia: A Network Meta-Analysis. Cochrane Database Syst. Rev. 2020, 10, CD012859. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, J.E.; Bonsel, G.J.; Moons, K.G.; Kalkman, C.J. Effect of Postoperative Experiences on Willingness to Pay to Avoid Postoperative Pain, Nausea, and Vomiting. Anesthesiology 2006, 104, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Sloan, F.; Dear, G.d.L.; El-Moalem, H.E.; Lubarsky, D.A. How Much Are Patients Willing to Pay to Avoid Postoperative Nausea and Vomiting? Anesth. Analg. 2001, 92, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Watcha, M.F. The Cost-Effective Management of Postoperative Nausea and Vomiting. Anesthesiology 2000, 92, 931–933. [Google Scholar] [CrossRef]
- Hill, R.P.; Lubarsky, D.A.; Phillips-Bute, B.; Fortney, J.T.; Creed, M.R.; Glass, P.S.; Gan, T.J. Cost-Effectiveness of Prophylactic Antiemetic Therapy with Ondansetron, Droperidol, or Placebo. Anesthesiology 2000, 92, 958–967. [Google Scholar] [CrossRef]
- Eberhart, L.H.J.; Morin, A.M.; Hoerle, S.; Wulf, H.; Geldner, G. Droperidol and Dolasetron Alone or in Combination for Prevention of Postoperative Nausea and Vomiting after Vitrectomy. Ophthalmology 2004, 111, 1569–1575. [Google Scholar] [CrossRef]
- Kienbaum, P.; Schaefer, M.S.; Weibel, S.; Schlesinger, T.; Meybohm, P.; Eberhart, L.H.; Kranke, P. Update on PONV-What is new in prophylaxis and treatment of postoperative nausea and vomiting?: Summary of recent consensus recommendations and Cochrane reviews on prophylaxis and treatment of postoperative nausea and vomiting. Anaesthesist 2022, 71, 123–128. [Google Scholar] [CrossRef]
- Ongel, E.; Erdag, E.; Adiyeke, E.; Bakan, N. Acupressure Versus Ondansetron Usage for Postoperative Nausea and Vomiting After Gynecologic Surgeries. Cureus 2023, 15, e36862. [Google Scholar] [CrossRef]
- Karsten, M.; Prince, D.; Robinson, R.; Stout-Aguilar, J. Effects of Peppermint Aromatherapy on Postoperative Nausea and Vomiting. J. Perianesth. Nurs. 2020, 35, 615–618. [Google Scholar] [CrossRef]
- Fearrington, M.A.; Qualls, B.W.; Carey, M.G. Essential Oils to Reduce Postoperative Nausea and Vomiting. J. Perianesth. Nurs. 2019, 34, 1047–1053. [Google Scholar] [CrossRef]
- Lu, C.; Chen, X.; Yan, X.; He, J.; Nie, Z. The Preventive and Relieving Effects of Ginger on Postoperative Nausea and Vomiting: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Nurs. Stud. 2022, 125, 104094. [Google Scholar] [CrossRef]
- Dursun Ergezen, F.; Özer, Z.; Kol, E. Effectiveness of Music Intervention on Postoperative Nausea and Vomiting: A Systematic Review and Meta-Analysis. J. Perianesth. Nurs. 2022, 37, 717–727. [Google Scholar] [CrossRef]
- Arslan, H.N.; Çelik, S.Ş. Nonpharmacological Nursing Interventions in Postoperative Nausea and Vomiting: A Systematic Review. J. Perianesth. Nurs. 2024, 39, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, Y.; Zhu, Y.-J.; Shan, X.-S.; Liu, H.; Ji, F.-H.; Peng, K. Comparison of Opioid-Free and Opioid-Inclusive Propofol Anaesthesia for Thyroid and Parathyroid Surgery: A Randomised Controlled Trial. Anaesthesia 2024, 79, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Williams, B.A. Postoperative Nausea and Vomiting in Ambulatory Regional Anesthesia. Int. Anesthesiol. Clin. 2011, 49, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.A.B.; Donachie, P.H.J.; Williamson, T.H.; Sparrow, J.M.; Johnston, R.L. The Royal College of Ophthalmologists’ National Ophthalmology Database Study of Vitreoretinal Surgery: Report 5, Anaesthetic Techniques. Br. J. Ophthalmol. 2016, 100, 246–252. [Google Scholar] [CrossRef]
- Mandelcorn, M.; Taback, N.; Mandelcorn, E.; Ananthanarayan, C. Risk Factors for Pain and Nausea Following Retinal and Vitreous Surgery under Conscious Sedation. Can. J. Ophthalmol. 1999, 34, 281–285. [Google Scholar]
- Ghali, A.M.; El Btarny, A.M. The Effect on Outcome of Peribulbar Anaesthesia in Conjunction with General Anesthesia for Vitreoretinal Surgery. Anaesthesia 2010, 65, 249–253. [Google Scholar] [CrossRef]
- Bayerl, K.; Boost, K.A.; Wolf, A.; Kampik, A.; Schaumberger, M.; Haritoglou, C. A 23-gauge pars plana vitrectomy after induction of general anesthesia: Effect of additional retrobulbar anesthesia on postoperative pain. Ophthalmologe 2014, 111, 1194–1200. [Google Scholar] [CrossRef]
- Schönfeld, C.-L.; Hierneis, S.; Kampik, A. Preemptive Analgesia with Ropivacaine for Pars Plana Vitrectomy: Randomized Controlled Trial on Efficacy and Required Dose. Retina 2012, 32, 912–917. [Google Scholar] [CrossRef]
- Henzler, D.; Müller-Kaulen, B.; Steinhorst, U.H.; Broermann, H.; Piepenbrock, S. The combination of retrobulbar block with general anaesthesia may lead to pre-emptive analgesia in patients undergoing pars plana vitrectomy. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 2002, 37, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Misiołek, H.; Cettler, M.; Woroń, J.; Wordliczek, J.; Dobrogowski, J.; Mayzner-Zawadzka, E. The 2014 Guidelines for Post-Operative Pain Management. Anaesthesiol. Intensive Ther. 2014, 46, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Chong, M.S. Preemptive Analgesia—Treating Postoperative Pain by Preventing the Establishment of Central Sensitization. Anesth. Analg. 1993, 77, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Bharti, N.; Chari, P.; Kumar, P. Effect of Sevoflurane versus Propofol-Based Anesthesia on the Hemodynamic Response and Recovery Characteristics in Patients Undergoing Microlaryngeal Surgery. Saudi J. Anaesth. 2012, 6, 380–384. [Google Scholar] [CrossRef]
- Gruenewald, M.; Ilies, C. Monitoring the Nociception-Anti-Nociception Balance. Best Pract. Res. Clin. Anaesthesiol. 2013, 27, 235–247. [Google Scholar] [CrossRef]
- Struys, M.M.R.F.; Vanpeteghem, C.; Huiku, M.; Uutela, K.; Blyaert, N.B.K.; Mortier, E.P. Changes in a Surgical Stress Index in Response to Standardized Pain Stimuli during Propofol-Remifentanil Infusion. Br. J. Anaesth. 2007, 99, 359–367. [Google Scholar] [CrossRef]
- Wennervirta, J.; Hynynen, M.; Koivusalo, A.-M.; Uutela, K.; Huiku, M.; Vakkuri, A. Surgical Stress Index as a Measure of Nociception/Antinociception Balance during General Anesthesia. Acta Anaesthesiol. Scand. 2008, 52, 1038–1045. [Google Scholar] [CrossRef]
- Ahonen, J.; Jokela, R.; Uutela, K.; Huiku, M. Surgical Stress Index Reflects Surgical Stress in Gynaecological Laparoscopic Day-Case Surgery. Br. J. Anaesth. 2007, 98, 456–461. [Google Scholar] [CrossRef]
- Ilies, C.; Gruenewald, M.; Ludwigs, J.; Thee, C.; Höcker, J.; Hanss, R.; Steinfath, M.; Bein, B. Evaluation of the Surgical Stress Index during Spinal and General Anaesthesia. Br. J. Anaesth. 2010, 105, 533–537. [Google Scholar] [CrossRef]
- Gruenewald, M.; Meybohm, P.; Ilies, C.; Höcker, J.; Hanss, R.; Scholz, J.; Bein, B. Influence of Different Remifentanil Concentrations on the Performance of the Surgical Stress Index to Detect a Standardized Painful Stimulus during Sevoflurane Anaesthesia. Br. J. Anaesth. 2009, 103, 586–593. [Google Scholar] [CrossRef]
- Ledowski, T.; Pascoe, E.; Ang, B.; Schmarbeck, T.; Clarke, M.W.; Fuller, C.; Kapoor, V. Monitoring of Intra-Operative Nociception: Skin Conductance and Surgical Stress Index versus Stress Hormone Plasma Levels. Anaesthesia 2010, 65, 1001–1006. [Google Scholar] [CrossRef]
- Bergmann, I.; Göhner, A.; Crozier, T.A.; Hesjedal, B.; Wiese, C.H.; Popov, A.F.; Bauer, M.; Hinz, J.M. Surgical Pleth Index-Guided Remifentanil Administration Reduces Remifentanil and Propofol Consumption and Shortens Recovery Times in Outpatient Anaesthesia. Br. J. Anaesth. 2013, 110, 622–628. [Google Scholar] [CrossRef]
- Upton, H.D.; Ludbrook, G.L.; Wing, A.; Sleigh, J.W. Intraoperative “Analgesia Nociception Index”-Guided Fentanyl Administration During Sevoflurane Anesthesia in Lumbar Discectomy and Laminectomy: A Randomized Clinical Trial. Anesth. Analg. 2017, 125, 81–90. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Lyssek-Boroń, A.; Krysik, K.; Majer, D.; Zmarzły, N.; Grabarek, B.O. Evaluating the Efficacy of Pre-Emptive Peribulbar Blocks with Different Local Anesthetics or Paracetamol Using the Adequacy of Anesthesia Guidance for Vitreoretinal Surgeries: A Preliminary Report. Biomedicines 2024, 12, 2303. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Kawka, M.; Krawczyk, L.; Niewiadomska, E.; Dobrowolski, D.; Rejdak, R.; Król, S.; Żak, J.; et al. Preventive Analgesia, Hemodynamic Stability, and Pain in Vitreoretinal Surgery. Medicina 2021, 57, 262. [Google Scholar] [CrossRef] [PubMed]
- Marczak, K.; Stasiowski, M.J.; Lyssek-Boroń, A.; Zmarzły, N. Adverse Events Following Vitreoretinal Surgeries Under Adequacy of Anesthesia with Combined Paracetamol/Metamizole—Additional Report. J. Clin. Med. 2025, 14, 6261. [Google Scholar] [CrossRef] [PubMed]
- Pluta, A.; Stasiowski, M.J.; Lyssek-Boroń, A.; Król, S.; Krawczyk, L.; Niewiadomska, E.; Żak, J.; Kawka, M.; Dobrowolski, D.; Grabarek, B.O.; et al. Adverse Events during Vitrectomy under Adequacy of Anesthesia-An Additional Report. J. Clin. Med. 2021, 10, 4172. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Król, S.; Krawczyk, L.; Niewiadomska, E.; Żak, J.; Kawka, M.; Dobrowolski, D.; Grabarek, B.O.; et al. Adverse Events during Vitreoretinal Surgery under Adequacy of Anesthesia Guidance-Risk Factor Analysis. Pharmaceuticals 2022, 15, 237. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Niewiadomska, E.; Krawczyk, L.; Dobrowolski, D.; Grabarek, B.O.; Kawka, M.; Rejdak, R.; Szumera, I.; et al. Adequacy of Anaesthesia for Nociception Detection during Vitreoretinal Surgery. Life 2023, 13, 505. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Lyssek-Boroń, A.; Zmarzły, N.; Marczak, K.; Grabarek, B.O. The Adequacy of Anesthesia Guidance for Vitreoretinal Surgeries with Preemptive Paracetamol/Metamizole. Pharmaceuticals 2024, 17, 129. [Google Scholar] [CrossRef]
- Owczuk, R. Guidelines for General Anaesthesia in the Elderly of the Committee on Quality and Safety in Anaesthesia, Polish Society of Anaesthesiology and Intensive Therapy. Anaesthesiol. Intensive Ther. 2013, 45, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.C. Techniques of Orbital Regional Anaesthesia. Br. J. Anaesth. 1995, 75, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.; Duława, A.; Szumera, I.; Marciniak, R.; Niewiadomska, E.; Kaspera, W.; Krawczyk, L.; Ładziński, P.; Grabarek, B.O.; Jałowiecki, P. Variations in Values of State, Response Entropy and Haemodynamic Parameters Associated with Development of Different Epileptiform Patterns during Volatile Induction of General Anaesthesia with Two Different Anaesthetic Regimens Using Sevoflurane in Comparison with Intravenous Induct: A Comparative Study. Brain Sci. 2020, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.; Missir, A.; Pluta, A.; Szumera, I.; Stasiak, M.; Szopa, W.; Błaszczyk, B.; Możdżyński, B.; Majchrzak, K.; Tymowski, M.; et al. Influence of Infiltration Anaesthesia on Perioperative Outcomes Following Lumbar Discectomy under Surgical Pleth Index-Guided General Anaesthesia: A Preliminary Report from a Randomised Controlled Prospective Trial. Adv. Med. Sci. 2020, 65, 149–155. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Szumera, I.; Wardas, P.; Król, S.; Żak, J.; Missir, A.; Pluta, A.; Niewiadomska, E.; Krawczyk, L.; Jałowiecki, P.; et al. Adequacy of Anesthesia and Pupillometry for Endoscopic Sinus Surgery. J. Clin. Med. 2021, 10, 4683. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Rajan, N.; Joshi, G.P. Management of Postoperative Nausea and Vomiting in Adults: Current Controversies. Curr. Opin. Anaesthesiol. 2021, 34, 695–702. [Google Scholar] [CrossRef]
- Reibaldi, M.; Longo, A.; Romano, M.R.; Cennamo, G.; Mariotti, C.; Boscia, F.; Bonfiglio, V.; Avitabile, T. Delayed Suprachoroidal Hemorrhage After Pars Plana Vitrectomy: Five-Year Results of a Retrospective Multicenter Cohort Study. Am. J. Ophthalmol. 2015, 160, 1235–1242.e1. [Google Scholar] [CrossRef]
- Gupta, N.; Kumar, R.; Kumar, S.; Sehgal, R.; Sharma, K.R. A Prospective Randomised Double Blind Study to Evaluate the Effect of Peribulbar Block or Topical Application of Local Anaesthesia Combined with General Anaesthesia on Intra-Operative and Postoperative Complications during Paediatric Strabismus Surgery. Anaesthesia 2007, 62, 1110–1113. [Google Scholar] [CrossRef]
- Chhabra, A.; Pandey, R.; Khandelwal, M.; Subramaniam, R.; Gupta, S. Anesthetic Techniques and Postoperative Emesis in Pediatric Strabismus Surgery. Reg. Anesth. Pain. Med. 2005, 30, 43–47. [Google Scholar] [CrossRef]
- Graf, B.M.; Abraham, I.; Eberbach, N.; Kunst, G.; Stowe, D.F.; Martin, E. Differences in Cardiotoxicity of Bupivacaine and Ropivacaine Are the Result of Physicochemical and Stereoselective Properties. Anesthesiology 2002, 96, 1427–1434. [Google Scholar] [CrossRef]
- Smith, H.S. Potential Analgesic Mechanisms of Acetaminophen. Pain. Physician 2009, 12, 269–280. [Google Scholar] [CrossRef]
- Graham, G.G.; Scott, K.F. Mechanism of Action of Paracetamol. Am. J. Ther. 2005, 12, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Walker, E.A.; Sterious, S.N. Opioid Receptors and Acetaminophen (Paracetamol). Eur. J. Pharmacol. 2004, 503, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Roca-Vinardell, A.; Ortega-Alvaro, A.; Gibert-Rahola, J.; Micó, J.A. The Role of 5-HT1A/B Autoreceptors in the Antinociceptive Effect of Systemic Administration of Acetaminophen. Anesthesiology 2003, 98, 741–747. [Google Scholar] [CrossRef]
- Bujalska, M. Effect of Nitric Oxide Synthase Inhibition on Antinociceptive Action of Different Doses of Acetaminophen. Pol. J. Pharmacol. 2004, 56, 605–610. [Google Scholar] [PubMed]
- Ottani, A.; Leone, S.; Sandrini, M.; Ferrari, A.; Bertolini, A. The Analgesic Activity of Paracetamol Is Prevented by the Blockade of Cannabinoid CB1 Receptors. Eur. J. Pharmacol. 2006, 531, 280–281. [Google Scholar] [CrossRef]
- Brodner, G.; Gogarten, W.; Van Aken, H.; Hahnenkamp, K.; Wempe, C.; Freise, H.; Cosanne, I.; Huppertz-Thyssen, M.; Ellger, B. Efficacy of Intravenous Paracetamol Compared to Dipyrone and Parecoxib for Postoperative Pain Management after Minor-to-Intermediate Surgery: A Randomised, Double-Blind Trial. Eur. J. Anaesthesiol. 2011, 28, 125–132. [Google Scholar] [CrossRef]
- Doleman, B.; Read, D.; Lund, J.N.; Williams, J.P. Preventive Acetaminophen Reduces Postoperative Opioid Consumption, Vomiting, and Pain Scores After Surgery: Systematic Review and Meta-Analysis. Reg. Anesth. Pain. Med. 2015, 40, 706–712. [Google Scholar] [CrossRef]
- Toms, L.; McQuay, H.J.; Derry, S.; Moore, R.A. Single Dose Oral Paracetamol (Acetaminophen) for Postoperative Pain in Adults. Cochrane Database Syst. Rev. 2008, 2008, CD004602. [Google Scholar] [CrossRef]
- Apfel, C.C.; Turan, A.; Souza, K.; Pergolizzi, J.; Hornuss, C. Intravenous Acetaminophen Reduces Postoperative Nausea and Vomiting: A Systematic Review and Meta-Analysis. Pain 2013, 154, 677–689. [Google Scholar] [CrossRef]
- Oscier, C.D.; Milner, Q.J.W. Peri-Operative Use of Paracetamol. Anaesthesia 2009, 64, 65–72. [Google Scholar] [CrossRef]
- McNicol, E.D.; Tzortzopoulou, A.; Cepeda, M.S.; Francia, M.B.D.; Farhat, T.; Schumann, R. Single-Dose Intravenous Paracetamol or Propacetamol for Prevention or Treatment of Postoperative Pain: A Systematic Review and Meta-Analysis. Br. J. Anaesth. 2011, 106, 764–775. [Google Scholar] [CrossRef]
- Steffen, P.; Schuhmacher, I.; Weichel, T.; Georgieff, M.; Seeling, W. Differential administration of non-opioids in postoperative analgesia, I. Quantification of the analgesic effect of metamizole using patient-controlled analgesia. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 1996, 31, 143–147. [Google Scholar] [CrossRef]
- Zukowski, M.; Kotfis, K. Safety of metamizole and paracetamol for acute pain treatment. Anestezjol. Intens. Ter. 2009, 41, 170–175. [Google Scholar]
- Konijnenbelt-Peters, J.; van der Heijden, C.; Ekhart, C.; Bos, J.; Bruhn, J.; Kramers, C. Metamizole (Dipyrone) as an Alternative Agent in Postoperative Analgesia in Patients with Contraindications for Nonsteroidal Anti-Inflammatory Drugs. Pain. Pract. 2017, 17, 402–408. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.; Chandrasena, C.; Schug, S.A. Nonopioid Analgesics for Postoperative Pain Management. Curr. Opin. Anaesthesiol. 2014, 27, 513–519. [Google Scholar] [CrossRef]
- Feng, C.-D.; Xu, Y.; Chen, S.; Song, N.; Meng, X.-W.; Liu, H.; Ji, F.-H.; Peng, K. Opioid-Free Anaesthesia Reduces Postoperative Nausea and Vomiting after Thoracoscopic Lung Resection: A Randomised Controlled Trial. Br. J. Anaesth. 2024, 132, 267–276. [Google Scholar] [CrossRef]
- Morel, J.; Pascal, J.; Charier, D.; De Pasquale, V.; Gain, P.; Auboyer, C.; Molliex, S. Preoperative Peribulbar Block in Patients Undergoing Retinal Detachment Surgery under General Anesthesia: A Randomized Double-Blind Study. Anesth. Analg. 2006, 102, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Sadrolsadat, S.H.; Yousefshahi, F.; Ostadalipour, A.; Mohammadi, F.Z.; Makarem, J. Effect of Intravenous Acetaminophen on Postoperative Pain in Vitrectomy: A Randomized, Double-Blind, Clinical Trial. Anesth. Pain. Med. 2017, 7, e13639. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Król, S.; Wodecki, P.; Zmarzły, N.; Grabarek, B.O. Adequacy of Anesthesia Guidance for Combined General/Epidural Anesthesia in Patients Undergoing Open Abdominal Infrarenal Aortic Aneurysm Repair; Preliminary Report on Hemodynamic Stability and Pain Perception. Pharmaceuticals 2024, 17, 1497. [Google Scholar] [CrossRef] [PubMed]
- McCracken, G.C.; Montgomery, J. Postoperative Nausea and Vomiting after Unrestricted Clear Fluids before Day Surgery: A Retrospective Analysis. Eur. J. Anaesthesiol. 2018, 35, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.Z.; Taguchi, A.; Holtmann, B.; Kurz, A. Effect of Supplemental Pre-Operative Fluid on Postoperative Nausea and Vomiting. Anaesthesia 2003, 58, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Zmarzły, N.; Grabarek, B.O.; Gąsiorek, J. Postoperative Nausea and Vomiting Following Endoscopic Sinus Surgery under the Guidance of Adequacy of Anesthesia or Pupillometry with Intravenous Propofol/Remifentanil. Pharmaceuticals 2024, 17, 2. [Google Scholar] [CrossRef]
- Heinke, W.; Frank, T.; Meier, P.; Wiegel, M.; Korth, D. Postoperative vomiting after pars plana vitrectomy. Anaesthesiol. Reanim. 1996, 21, 47–50. [Google Scholar]
- Horn, C.C.; Wallisch, W.J.; Homanics, G.E.; Williams, J.P. Pathophysiological and Neurochemical Mechanisms of Postoperative Nausea and Vomiting. Eur. J. Pharmacol. 2014, 722, 55–66. [Google Scholar] [CrossRef]
- Reibaldi, M.; Fallico, M.; Longo, A.; Avitabile, T.; Astuto, M.; Murabito, P.; Minardi, C.; Bonfiglio, V.; Boscia, F.; Furino, C.; et al. Efficacy of Three Different Prophylactic Treatments for Postoperative Nausea and Vomiting after Vitrectomy: A Randomized Clinical Trial. J. Clin. Med. 2019, 8, 391. [Google Scholar] [CrossRef]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef]
- Apfel, C.C.; Läärä, E.; Koivuranta, M.; Greim, C.A.; Roewer, N. A Simplified Risk Score for Predicting Postoperative Nausea and Vomiting: Conclusions from Cross-Validations between Two Centers. Anesthesiology 1999, 91, 693–700. [Google Scholar] [CrossRef]
- Koivuranta, M.; Läärä, E.; Snåre, L.; Alahuhta, S. A Survey of Postoperative Nausea and Vomiting. Anaesthesia 1997, 52, 443–449. [Google Scholar] [CrossRef]
- Nitahara, K.; Sugi, Y.; Shono, S.; Hamada, T.; Higa, K. Risk Factors for Nausea and Vomiting Following Vitrectomy in Adults. Eur. J. Anaesthesiol. 2007, 24, 166–170. [Google Scholar] [CrossRef]
- Gola, W.; Bialka, S.; Owczarek, A.J.; Misiolek, H. Effectiveness of Fascia Iliaca Compartment Block after Elective Total Hip Replacement: A Prospective, Randomized, Controlled Study. Int. J. Environ. Res. Public. Health 2021, 18, 4891. [Google Scholar] [CrossRef] [PubMed]
- Foo, I.; Macfarlane, A.J.R.; Srivastava, D.; Bhaskar, A.; Barker, H.; Knaggs, R.; Eipe, N.; Smith, A.F. The Use of Intravenous Lidocaine for Postoperative Pain and Recovery: International Consensus Statement on Efficacy and Safety. Anaesthesia 2021, 76, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.W.-S.; Schraag, S. The Use of Intravenous Lidocaine in Perioperative Medicine: Anaesthetic, Analgesic and Immune-Modulatory Aspects. J. Clin. Med. 2022, 11, 3543. [Google Scholar] [CrossRef] [PubMed]
- Pujari, V.S.; Bhandary, A.S.; Bevinaguddaiah, Y.; Shivanna, S. Successful Lipid Rescue of Local Anesthetic Systemic Toxicity Following Peribulbar Block. Med. J. Dr. D.Y. Patil. Vidyapeeth 2015, 8, 807. [Google Scholar] [CrossRef]
- Lee, S.H.; Sohn, J.-T. Mechanisms Underlying Lipid Emulsion Resuscitation for Drug Toxicity: A Narrative Review. Korean J. Anesthesiol. 2023, 76, 171–182. [Google Scholar] [CrossRef]
- Doggrell, S.A.; Hancox, J.C. Cardiac Safety Concerns for Ondansetron, an Antiemetic Commonly Used for Nausea Linked to Cancer Treatment and Following Anaesthesia. Expert. Opin. Drug Saf. 2013, 12, 421–431. [Google Scholar] [CrossRef]
- Bartlett, R.; Hartle, A.J. Routine Use of Dexamethasone for Postoperative Nausea and Vomiting: The Case Against. Anaesthesia 2013, 68, 892–896. [Google Scholar] [CrossRef]
- Lavand’homme, P.; Kehlet, H. Benefits versus Harm of Intraoperative Glucocorticoid for Postoperative Nausea and Vomiting Prophylaxis. Br. J. Anaesth. 2023, 131, 8–10. [Google Scholar] [CrossRef]
- Low, Y.; White, W.D.; Habib, A.S. Postoperative Hyperglycemia after 4- vs 8-10-Mg Dexamethasone for Postoperative Nausea and Vomiting Prophylaxis in Patients with Type II Diabetes Mellitus: A Retrospective Database Analysis. J. Clin. Anesth. 2015, 27, 589–594. [Google Scholar] [CrossRef]
- Schlesinger, T.; Meybohm, P.; Kranke, P. Postoperative Nausea and Vomiting: Risk Factors, Prediction Tools, and Algorithms. Curr. Opin. Anaesthesiol. 2023, 36, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Yaşlı, S.O.; Canpolat, D.G.; Dogruel, F.; Demirbaş, A.E. Is Postoperative Pain Associated with Nausea and Vomiting Following Orthognathic Surgery? J. Oral. Maxillofac. Surg. 2024, 82, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Groene, P.; Eisenlohr, J.; Zeuzem, C.; Dudok, S.; Karcz, K.; Hofmann-Kiefer, K. Postoperative Nausea and Vomiting in Bariatric Surgery in Comparison to Non-Bariatric Gastric Surgery. Wideochir Inne Tech. Maloinwazyjne 2019, 14, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, A.A.; Lambourne, A.; Yazji, N.S.; Laghari, N.A. Vomiting after Ophthalmic Surgery. Effects of Intra-Operative Antiemetics and Postoperative Oral Fluid Restriction. Anaesthesia 1987, 42, 270–276. [Google Scholar] [CrossRef]
- van den Berg, A.A.; Lambourne, A.; Clyburn, P.A. The Oculo-Emetic Reflex. A Rationalisation of Postophthalmic Anaesthesia Vomiting. Anaesthesia 1989, 44, 110–117. [Google Scholar] [CrossRef]
- Allison, C.E.; De Lange, J.J.; Koole, F.D.; Zuurmond, W.W.; Ros, H.H.; van Schagen, N.T. A Comparison of the Incidence of the Oculocardiac and Oculorespiratory Reflexes during Sevoflurane or Halothane Anesthesia for Strabismus Surgery in Children. Anesth. Analg. 2000, 90, 306–310. [Google Scholar] [CrossRef]
- Deb, K.; Subramaniam, R.; Dehran, M.; Tandon, R.; Shende, D. Safety and Efficacy of Peribulbar Block as Adjunct to General Anaesthesia for Paediatric Ophthalmic Surgery. Paediatr. Anaesth. 2001, 11, 161–167. [Google Scholar] [CrossRef]
- Bröking, K. Pitfalls of anesthesiologic management in paediatric strabismus surgery. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 2011, 46, 88–93. [Google Scholar] [CrossRef]
- Shende, D.; Sadhasivam, S.; Madan, R. Effects of Peribulbar Bupivacaine as an Adjunct to General Anaesthesia on Peri-Operative Outcome Following Retinal Detachment Surgery. Anaesthesia 2000, 55, 970–975. [Google Scholar] [CrossRef]
- Ledowski, T.; Burke, J.; Hruby, J. Surgical Pleth Index: Prediction of Postoperative Pain and Influence of Arousal. Br. J. Anaesth. 2016, 117, 371–374. [Google Scholar] [CrossRef]
- Ilies, C.; Ludwigs, J.; Gruenewald, M.; Thee, C.; Hanf, J.; Hanss, R.; Steinfath, M.; Bein, B. The Effect of Posture and Anaesthetic Technique on the Surgical Pleth Index. Anaesthesia 2012, 67, 508–513. [Google Scholar] [CrossRef]
| Intraoperative Data N (%) | Total N = 175 (100%) | BL Group n = 42 (24%) | BPV Group n = 45 (25.7%) | RPV Group n = 43 (24.6%) | P Group n = 45 (25.7%) | p-Value | 95% CI (BL/BPV/RPV/P) |
|---|---|---|---|---|---|---|---|
| PONV in the PACU (early PONV) | 7 (4%) | 1 (2.9%) | 2 (4.4%) | 4 (9.3%) | 0 (0%) | 0.1 NS | 0.1–12.6/0.5–15.2/2.6–22.1/0–7.9 |
| Nausea in the PACU (early nausea) | 2 (1.1%) | 1 (2.4%) | 0 (0%) | 1 (2.3%) | 0 (0%) | 0.4 NS | 0.1–12.6/0–7.9/0.1–12.3/0–7.9 |
| Vomiting in the PACU (early vomiting) | 6 (3.4%) | 1 (2.4%) | 2 (4.4%) | 3 (7%) | 0 (0%) | 0.3 NS | 0.1–12.6/0.5–15.2/1.5–19.1/0–7.9 |
| Retching in the PACU (early severe unproductive vomiting) | 1 (0.6%) | 0 (0%) | 0 (0%) | 1 (2.3%) | 0 (0%) | 0.5 NS | 0–8.4/0–7.9/0.1–12.3/0–7.9 |
| Both nausea and vomiting in the PACU (early nausea and vomiting/retching) | 2 (1.1%) | 1 (2.4%) | 0 (0%) | 1 (2.3%) | 0 (0%) | 0.4 NS | 0.1–12.6/0–7.9/0.1–12.3/0–7.9 |
| IPPP (acute + moderate pain perception) | 18 (10.2%) | 5 (11.9%) | 1 (2.2%) | 6 (14%) | 6 (13%) | 0.2 NS | 4–25.6/0.1–12.9/5.3–27.9/5.1–26.8 |
| OCR | 20 (11.4%) | 5 (11.9%) | 6 (13.3%) | 4 (9.3%) | 5 (11.1%) | 0.97 NS | 4–25.6/5.1–26.8/2.6–22.1/3.7–24.1 |
| OER (OCR + PONV) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | - | 0–8.4/0–7.9/0–8.2/0–7.9 |
| Intraoperative Data | Total N = 175 (100%) | BL Group n = 42 (24%) | BPV Group n = 45 (25.7%) | RPV Group n = 43 (24.6%) | P Group n = 45 (25.7%) | p-Value | 95% CI (BL/BPV/RPV/P) |
|---|---|---|---|---|---|---|---|
| Apfel score X ± Sd Me (IQR) | 1.49 ± 0.7 2 (1) | 1.5 ± 0.7 1.5 (1) | 1.4 ± 0.7 1 (1) | 1.4 ± 0.9 1 (1) | 1.7 ± 0.5 2 (1) | 0.1 NS | 1–2/1–2/1–2/2–2 |
| Apfel (%) X ± Sd Me (IQR) | 0.3 ± 0.12 0.4 (0.2) | 0.3 ± 0.12 0.3 (0.2) | 0.3 ± 0.12 0.2 (0.2) | 0.3 ± 0.15 0.2 (0.2) | 0.3 ± 0.1 0.4 (0.2) | 0.07 NS | 0.2–0.4/0.2–0.4/0.2–0.4/0.4–0.4 |
| Sex Female/Male n (%) |
99/76
56.57%/43.4 |
26/16
61.9%/38.1% |
23/22
51.1%/48.9% |
19/24
44.2%/55.8% |
31/14
68.9%/31.1% | 0.09 NS | 45.6–76.4/35.8–66.3/29.1–60.1/53.4–81.8 |
| Motion sickness Yes/No n (%) | 11/164 93.7%/6.3% | 2/40 4.8%/95.2% | 2/43 4.4%/95.6% | 5/38 11.6%/88.4% | 2/43 4.4%/95.6% | 0.5 NS | 0.6–16.2/0.5–15.2/3.9–25.1/0.5–15.2 |
| History of PONV Yes/No n (%) | 0/175 0%/100% | 0/42 0%/100% | 0/45 0%/100% | 0/43 0%/100% | 0/45 0%/100% | - | 0–8.4/0–7.9/0–8.2/0–7.9 |
| Smoking Yes/No n (%) | 25/150 14.3%/85.7% | 7/35 16.7%/83.3% | 7/38 15.6%/84.4% | 9/34 20.9%/79.1% | 2/43 4.4%/95.6% | 0.1 NS | 7–31.4/6.5–29.5/10–36/0.5–15.2 |
| Postoperative use of opioid drugs Yes/No n (%) | 0/175 0%/100% | 0/42 0%/100% | 0/45 0%/100% | 0/43 0%/100% | 0/45 0%/100% | - | 0–8.4/0–7.9/0–8.2/0–7.9 |
| Apfel Score [Point] N (%) | 0 (10% Risk of PONV) | 1 (21% Risk of PONV) | 2 (39% Risk of PONV) | 3 (61% Risk of PONV) | p-Value | 95% CI |
|---|---|---|---|---|---|---|
| Total N = 175 (100%) | 15 (8.6%) | 72 (41.1%) | 80 (45.7%) | 8 (4.6%) | 0 vs. 1, p < 0.001; 0 vs. 2, p < 0.001; 1 vs. 3, p < 0.001; 2 vs. 3, p < 0.001 | 1 vs. 0, 24–41; 2 vs. 0, 29–46; 1 vs. 3, 28.7–44.5; 2 vs. 3, 33–49 |
| PONV n = 7 (4%) | 1 (14.3%) | 4 (57.1%) | 2 (28.6%) | 0 (0%) | 0.6 NS | 0 risk factors: 0.2–31.9 1 risk factor: 1.5–13.6 2 risk factors: 0.3–8.7 3 risk factors: 0–36.9 |
| No-PONV n = 168 (96%) | 14 (8.3%) | 68 (40.5%) | 78 (46.4%) | 8 (4.8%) | ||
| BL group | 2 (4.8%) | 19 (45.2%) | 19 (45.2%) | 2 (4.8%) | BPV vs. P, p = 0.03; RPV vs. P, p = 0.02 | BPV vs. P: 0, 5–26; 1, −9–31; 2, −47–−7; 3, −6–6; RPV vs. P: 0, 4–24; 1, −12–29; 2, −50–−10; 3, −3–17 |
| BPV group | 7 (15.6%) | 20 (44.4%) | 17 (37.8%) | 1 (2.2%) | ||
| RPV group | 6 (13.9%) | 18 (41.9%) | 15 (34.9%) | 4 (9.3%) | ||
| P group | 0 (0%) | 15 (33.3%) | 29 (64.4%) | 1 (2.2%) | ||
| BL + BPV + RPV group | 15 (11.5%) | 57 (43.9%) | 51 (39.2%) | 7 (5.4%) | 0 vs. 1, p < 0.001; 0 vs. 2, p < 0.001; 1 vs. 3, p < 0.001; 2 vs. 3, p < 0.001 | 1 vs. 0, 22–43; 2 vs. 0, 18–38; 1 vs. 3, 29–49; 2 vs. 3, 25–43 |
| Factors | PONV | No-PONV | p-Value | 95% CI |
|---|---|---|---|---|
| Non-DM | 6 (4.8%) | 119 (95.2%) | 0.7 NS | 0.3–115.9 |
| Overall DM | 1 (2%) | 49 (98%) | ||
| Insulin-dependent | 1 (3.4%) | 28 (96.6%) | 1.0 NS | 0.02-inf |
| Insulin-independent | 0 (0%) | 21 (100%) | ||
| Normal weight | 1 (1.96%) | 50 (98.04%) | 0.6 NS | Normal: 0.05–10.5; Overweight: 1.6–14.2; Obese: 0.5–14.3 |
| Overweight | 4 (5.8%) | 65 (94.2%) | ||
| Obesity | 2 (4%) | 46 (96%) | ||
| IPPP | 0 (%) | 18 (100%) | 1.0 NS | 0–6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majer, D.; Stasiowski, M.J.; Lyssek-Boroń, A.; Krysik, K.; Zmarzły, N. Adequacy of Anesthesia Guidance Combined with Peribulbar Blocks Shows Potential Benefit in High-Risk PONV Patients Undergoing Vitreoretinal Surgeries. J. Clin. Med. 2025, 14, 8081. https://doi.org/10.3390/jcm14228081
Majer D, Stasiowski MJ, Lyssek-Boroń A, Krysik K, Zmarzły N. Adequacy of Anesthesia Guidance Combined with Peribulbar Blocks Shows Potential Benefit in High-Risk PONV Patients Undergoing Vitreoretinal Surgeries. Journal of Clinical Medicine. 2025; 14(22):8081. https://doi.org/10.3390/jcm14228081
Chicago/Turabian StyleMajer, Dominika, Michał J. Stasiowski, Anita Lyssek-Boroń, Katarzyna Krysik, and Nikola Zmarzły. 2025. "Adequacy of Anesthesia Guidance Combined with Peribulbar Blocks Shows Potential Benefit in High-Risk PONV Patients Undergoing Vitreoretinal Surgeries" Journal of Clinical Medicine 14, no. 22: 8081. https://doi.org/10.3390/jcm14228081
APA StyleMajer, D., Stasiowski, M. J., Lyssek-Boroń, A., Krysik, K., & Zmarzły, N. (2025). Adequacy of Anesthesia Guidance Combined with Peribulbar Blocks Shows Potential Benefit in High-Risk PONV Patients Undergoing Vitreoretinal Surgeries. Journal of Clinical Medicine, 14(22), 8081. https://doi.org/10.3390/jcm14228081






