Sessile Serrated Lesions in Inflammatory Bowel Disease: Hidden Players in Colitis-Associated Colorectal Cancer?
Abstract
1. Introduction
2. Materials and Methods
Search Strategy
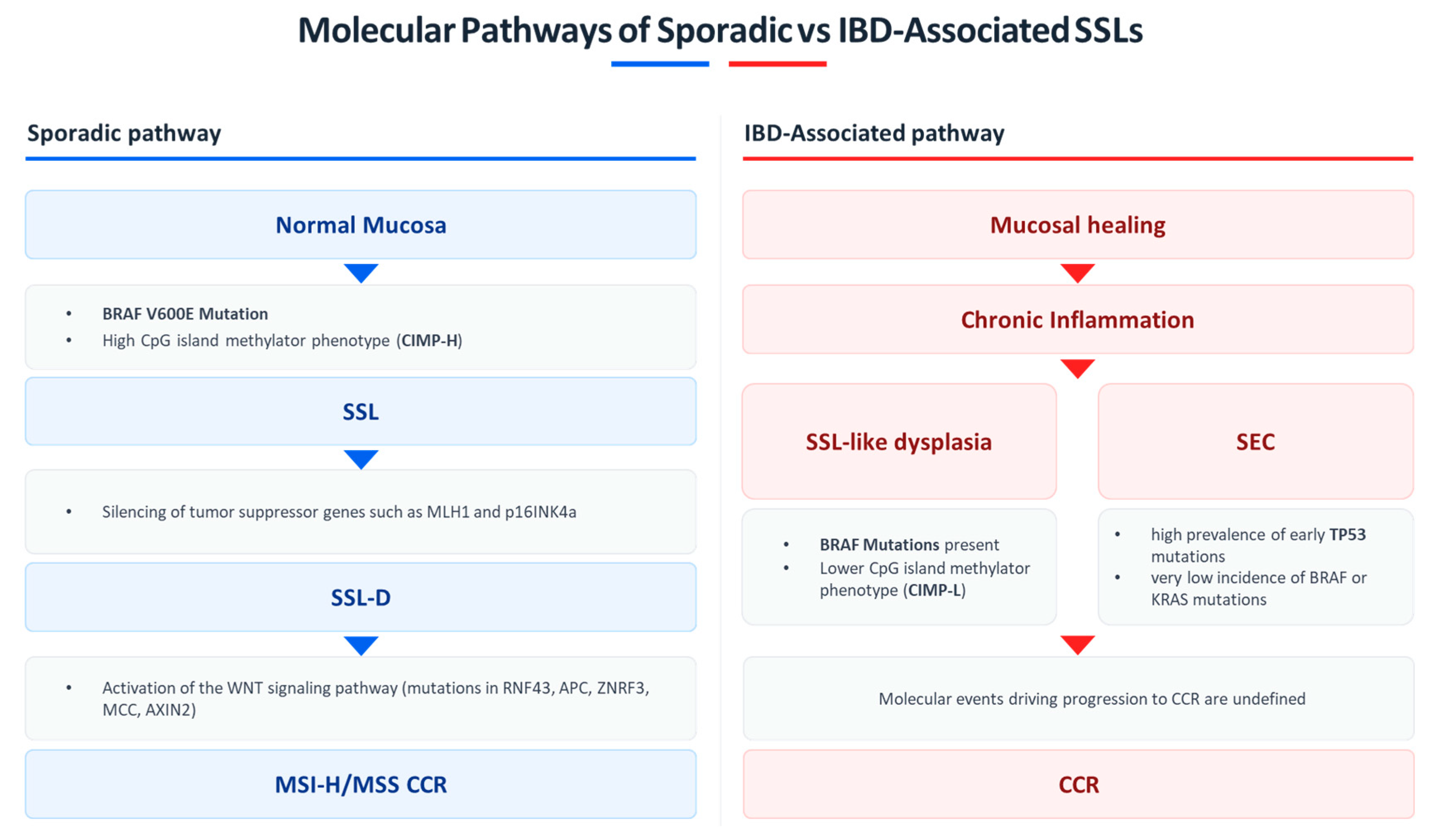
3. Histopathology and Molecular Features of SSLs
3.1. Sporadic Serrated Lesions
3.2. Serrated Lesions in Inflammatory Bowel Disease
4. Prevalence and Detection
4.1. Reported Rates in IBD Cohorts
4.2. Endoscopic Challenges
4.3. Differences by IBD Subtype
5. Neoplastic Potential and Risk of Progression
5.1. Evidence Linking SSLs to Dysplasia/Carcinoma in IBD
5.2. Evidence from Surveillance Cohorts
5.3. Malignant Potential: Established Risks and Remaining Gaps
6. Surveillance and Management Implications
6.1. Are SSLs in IBD Addressed by Current Guidelines?
6.2. Endoscopic Resection Strategies
6.3. Post-Resection Surveillance and Uncertainties
7. Knowledge Gaps
Diagnostic Variability and Its Implications for Surveillance Practice
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACG | American College of Gastroenterology |
| AE | Adverse event |
| AGA | American Gastroenterological Association |
| AI | Artificial intelligence |
| APC | Adenomatous polyposis coli (gene) |
| AXIN2 | Axis inhibition protein 2 (gene) |
| BLI | Blue light imaging |
| BRAF | v-RAF murine sarcoma viral oncogene homolog B1 (gene) |
| BSG | British Society of Gastroenterology |
| CE | Chromoendoscopy |
| CD | Crohn’s disease |
| CIN | Chromosomal instability |
| CIMP | CpG island methylator phenotype |
| CIMP-H | CpG island methylator phenotype–high |
| CIMP-L | CpG island methylator phenotype–low |
| CRC | Colorectal cancer |
| CRN | Colorectal neoplasia |
| DCE | Dye-based chromoendoscopy |
| ECCO | European Crohn’s and Colitis Organisation |
| EMR | Endoscopic mucosal resection |
| ER | Endoscopic resection |
| ESGE | European Society of Gastrointestinal Endoscopy |
| ESD | Endoscopic submucosal dissection |
| FBXW7 | F-box/WD repeat-containing protein 7 (gene) |
| HD-WLE | High-definition white-light endoscopy |
| HGD | High-grade dysplasia |
| HP | Hyperplastic polyp |
| HR-CAN | High-risk colorectal advanced neoplasia |
| IBD | Inflammatory bowel disease |
| IBD-CRC | IBD-associated colorectal cancer |
| LGD | Low-grade dysplasia |
| LST-NG | Laterally spreading tumor—non-granular type |
| MCC | Mutated in colorectal cancers (gene) |
| MDT | Multidisciplinary team |
| MLH1 | MutL homolog 1 (gene) |
| Mo | months |
| MSI-H | Microsatellite instability–high |
| MSS | Microsatellite stable |
| NBI | Narrow band imaging |
| NGS | Next-generation sequencing |
| NOS | Not otherwise specified |
| OS | Overall survival |
| PSC | Primary sclerosing cholangitis |
| Pts | patients |
| QA | Quality assurance |
| R0 | Complete (negative-margin) resection |
| R1 | Incomplete (positive-margin) resection |
| RNF43 | Ring Finger Protein 43 (gene) |
| SCENIC | Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in IBD Consensus |
| SEC | Serrated epithelial change |
| SD-NOS | Serrated dysplasia—not otherwise specified |
| sCRC | Sporadic colorectal cancer |
| SMOC1 | SPARC-related modular calcium-binding protein 1 (gene) |
| SP | Serrated polyp |
| SPU | Serrated polyp, unclassified |
| SSA/P | Sessile serrated adenoma/polyp (historical term, mapped as SSL) |
| SSP | Sessile serrated polyp (historical term, mapped as SSL) |
| SSL | Sessile serrated lesion |
| SSL-D | Sessile serrated lesion with dysplasia |
| TSA | Traditional serrated adenoma |
| UC | Ulcerative colitis |
| U-EMR | Underwater endoscopic mucosal resection |
| U-ESD | Underwater endoscopic submucosal dissection |
| VCE | Virtual chromoendoscopy |
| WHO | World Health Organization |
| WNT | Wingless/integrated signaling pathway |
| ZNRF3 | Zinc and ring finger 3 (gene) |
References
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Grillo, T.G.; De Oliveira, E.C.S.; Di Stasi, L.C.; Sassaki, L.Y. Gut Microbiota, Inflammatory Bowel Disease and Colorectal Cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.K.; Feuerstein, J.D.; Nguyen, G.C.; Velayos, F.S. AGA Clinical Practice Update on Endoscopic Surveillance and Management of Colorectal Dysplasia in Inflammatory Bowel Diseases: Expert Review. Gastroenterology 2021, 161, 1043–1051.e4. [Google Scholar] [CrossRef] [PubMed]
- Jess, T.; Gamborg, M.; Matzen, P.; Munkholm, P.; Sørensen, T.I.A. Increased Risk of Intestinal Cancer in Crohn’s Disease: A Meta-Analysis of Population-Based Cohort Studies. Am. J. Gastroenterol. 2005, 100, 2724–2729. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Gönczi, L.; Lakatos, P.L.; Burisch, J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohn’s Colitis 2021, 15, 1573–1587. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Yio, X. Inflammation and Cancer—IV. Colorectal Cancer in Inflammatory Bowel Disease: The Role of Inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G7–G17. [Google Scholar] [CrossRef]
- Baker, K.T.; Salk, J.J.; Brentnall, T.A.; Risques, R.A. Precancer in Ulcerative Colitis: The Role of the Field Effect and Its Clinical Implications. Carcinogenesis 2018, 39, 11–20. [Google Scholar] [CrossRef]
- Baker, A.M.; Cross, W.; Curtius, K.; Al Bakir, I.; Choi, C.H.R.; Davis, H.L.; Temko, D.; Biswas, S.; Martinez, P.; Williams, M.J.; et al. Evolutionary History of Human Colitis-Associated Colorectal Cancer. Gut 2019, 68, 985–995. [Google Scholar] [CrossRef]
- Robles, A.I.; Traverso, G.; Zhang, M.; Roberts, N.J.; Khan, M.A.; Joseph, C.; Lauwers, G.Y.; Selaru, F.M.; Popoli, M.; Pittman, M.E.; et al. Whole-Exome Sequencing Analyses of Inflammatory Bowel Disease-Associated Colorectal Cancers. Gastroenterology 2016, 150, 931–943. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Gay, L.; Otto, G.A.; White, E.; Iwanik, K.; Palmer, G.; Yelensky, R.; Lipson, D.M.; Chmielecki, J.; et al. Comprehensive Genomic Profiling of Carcinoma of Unknown Primary Site: New Routes to Targeted Therapies. JAMA Oncol. 2015, 1, 40–49. [Google Scholar] [CrossRef]
- Crockett, S.D.; Nagtegaal, I.D. Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology 2019, 157, 949–966.e4. [Google Scholar] [CrossRef]
- Iacucci, M.; Hassan, C.; Fort Gasia, M.; Urbanski, S.; Gui, X.; Eksteen, B.; Eustace, G.; Kaplan, G.G.; Panaccione, R. Serrated Adenoma Prevalence in Inflammatory Bowel Disease Surveillance Colonoscopy, and Characteristics Revealed by Chromoendoscopy and Virtual Chromoendoscopy. Can. J. Gastroenterol. Hepatol. 2014, 28, 589–594. [Google Scholar] [CrossRef]
- Bell, S.M.; Kelly, S.A.; Hoyle, J.A.; Lewis, F.A.; Dixon, M.F.; Quirke, P.; Taylor, G.R.; Thompson, H. C-Ki-Ras Gene Mutations in Dysplasia and Carcinomas Complicating Ulcerative Colitis. Br. J. Cancer 1991, 64, 174–178. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Bleijenberg, A.; Balaguer, F.; IJspeert, J.E.G.; Bleijenberg, A.G.C.; Pellisé, M.; Carballal, S.; Rivero, L.; Latchford, A. Update on the World Health Organization Criteria for Diagnosis of Serrated Polyposis Syndrome. Gastroenterology 2020, 158, 1520–1523. [Google Scholar] [CrossRef] [PubMed]
- Bleijenberg, A.G.C.; IJspeert, J.E.G.; Hazewinkel, Y.; Boparai, K.S.; Oppeneer, S.C.; Bastiaansen, B.A.J.; Dekker, E. The Long-Term Outcomes and Natural Disease Course of Serrated Polyposis Syndrome: Over 10 Years of Prospective Follow-up in a Specialized Center. Gastrointest. Endosc. 2020, 92, 1098–1107.e1. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Z.; Sha, F.; Qi, X.; He, Z.; Szeto, C.H.; Yang, Z.; Tang, J. Prevalence of Incidental Colorectal Cancer and Polyps in Autopsies of Different Populations: A Systematic Review with Meta-Regression Analysis. Eur. J. Epidemiol. 2023, 38, 939–955. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, T.; Yamada, Y.; Diaz-Meco, M.T.; Moscat, J.; Nakanishi, Y. Sessile Serrated Lesions with Dysplasia: Is It Possible to Nip Them in the Bud? J. Gastroenterol. 2023, 58, 705–717. [Google Scholar] [CrossRef]
- Peruhova, M.; Peshevska-Sekulovska, M.; Krastev, B.; Panayotova, G.; Georgieva, V.; Konakchieva, R.; Nikolaev, G.; Velikova, T.V. What Could MicroRNA Expression Tell Us More about Colorectal Serrated Pathway Carcinogenesis? World J. Gastroenterol. 2020, 26, 6556–6571. [Google Scholar] [CrossRef]
- Mezzapesa, M.; Losurdo, G.; Celiberto, F.; Rizzi, S.; D’amati, A.; Piscitelli, D.; Ierardi, E.; Di Leo, A. Serrated Colorectal Lesions: An Up-to-Date Review from Histological Pattern to Molecular Pathogenesis. Int. J. Mol. Sci. 2022, 23, 4461. [Google Scholar] [CrossRef]
- Wang, J.D.; Xu, G.S.; Hu, X.L.; Li, W.Q.; Yao, N.; Han, F.Z.; Zhang, Y.; Qu, J. The Histologic Features, Molecular Features, Detection and Management of Serrated Polyps: A Review. Front. Oncol. 2024, 14, 1356250. [Google Scholar] [CrossRef]
- Sullivan, B.A.; Noujaim, M.; Roper, J. Cause, Epidemiology, and Histology of Polyps and Pathways to Colorectal Cancer. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 177–194. [Google Scholar] [CrossRef]
- Gui, H.; Husson, M.A.; Mannan, R. Correlations of Morphology and Molecular Alterations in Traditional Serrated Adenoma. World J. Gastrointest. Pathophysiol. 2020, 11, 78–83. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, G.H. Evolving Pathologic Concepts of Serrated Lesions of the Colorectum. J. Pathol. Transl. Med. 2020, 54, 276–289. [Google Scholar] [CrossRef]
- Choi, W.T.; Yozu, M.; Miller, G.C.; Shih, A.R.; Kumarasinghe, P.; Misdraji, J.; Harpaz, N.; Lauwers, G.Y. Nonconventional Dysplasia in Patients with Inflammatory Bowel Disease and Colorectal Carcinoma: A Multicenter Clinicopathologic Study. Mod. Pathol. 2020, 33, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Rabinovitch, P.S.; Mattis, A.N.; Lauwers, G.Y.; Choi, W.T. Non-Conventional Dysplasia in Inflammatory Bowel Disease Is More Frequently Associated with Advanced Neoplasia and Aneuploidy than Conventional Dysplasia. Histopathology 2021, 78, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Waters, K.M.; Singhi, A.D.; Montgomery, E.A. Exploring the Spectrum of Serrated Epithelium Encountered in Inflammatory Bowel Disease. Hum. Pathol. 2023, 132, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Waters, K.M.; Makhoul, E.P.; Parian, A.; Lazarev, M.G.; Proksell, S.S.; Dueker, J.M.; Schwartz, M.B.; Wald, A.I.; Nikiforova, M.N.; et al. Targeted Next-Generation Sequencing Supports Serrated Epithelial Change as an Early Precursor to Inflammatory Bowel Disease–Associated Colorectal Neoplasia. Hum. Pathol. 2021, 112, 9–19. [Google Scholar] [CrossRef]
- Parian, A.M.; Limketkai, B.N.; Chowdhury, R.; Brewer, G.G.; Salem, G.; Falloon, K.; Selaru, F.; Melia, J.; Lazarev, M.G. Serrated Epithelial Change Is Associated with High Rates of Neoplasia in Ulcerative Colitis Patients: A Case-Controlled Study and Systematic Review with Meta-Analysis. Inflamm. Bowel Dis. 2021, 27, 1475–1481. [Google Scholar] [CrossRef]
- Ko, H.M.; Harpaz, N.; Mcbride, R.B.; Cui, M.; Ye, F.; Zhang, D.; Ullman, T.A.; Polydorides, A.D. Serrated Colorectal Polyps in Inflammatory Bowel Disease. Mod. Pathol. 2015, 28, 1584–1593. [Google Scholar] [CrossRef]
- Nishio, M.; Kunisaki, R.; Shibata, W.; Ajioka, Y.; Hirasawa, K.; Takase, A.; Chiba, S.; Inayama, Y.; Ueda, W.; Okawa, K.; et al. Serrated Polyps in Patients with Ulcerative Colitis: Unique Clinicopathological and Biological Characteristics. PLoS ONE 2023, 18, e0282204. [Google Scholar] [CrossRef]
- Johnson, D.H.; Khanna, S.; Smyrk, T.C.; Loftus, E.V.J.; Anderson, K.S.; Mahoney, D.W.; Ahlquist, D.A.; Kisiel, J.B. Detection Rate and Outcome of Colonic Serrated Epithelial Changes in Patients with Ulcerative Colitis or Crohn’s Colitis. Aliment. Pharmacol. Ther. 2014, 39, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.H.; Iacucci, M.; Gasia, M.F.; Ghosh, S.; Panaccione, R.; Urbanski, S. Prevalence and Anatomic Distribution of Serrated and Adenomatous Lesions in Patients with Inflammatory Bowel Disease. Can. J. Gastroenterol. Hepatol. 2017, 2017, 5490803. [Google Scholar] [CrossRef]
- Yeaman, F.; Thin, L. The Yield of Dysplasia and Serrated Lesions in a Single-Centre Tertiary Inflammatory Bowel Disease Cohort. Therap. Adv. Gastroenterol. 2023, 16, 17562848231167280. [Google Scholar] [CrossRef]
- de Jong, M.E.; Nagtegaal, I.D.; Vos, S.; van der Post, R.S.; van Herwaarden, Y.; Derikx, L.A.A.P.; Hoentjen, F. Increased Colorectal Neoplasia Risk in Patients with Inflammatory Bowel Disease and Serrated Polyps with Dysplasia. Dig. Dis. Sci. 2022, 67, 5647–5656. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.E.; Achkar, J.P.; Macaron, C.; Lee, L.; Liu, X.; Pai, R.K.; Lopez, R.; Burke, C.A.; Allende, D.S. The Significance of Sessile Serrated Polyps in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Universität, F.S. Updated S3-Guideline Ulcerative Colitis. German Society for Digestive and Metabolic Diseases (DGVS) Aktualisierte S3-Leitlinie Colitis Ulcerosa der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS). Z. Gastroenterol. 2019, 56, 162–241. [Google Scholar]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial Diagnosis, Monitoring of Known IBD, Detection of Complications. J. Crohn’s Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Laine, L.; Kaltenbach, T.; Barkun, A.; McQuaid, K.R.; Subramanian, V.; Soetikno, R. SCENIC International Consensus Statement on Surveillance and Management of Dysplasia in Inflammatory Bowel Disease. Gastroenterology 2015, 148, 639–651.e28. [Google Scholar] [CrossRef]
- Dekker, E.; Nass, K.J.; Iacucci, M.; Murino, A.; Sabino, J.; Bugajski, M.; Carretero, C.; Cortas, G.; Despott, E.J.; East, J.E.; et al. Performance Measures for Colonoscopy in Inflammatory Bowel Disease Patients: European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy 2022, 54, 904–915. [Google Scholar] [CrossRef]
- Iacucci, M.; Furfaro, F.; Matsumoto, T.; Uraoka, T.; Smith, S.; Ghosh, S.; Kiesslich, R. Advanced Endoscopic Techniques in the Assessment of Inflammatory Bowel Disease: New Technology, New Era. Gut 2019, 68, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Ono, A.; Sekiguchi, M.; Fujii, T.; Saito, Y. Advances in Image Enhancement in Colonoscopy for Detection of Adenomas. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Bisschops, R.; East, J.E.; Hassan, C.; Hazewinkel, Y.; Kamiński, M.F.; Neumann, H.; Pellisé, M.; Antonelli, G.; Bustamante Balen, M.; Coron, E.; et al. Correction: Advanced Imaging for Detection and Differentiation of Colorectal Neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2019. Endoscopy 2019, 51, 1155–1179, reprinted in Endoscopy 2019, 51, C6. https://doi.org/10.1055/a-1074-5788. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Q.; Li, X.; Bai, T.; Hou, X.; Li, G.; Song, J. The Effect of the Second Forward View on the Detection Rate of Sessile Serrated Lesions in the Proximal Colon: A Single-Center Prospective Randomized Controlled Study. Clin. Transl. Gastroenterol. 2024, 16, e00805. [Google Scholar] [CrossRef]
- Vemulapalli, K.C.; Lahr, R.E.; Rex, D.K. Most Large Colorectal Polyps Missed by Gastroenterology Fellows at Colonoscopy Are Sessile Serrated Lesions. Endosc. Int. Open 2022, 10, E659–E663. [Google Scholar] [CrossRef]
- Rubin, D.C.; Shaker, A.; Levin, M.S. Chronic Intestinal Inflammation: Inflammatory Bowel Disease and Colitis-Associated Colon Cancer. Front. Immunol. 2012, 3, 107. [Google Scholar] [CrossRef]
- Bae, S.I.; Kim, Y.S. Colon Cancer Screening and Surveillance in Inflammatory Bowel Disease. Clin. Endosc. 2014, 47, 509–515. [Google Scholar] [CrossRef]
- Naymagon, S.; Ullman, T.A. Chromoendoscopy and Dysplasia Surveillance in Inflammatory Bowel Disease: Past, Present, and Future. Gastroenterol. Hepatol. 2015, 11, 304–311. [Google Scholar]
- Konijeti, G.G.; Shrime, M.G.; Ananthakrishnan, A.N.; Chan, A.T. Cost-Effectiveness Analysis of Chromoendoscopy for Colorectal Cancer Surveillance in Patients with Ulcerative Colitis. Gastrointest. Endosc. 2014, 79, 455–465. [Google Scholar] [CrossRef]
- Roelandt, P.; Demedts, I.; Willekens, H.; Bessissow, T.; Braeye, L.; Coremans, G.; Cuyle, P.J.; Ferrante, M.; Gevers, A.M.; Hiele, M.; et al. Impact of Endoscopy System, High Definition, and Virtual Chromoendoscopy in Daily Routine Colonoscopy: A Randomized Trial. Endoscopy 2019, 51, 237–243. [Google Scholar] [CrossRef]
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Al Sulais, E.; Axelrad, J.E.; Balendran, K.; Burisch, J.; De Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J. Crohn’s Colitis 2023, 17, 827–854. [Google Scholar] [CrossRef]
- East, J.E.; Gordon, M.; Nigam, G.B.; Sinopoulou, V.; Bateman, A.C.; Din, S.; Iacucci, M.; Kabir, M.; Lamb, C.A.; Wilson, A.; et al. British Society of Gastroenterology Guidelines on Colorectal Surveillance in Inflammatory Bowel Disease. Gut 2025, 4, 1–34. [Google Scholar] [CrossRef]
- Brcic, I.; Dawson, H.; Gröchenig, H.P.; Högenauer, C.; Kashofer, K. Serrated Lesions in Inflammatory Bowel Disease: Genotype-Phenotype Correlation. Int. J. Surg. Pathol. 2021, 29, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kappelman, M.D.; Farkas, D.K.; Long, M.D.; Erichsen, R.; Sandler, R.S.; Sørensen, H.T.; Baron, J.A. Risk of Cancer in Patients with Inflammatory Bowel Diseases: A Nationwide Population-Based Cohort Study with 30 Years of Follow-up Evaluation. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2014, 12, 265–273.e1. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, M.W.M.D.; van Oijen, M.G.H.; van der Heijden, G.J.M.G.; Vleggaar, F.P.; Siersema, P.D.; Oldenburg, B. Declining Risk of Colorectal Cancer in Inflammatory Bowel Disease: An Updated Meta-Analysis of Population-Based Cohort Studies. Inflamm. Bowel Dis. 2013, 19, 789–799. [Google Scholar] [CrossRef]
- Duricova, D.; Pedersen, N.; Elkjaer, M.; Gamborg, M.; Munkholm, P.; Jess, T. Overall and Cause-Specific Mortality in Crohn’s Disease: A Meta-Analysis of Population-Based Studies. Inflamm. Bowel Dis. 2010, 16, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Bogach, J.; Pond, G.; Eskicioglu, C.; Seow, H. Corrigendum to Age-Related Survival Differences in Patients with Inflammatory Bowel Disease-Associated Colorectal Cancer: A Population-Based Cohort Study. Inflamm. Bowel Dis. 2019, 25, 1957–1965, Erratum in Inflamm. Bowel Dis. 2019, 25, e169. [Google Scholar] [CrossRef]
- Leggett, B.; Whitehall, V. Role of the Serrated Pathway in Colorectal Cancer Pathogenesis. Gastroenterology 2010, 138, 2088–2100. [Google Scholar] [CrossRef]
- East, J.E.; Vieth, M.; Rex, D.K. Serrated Lesions in Colorectal Cancer Screening: Detection, Resection, Pathology and Surveillance. Gut 2015, 64, 991–1000. [Google Scholar] [CrossRef]
- Musquer, N. French Comment on Article: Prevalence, Distribution and Risk of Sessile Serrated Adenomas/Polyps at a Center with a High Adenoma Detection Rate and Experienced Pathologists. Endoscopy 2016, 48, 784. [Google Scholar] [CrossRef]
- He, X.; Hang, D.; Wu, K.; Nayor, J.; Drew, D.A.; Giovannucci, E.L.; Ogino, S.; Chan, A.T.; Song, M. Long-Term Risk of Colorectal Cancer After Removal of Conventional Adenomas and Serrated Polyps. Gastroenterology 2020, 158, 852–861.e4. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Olén, O.; Söderling, J.; Roelstraete, B.; Khalili, H.; Song, M.; Faye, A.; Eberhardson, M.; Halfvarson, J.; Ludvigsson, J.F. Inflammatory Bowel Disease and Risk of Colorectal Polyps: A Nationwide Population-Based Cohort Study From Sweden. J. Crohn’s Colitis 2023, 17, 1395–1409. [Google Scholar] [CrossRef]
- Nardone, O.M.; Zammarchi, I.; Santacroce, G.; Ghosh, S.; Iacucci, M. Inflammation-Driven Colorectal Cancer Associated with Colitis: From Pathogenesis to Changing Therapy. Cancers 2023, 15, 2389. [Google Scholar] [CrossRef]
- Zhang, R.; Lauwers, G.Y.; Choi, W.T. Increased Risk of Non-Conventional and Invisible Dysplasias in Patients with Primary Sclerosing Cholangitis and Inflammatory Bowel Disease. J. Crohn’s Colitis 2022, 16, 1825–1834. [Google Scholar] [CrossRef]
- Vennelaganti, S.; Cuatrecasas, M.; Vennalaganti, P.; Kennedy, K.F.; Srinivasan, S.; Patil, D.T.; Plesec, T.; Lanas, A.; Hörndler, C.; Andraws, N.; et al. Interobserver Agreement Among Pathologists in the Differentiation of Sessile Serrated From Hyperplastic Polyps. Gastroenterology 2021, 160, 452–454.e1. [Google Scholar] [CrossRef] [PubMed]
- Bastos Junior, C.D.S.; Pannain, V.L.N.; Caroli-Bottino, A. Morphological Characteristics, Classifications and Difficulties in the Use of Diagnostic Criteria for Serrated Lesions of the Large Intestine. J. Coloproctology 2021, 41, 430–437. [Google Scholar] [CrossRef]
- Medawar, E.; Djinbachian, R.; Crainic, I.P.; Safih, W.; Battat, R.; Mccurdy, J.; Lakatos, P.L.; von Renteln, D. Serrated Polyps in Inflammatory Bowel Disease Indicate a Similar Risk of Metachronous Colorectal Neoplasia as in the General Population. Dig. Dis. Sci. 2024, 69, 2595–2610. [Google Scholar] [CrossRef]
- East, J.E.; Atkin, W.S.; Bateman, A.C.; Clark, S.K.; Dolwani, S.; Ket, S.N.; Leedham, S.J.; Phull, P.S.; Rutter, M.D.; Shepherd, N.A.; et al. British Society of Gastroenterology Position Statement on Serrated Polyps in the Colon and Rectum. Gut 2017, 66, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Annese, V.; Daperno, M.; Rutter, M.D.; Amiot, A.; Bossuyt, P.; East, J.; Ferrante, M.; Götz, M.; Katsanos, K.H.; Kießlich, R.; et al. European Evidence Based Consensus for Endoscopy in Inflammatory Bowel Disease. J. Crohn’s Colitis 2013, 7, 982–1018. [Google Scholar] [CrossRef]
- Ferlitsch, M.; Hassan, C.; Bisschops, R.; Bhandari, P.; Dinis-Ribeiro, M.; Risio, M.; Paspatis, G.A.; Moss, A.; Libânio, D.; Lorenzo-Zúñiga, V.; et al. Colorectal Polypectomy and Endoscopic Mucosal Resection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2024. Endoscopy 2024, 56, 516–545. [Google Scholar] [CrossRef]
- Moran, G.W.; Gordon, M.; Sinopolou, V.; Radford, S.J.; Darie, A.-M.; Vuyyuru, S.K.; Alrubaiy, L.; Arebi, N.; Blackwell, J.; Butler, T.D.; et al. British Society of Gastroenterology Guidelines on Inflammatory Bowel Disease in Adults: 2025. Gut 2025, 74, s1–s101. [Google Scholar] [CrossRef] [PubMed]
- Maselli, R.; de Sire, R.; Massimi, D.; Franchellucci, G.; Busacca, A.; Castiglione, F.; Rispo, A.; Hassan, C.; Armuzzi, A.; Repici, A. Advancements in Endoscopic Resection for Colitis-Associated Colorectal Neoplasia in Inflammatory Bowel Disease: Turning Visible into Resectable. Diagnostics 2024, 14, 9. [Google Scholar] [CrossRef]
- Bak, M.T.J.; Albéniz, E.; East, J.E.; Coelho-Prabhu, N.; Suzuki, N.; Saito, Y.; Matsumoto, T.; Banerjee, R.; Kaminski, M.F.; Kiesslich, R.; et al. Endoscopic Management of Patients with High-Risk Colorectal Colitis–Associated Neoplasia: A Delphi Study. Gastrointest. Endosc. 2023, 97, 767–779.e6. [Google Scholar] [CrossRef]
- Hirai, M.; Yanai, S.; Kunisaki, R.; Nishio, M.; Watanabe, K.; Sato, T.; Ishihara, S.; Anzai, H.; Hisabe, T.; Yasukawa, S.; et al. Effectiveness of Endoscopic Resection for Colorectal Neoplasms in Ulcerative Colitis: A Multicenter Registration Study. Gastrointest. Endosc. 2023, 98, 806–812. [Google Scholar] [CrossRef]
- Iacopini, F.; Saito, Y.; Yamada, M.; Grossi, C.; Rigato, P.; Costamagna, G.; Gotoda, T.; Matsuda, T.; Scozzarro, A. Curative Endoscopic Submucosal Dissection of Large Nonpolypoid Superficial Neoplasms in Ulcerative Colitis (with Videos). Gastrointest. Endosc. 2015, 82, 734–738. [Google Scholar] [CrossRef]
- Kasuga, K.; Yamada, M.; Shida, D.; Tagawa, T.; Takamaru, H.; Sekiguchi, M.; Sakamoto, T.; Uraoka, T.; Sekine, S.; Kanemitsu, Y.; et al. Treatment Outcomes of Endoscopic Submucosal Dissection and Surgery for Colorectal Neoplasms in Patients with Ulcerative Colitis. United Eur. Gastroenterol. J. 2021, 9, 964–972. [Google Scholar] [CrossRef]
- Ngamruengphong, S.; Aihara, H.; Friedland, S.; Nishimura, M.; Faleck, D.; Benias, P.; Yang, D.; Draganov, P.V.; Kumta, N.A.; Borman, Z.A.; et al. Endoscopic Submucosal Dissection for Colorectal Dysplasia in Inflammatory Bowel Disease: A US Multicenter Study. Endosc. Int. Open 2022, 10, E354–E360. [Google Scholar] [CrossRef]
- Nishio, M.; Hirasawa, K.; Ozeki, Y.; Sawada, A.; Ikeda, R.; Fukuchi, T.; Kobayashi, R.; Makazu, M.; Sato, C.; Kunisaki, R.; et al. An Endoscopic Treatment Strategy for Superficial Tumors in Patients with Ulcerative Colitis. J. Gastroenterol. Hepatol. 2021, 36, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbach, T.; Holmes, I.; Nguyen-Vu, T.; Malvar, C.; Balitzer, D.; Fong, D.; Fu, A.; Shergill, A.; McQuaid, K.; Soetikno, R. Longitudinal Outcomes of the Endoscopic Resection of Nonpolypoid Dysplastic Lesions in Patients with Inflammatory Bowel Disease. Gastrointest. Endosc. 2023, 97, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Maselli, R.; De Sire, R.; Barbaro, F.; Cecinato, P.; Andrisani, G.; Rosa-Rizzotto, E.; Sferrazza, S.; Fiori, G.; Azzolini, F.; Pugliese, F.; et al. Outcomes of Endoscopic Submucosal Dissection for High-Risk Colorectal Colitis-Associated Neoplasia in Inflammatory Bowel Disease. Endoscopy 2025, 57, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Bordillon, P.; Pioche, M.; Wallenhorst, T.; Rivory, J.; Legros, R.; Albouys, J.; Lepetit, H.; Rostain, F.; Dahan, M.; Ponchon, T.; et al. Double-Clip Traction for Colonic Endoscopic Submucosal Dissection: A Multicenter Study of 599 Consecutive Cases (with Video). Gastrointest. Endosc. 2021, 94, 333–343. [Google Scholar] [CrossRef]
- Grimaldi, J.; Masgnaux, L.-J.; Lafeuille, P.; de Cristofaro, E.; Rivory, J.; Ponchon, T.; Yzet, C.; Wallenhorst, T.; Alexandru, L.; Legros, R.; et al. Endoscopic Submucosal Dissection with Adaptive Traction Strategy: First Prospective Multicenter Study (with Video). Gastrointest. Endosc. 2024, 100, 517–523. [Google Scholar] [CrossRef]
- de Sire, R.; Capogreco, A.; Massimi, D.; Alfarone, L.; Brandaleone, L.; Vadalà, V.; Minini, F.; Marco, S.; Facciorusso, A.; Alkandari, A.; et al. Underwater Endoscopic Submucosal Dissection for Large Non-Pedunculated Colorectal Polyps. Gut 2025, 74, 1945–1948. [Google Scholar] [CrossRef]
- de Sire, R.; Alfarone, L.; Capogreco, A.; Massimi, D.; Vadalà, V.; Brandaleone, L.; Spadaccini, M.; Alkandari, A.; Bhandari, P.; Rosch, T.; et al. A Novel Thin-Needle Knife with High-Pressure Waterjet for Colorectal Underwater Endoscopic Submucosal Dissection. Gastrointest. Endosc. 2025, in press. [Google Scholar] [CrossRef]
- Belderbos, T.D.G.; Leenders, M.; Moons, L.M.G.; Siersema, P.D. Local Recurrence after Endoscopic Mucosal Resection of Nonpedunculated Colorectal Lesions: Systematic Review and Meta-Analysis. Endoscopy 2014, 46, 388–402. [Google Scholar] [CrossRef]
- Rex, D.K.; Ahnen, D.J.; Baron, J.A.; Batts, K.P.; Burke, C.A.; Burt, R.W.; Goldblum, J.R.; Guillem, J.G.; Kahi, C.J.; Kalady, M.F.; et al. Serrated Lesions of the Colorectum: Review and Recommendations from an Expert Panel. Am. J. Gastroenterol. 2012, 107, 1315–1329. [Google Scholar] [CrossRef]
- IJspeert, J.E.G.; Bevan, R.; Senore, C.; Kaminski, M.F.; Kuipers, E.J.; Mroz, A.; Bessa, X.; Cassoni, P.; Hassan, C.; Repici, A.; et al. Detection Rate of Serrated Polyps and Serrated Polyposis Syndrome in Colorectal Cancer Screening Cohorts: A European Overview. Gut 2017, 66, 1225–1232. [Google Scholar] [CrossRef]
- Leoncini, G.; Donato, F.; Reggiani-Bonetti, L.; Salviato, T.; Cadei, M.; Daperno, M.; Principi, M.B.; Armuzzi, A.; Caprioli, F.; Canavese, G.; et al. Diagnostic Interobserver Variability in Crohn’s Disease- and Ulcerative Colitis-Associated Dysplasia: A Multicenter Digital Survey from the IG-IBD Pathologists Group. Tech. Coloproctol. 2021, 25, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Batts, K.P.; Atwaibi, M.; Weinberg, D.I.; McCabe, R.P. Significance of Serrated Epithelial Change in Inflammatory Bowel Disease. Postgrad. Med. 2021, 133, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Cannarozzi, A.L.; Massimino, L.; Latiano, A.; Parigi, T.L.; Giuliani, F.; Bossa, F.; Di Brina, A.L.; Ungaro, F.; Biscaglia, G.; Danese, S.; et al. Artificial Intelligence: A New Tool in the Pathologist’s Armamentarium for the Diagnosis of IBD. Comput. Struct. Biotechnol. J. 2024, 23, 3407–3417. [Google Scholar] [CrossRef]
- Choi, W.T.; Wen, K.W.; Rabinovitch, P.S.; Huang, D.; Mattis, A.N.; Gill, R.M. DNA Content Analysis of Colorectal Serrated Lesions Detects an Aneuploid Subset of Inflammatory Bowel Disease-Associated Serrated Epithelial Change and Traditional Serrated Adenomas. Histopathology 2018, 73, 464–472. [Google Scholar] [CrossRef]
- Gupta, S.; Lieberman, D.; Anderson, J.C.; Burke, C.A.; Jason, A.; Kaltenbach, T.; Robertson, D.J.; Shaukat, A.; Rex, D.K.; Diego, S.; et al. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020, 158, 1131–1153. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbach, T.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Gupta, S.; Lieberman, D.; Robertson, D.J.; Shaukat, A.; Syngal, S.; Rex, D.K. Endoscopic Removal of Colorectal Lesions—Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020, 158, 1095–1129. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Siggens, K.; Iwadate, Y.; Maeda, N.; Htet, H.; Bhandari, P. New AI Model for Neoplasia Detection and Characterisation in Inflammatory Bowel Disease. Gut 2024, 73, 725–728. [Google Scholar] [CrossRef] [PubMed]
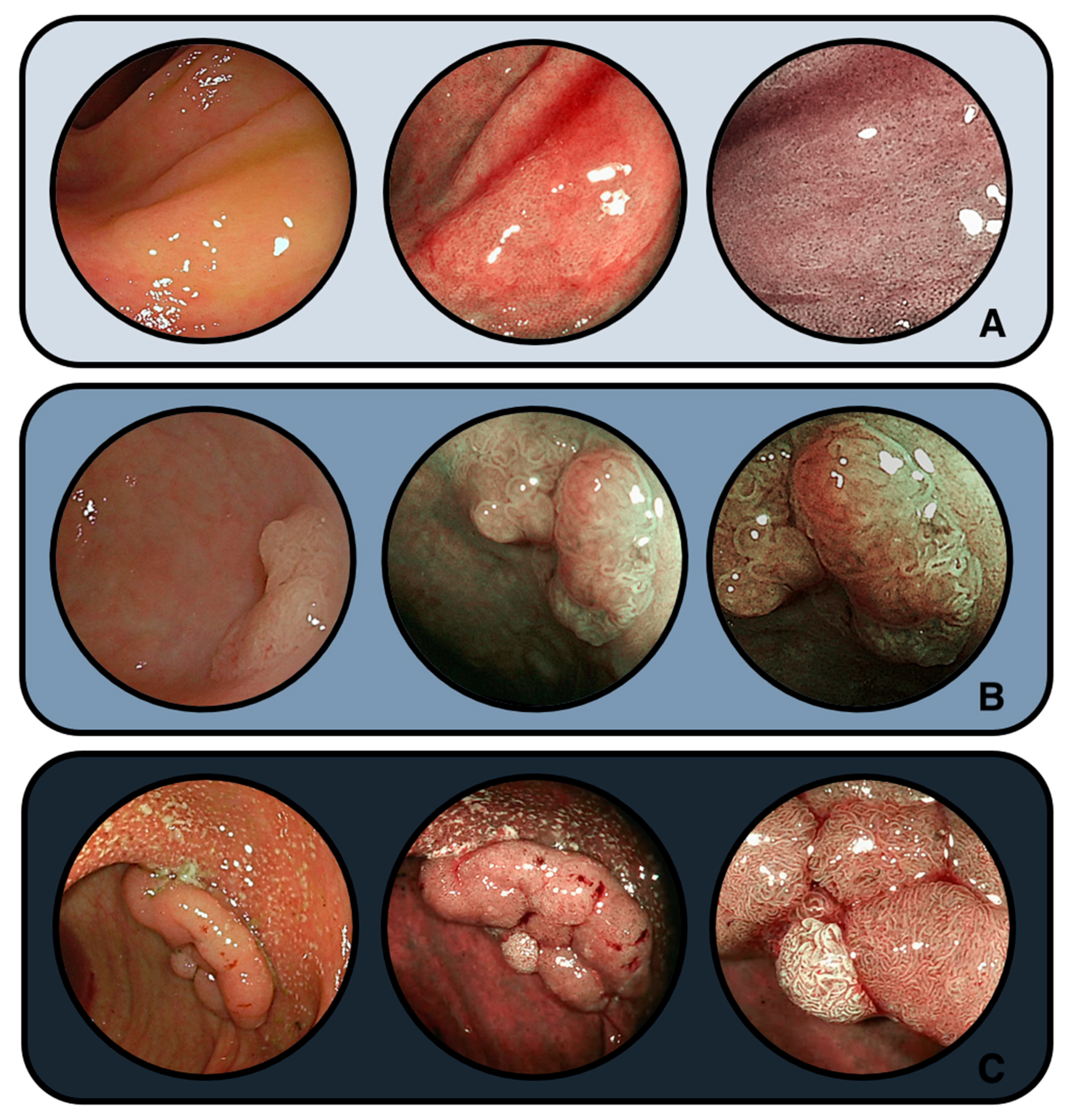

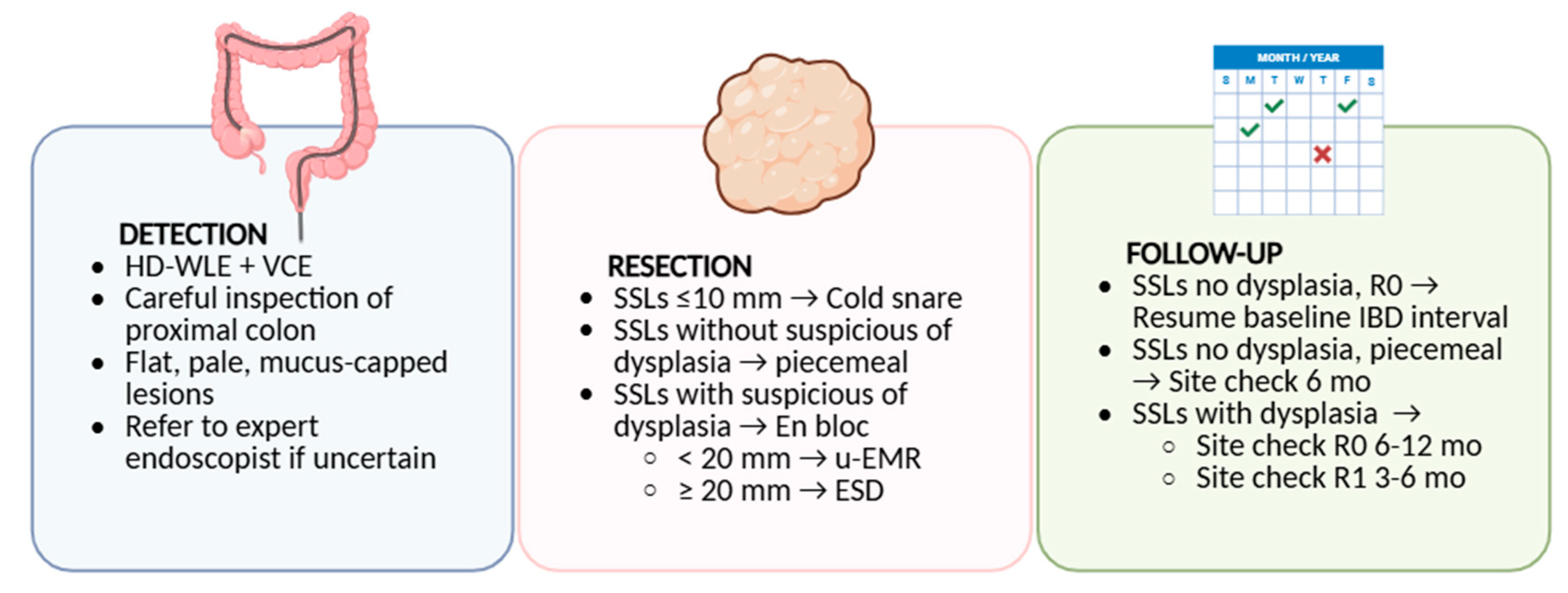
| Guideline (Year) | Primary Focus | Surveillance Policy in IBD | Resection/Management of Visible Lesions | Position on SSLs in IBD and Post-resection Follow-Up |
|---|---|---|---|---|
| SCENIC Consensus (2015) [40] | Dysplasia surveillance & management in IBD; imaging standards | Begin 8–10 years after extensive colitis; risk-stratified thereafter; prefer HD-WLE and DCE in expert settings | Resection of all visible lesions; colectomy for unresectable or flat/invisible dysplasia | SSLs not addressed (pre-WHO 2019); no SSL-specific intervals |
| ESGE Performance Measures (2022) [41] | Quality standards for colonoscopy in IBD | Not interval-focused; emphasizes HD imaging, complete mucosal inspection, documentation, service-level QA | Not a resection guideline | Highlights need to detect subtle/flat lesions (incl. serrated) but no SSL-specific metrics or intervals |
| ECCO Guidelines (2023) [52] | IBD & malignancies | Intervals based on extent, cumulative inflammatory burden, PSC, prior dysplasia; MDT review | Visible dysplasia resect if complete (R0) is feasible; management individualized | SSLs acknowledged within dysplasia spectrum; after R0, surveillance reverts to baseline IBD risk; no SSL-specific intervals; evidence gap noted |
| ESGE Polypectomy/EMR Update (2024) [71] | Technical guidance for resection (general population) | Not IBD-specific for intervals; advises site-check after piecemeal EMR | SSL ≤ 9 mm: cold snare with 1–2 mm margin; 10–19 mm: EMR/U-EMR; ≥20 mm: piecemeal EMR; selective ESD in expert centers | Provides detailed SSL resection strategy; commonly extrapolated to IBD; pragmatic 6–12 months site-check after piecemeal or dysplasia-containing lesions |
| BSG Surveillance Guideline (2025) [53] | Colorectal surveillance framework in IBD | Structured risk tiers (e.g., annual for high-risk such as PSC/previous dysplasia; 3–5 years for lower-risk categories) with QA metrics | Prefer HD imaging; resect visible lesions when feasible; MDT pathways | SSLs acknowledged but not an independent risk modifier; no SSL-specific intervals after complete resection |
| BSG IBD Guideline (2025) [72] | Comprehensive adult IBD management (incl. malignancy risk) | Adopts risk-stratified surveillance as above | MDT-based management of dysplasia | Notes serrated lesions but no tailored SSL algorithms |
| First Author | Study Design | Population | Intervention | Key Outcomes | Follow-Up |
|---|---|---|---|---|---|
| Hirai et al. [75] | Retrospective multicenter | UC, 238 colorectal lesions | EMR (142, incl. 22 SSL); ESD (96, incl. 12 SSL) | Perforation 2.5% (higher in ESD: 6.3%); recurrence 2.7%; metachronous neoplasia 6.1%; OS higher in ER group | Median 34.7 mo |
| Iacopini et al. [76] | Prospective multicenter case series | UC, 9 pts with large (>20 mm) lesions | ESD en bloc in 8/9; curative in 7 (incl. 1 SSL) | No invisible dysplasia/cancer; technically challenging due to fibrosis | Median 24 mo |
| Kasuga et al. [77] | Single-center retrospective | UC, 9 pts, 11 lesions | ESD (incl. 1 SSL); compared to colectomy (19 lesions) | En bloc 91%; curative 82%; feasible despite fibrosis/scarring | Median 25 mo |
| Ngamruengphong et al. [78] | Retrospective multicenter | IBD, 41 pts, 45 lesions | ESD (incl. 4 serrated adenomas/polyps) | En bloc 96%; R0 76%; 1 perforation (2.4%); recurrence 2.6%; metachronous 31% | Median 18 mo |
| Nishio et al. [79] | Retrospective single-center | UC, 74 pts, 102 lesions | ESD (39 lesions); EMR (63 lesions); 19 SPs | R0: ESD 97% vs. EMR 80%; 4 perforations; no recurrence; metachronous HGD in 3 pts | Mean 12 mo |
| Kaltenbach et al. [80] | Retrospective multicenter | IBD, 326 pts; 63 nonpolypoid lesions | ER (EMR/ESD/standard); 14 SSL | Success 96.8%; AE 1.5%; recurrence 6.3% | Mean 14.1 ± 26.1 mo |
| Maselli et al. [81] | Retrospective multicenter | IBD, 91 pts; 96 HR-CANs (14.6% SSL) | ESD (82.3%) or h-ESD (17.7%) | En bloc 95.8%; R0 85.4%; curative 83.3%; AE 12.5%; recurrence 3.1%; metachronous 3.1% | Mean 23.4 mo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sire, R.; De Deo, D.; Mercurio, M.; Franchellucci, G.; Calabrese, G.; Bonacci, L.; Sollai Pinna, M.; Bezzio, C.; Armuzzi, A.; Hassan, C.; et al. Sessile Serrated Lesions in Inflammatory Bowel Disease: Hidden Players in Colitis-Associated Colorectal Cancer? J. Clin. Med. 2025, 14, 8042. https://doi.org/10.3390/jcm14228042
de Sire R, De Deo D, Mercurio M, Franchellucci G, Calabrese G, Bonacci L, Sollai Pinna M, Bezzio C, Armuzzi A, Hassan C, et al. Sessile Serrated Lesions in Inflammatory Bowel Disease: Hidden Players in Colitis-Associated Colorectal Cancer? Journal of Clinical Medicine. 2025; 14(22):8042. https://doi.org/10.3390/jcm14228042
Chicago/Turabian Stylede Sire, Roberto, Diletta De Deo, Miriana Mercurio, Gianluca Franchellucci, Giulio Calabrese, Livio Bonacci, Mauro Sollai Pinna, Cristina Bezzio, Alessandro Armuzzi, Cesare Hassan, and et al. 2025. "Sessile Serrated Lesions in Inflammatory Bowel Disease: Hidden Players in Colitis-Associated Colorectal Cancer?" Journal of Clinical Medicine 14, no. 22: 8042. https://doi.org/10.3390/jcm14228042
APA Stylede Sire, R., De Deo, D., Mercurio, M., Franchellucci, G., Calabrese, G., Bonacci, L., Sollai Pinna, M., Bezzio, C., Armuzzi, A., Hassan, C., Repici, A., Castiglione, F., Ardizzone, S., & Maselli, R. (2025). Sessile Serrated Lesions in Inflammatory Bowel Disease: Hidden Players in Colitis-Associated Colorectal Cancer? Journal of Clinical Medicine, 14(22), 8042. https://doi.org/10.3390/jcm14228042







