Association of qEEG TAR and TBR During Eyes-Open and Eyes-Closed with Plasma Oligomeric Amyloid-β Levels in an Aging Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Demographics of the Participants
2.2. Assessment of Amyloid-β Oligomerization in Plasma
2.3. EEG Signals Acquisition and Pre-Processing
2.4. Neuropsychological Screening Batteries
2.5. Statistical Analysis
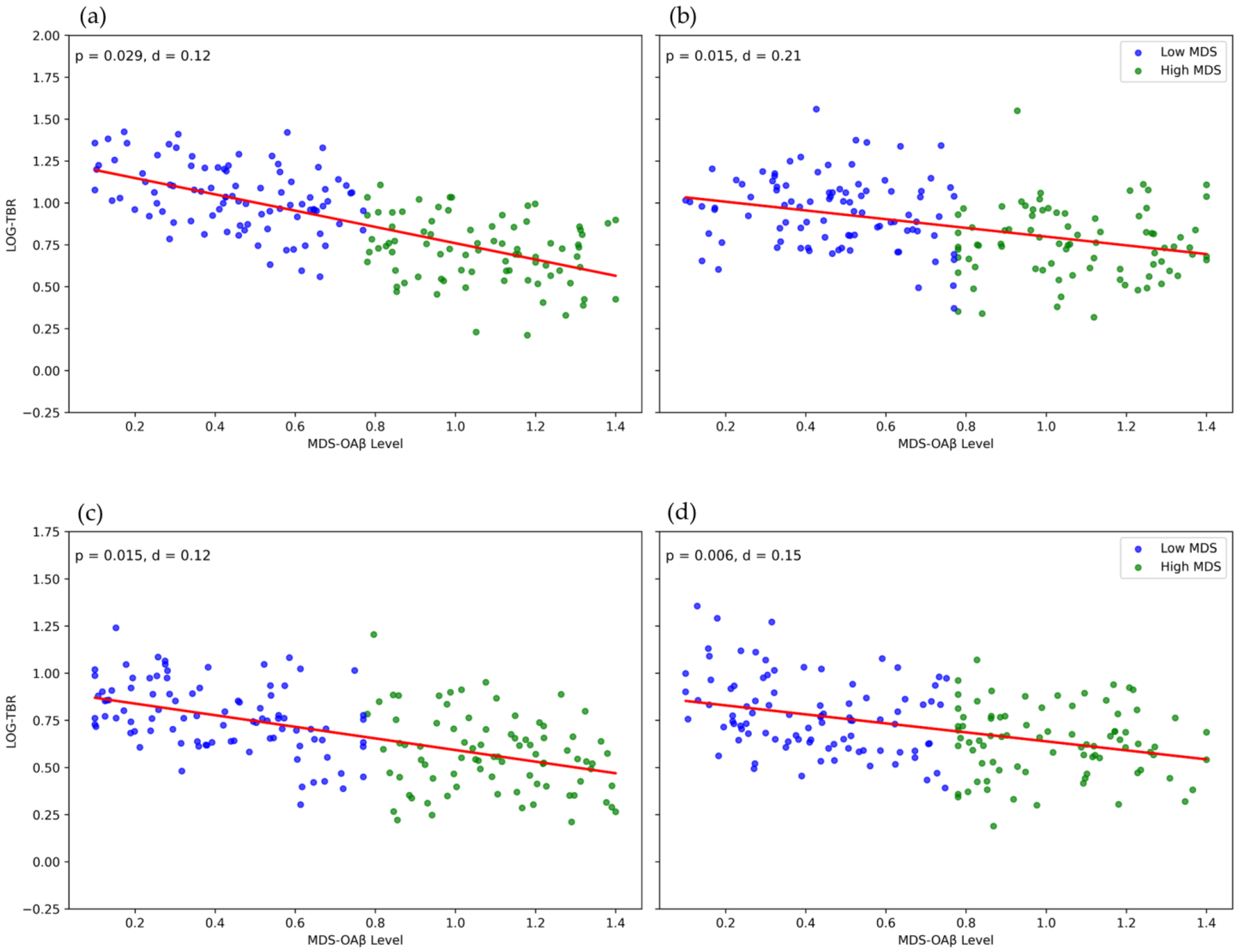
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, E.; Zhang, Y.; Wang, J.Z. Updates in Alzheimer’s disease: From basic research to diagnosis and therapies. Transl. Neurodegener. 2024, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Myrou, A.; Barmpagiannos, K.; Ioakimidou, A.; Savopoulos, C. Molecular Biomarkers in Neurological Diseases: Advances in Diagnosis and Prognosis. Int. J. Mol. Sci. 2025, 26, 2231. [Google Scholar] [CrossRef] [PubMed]
- Iliyasu, M.O.; Musa, S.A.; Oladele, S.B.; Iliya, A.I. Amyloid-beta aggregation implicates multiple pathways in Alzheimer’s disease: Understanding the mechanisms. Front. Neurosci. 2023, 17, 1081938. [Google Scholar] [CrossRef]
- Palop, J.J.; Mucke, L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010, 13, 812–818. [Google Scholar] [CrossRef]
- Zhang, X.; Song, W. The role of APP and BACE1 trafficking in APP processing and amyloid-β generation. Alzheimer’s Res. Ther. 2013, 5, 46. [Google Scholar] [CrossRef]
- Zhao, L.N.; Long, H.W.; Mu, Y.; Chew, L.Y. The toxicity of amyloid β oligomers. Int. J. Mol. Sci. 2012, 13, 7303–7327. [Google Scholar] [CrossRef]
- Dean, D.N.; Pate, K.M.; Moss, M.A.; Rangachari, V. Conformational Dynamics of Specific Aβ Oligomers Govern Their Ability To Replicate and Induce Neuronal Apoptosis. Biochemistry 2016, 55, 2238–2250. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; von Arnim, C.A.F.; Burnie, N.; Bozeat, S.; Cummings, J. Biomarkers in Alzheimer’s disease: Role in early and differential diagnosis and recognition of atypical variants. Alzheimer’s Res. Ther. 2023, 15, 175. [Google Scholar] [CrossRef]
- Dulewicz, M.; Kulczyńska-Przybik, A.; Mroczko, P.; Kornhuber, J.; Lewczuk, P.; Mroczko, B. Biomarkers for the Diagnosis of Alzheimer’s Disease in Clinical Practice: The Role of CSF Biomarkers during the Evolution of Diagnostic Criteria. Int. J. Mol. Sci. 2022, 23, 8598. [Google Scholar] [CrossRef]
- Canevelli, M.; Remoli, G.; Bacigalupo, I.; Valletta, M.; Toccaceli Blasi, M.; Sciancalepore, F.; Bruno, G.; Cesari, M.; Vanacore, N. Use of Biomarkers in Ongoing Research Protocols on Alzheimer’s Disease. J. Pers. Med. 2020, 10, 68. [Google Scholar] [CrossRef]
- Lian, P.; Guo, Y.; Yu, J. Biomarkers in Alzheimer’s disease: Emerging trends and clinical implications. Chin. Med. J. 2025, 138, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Leuzy, A.; Bollack, A.; Pellegrino, D.; Teunissen, C.E.; La Joie, R.; Rabinovici, G.D.; Franzmeier, N.; Johnson, K.; Barkhof, F.; Shaw, L.M.; et al. Considerations in the clinical use of amyloid PET and CSF biomarkers for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2025, 21, e14528. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Blennow, K.; Zetterberg, H.; Dage, J. Blood biomarkers for Alzheimer’s disease in clinical practice and trials. Nat. Aging 2023, 3, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Simfukwe, C.; Han, S.H.; Jeong, H.T.; Youn, Y.C. qEEG as Biomarker for Alzheimer’s Disease: Investigating Relative PSD Difference and Coherence Analysis. Neuropsychiatr. Dis. Treat. 2023, 19, 2423–2437. [Google Scholar] [CrossRef]
- Arslan, B.; Zetterberg, H.; Ashton, N.J. Blood-based biomarkers in Alzheimer’s disease-moving towards a new era of diagnostics. Clin. Chem. Lab. Med. 2024, 62, 1063–1069. [Google Scholar] [CrossRef]
- Dominguez, J.C.; Yu, J.R.T.; De Guzman, M.F.; Ampil, E.; Guevarra, A.C.; Joson, M.L.; Reandelar, M.; Martinez, M.S., Jr.; Ligsay, A.; Ocampo, F.; et al. Multimer Detection System-Oligomerized Amyloid Beta (MDS-OAβ): A Plasma-Based Biomarker Differentiates Alzheimer’s Disease from Other Etiologies of Dementia. Int. J. Alzheimer’s Dis. 2022, 2022, 9960832. [Google Scholar] [CrossRef]
- Wang, S.M.; Kang, D.W.; Um, Y.H.; Kim, S.; Lee, C.U.; Scheltens, P.; Lim, H.K. Plasma oligomer beta-amyloid is associated with disease severity and cerebral amyloid deposition in Alzheimer’s disease spectrum. Alzheimer’s Res. Ther. 2024, 16, 55. [Google Scholar] [CrossRef]
- Nah, E.H.; Cho, S.; Park, H.; Noh, D.; Hwang, I.; Cho, H.I. Reference interval and the role of plasma oligomeric beta amyloid in screening of risk groups for cognitive dysfunction at health checkups. J. Clin. Lab. Anal. 2021, 35, e23933. [Google Scholar] [CrossRef]
- Youn, Y.C.; Lee, B.S.; Kim, G.J.; Ryu, J.S.; Lim, K.; Lee, R.; Suh, J.; Park, Y.H.; Pyun, J.M.; Ryu, N.; et al. Blood Amyloid-β Oligomerization as a Biomarker of Alzheimer’s Disease: A Blinded Validation Study. J. Alzheimer’s Dis. 2020, 75, 493–499. [Google Scholar] [CrossRef]
- Lee, H.; Ugay, D.; Hong, S.; Kim, Y. Alzheimer’s Disease Diagnosis Using Misfolding Proteins in Blood. Dement. Neurocognitive Disord. 2020, 19, 1–18. [Google Scholar] [CrossRef]
- Meng, X.; Li, T.; Wang, X.; Lv, X.; Sun, Z.; Zhang, J.; Su, F.; Kang, S.; Kim, S.; An, S.S.A.; et al. Association between increased levels of amyloid-β oligomers in plasma and episodic memory loss in Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 89. [Google Scholar] [CrossRef]
- Pyun, J.M.; Ryu, J.S.; Lee, R.; Shim, K.H.; Youn, Y.C.; Ryoo, N.; Han, S.W.; Park, Y.H.; Kang, S.; An, S.S.A.; et al. Plasma Amyloid-β Oligomerization Tendency Predicts Amyloid PET Positivity. Clin. Interv. Aging 2021, 16, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Babapour Mofrad, R.; Scheltens, P.; Kim, S.; Kang, S.; Youn, Y.C.; An, S.S.A.; Tomassen, J.; van Berckel, B.N.M.; Visser, P.J.; van der Flier, W.M.; et al. Plasma amyloid-β oligomerization assay as a pre-screening test for amyloid status. Alzheimer’s Res. Ther. 2021, 13, 133. [Google Scholar] [CrossRef]
- Simfukwe, C.; An, S.S.A.; Youn, Y.C. Time-Frequency Domain Analysis of Quantitative Electroencephalography as a Biomarker for Dementia. Diagnostics 2025, 15, 1509. [Google Scholar] [CrossRef]
- Simfukwe, C.; An, S.S.A.; Youn, Y.C. Investigating Gamma Frequency Band PSD in Alzheimer’s Disease Using qEEG from Eyes-Open and Eyes-Closed Resting States. J. Clin. Med. 2025, 14, 4256. [Google Scholar] [CrossRef] [PubMed]
- Simfukwe, C.; An, S.S.A.; Youn, Y.C. Difference between eyes-open and eyes-closed resting state quantitative electroencephalography (qEEG) for predicting cognitive impairment using deep learning. Appl. Neuropsychol. Adult 2025. [Google Scholar] [CrossRef]
- Cassani, R.; Estarellas, M.; San-Martin, R.; Fraga, F.J.; Falk, T.H. Systematic Review on Resting-State EEG for Alzheimer’s Disease Diagnosis and Progression Assessment. Dis. Markers 2018, 2018, 5174815. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; Chen, X.; Yin, B.; Li, X.; Xie, P. Alzheimer’s disease diagnosis using rhythmic power changes and phase differences: A low-density EEG study. Front. Aging Neurosci. 2025, 16, 1485132. [Google Scholar] [CrossRef]
- Musaeus, C.S.; Engedal, K.; Høgh, P.; Jelic, V.; Mørup, M.; Naik, M.; Oeksengaard, A.R.; Snaedal, J.; Wahlund, L.O.; Waldemar, G.; et al. EEG Theta Power Is an Early Marker of Cognitive Decline in Dementia due to Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 1359–1371. [Google Scholar] [CrossRef]
- Zawiślak-Fornagiel, K.; Ledwoń, D.; Bugdol, M.; Grażyńska, A.; Ślot, M.; Tabaka-Pradela, J.; Bieniek, I.; Siuda, J. Quantitative EEG Spectral and Connectivity Analysis for Cognitive Decline in Amnestic Mild Cognitive Impairment. J. Alzheimer’s Dis. 2024, 97, 1235–1247. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Y. The role of quantitative EEG biomarkers in Alzheimer’s disease and mild cognitive impairment: Applications and insights. Front. Aging Neurosci. 2025, 17, 1522552. [Google Scholar] [CrossRef]
- Papaliagkas, V. The Role of Quantitative EEG in the Diagnosis of Alzheimer’s Disease. Diagnostics 2025, 15, 1965. [Google Scholar] [CrossRef]
- Alam, R.U.; Zhao, H.; Goodwin, A.; Kavehei, O.; McEwan, A. Differences in Power Spectral Densities and Phase Quantities Due to Processing of EEG Signals. Sensors 2020, 20, 6285. [Google Scholar] [CrossRef]
- Astfalck, L.C.; Sykulski, A.M.; Cripps, E.J. Debiasing Welch’s method for spectral density estimation. Biometrika 2024, 111, 1313–1329. [Google Scholar] [CrossRef]
- Azami, H.; Zrenner, C.; Brooks, H.; Zomorrodi, R.; Blumberger, D.M.; Fischer, C.E.; Flint, A.; Herrmann, N.; Kumar, S.; Lanctôt, K.; et al. PACt-MD Study Group Beta to theta power ratio in EEG periodic components as a potential biomarker in mild cognitive impairment and Alzheimer’s dementia. Alzheimer’s Res. Ther. 2023, 15, 133. [Google Scholar] [CrossRef]
- Meghdadi, A.H.; Stevanović Karić, M.; McConnell, M.; Rupp, G.; Richard, C.; Hamilton, J.; Salat, D.; Berka, C. Resting state EEG biomarkers of cognitive decline associated with Alzheimer’s disease and mild cognitive impairment. PLoS ONE 2021, 16, e0244180. [Google Scholar] [CrossRef]
- Bae, H.; Kang, M.J.; Ha, S.W.; Jeong, D.E.; Lee, K.; Lim, S.; Min, J.Y.; Min, K.B. Association of plasma amyloid-β oligomerization with theta/beta ratio in older adults. Front. Aging Neurosci. 2023, 15, 1291881. [Google Scholar] [CrossRef]
- Baik, K.; Jung, J.H.; Jeong, S.H.; Chung, S.J.; Yoo, H.S.; Lee, P.H.; Sohn, Y.H.; Kang, S.W.; Ye, B.S. Implication of EEG theta/alpha and theta/beta ratio in Alzheimer’s and Lewy body disease. Sci. Rep. 2022, 12, 18706. [Google Scholar] [CrossRef] [PubMed]
- Duits, F.H.; Nilsson, J.; Zetterberg, H.; Blennow, K.; van der Flier, W.M.; Teunissen, C.E.; Brinkmalm, A. Serial Cerebrospinal Fluid Sampling Reveals Trajectories of Potential Synaptic Biomarkers in Early Stages of Alzheimer’s Disease. J. Alzheimer’s Dis. 2024, 100, S103–S114. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, R.; Mao, K.; Deng, M.; Li, Z. The Role of Glial Cells in Synaptic Dysfunction: Insights into Alzheimer’s Disease Mechanisms. Aging Dis. 2024, 15, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Kurucu, H.; Colom-Cadena, M.; Davies, C.; Wilkins, L.; King, D.; Rose, J.; Tzioras, M.; Tulloch, J.H.; Smith, C.; Spires-Jones, T.L. Inhibitory synapse loss and accumulation of amyloid beta in inhibitory presynaptic terminals in Alzheimer’s disease. Eur. J. Neurol. 2022, 29, 1311–1323. [Google Scholar] [CrossRef]
- John, A.; Reddy, P.H. Synaptic basis of Alzheimer’s disease: Focus on synaptic amyloid beta, P-tau and mitochondria. Ageing Res. Rev. 2021, 65, 101208. [Google Scholar] [CrossRef] [PubMed]
- Jamerlan, A.M.; Shim, K.H.; Sharma, N.; An, S.S.A. Multimer Detection System: A Universal Assay System for Differentiating Protein Oligomers from Monomers. Int. J. Mol. Sci. 2025, 26, 1199. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.J.; Yang, D.W. The Seoul Neuropsychological Screening Battery (SNSB) for Comprehensive Neuropsychological Assessment. Dement. Neurocognitive Disord. 2023, 22, 1–15. [Google Scholar] [CrossRef]
- Ahn, H.J.; Chin, J.; Park, A.; Lee, B.H.; Suh, M.K.; Seo, S.W.; Na, D.L. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): A useful tool for assessing and monitoring cognitive impairments in dementia patients. J. Korean Med. Sci. 2010, 25, 1071–1076. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, J.Y.; Hong, D.; Kim, D.; Min, J.Y.; Min, K.B. Sex differences in the association between sarcopenia and mild cognitive impairment in the older Korean population. BMC Geriatr. 2023, 23, 332. [Google Scholar] [CrossRef]
- Du, H.; Guo, L.; Yan, S.S. Synaptic mitochondrial pathology in Alzheimer’s disease. Antioxid. Redox Signal. 2012, 16, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zou, L.; Xiong, M.; Chen, J.; Zhang, X.; Yu, T.; Li, Y.; Liu, C.; Chen, G.; Wang, Z.; et al. A synapsin Ⅰ cleavage fragment contributes to synaptic dysfunction in Alzheimer’s disease. Aging Cell 2022, 21, e13619. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Schmidt, M.T.; Kanda, P.A.; Basile, L.F.; da Silva Lopes, H.F.; Baratho, R.; Demario, J.L.; Jorge, M.S.; Nardi, A.E.; Machado, S.; Ianof, J.N.; et al. Index of alpha/theta ratio of the electroencephalogram: A new marker for Alzheimer’s disease. Front. Aging Neurosci. 2013, 5, 60. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Li, R.; Yuan, X.; Liu, M.; Zhang, W.; Li, Y. Multiple cross-frequency coupling analysis of resting-state EEG in patients with mild cognitive impairment and Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1142085. [Google Scholar] [CrossRef] [PubMed]
- Smailovic, U.; Koenig, T.; Kåreholt, I.; Andersson, T.; Kramberger, M.G.; Winblad, B.; Jelic, V. Quantitative EEG power and synchronization correlate with Alzheimer’s disease CSF biomarkers. Neurobiol. Aging 2018, 63, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Del Percio, C.; Lizio, R.; Lopez, S.; Noce, G.; Carpi, M.; Jakhar, D.; Soricelli, A.; Salvatore, M.; Yener, G.; Güntekin, B.; et al. Resting-State EEG Alpha Rhythms Are Related to CSF Tau Biomarkers in Prodromal Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 356. [Google Scholar] [CrossRef]
- Kremen, W.S.; Elman, J.A.; Panizzon, M.S.; Eglit, G.M.L.; Sanderson-Cimino, M.; Williams, M.E.; Lyons, M.J.; Franz, C.E. Cognitive Reserve and Related Constructs: A Unified Framework Across Cognitive and Brain Dimensions of Aging. Front. Aging Neurosci. 2022, 14, 834765. [Google Scholar] [CrossRef]
- Kelly, J.F.; Furukawa, K.; Barger, S.W.; Rengen, M.R.; Mark, R.J.; Blanc, E.M.; Roth, G.S.; Mattson, M.P. Amyloid beta-peptide disrupts carbachol-induced muscarinic cholinergic signal transduction in cortical neurons. Proc. Natl. Acad. Sci. USA 1996, 93, 6753–6758. [Google Scholar] [CrossRef] [PubMed]
- D’Atri, A.; Gorgoni, M.; Scarpelli, S.; Cordone, S.; Alfonsi, V.; Marra, C.; Ferrara, M.; Rossini, P.M.; De Gennaro, L. Relationship between Cortical Thickness and EEG Alterations during Sleep in the Alzheimer’s Disease. Brain Sci. 2021, 11, 1174. [Google Scholar] [CrossRef]
- Thammasart, S.; Namchaiw, P.; Pasuwat, K.; Tonsomboon, K.; Khantachawana, A. Neuroprotective Potential of Photobiomodulation Therapy: Mitigating Amyloid-Beta Accumulation and Modulating Acetylcholine Levels in an In Vitro Model of Alzheimer’s Disease. Photobiomodulation Photomed. Laser Surg. 2024, 42, 524–533. [Google Scholar] [CrossRef]
- Ribarič, S. Detecting Early Cognitive Decline in Alzheimer’s Disease with Brain Synaptic Structural and Functional Evaluation. Biomedicines 2023, 11, 355. [Google Scholar] [CrossRef]
| Characteristic | Total (n = 174) | MDS Low (<0.78 ng/mL) (n = 92) | MDS High (≥0.78 ng/mL) (n = 82) |
|---|---|---|---|
| Age group (years) n (%) | |||
| <60 | 13 (7.47) | 10 (10.87) | 3 (3.66) |
| 60–64 | 8 (4.60) | 4 (4.35) | 4 (4.88) |
| 65–69 | 23 (13.22) | 14 (15.22) | 9 (10.98) |
| 70–74 | 27 (15.52) | 14 (15.22) | 13 (15.85) |
| 75–79 | 46 (26.44) | 25 (27.17) | 21 (25.61) |
| 80–84 | 39 (22.41) | 16 (17.39) | 23 (28.05) |
| ≥85 | 18 (10.34) | 9 (9.78) | 9 (10.98) |
| Sex—n (%) | |||
| Female | 123 (70.69) | 65 (70.65) | 58 (70.73) |
| Male | 51 (29.31) | 27 (29.35) | 24 (29.27) |
| Education level—n (%) | |||
| Below elementary | 42 (24.14) | 25 (27.17) | 17 (20.73) |
| Middle school | 47 (27.01) | 25 (27.17) | 22 (26.83) |
| High school | 27 (15.52) | 12 (13.04) | 15 (18.29) |
| Over college | 58 (33.33) | 30 (32.61) | 28 (34.15) |
| Continuous variables (Mean ± SD) | |||
| Age, years | 74.87 ± 8.52 | 73.79 ± 9.01 | 76.07 ± 7.82 |
| Education, years | 10.89 ± 4.44 | 10.74 ± 4.49 | 11.04 ± 4.41 |
| Neuropsychological Test | Low-MDS Mean (SD) | High-MDS Mean (SD) | p-Value |
|---|---|---|---|
| K-Mini-Mental State Examination | 23.75 (4.54) | 23.02 (5.11) | 0.32 |
| Rey Complex Figure Test Copy | 27.91 (10.26) | 26.44 (10.91) | 0.36 |
| Seoul Verbal Learning Test Recall | 13.99 (5.83) | 13.15 (5.14) | 0.31 |
| Digit Span Forward | 7.27 (1.47) | 7.04 (1.46) | 0.29 |
| Digit Span Backward | 3.86 (1.75) | 3.50 (1.29) | 0.12 |
| K-Boston Naming Test | 9.50 (3.18) | 9.04 (3.22) | 0.34 |
| Lobe | Total Mean (SD) | Low-MDS Mean (SD) | High-MDS Mean (SD) | p-Value | |
|---|---|---|---|---|---|
| TAR | Central | 0.04 (0.23) | 0.03 (0.24) | 0.04 (0.22) | 0.86 |
| Frontal | 0.21 (0.20) | 0.20 (0.20) | 0.21 (0.19) | 0.91 | |
| Occipital | −0.15 (0.33) | −0.16 (0.35) | −0.13 (0.30) | 0.58 | |
| Parietal | 0.01 (0.25) | 1.00 × 10−3 (0.27) | 0.03 (0.24) | 0.56 | |
| Temporal | 0.04 (0.24) | 0.04 (0.26) | 0.04 (0.21) | 0.96 | |
| TBR | Central | 0.55 (0.30) | 0.57 (0.29) | 0.53 (0.32) | 0.39 |
| Frontal | 0.77 (0.26) | 0.78 (0.25) | 0.75 (0.26) | 0.41 | |
| Occipital | 0.70 (0.35) | 0.72 (0.36) | 0.66 (0.33) | 0.25 | |
| Parietal | 0.59 (0.33) | 0.62 (0.33) | 0.57 (0.33) | 0.31 | |
| Temporal | 0.74 (0.31) | 0.77 (0.31) | 0.71 (0.32) | 0.22 |
| Lobar Region | r (EO) | p (EO) | r (EC) | p (EC) | |
|---|---|---|---|---|---|
| TAR | Frontal | −0.01 | 0.39 | −0.01 | 0.59 |
| Central | −0.01 | 0.39 | −0.02 | 0.25 | |
| Parietal | −2.00 × 10−3 | 0.93 | −9.00 × 10−3 | 0.65 | |
| Occipital | 0.01 | 0.72 | −0.014 | 0.66 | |
| Temporal | −0.02 | 0.35 | −0.027 | 0.14 | |
| TBR | Frontal | −0.04 | 0.02 * | −0.03 | 0.11 |
| Central | −0.06 | 0.02 * | −0.06 | 0.01 * | |
| Parietal | −0.053 | 0.04 * | −0.07 | 9.00 × 10−3 | |
| Occipital | −0.06 | 0.08 | −0.06 | 0.07 | |
| Temporal | −0.06 | 0.01 * | −0.05 | 0.03 * |
| Lobar Region | Unadjusted Model | Adjusted Model | |
|---|---|---|---|
| (a) EO | |||
| TAR | Frontal | β = −0.01 (SE = 0.01), p = 0.39 | β = −0.01 (SE = 0.01), p = 0.43 |
| Central | β = −0.02 (SE = 0.02), p = 0.39 | β = −0.01 (SE = 0.02), p = 0.44 | |
| Parietal | β = −2.00 × 10−3 (SE = 0.02), p = 0.927 | β = −1.00 × 10−3 (SE = 0.02), p = 0.97 | |
| Occipital | β = 0.01 (SE = 0.03), p = 0.72 | β = 0.01 (SE = 0.03), p = 0.67 | |
| Temporal | β = −0.02 (SE = 0.02), p = 0.35 | β = −0.01 (SE = 0.02), p = 0.40 | |
| TBR | Frontal | β = −0.04 (SE = 0.02), p = 0.02 * | β = −0.04 (SE = 0.02), p = 0.03 * |
| Central | β = −0.06 (SE = 0.02), p = 0.03 * | β = −0.05 (SE = 0.02), p = 0.03 * | |
| Parietal | β = −0.05 (SE = 0.03), p = 0.05 * | β = −0.05 (SE = 0.03), p = 0.06 | |
| Occipital | β = −0.06 (SE = 0.03), p = 0.08 | β = −0.05 (SE = 0.03), p = 0.09 | |
| Temporal | β = −0.06 (SE = 0.02), p = 0.01 * | β = −0.05 (SE = 0.02), p = 0.02 * | |
| (b) EC | |||
| TAR | Frontal | β = −8.00 × 10−3 (SE = 0.01), p = 0.59 | β = −7.00 × 10−3 (SE = 0.01), p = 0.64 |
| Central | β = −0.02 (SE = 0.02), p = 0.25 | β = −0.02 (SE = 0.02), p = 0.25 | |
| Parietal | β = −9.00 × 10−3 (SE = 0.02), p = 0.65 | β = −0.01 (SE = 0.02), p = 0.64 | |
| Occipital | β = −0.01 (SE = 0.03), p = 0.66 | β = −0.02 (SE = 0.03), p = 0.55 | |
| Temporal | β = −0.03 (SE = 0.02), p = 0.14 | β = −0.03 (SE = 0.02), p = 0.11 | |
| TBR | Frontal | β = −0.03 (SE = 0.02), p = 0.11 | β = −0.03 (SE = 0.02), p = 0.14 |
| Central | β = −0.06 (SE = 0.03), p = 0.01 * | β = −0.06 (SE = 0.02), p = 0.02 * | |
| Parietal | β = −0.07 (SE = 0.03), p = 0.01 * | β = −0.07 (SE = 0.03), p = 6.00 × 10−3 * | |
| Occipital | β = −0.06 (SE = 0.03), p = 0.07 | β = −0.07 (SE = 0.03), p = 0.04 * | |
| Temporal | β = −0.05 (SE = 0.02), p = 0.03 * | β = −0.05 (SE = 0.02), p = 0.02 * | |
| Lobar Region | Education Group | Unadjusted Model | Adjusted Model |
|---|---|---|---|
| (a) EO | |||
| Central | Education ≤ 6 | β = −0.18 (SE = 0.07), p = 6.1 × 10−3 * | β = −0.19 (SE = 0.06), p = 3.4 × 10−3 * |
| Education 7–12 | β = 0.03 (SE = 0.06), p = 0.63 | β = −0.01 (SE = 0.06), p = 0.87 | |
| Education > 12 | β = −0.07 (SE = 0.06), p = 0.23 | β = −0.07 (SE = 0.06), p = 0.22 | |
| Temporal | Education ≤ 6 | β = −0.18 (SE = 0.06), p = 3.7 × 10−3 * | β = −0.19 (SE = 0.06), p = 1.8 × 10−3 * |
| Education 7–12 | β = −0.02 (SE = 0.05), p = 0.69 | β = −0.03 (SE = 0.05), p = 0.62 | |
| Education > 12 | β = −0.02 (SE = 0.05), p = 0.70 | β = −0.02 (SE = 0.05), p = 0.70 | |
| (b) EC | |||
| Central | Education ≤ 6 | β = −0.12 (SE = 0.06), p = 0.07 | β = −0.13 (SE = 0.06), p = 0.05 * |
| Education 7–12 | β = −0.05 (SE = 0.06), p = 0.40 | β = −0.11 (SE = 0.05), p = 0.06 | |
| Education > 12 | β = −0.11 (SE = 0.07), p = 0.11 | β = −0.12 (SE = 0.07), p = 0.08 | |
| Parietal | Education ≤ 6 | β = −0.05 (SE = 0.07), p = 0.46 | β = −0.08 (SE = 0.07), p = 0.27 |
| Education 7–12 | β = 0.01 (SE = 0.08), p = 0.92 | β = −0.03 (SE = 0.07), p = 0.68 | |
| Education > 12 | β = −0.06 (SE = 0.07), p = 0.40 | β = −0.09 (SE = 0.07), p = 0.20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simfukwe, C.; An, S.S.A.; Youn, Y.C.; Kang, J. Association of qEEG TAR and TBR During Eyes-Open and Eyes-Closed with Plasma Oligomeric Amyloid-β Levels in an Aging Population. J. Clin. Med. 2025, 14, 8069. https://doi.org/10.3390/jcm14228069
Simfukwe C, An SSA, Youn YC, Kang J. Association of qEEG TAR and TBR During Eyes-Open and Eyes-Closed with Plasma Oligomeric Amyloid-β Levels in an Aging Population. Journal of Clinical Medicine. 2025; 14(22):8069. https://doi.org/10.3390/jcm14228069
Chicago/Turabian StyleSimfukwe, Chanda, Seong Soo A. An, Young Chul Youn, and Jeena Kang. 2025. "Association of qEEG TAR and TBR During Eyes-Open and Eyes-Closed with Plasma Oligomeric Amyloid-β Levels in an Aging Population" Journal of Clinical Medicine 14, no. 22: 8069. https://doi.org/10.3390/jcm14228069
APA StyleSimfukwe, C., An, S. S. A., Youn, Y. C., & Kang, J. (2025). Association of qEEG TAR and TBR During Eyes-Open and Eyes-Closed with Plasma Oligomeric Amyloid-β Levels in an Aging Population. Journal of Clinical Medicine, 14(22), 8069. https://doi.org/10.3390/jcm14228069







