Anticoagulation in Frail Older Adults with Non-Valvular Atrial Fibrillation: Clinical Challenges and Personalized Approach
Abstract
1. Introduction
2. Methods
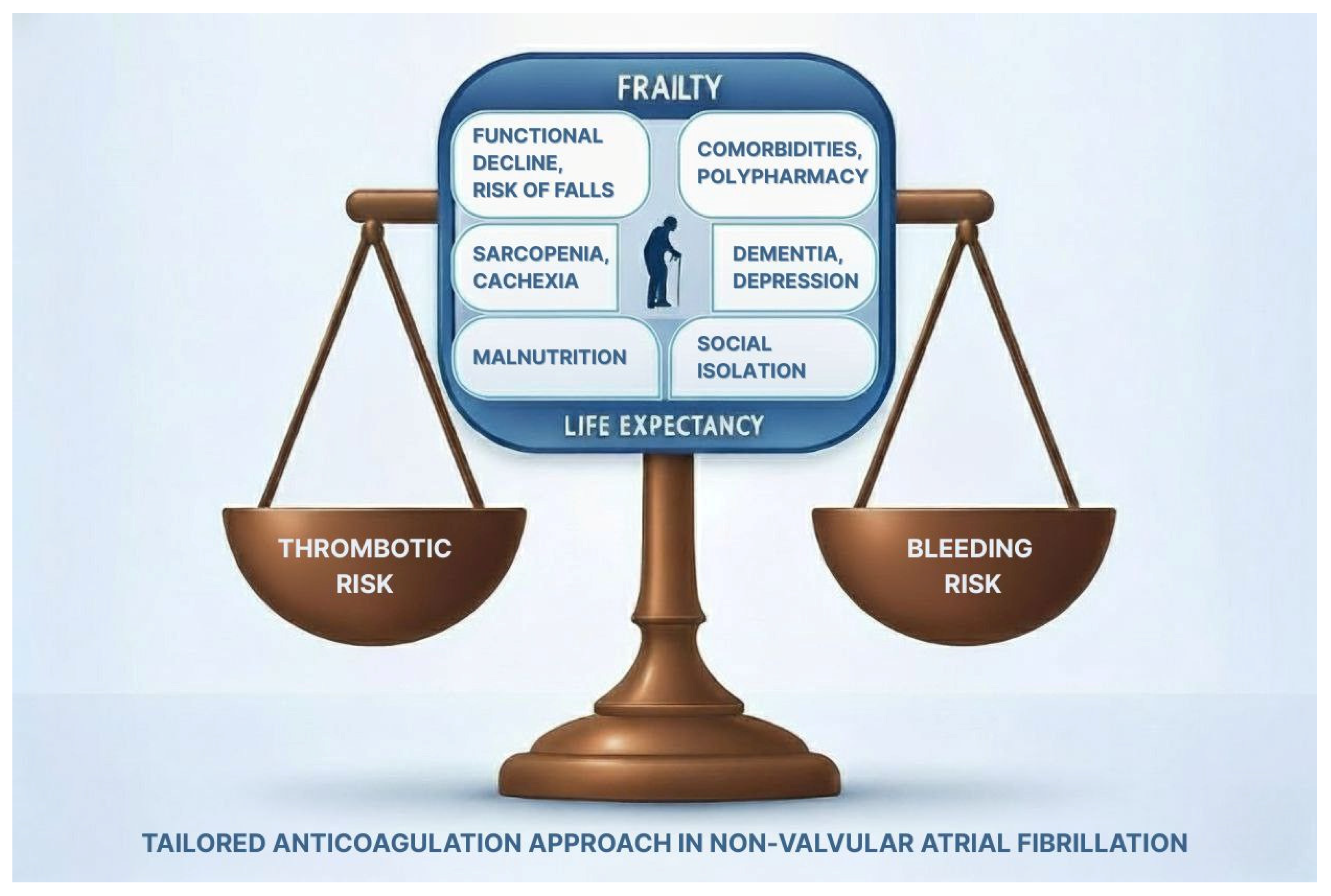
3. Frailty: Definition and Assessment
4. Anticoagulation in Frail Older Adults with Atrial Fibrillation
| First Author, Year | Country | Study Population | Mean Age | Frailty Definition | Main Results | Conclusion |
|---|---|---|---|---|---|---|
| Kim, 2022 [37] | Korea | 83,635 frail patients with AF
| 78.5 years old | ≥5 Hospital Frailty Risk Score | OAC use vs. non-use Outcome HR (95% CI): NACE 0.70 (0.66–0.74) Ischemic Stroke 0.91 (0.85–0.97) Major bleeding 1.05 (0.99–1.11) CV death 0.40 (0.36–0.44) | OAC treatment was associated with a positive net clinical outcome. |
| Søgaard, 2024 [38] | Denmark | 36,223 frail patients with AF
| 79 years old | ≥5 Hospital Frailty Risk Score | NCB 0.70% (95% CI, 0.32–1.08%) | The NCB was in favor of anticoagulation, but decreased with advancing age and increasing frailty. |
5. Bleeding Risk and Integrated Approach in Older Adults with AF
6. Selection of the Anticoagulant Agent
6.1. Vitamin K Antagonists (VKAs)
6.2. Direct Oral Anticoagulants (DOACs)
| First Author, Year | Study Design | Study Population | Frailty Definition | Results |
|---|---|---|---|---|
| Martinez, 2018 [99] | Retrospective study | Three cohorts of frail patients:
| Johns Hopkins Claims-based Frailty Indicator score ≥0.20 | Rivaroxaban, but not apixaban or dabigatran, was associated with a significant hazard reduction of stroke or systemic embolism compared with warfarin. No significant differences in major bleeding were found between DOAS and warfarin. |
| Wilkinson, 2020 [98] | Post hoc analysis of the ENGAGE TIMI 48 RCT | 20,867 patients randomized at edoxaban vs. warfarin | Frailty index
| Edoxaban was associated with similar efficacy to warfarin in every frailty category and was associated with lower rates of bleeding except in those with severe frailty. |
| Kim, 2021 [100] | Retrospective cohort study | 3 cohorts of dabigatran (n = 81,863) vs. warfarin (n = 256,722); rivaroxaban (n = 185,011) vs. warfarin (n = 228,028); apixaban (n = 222,478) vs. warfarin (n = 206,031) | Claims-based frailty index
| Apixaban was associated with lower rates of the composite endpoint of death, ischemic stroke, or major bleeding across all frailty levels. Dabigatran and rivaroxaban were associated with lower event rates only among nonfrail patients. This beneficial association for apixaban vs. warfarin in the frail subgroup appeared to be mainly driven by a large reduction in major bleeding. |
| Lip, 2021 [101] | Subgroup analysis of the ARISTOPHANES study (retrospective study) | 150,847 patients, grouped into six cohorts:
| Claims-based frailty index ≥ 0.20 | DOAC–warfarin comparison Apixaban and rivaroxaban were associated with a lower risk of stroke/systemic embolism compared with warfarin. Regarding major bleeding, apixaban and dabigatran were associated with a lower risk, while rivaroxaban was associated with a higher risk compared with warfarin. DOAC–DOAC comparison Regarding stroke and systemic embolisms, apixaban presented a similar risk compared with dabigatran and a lower risk compared with rivaroxaban, while dabigatran was associated with a similar risk compared with rivaroxaban. Regarding major and gastrointestinal bleeding, apixaban was associated with a lower risk compared with dabigatran and rivaroxaban, while dabigatran was associated with a lower risk compared with rivaroxaban. Overall, apixaban was the OAC with the best safety profile. |
| Zheng, 2022 [104] | Meta-analysis of four studies [26,27,28,29] | A total of 835,520 patients | Compared with warfarin, DOACs were significantly associated with reduced risks of
| |
| Lin, 2023 [102] | Retrospective cohort study | 45,950 apixaban, 45,320 rivaroxaban, and 45,281 warfarin initiators | claims-based frailty index
| Apixaban was associated with lower rates of the composite endpoint of ischemic stroke, systemic embolism, major bleeding, or death than rivaroxaban and warfarin, especially for those with frailty |
| Søgaard, 2024 [103] | Retrospective cohort study | 32,048 frail patients, divided into three groups
| Hospital Frailty Risk Score ≥ 5 | A similar thromboembolic risk was found between DOACs (either standard or reduced dose) and warfarin. Major bleeding was significantly lower with both standard and reduced DOAC doses compared with warfarin. |
7. Appropriate Dose
8. Switching from VKAs to DOACs
9. Knowledge Gaps and Future Directions
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AF | Atrial Fibrillation |
| DOACs | Direct Oral Anticoagulants |
| VKAs | Vitamin K Antagonists |
References
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.; Franco, O.H.; Hofman, A.; Witteman, J.C.; Stricker, B.H.; Heeringa, J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef]
- Khurshid, S.; Ashburner, J.M.; Ellinor, P.T.; McManus, D.D.; Atlas, S.J.; Singer, D.E.; Lubitz, S.A. Prevalence and Incidence of Atrial Fibrillation Among Older Primary Care Patients. JAMA Netw. Open 2023, 6, e2255838. [Google Scholar] [CrossRef]
- Testa, C.; Salvi, M.; Zucchini, I.; Cattabiani, C.; Giallauria, F.; Petraglia, L.; Leosco, D.; Lauretani, F.; Maggio, M. Atrial Fibrillation as a Geriatric Syndrome: Why Are Frailty and Disability Often Confused? A Geriatric Perspective from the New Guidelines. Int. J. Environ. Res. Public. Health 2025, 22, 179. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Rockwood, K. Frailty in Older Adults. N. Engl. J. Med. 2024, 391, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Du, X.; Ma, C.S. Atrial fibrillation and frailty. J. Geriatr. Cardiol. 2020, 17, 105–109. [Google Scholar] [CrossRef]
- Proietti, M.; Romiti, G.F.; Raparelli, V.; Diemberger, I.; Boriani, G.; Dalla Vecchia, L.A.; Bellelli, G.; Marzetti, E.; Lip, G.Y.; Cesari, M. Frailty prevalence and impact on outcomes in patients with atrial fibrillation: A systematic review and meta-analysis of 1,187,000 patients. Ageing Res. Rev. 2022, 79, 101652. [Google Scholar] [CrossRef]
- Trevisan, C.; Ceolin, C.; Vetrano, D.L.; Petrovic, M.; Lip, G.Y.H.; Buchan, I.; Rui, M.; Sergi, G.; Maggi, S.; Noale, M.; et al. Frailty increases the risk of hospitalization for atrial fibrillation in older adults: A population-based cohort study. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 104159. [Google Scholar] [CrossRef] [PubMed]
- Candeloro, M.; Di Nisio, M.; Potere, N.; Di Pizio, L.; Secinaro, E.; De Flaviis, C.; Federici, C.; Guglielmi, M.D.; Pardi, S.; Schulman, S.; et al. Frailty phenotype as a predictor of bleeding and mortality in ambulatory patients receiving direct oral anticoagulants. J. Am. Geriatr. Soc. 2022, 70, 3503–3512. [Google Scholar] [CrossRef]
- Soler-Espejo, E.; Zazo-Luengo, B.Á.; Rivera-Caravaca, J.M.; López-Gávez, R.; Esteve-Pastor, M.A.; Lip, G.Y.H.; Marín, F.; Roldán, V. Poor clinical outcomes associated to multimorbidity, frailty and malnutrition in patients with atrial fibrillation. J. Nutr. Health Aging 2025, 29, 100430. [Google Scholar] [CrossRef]
- Okoye, C.; Qiu, C.; Xia, X.; Lip, G.Y.H.; Bellelli, G.; Welmer, A.K.; Calderón-Larrañaga, A.; Vetrano, D.L. Atrial fibrillation accelerates functional decline in older adults: A 15-year follow-up population-based study. Europace 2024, 26, euae173. [Google Scholar] [CrossRef]
- Oqab, Z.; Pournazari, P.; Sheldon, R.S. What is the Impact of Frailty on Prescription of Anticoagulation in Elderly Patients with Atrial Fibrillation? A Systematic Review and Meta-Analysis. J. Atr. Fibrillation 2018, 10, 1870. [Google Scholar] [CrossRef]
- Proietti, M.; Romiti, G.F.; Vitolo, M.; Harrison, S.L.; Lane, D.A.; Fauchier, L.; Marin, F.; Näbauer, M.; Potpara, T.S.; Dan, G.A.; et al. Epidemiology and impact of frailty in patients with atrial fibrillation in Europe. Age Ageing 2022, 51, afac192. [Google Scholar] [CrossRef]
- Orlandi, M.; Dover, D.C.; Sandhu, R.K.; Hawkins, N.M.; Kaul, P.; McAlister, F.A. The Introduction of Direct Oral Anticoagulants Has Not Resolved Treatment Gaps for Frail Patients with Nonvalvular Atrial Fibrillation. Can. J. Cardiol. 2022, 38, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Romiti, G.F.; Proietti, M.; Bonini, N.; Ding, W.Y.; Boriani, G.; Huisman, M.V.; Lip, G.Y.H. GLORIA-AF Investigators. Clinical Complexity Domains Anticoagulation Outcomes in Patients with Atrial Fibrillation: A Report from the GLORIA-AF Registry Phase II and III. Thromb. Haemost. 2022, 122, 2030–2041. [Google Scholar] [CrossRef]
- Ko, D.; Lin, K.J.; Bessette, L.G.; Lee, S.B.; Walkey, A.J.; Cheng, S.; Kim, E.; Glynn, R.J.; Kim, D.H. Trends in Use of Oral Anticoagulants in Older Adults with Newly Diagnosed Atrial Fibrillation, 2010–2020. JAMA Netw. Open 2022, 5, e2242964. [Google Scholar] [CrossRef] [PubMed]
- Díez-Villanueva, P.; Cosín-Sales, J.; Roldán-Schilling, V.; Barrios, V.; Riba-Artés, D.; Gavín-Sebastián, O. RE_BELD Spanish Investigator’s Group. Use of Direct Acting Oral Anticoagulants in Elderly Patients with Atrial Fibrillation: A Multicenter, Cross-Sectional Study in Spain. J. Clin. Med. 2023, 12. [Google Scholar] [CrossRef]
- Bucci, T.; Romiti, G.F.; Ishiguchi, H.; Gerra, L.; Mantovani, M.; Huang, B.; Proietti, M.; Lip, G.Y.H. Adverse events in clinically complex elderly patients with atrial fibrillation according to oral anticoagulation status. eClinicalMedicine 2024, 78, 102974. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chao, K.; Wang, Z.; Xue, R.; Zhang, X.; Wang, D. Accelerated biological aging and risk of atrial fibrillation: A cohort study. Heart Rhythm. 2025, 22, 2507–2514. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Mitnitski, A.; Song, X.; Steen, B.; Skoog, I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J. Am. Geriatr. Soc. 2006, 54, 975–979. [Google Scholar] [CrossRef]
- Rockwood, K.; Theou, O. Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources. Can. Geriatr. J. 2020, 23, 210–215. [Google Scholar] [CrossRef]
- Parks, A.L.; Frankel, D.S.; Kim, D.H.; Ko, D.; Kramer, D.B.; Lydston, M.; Fang, M.C.; Shah, S.J. Management of atrial fibrillation in older adults. BMJ 2024, 386, e076246. [Google Scholar] [CrossRef] [PubMed]
- Umegaki, H. Frailty, multimorbidity, and polypharmacy: Proposal of the new concept of the geriatric triangle. Geriatr. Gerontol. Int. 2025, 25, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.; Lucerna, M.; Pecen, L.; Siller-Matula, J.M.; Cavallari, I.; Kirchhof, P.; De Caterina, R. Thromboembolic Risk, Bleeding Outcomes and Effect of Different Antithrombotic Strategies in Very Elderly Patients with Atrial Fibrillation: A Sub-Analysis From the PREFER in AF (PREvention oF Thromboembolic Events-European Registry in Atrial Fibrillation). J. Am. Heart Assoc. 2017, 6, e005657. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156, Erratum in Circulation 2024, 149, e167. https://doi.org/10.1161/CIR.0000000000001207. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Bonanad, C.; Formiga, F.; Anguita, M.; Petidier, R.; Gullón, A. Oral Anticoagulant Use and Appropriateness in Elderly Patients with Atrial Fibrillation in Complex Clinical Conditions: ACONVENIENCE Study. J. Clin. Med. 2022, 11, 7423. [Google Scholar] [CrossRef]
- Pundi, K.; Perino, A.C.; Fan, J.; Din, N.; Szummer, K.; Heidenreich, P.; Turakhia, M.P. Association of CHA2DS2-VASc and HAS-BLED to frailty and frail outcomes: From the TREAT-AF study. Am. Heart J. 2023, 261, 85–94. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Rasmussen, P.V.; Sakthivel, T.; Dalgaard, F.; Gislason, G.H.; Pallisgaard, J.L.; Hansen, M.L. Treatment patterns for oral anticoagulants in older patients with atrial fibrillation: A retrospective, cross-sectional, nationwide study from Denmark. BMJ Open 2022, 12, e062353. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Igras, E.; Ramesh, A.; Power, B.; O’Connor, K.; Liston, R. Assessing the Appropriateness of Oral Anticoagulation for Atrial Fibrillation in Advanced Frailty: Use of Stroke and Bleeding Risk-Prediction Models. J. Frailty Aging 2017, 6, 46–52. [Google Scholar] [CrossRef]
- Shah, S.J.; Singer, D.E.; Fang, M.C.; Reynolds, K.; Go, A.S.; Eckman, M.H. Net Clinical Benefit of Oral Anticoagulation Among Older Adults with Atrial Fibrillation. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e006212. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.S.; Sung, J.H.; Jang, E.; Yu, H.T.; Kim, T.H.; Uhm, J.S.; Kim, J.Y.; Pak, H.N.; Lee, M.H.; et al. Effectiveness and Safety of Anticoagulation Therapy in Frail Patients with Atrial Fibrillation. Stroke 2022, 53, 1873–1882, Erratum in Stroke 2022, 53, e533. https://doi.org/10.1161/STR.0000000000000421. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, M.; Jensen, M.; Højen, A.A.; Larsen, T.B.; Lip, G.Y.H.; Ording, A.G.; Nielsen, P.B. Net Clinical Benefit of Oral Anticoagulation Among Frail Patients with Atrial Fibrillation: Nationwide Cohort Study. Stroke 2024, 55, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, E.; Presta, R.; Okoye, C.; Filippini, C.; Raspo, S.; Bruno, G.; Marabotto, M.; Monzani, F.; Bo, M. Predictors and Outcomes of Oral Anticoagulant Deprescribing in Geriatric Inpatients with Atrial Fibrillation: A Retrospective Multicenter Cohort Study. J. Am. Med. Dir. Assoc. 2024, 25, 545–551.e4. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Camera, M.; Gallieni, M.; Gianturco, L.; Gidaro, A.; Piemontese, C.; Pizzetti, G.; Redaelli, F.; Scimeca, B.; Tadeo, C.S.; et al. Use and Prescription of Direct Oral Anticoagulants in Older and Frail Patients with Atrial Fibrillation: A Multidisciplinary Consensus Document. J. Pers. Med. 2022, 12, 469. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Nadarajah, R.; Nakao, Y.M.; Nakao, K.; Wilkinson, C.; Mamas, M.A.; Camm, A.J.; Gale, C.P. Temporal trends and patterns in atrial fibrillation incidence: A population-based study of 3·4 million individuals. Lancet Reg. Health Eur. 2022, 17, 100386. [Google Scholar] [CrossRef]
- Proietti, M.; Marzona, I.; Vannini, T.; Tettamanti, M.; Fortino, I.; Merlino, L.; Basili, S.; Mannucci, P.M.; Boriani, G.; Lip, G.Y.H.; et al. Long-Term Relationship Between Atrial Fibrillation, Multimorbidity and Oral Anticoagulant Drug Use. Mayo Clin. Proc. 2019, 94, 2427–2436. [Google Scholar] [CrossRef]
- Proietti, M.; Esteve-Pastor, M.A.; Rivera-Caravaca, J.M.; Roldán, V.; Roldán Rabadán, I.; Muñiz, J.; Cequier, Á.; Bertomeu-Martínez, V.; Badimón, L.; Anguita, M.; et al. Relationship between multimorbidity and outcomes in atrial fibrillation. Exp. Gerontol. 2021, 153, 111482. [Google Scholar] [CrossRef]
- Rasmussen, P.V.; Pallisgaard, J.L.; Hansen, M.L.; Gislason, G.H.; Torp-Pedersen, C.; Ruwald, M.; Alexander, K.P.; Lopes, R.D.; Al-Khatib, S.M.; Dalgaard, F. Treatment of older patients with atrial fibrillation by morbidity burden. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 23–30. [Google Scholar] [CrossRef]
- Kumar, M.; Lopetegui-Lia, N.; Malouf, C.A.; Almnajam, M.; Coll, P.P.; Kim, A.S. Atrial fibrillation in older adults with cancer. J. Geriatr. Cardiol. 2022, 19, 1–8. [Google Scholar] [CrossRef]
- Ding, W.Y.; Potpara, T.S.; Blomström-Lundqvist, C.; Boriani, G.; Marin, F.; Fauchier, L.; Lip, G.Y.H. ESC-EHRA EORP-AF Long-Term General Registry Investigators. Impact of renal impairment on atrial fibrillation: ESC-EHRA EORP-AF Long-Term General Registry. Eur. J. Clin. Investig. 2022, 52, e13745. [Google Scholar] [CrossRef] [PubMed]
- Westenbrink, B.D.; Alings, M.; Connolly, S.J.; Eikelboom, J.; Ezekowitz, M.D.; Oldgren, J.; Yang, S.; Pongue, J.; Yusuf, S.; Wallentin, L.; et al. Anemia predicts thromboembolic events, bleeding complications and mortality in patients with atrial fibrillation: Insights from the RE-LY trial. J. Thromb. Haemost. 2015, 13, 699–707. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Liu, X.; Lip, G.Y.H.; Guo, L.; Zhu, W. Effect of Oral Anticoagulants in Atrial Fibrillation Patients with Polypharmacy: A Meta-analysis. Thromb. Haemost. 2025, 125, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Amrouch, C.; Vetrano, D.L.; Damiano, C.; Dai, L.; Calderón-Larrañaga, A.; Grymonprez, M.; Proietti, M.; Lip, G.Y.H.; Johnsen, S.P.; Wastesson, J.W.; et al. Potentially inappropriate prescribing in polymedicated older adults with atrial fibrillation and multimorbidity: A Swedish national register-based cohort study. Front. Pharmacol. 2024, 15, 1476464. [Google Scholar] [CrossRef]
- Rivard, L.; Friberg, L.; Conen, D.; Healey, J.S.; Berge, T.; Boriani, G.; Brandes, A.; Calkins, H.; Camm, A.J.; Yee Chen, L.; et al. Atrial Fibrillation and Dementia: A Report From the AF-SCREEN International Collaboration. Circulation 2022, 145, 392–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gu, Z.C.; Shen, L.; Pan, M.M.; Yan, Y.D.; Pu, J.; Liu, X.Y.; Lin, H.W. Non-vitamin K Antagonist Oral Anticoagulants and Cognitive Impairment in Atrial Fibrillation: Insights From the Meta-Analysis of Over 90,000 Patients of Randomized Controlled Trials and Real-World Studies. Front. Aging Neurosci. 2018, 10, 258. [Google Scholar] [CrossRef]
- Lee, S.R.; Choi, E.K.; Park, S.H.; Jung, J.H.; Han, K.D.; Oh, S.; Lip, G.Y.H. Comparing Warfarin and 4 Direct Oral Anticoagulants for the Risk of Dementia in Patients with Atrial Fibrillation. Stroke 2021, 52, 3459–3468. [Google Scholar] [CrossRef]
- Agarwal, A.; Mostafa, M.A.; Ahmad, M.I.; Soliman, E.Z. Exploring the Link between Anticoagulation, Cognitive Impairment and Dementia in Atrial Fibrillation: A Systematic Review. J. Clin. Med. 2024, 13, 2418. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Yeh, J.T.; Wu, M.L.; Yeh, W.Y.; Lip, G.Y.H.; Chiang, C.E.; Chen, C.H.; Cheng, H.M. Oral anticoagulants and cognitive impairment in patients with atrial fibrillation: A systematic review with meta-analysis and trial sequential analysis. Thromb. Res. 2024, 238, 132–140. [Google Scholar] [CrossRef]
- Presented by Dr. Lena Rivard at the American Heart Association Scientific Sessions, Chicago, IL, USA, 16 November 2024. Available online: https://www.acc.org/latest-in-cardiology/clinical-trials/2024/11/15/15/17/brain-af (accessed on 9 November 2025).
- Rocheeld, J.; Kusayev, J.; Frishman, W.H.; Aronow, W.S. Anticoagulation in Atrial Fibrillation and Dementia. Cardiol Rev. 2025. [Google Scholar] [CrossRef]
- Pino, M.D.; Rivero, P.; Taylor, A.; Gabriel, R. Impact of depression and cardiovascular risk factors on cognitive impairment in patients with atrial fibrillation: A Systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2025, 128, 105601. [Google Scholar] [CrossRef] [PubMed]
- Lapa, M.E.; Swabe, G.M.; Magnani, J.W. Association of Depression and Adherence to Oral Anticoagulation in Patients with Atrial Fibrillation. J. Am. Heart Assoc. 2023, 12, e031281. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, Y.; Ge, T.; Liu, Y.; Chen, Y.; Niu, G.; Yuan, Y. Association of Social Disconnection with the Incidence and Prognosis of Atrial Fibrillation: A Multistate Analysis. J. Am. Heart Assoc. 2025, 14, e039885. [Google Scholar] [CrossRef]
- Ceolin, C.; Mizzon, E.; Noale, M.; Ravelli, A.; Pigozzo, S.; Curreri, C.; Zanforlini, B.M.; Manzato, E.; Coin, A.; Devita, M.; et al. The Impact of Atrial Fibrillation on Physical Performance in Older Adults: A Longitudinal Study in Relation to Cognitive Function. J. Am. Med. Dir. Assoc. 2025, 26, 105764. [Google Scholar] [CrossRef] [PubMed]
- Romiti, G.F.; Corica, B.; Bucci, T.; Boriani, G.; Olshansky, B.; Chao, T.F.; Huisman, M.V.; Proietti, M.; Lip, G.Y.H. GLORIA-AF Investigators. History of falls in patients with atrial fibrillation and risk of major outcomes: Analysis from the Prospective GLORIA-AF Registry. Geroscience 2025. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, S.; Xu, C.; Li, B.; Lai, Y.; Zhan, R.; Guo, Y.; Ma, Y.; Liao, X.; Wu, X.; et al. Malnutrition increases the risk of atrial fibrillation. BMC Cardiovasc. Disord. 2025, 25, 678. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, Y.; Ding, J.; Ma, Q.; Zhang, D.; Huang, W.; Xing, Y. Effect of nutritional status on adverse clinical events in elderly patients with nonvalvular atrial fibrillation: A retrospective cohort study. Ann. Noninvasive Electrocardiol. 2024, 29, e13130. [Google Scholar] [CrossRef]
- Lip, G.Y.H. The ABC pathway: An integrated approach to improve AF management. Nat. Rev. Cardiol. 2017, 14, 627–628. [Google Scholar] [CrossRef]
- Proietti, M.; Romiti, G.F.; Olshansky, B.; Lane, D.A.; Lip, G.Y.H. Improved Outcomes by Integrated Care of Anticoagulated Patients with Atrial Fibrillation Using the Simple ABC (Atrial Fibrillation Better Care) Pathway. Am. J. Med. 2018, 131, 1359–1366.e6. [Google Scholar] [CrossRef]
- Guo, Y.; Romiti, G.F.; Proietti, M.; Bonini, N.; Zhang, H.; Lip, G.Y.H. mAF-App II Trial Investigators. Mobile health technology integrated care in older atrial fibrillation patients: A subgroup analysis of the mAFA-II randomised clinical trial. Age Ageing 2022, 51, afac245. [Google Scholar] [CrossRef]
- Johnsen, S.P.; Proietti, M.; Maggioni, A.P.; Lip, G.Y.H. A multinational European network to implement integrated care in elderly multimorbid atrial fibrillation patients: The AFFIRMO Consortium. Eur. Heart J. 2022, 43, 2916–2918. [Google Scholar] [CrossRef] [PubMed]
- Heidbuchel, H.; Van Gelder, I.C.; Desteghe, L.; EHRA-PATHS Investigators. ESC and EHRA lead a path towards integrated care for multimorbid atrial fibrillation patients: The Horizon 2020 EHRA-PATHS project. Eur. Heart J. 2022, 43, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Stuby, J.; Haschke, M.; Tritschler, T.; Aujesky, D. Oral anticoagulant therapy in older adults. Thromb. Res. 2024, 238, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Spruit, J.R.; de Vries, T.A.C.; Hemels, M.E.W.; Pisters, R.; de Groot, J.R.; Jansen, R.W.M.M. Direct Oral Anticoagulants in Older and Frail Patients with Atrial Fibrillation: A Decade of Experience. Drugs Aging 2024, 41, 725–740. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Wallentin, L.; Connolly, S.J.; Ezekowitz, M.; Healey, J.S.; Oldgren, J.; Yang, S.; Alings, M.; Kaatz, S.; Hohnloser, S.H.; et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: An analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011, 123, 2363–2372. [Google Scholar] [CrossRef]
- Halperin, J.L.; Hankey, G.J.; Wojdyla, D.M.; Piccini, J.P.; Lokhnygina, Y.; Patel, M.R.; Breithardt, G.; Singer, D.E.; Becker, R.C.; Hacke, W.; et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014, 130, 138–146. [Google Scholar] [CrossRef]
- Kato, E.T.; Giugliano, R.P.; Ruff, C.T.; Koretsune, Y.; Yamashita, T.; Kiss, R.G.; Nordio, F.; Murphy, S.A.; Kimura, T.; Jin, J.; et al. Efficacy and Safety of Edoxaban in Elderly Patients with Atrial Fibrillation in the ENGAGE AF-TIMI 48 Trial. J. Am. Heart Assoc. 2016, 5, e003432. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, S.; Atar, D.; Yang, H.; De Caterina, R.; Erol, C.; Garcia, D.; Granger, C.B.; Hanna, M.; Held, C.; Husted, S.; et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: Observations from the ARISTOTLE trial. Eur. Heart J. 2014, 35, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Kim, H.J.; Kim, T.H.; Uhm, J.S.; Joung, B.; Lee, M.H.; Pak, H.N. Non-vitamin K antagonist oral anticoagulants have better efficacy and equivalent safety compared to warfarin in elderly patients with atrial fibrillation: A systematic review and meta-analysis. J. Cardiol. 2018, 72, 105–112. [Google Scholar] [CrossRef]
- Caldeira, D.; Nunes-Ferreira, A.; Rodrigues, R.; Vicente, E.; Pinto, F.J.; Ferreira, J.J. Non-vitamin K antagonist oral anticoagulants in elderly patients with atrial fibrillation: A systematic review with meta-analysis and trial sequential analysis. Arch Gerontol. Geriatr. 2019, 81, 209–214. [Google Scholar] [CrossRef]
- Malik, A.H.; Yandrapalli, S.; Aronow, W.S.; Panza, J.A.; Cooper, H.A. Meta-Analysis of Direct-Acting Oral Anticoagulants Compared with Warfarin in Patients >75 Years of Age. Am. J. Cardiol. 2019, 123, 2051–2057. [Google Scholar] [CrossRef]
- Deng, K.; Cheng, J.; Rao, S.; Xu, H.; Li, L.; Gao, Y. Efficacy and Safety of Direct Oral Anticoagulants in Elderly Patients with Atrial Fibrillation: A Network Meta-Analysis. Front. Med. 2020, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Bonanad, C.; García-Blas, S.; Torres Llergo, J.; Fernández-Olmo, R.; Díez-Villanueva, P.; Ariza-Solé, A.; Martínez-Sellés, M.; Raposeiras, S.; Ayesta, A.; Bertomeu-González, V.; et al. Direct Oral Anticoagulants versus Warfarin in Octogenarians with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 5268. [Google Scholar] [CrossRef]
- Carnicelli, A.P.; Hong, H.; Connolly, S.J.; Eikelboom, J.; Giugliano, R.P.; Morrow, D.A.; Patel, M.R.; Wallentin, L.; Alexander, J.H.; Cecilia Bahit, M.; et al. Direct Oral Anticoagulants Versus Warfarin in Patients with Atrial Fibrillation: Patient-Level Network Meta-Analyses of Randomized Clinical Trials with Interaction Testing by Age and Sex. Circulation 2022, 145, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Chen, Y.F.; Yeh, W.Y.; Yeh, J.T.; Yang, T.H.; Chou, C.Y.; Chang, Y.L.; Wang, W.T.; Chiang, C.E.; Chen, C.H.; et al. Optimal stroke preventive strategy for patients aged 80 years or older with atrial fibrillation: A systematic review with traditional and network meta-analysis. Age Ageing 2022, 51, afac292. [Google Scholar] [CrossRef]
- Lin, D.S.; Lo, H.Y.; Huang, K.C.; Lin, T.T.; Lee, J.K. Efficacy and Safety of Direct Oral Anticoagulants for Stroke Prevention in Older Patients with Atrial Fibrillation: A Network Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2023, 12, e030380. [Google Scholar] [CrossRef] [PubMed]
- Doni, K.; Bühn, S.; Weise, A.; Mann, N.K.; Hess, S.; Sönnichsen, A.; Salem, S.; Pieper, D.; Thürmann, P.; Mathes, T. Safety outcomes of direct oral anticoagulants in older adults with atrial fibrillation: A systematic review and meta-analysis of (subgroup analyses from) randomized controlled trials. Geroscience 2024, 46, 923–944. [Google Scholar] [CrossRef]
- Deitelzweig, S.; Keshishian, A.; Li, X.; Kang, A.; Dhamane, A.D.; Luo, X.; Balachander, N.; Rosenblatt, L.; Mardekian, J.; Pan, X.; et al. Comparisons between oral anticoagulants among older nonvalvular atrial fibrillation patients. J. Am. Geriatr. Soc. 2019, 67, 1662–1671. [Google Scholar] [CrossRef]
- Lau, W.C.; Torre, C.O.; Man, K.K.; Stewart, H.M.; Seager, S.; Van Zandt, M.; Reich, C.; Li, J.; Brewster, J.; Lip, G.Y.; et al. Comparative effectiveness and safety between apixaban, dabigatran, edoxaban, and rivaroxaban among patients with atrial fibrillation: A multinational population-based cohort study. Ann. Intern. Med. 2022, 175, 1515–1524. [Google Scholar] [CrossRef]
- Yamashita, T.; Suzuki, S.; Inoue, H.; Akao, M.; Atarashi, H.; Ikeda, T.; Okumura, K.; Koretsune, Y.; Shimizu, W.; Tsutsui, H.; et al. Two-year outcomes of more than 30 000 elderly patients with atrial fibrillation: Results from the All Nippon AF In the Elderly (ANAFIE) Registry. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 202–213. [Google Scholar] [CrossRef]
- Lee, J.Z. Anticoagulation in Atrial Fibrillation Patients with Frailty. JACC Case Rep. 2024, 29 (Suppl. S22), 102687. [Google Scholar] [CrossRef]
- Chiv, R.; Beradid, S.; Suissa, S.; Renoux, C. Effectiveness and Safety of Edoxaban Compared with Apixaban in Elderly Patients with Nonvalvular Atrial Fibrillation: A Real-World Population-Based Cohort Study. Stroke 2024, 55, 1161–1170. [Google Scholar] [CrossRef]
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2023, 71, 2052–2081. [Google Scholar] [CrossRef]
- Alshehri, A.M.; Alfehaid, L.; Alhawas, S.; Alsuwaylihi, A.; Alarifi, A.; Al Yami, M.S. Comparison of Safety and Effectiveness Between Direct Oral Anticoagulants and Vitamin K Antagonists in Dementia Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 5758. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.P.; Brouwer, M.A.; Mulder, H.; Vinereanu, D.; Lopes, R.D.; Proietti, M.; Al-Khatib, S.M.; Hijazi, Z.; Halvorsen, S.; Hylek, E.M.; et al. Outcomes of apixaban versus warfarin in patients with atrial fibrillation multi-morbidity: Insights from the ARISTOTLE trial. Am. Heart J. 2019, 208, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Jaspers Focks, J.; Brouwer, M.A.; Wojdyla, D.M.; Thomas, L.; Lopes, R.D.; Washam, J.B.; Lanas, F.; Xavier, D.; Husted, S.; Wallentin, L.; et al. Polypharmacy and effects of apixaban versus warfarin in patients with atrial fibrillation: Post hoc analysis of the ARISTOTLE trial. BMJ 2016, 353, i2868. [Google Scholar] [CrossRef]
- Steffel, J.; Giugliano, R.P.; Braunwald, E.; Murphy, S.A.; Mercuri, M.; Choi, Y.; Aylward, P.; White, H.; Zamorano, J.L.; Antman, E.M.; et al. Edoxaban Versus Warfarin in Atrial Fibrillation Patients at Risk of Falling: ENGAGE AF-TIMI 48 Analysis. J. Am. Coll. Cardiol. 2016, 68, 1169–1178. [Google Scholar] [CrossRef]
- Wilkinson, C.; Wu, J.; Searle, S.D.; Todd, O.; Hall, M.; Kunadian, V.; Clegg, A.; Rockwood, K.; Gale, C.P. Clinical outcomes in patients with atrial fibrillation and frailty: Insights from the ENGAGE AF-TIMI 48 trial. BMC Med. 2020, 18, 401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinez, B.K.; Sood, N.A.; Bunz, T.J.; Coleman, C.I. Effectiveness and Safety of Apixaban, Dabigatran, and Rivaroxaban Versus Warfarin in Frail Patients with Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2018, 7, e008643. [Google Scholar] [CrossRef]
- Kim, D.H.; Pawar, A.; Gagne, J.J.; Bessette, L.G.; Lee, H.; Glynn, R.J.; Schneeweiss, S. Frailty and Clinical Outcomes of Direct Oral Anticoagulants Versus Warfarin in Older Adults with Atrial Fibrillation: A Cohort Study. Ann. Intern. Med. 2021, 174, 1214–1223. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Keshishian, A.V.; Kang, A.L.; Dhamane, A.D.; Luo, X.; Li, X.; Balachander, N.; Rosenblatt, L.; Mardekian, J.; Pan, X.; et al. Oral anticoagulants for nonvalvular atrial fibrillation in frail elderly patients: Insights from the ARISTOPHANES study. J. Intern. Med. 2021, 289, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.J.; Singer, D.E.; Ko, D.; Glynn, R.; Najafzadeh, M.; Lee, S.B.; Bessette, L.G.; Cervone, A.; DiCesare, E.; Kim, D.H. Frailty, Home Time, and Health Care Costs in Older Adults with Atrial Fibrillation Receiving Oral Anticoagulants. JAMA Netw. Open 2023, 6, e2342264. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, M.; Ording, A.G.; Skjøth, F.; Larsen, T.B.; Nielsen, P.B. Effectiveness and safety of direct oral anticoagulation vs. warfarin in frail patients with atrial fibrillation. Eur. Heart J. Cardiovasc. Pharmacother. 2024, 10, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zheng, Y.; Jiang, J.; Ma, J.; Zhu, W.; Cai, X. Effectiveness and Safety of DOACs vs. Warfarin in Patients with Atrial Fibrillation and Frailty: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 907197. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Sharma, M.; Cohen, A.T.; Demchuk, A.M.; Członkowska, A.; Lindgren, A.G.; Molina, C.A.; Bereczki, D.; Toni, D.; Seiffge, D.J.; et al. Andexanet for Factor Xa Inhibitor-Associated Acute Intracerebral Hemorrhage. N. Engl. J. Med. 2024, 390, 1745–1755. [Google Scholar] [CrossRef]
- Gembillo, G.; Soraci, L.; Santoro, D. Chronic kidney disease in geriatric patients: Estimating glomerular filtration rate in older patients with comorbidities. World J. Nephrol. 2025, 14, 105803. [Google Scholar] [CrossRef]
- Eidam, A.; Marji, J.; Benzinger, P.; Foerster, K.I.; Burhenne, J.; Czock, D.; Stoll, F.; Blank, A.; Mikus, G.; Haefeli, W.E.; et al. Frailty as a Marker for the Plasma Concentrations of Direct Oral Anticoagulants in Older Patients: Results of an Exploratory Study. Drugs Aging 2023, 40, 153–164. [Google Scholar] [CrossRef]
- Bendayan, M.; Mardigyan, V.; Williamson, D.; Chen-Tournoux, A.; Eintracht, S.; Rudski, L.; MacNamara, E.; Blostein, M.; Afilalo, M.; Afilalo, J. Muscle Mass and Direct Oral Anticoagulant Activity in Older Adults with Atrial Fibrillation. J. Am. Geriatr. Soc. 2021, 69, 1012–1018. [Google Scholar] [CrossRef]
- Sanghai, S.; Wong, C.; Wang, Z.; Clive, P.; Tran, W.; Waring, M.; Goldberg, R.; Hayward, R.; Saczynski, J.S.; McManus, D.D. Rates of Potentially Inappropriate Dosing of Direct-Acting Oral Anticoagulants and Associations with Geriatric Conditions Among Older Patients with Atrial Fibrillation: The SAGE-AF Study. J. Am. Heart Assoc. 2020, 9, e014108. [Google Scholar] [CrossRef]
- Decaix, T.; Kemache, K.; Gay, P.; Laprévote, O.; Ketz, F.; Pautas, É. Prevalence and factors associated with inappropriate dosing of apixaban and rivaroxaban in hospitalized older adults with atrial fibrillation: A cross-sectional study. Drugs Aging 2024, 41, 55–64. [Google Scholar] [CrossRef]
- De Vincentis, A.; Soraci, L.; Arena, E.; Sciacqua, A.; Armentaro, G.; Aucella, F.; Corsonello, A.; Aucella, F.; Antonelli Incalzi, R. Appropriateness of direct oral anticoagulant prescribing in older subjects with atrial fibrillation discharged from acute medical wards. Br. J. Clin. Pharmacol. 2024, 90, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Guenoun, M.; Cohen, S.; Villaceque, M.; Sharareh, A.; Schwartz, J.; Hoffman, O.; Dib, J.C.; Ouazana, L.; Assouline, S.; Parrens, E.; et al. Characteristics of patients with atrial fibrillation treated with direct oral anticoagulants and new insights into inappropriate dosing: Results from the French National Prospective Registry: PAFF. Europace 2023, 25, euad302. [Google Scholar] [CrossRef]
- Akao, M.; Inoue, H.; Yamashita, T.; Atarashi, H.; Ikeda, T.; Koretsune, Y.; Okumura, K.; Suzuki, S.; Tsutsui, H.; Toyoda, K.; et al. Relationship Between Direct Oral Anticoagulant Doses and Clinical Outcomes in Elderly Patients with Non-Valvular Atrial Fibrillation—ANAFIE Registry Sub-Analysis. Circ. J. 2023, 87, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.Q.; David, C.; Almeida, A.G.; Brito, D.; Pinto, F.J.; Caldeira, D. Clinical effects of off-label reduced doses of Direct Oral Anticoagulants: A systematic review and meta-analysis. Int. J. Cardiol. 2022, 362, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Sabbatinelli, J.; Protic, O.; Bonfigli, A.R.; Stronati, A.; Pavani, M.; Procopio, A.D.; Lattanzio, F.; Olivieri, F.; Antonicelli, R.; Testa, R. Safety and efficacy of direct oral anticoagulants in geriatric patients with non-valvular atrial fibrillation: A single-center retrospective study. Thromb. Res. 2023, 221, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, H.; Agasthi, P.; Shanbhag, A.; Mehta, R.A.; Rattanawong, P.; Allam, M.; Pujari, S.H.; Mookadam, F.; Freeman, W.K.; Srivathsan, K.; et al. Long-Term Clinical Outcomes of Underdosed Direct Oral Anticoagulants in Patients with Atrial Fibrillation and Atrial Flutter. Am. J. Med. 2021, 134, 788–796. [Google Scholar] [CrossRef]
- Alexander, J.H.; Andersson, U.; Lopes, R.D.; Hijazi, Z.; Hohnloser, S.H.; Ezekowitz, J.A.; Halvorsen, S.; Hanna, M.; Commerford, P.; Ruzyllo, W.; et al. Apixaban 5 mg Twice Daily and Clinical Outcomes in Patients with Atrial Fibrillation and Advanced Age, Low Body Weight, or High Creatinine: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2016, 1, 673–681. [Google Scholar] [CrossRef]
- Okumura, K.; Akao, M.; Yoshida, T.; Kawata, M.; Okazaki, O.; Akashi, S.; Eshima, K.; Tanizawa, K.; Fukuzawa, M.; Hayashi, T.; et al. Low-Dose Edoxaban in Very Elderly Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1735–1745. [Google Scholar] [CrossRef]
- Akashi, S.; Oguri, M.; Ikeno, E.; Manita, M.; Taura, J.; Watanabe, S.; Hayashi, T.; Akao, M.; Okumura, K.; Akishita, M.; et al. Outcomes and Safety of Very-Low-Dose Edoxaban in Frail Patients with Atrial Fibrillation in the ELDERCARE-AF Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2228500. [Google Scholar] [CrossRef]
- Akao, M.; Yamashita, T.; Fukuzawa, M.; Hayashi, T.; Okumura, K. Efficacy and Safety of Low-Dose Edoxaban by Body Weight in Very Elderly Patients with Atrial Fibrillation: A Subanalysis of the Randomized ELDERCARE-AF Trial. J. Am. Heart Assoc. 2024, 13, e031506. [Google Scholar] [CrossRef]
- Yoshida, T.; Nakamura, A.; Funada, J.; Amino, M.; Shimizu, W.; Fukuzawa, M.; Watanabe, S.; Hayashi, T.; Yamashita, T.; Okumura, K.; et al. Efficacy and Safety of Edoxaban 15 mg According to Renal Function in Very Elderly Patients with Atrial Fibrillation: A Subanalysis of the ELDERCARE-AF Trial. Circulation 2022, 145, 718–720. [Google Scholar] [CrossRef]
- Joosten, L.P.T.; van Doorn, S.; van de Ven, P.M.; Köhlen, B.T.G.; Nierman, M.C.; Koek, H.L.; Hemels, M.E.W.; Huisman, M.V.; Kruip, M.; Faber, L.M.; et al. Safety of Switching From a Vitamin K Antagonist to a Non-Vitamin K Antagonist Oral Anticoagulant in Frail Older Patients with Atrial Fibrillation: Results of the FRAIL-AF Randomized Controlled Trial. Circulation 2024, 149, 279–289. [Google Scholar] [CrossRef]
- Ingason, A.B.; Hreinsson, J.P.; Ágústsson, A.S.; Lund, S.H.; Rumba, E.; Pálsson, D.A.; Reynisson, I.E.; Guðmundsdóttir, B.R.; Önundarson, P.T.; Björnsson, E.S. Rivaroxaban Is Associated with Higher Rates of Gastrointestinal Bleeding Than Other Direct Oral Anticoagulants: A Nationwide Propensity Score-Weighted Study. Ann. Intern. Med. 2021, 174, 1493–1502. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Yao, X.; Abraham, N.S.; Sangaralingham, L.R.; McBane, R.D.; Shah, N.D. Direct Comparison of Dabigatran, Rivaroxaban, and Apixaban for Effectiveness and Safety in Nonvalvular Atrial Fibrillation. Chest 2016, 150, 1302–1312. [Google Scholar] [CrossRef]
- Nicolau, A.M.; Giugliano, R.P.; Zimerman, A.; Afilalo, J.; Gencer, B.; Steffel, J.; Palazzolo, M.G.; Eikelboom, J.W.; Granger, C.B.; Patel, M.R.; et al. Outcomes in Older Patients After Switching to a Newer Anticoagulant or Remaining on Warfarin: The COMBINE-AF Substudy. J. Am. Coll. Cardiol. 2025, 86, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Caso, V.; Connolly, S.J.; Fox, K.A.A.; Oldgren, J.; Jones, W.S.; Gorog, D.A.; Durdil, V.; Viethen, T.; Neumann, C.; et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): A multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 2022, 399, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Patel, M.R.; Steffel, J.; Ferdinand, K.; Van Gelder, I.C.; Russo, A.M.; Ma, C.S.; Goodman, S.G.; Oldgren, J.; Hammett, C.; et al. Asundexian versus Apixaban in Patients with Atrial Fibrillation. N. Engl. J. Med. 2025, 392, 23–32. [Google Scholar] [CrossRef]
- Ruff, C.T.; Goodrich, E.L.; Sabatine, M.S. Abelacimab versus Rivaroxaban in Patients with Atrial Fibrillation. N. Engl. J. Med. 2025, 392, 1556–1557. [Google Scholar] [CrossRef]
- Xue, Z.; Liao, S.; Fan, H.; Shen, Y.; Nie, Z. A Systematic Review of Factor XI/XIa Inhibitors Versus Direct Oral Anticoagulants in Patients with Atrial Fibrillation. Clin. Appl. Thromb. Hemost. 2025, 31, 10760296251335967. [Google Scholar] [CrossRef]
- Agarwal, S.; Munir, M.B.; Bansal, A.; DeSimone, C.V.; Baber, U.; Deshmukh, A.; Asad, Z.U.A. Impact of Frailty on In-Hospital Outcomes in Patients Who Underwent Percutaneous Left Atrial Appendage Occlusion. Am. J. Cardiol. 2023, 196, 19–21. [Google Scholar] [CrossRef]
- Darden, D.; Bilal Munir, M.; Zimmerman, S.; Eskander, M.; Pothineni, N.V.K.; Gopinathannair, R.; Kabra, R.; Lakkireddy, D.; Duong, T.; Han, F.T.; et al. Frailty and associated outcomes in patients undergoing percutaneous left atrial appendage occlusion: Findings from the NCDR LAAO registry. J. Interv. Card. Electrophysiol. 2024, 67, 625–635. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbri, E.; Maestri, L.; Muratori, P. Anticoagulation in Frail Older Adults with Non-Valvular Atrial Fibrillation: Clinical Challenges and Personalized Approach. J. Clin. Med. 2025, 14, 8079. https://doi.org/10.3390/jcm14228079
Fabbri E, Maestri L, Muratori P. Anticoagulation in Frail Older Adults with Non-Valvular Atrial Fibrillation: Clinical Challenges and Personalized Approach. Journal of Clinical Medicine. 2025; 14(22):8079. https://doi.org/10.3390/jcm14228079
Chicago/Turabian StyleFabbri, Elisa, Lorenzo Maestri, and Paolo Muratori. 2025. "Anticoagulation in Frail Older Adults with Non-Valvular Atrial Fibrillation: Clinical Challenges and Personalized Approach" Journal of Clinical Medicine 14, no. 22: 8079. https://doi.org/10.3390/jcm14228079
APA StyleFabbri, E., Maestri, L., & Muratori, P. (2025). Anticoagulation in Frail Older Adults with Non-Valvular Atrial Fibrillation: Clinical Challenges and Personalized Approach. Journal of Clinical Medicine, 14(22), 8079. https://doi.org/10.3390/jcm14228079






