Diagnostic Utility of CT Findings as Indicators for Bowel Resection in Strangulated Small Bowel Obstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.1.1. The Inclusion Criteria Were as Follows
- Diagnosis of SSBO based on clinical and radiological findings.
- Implementation of preoperative contrast-enhanced abdominal CT.
- Surgical intervention during the same hospital admission.
- Age ≥ 20 years.
2.1.2. The Exclusion Criteria Were as Follows
- 1.
- Patients who improved with non-surgical conservative treatment alone.
- 2.
- Absence of contrast-enhanced CT.
- 3.
- Incomplete clinical or imaging data.
- 4.
- Patients who underwent surgery more than 24 h after contrast-enhanced CT and in whom there were no suspicions of SSBO at the time of surgical decision.
2.2. CT Protocol
2.3. Preoperative CT Assessment by Radiologist
2.4. CT Findings Suggestive of SSBO
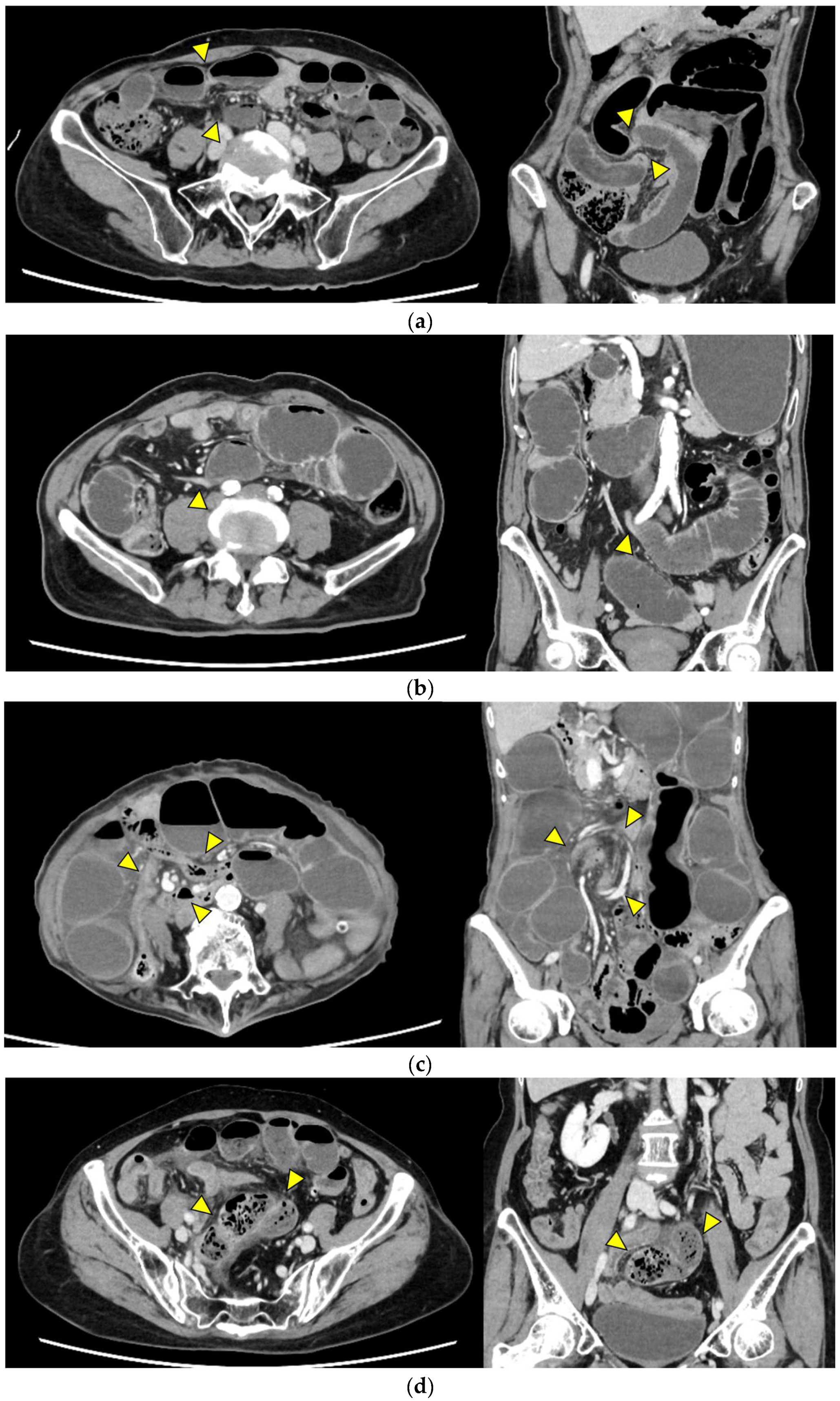
- Closed loop (Figure 1a): A dilated U- or C-shaped bowel segment with two transition points in close proximity, often associated with radial distribution of mesenteric vessels. This configuration may carry a high risk of rapid progression to strangulation and ischemia, requiring prompt surgical consideration.
- Beak sign (Figure 1b): Gradual tapering of the bowel lumen or contrast column at the obstruction point, typically indicating torsion or sharp angulation. This sign localizes the obstruction and often reflects mechanical blockage due to adhesions or volvulus, which may progress to ischemia if left untreated.
- Whirl sign (Figure 1c): Swirling of mesenteric vessels and fat, suggestive of volvulus or twisted mesentery. Presence of this sign strongly suggests torsion with compromised mesenteric blood flow.
- Small bowel feces sign (Figure 1d): Mixture of gas and particulate matter (resembling feces) within a dilated small bowel loop proximal to the obstruction, indicating delayed transit. This sign indicates subacute or prolonged obstruction, but does not necessarily imply ischemia.
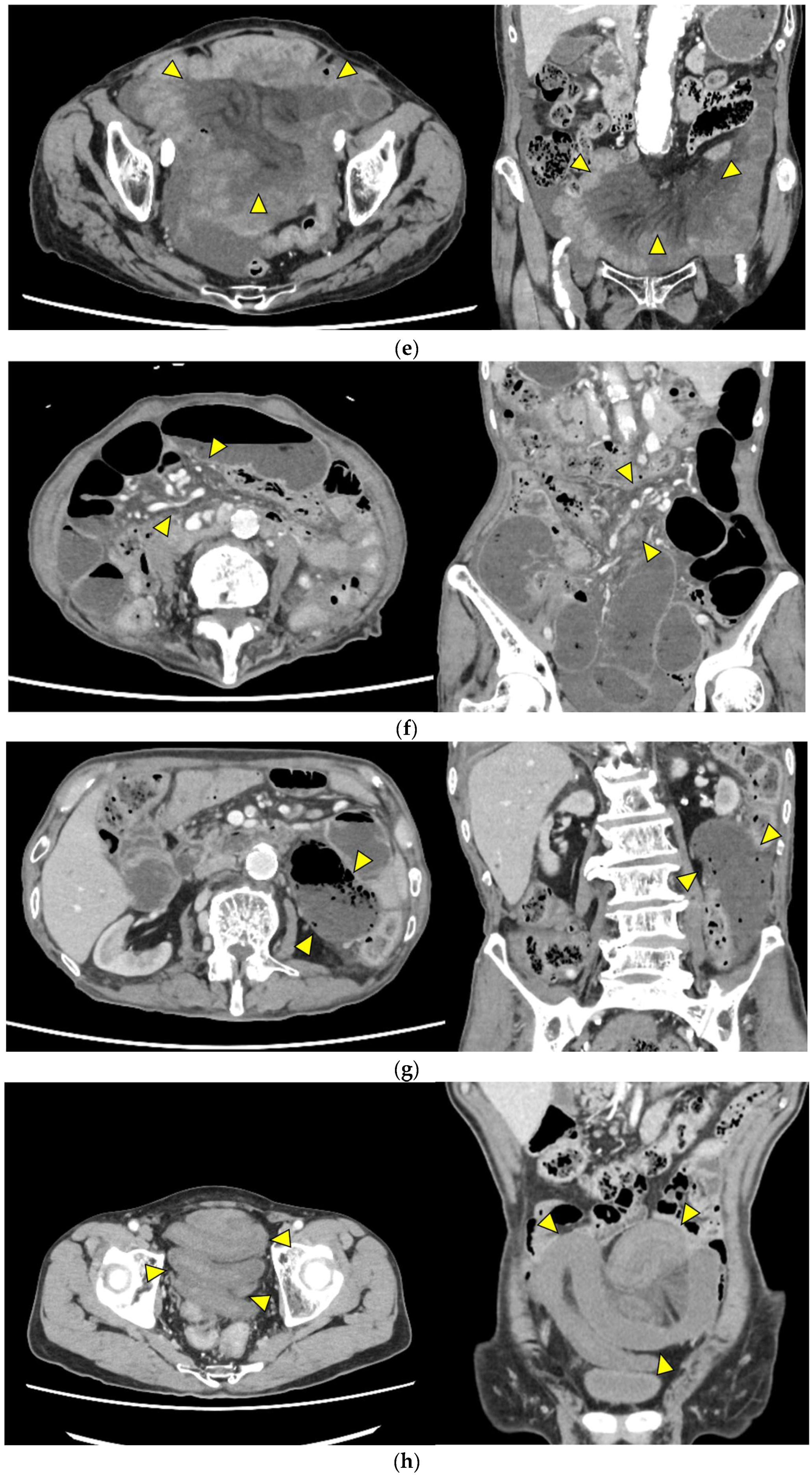
- Mesenteric edema (Figure 1e): Increased attenuation and stranding of mesenteric fat surrounding the affected loop, typically reflecting venous congestion. This is an early indicator of impaired venous outflow and increased risk of ischemia, requiring close monitoring and a low threshold for surgical intervention if clinical deterioration occurs.
- Mesenteric vessel engorgement (Figure 1f): Prominent or dilated mesenteric veins near the involved segment, suggestive of impaired venous outflow or strangulation. Venous congestion typically precedes arterial compromise, and the presence of this sign indicates evolving mesenteric ischemia.
- Absent bowel wall enhancement (Figure 1g): Complete absence of bowel wall enhancement on contrast-enhanced CT, which is highly suggestive of transmural infarction. This is a critical sign of irreversible ischemia and generally indicates the need for immediate surgical intervention.
- Blurred Kerckring folds (Figure 1h): Indistinct mucosal folds (valvulae conniventes) in dilated small bowel loops, often associated with ischemic edema. Blurring of mucosal folds reflects mucosal/submucosal injury from ischemia and impending bowel damage due to severe obstruction.
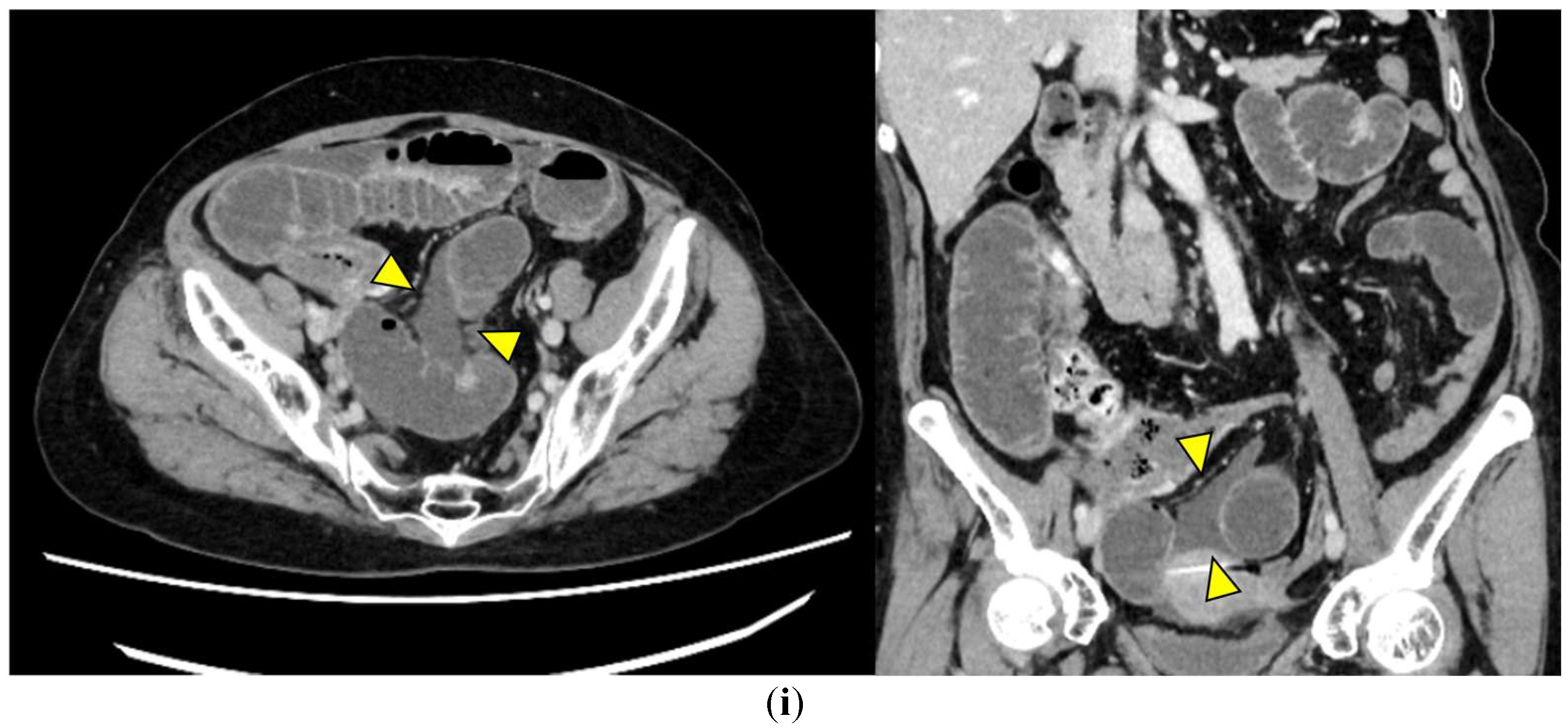
- Ascites (Figure 1i): Free peritoneal fluid, either localized around the affected loop or diffusely distributed, is frequently associated with advanced ischemia. Increasing ascites in the obstruction setting often indicates transmural ischemia or severe inflammation.
2.5. Surgical Procedure
2.6. Study Parameters
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Etiology of SSBO
3.3. Diagnostic Performance of CT Findings
3.4. Frequency of Preoperative CT Findings Between Groups
3.5. Multicollinearity Assessment
3.6. Multivariate Logistic Regression Analysis
3.7. ROC Curve Analysis
3.8. Exceptions in Imaging–Surgical Correlations
3.9. Interval Between CT and Surgery
4. Discussion
4.1. Overview of Key Findings
4.2. Clinical Significance of Other CT Findings
4.3. Discrepancies Between Intraoperative Decisions and CT Findings
4.4. Limitations
4.4.1. Study Design and Sample Size
4.4.2. Interobserver Variability
4.4.3. CT Acquisition Protocols
4.4.4. Assessment of Bowel Wall Enhancement
4.4.5. Interval Between CT and Surgery
4.4.6. Use of ICG Fluorescence Imaging
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA-PS | American Society of Anesthesiologists physical status |
| AUC | Area under the curve |
| BMI | Body mass index |
| CT | Computed tomography |
| ICG | Indocyanine green |
| ROC | Receiver operating characteristic |
| SSBO | Strangulated small bowel obstruction |
| VIF | Variance inflation factor |
Appendix A
| Protocol Type | Details (Iodine Dose, Tube Voltage) | Resection Group (n = 16) | Non-Resection Group (n = 36) |
|---|---|---|---|
| Single-phase | 384 mgI/kg, 100 kVp | 9 (56.3) | 18 (50.0) |
| 480 mgI/kg, 120 kVp | 0 (0) | 2 (5.6) | |
| Dual-phase | 480 mgI/kg, 100 kVp | 4 (25.0) | 12 (33.3) |
| 600 mgI/kg, 120 kVp | 1 (6.3) | 0 | |
| N/A | — | 2 (12.5) | 4 (11.1) |
References
- Xu, W.X.; Zhong, Q.H.; Cai, Y.; Zhan, C.H.; Chen, S.; Wang, H.; Lin, L.; Geng, Y.Q.; Hou, P.; Chen, X.Q.; et al. Prediction and management of strangulated bowel obstruction: A multi-dimensional model analysis. BMC Gastroenterol. 2022, 22, 304. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Fang, G.; Lin, J.; Xu, K.; Shi, H.; Zhuang, L. A Prediction Model for Recognizing Strangulated Small Bowel Obstruction. Gastroenterol. Res. Pract. 2018, 2018, 7164648. [Google Scholar] [CrossRef] [PubMed]
- Block, T.; Nilsson, T.K.; Björck, M.; Acosta, S. Diagnostic accuracy of plasma biomarkers for intestinal ischaemia. Scand. J. Clin. Lab. Investig. 2008, 68, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Murao, S.; Fujino, S.; Danno, K.; Takeda, T.; Yamamoto, K.; Higashiguchi, M.; Noguchi, K.; Hirao, T.; Oka, Y. Ischemia prediction score (IsPS) in patients with strangulated small bowel obstruction: A retrospective cohort study. BMC Gastroenterol. 2023, 23, 133. [Google Scholar] [CrossRef]
- Evennett, N.J.; Petrov, M.S.; Mittal, A.; Windsor, J.A. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J. Surg. 2009, 33, 1374–1383. [Google Scholar] [CrossRef]
- Millet, I.; Boutot, D.; Faget, C.; Pages-Bouic, E.; Molinari, N.; Zins, M.; Taourel, P. Assessment of Strangulation in Adhesive Small Bowel Obstruction on the Basis of Combined CT Findings: Implications for Clinical Care. Radiology 2017, 285, 798–808. [Google Scholar] [CrossRef]
- Millet, I.; Taourel, P.; Ruyer, A.; Molinari, N. Value of CT findings to predict surgical ischemia in small bowel obstruction: A systematic review and meta-analysis. Eur. Radiol. 2015, 25, 1823–1835. [Google Scholar] [CrossRef]
- Zalcman, M.; Sy, M.; Donckier, V.; Closset, J.; Gansbeke, D.V. Helical CT signs in the diagnosis of intestinal ischemia in small-bowel obstruction. AJR Am. J. Roentgenol. 2000, 175, 1601–1607. [Google Scholar] [CrossRef]
- Paulson, E.K.; Thompson, W.M. Review of small-bowel obstruction: The diagnosis and when to worry. Radiology 2015, 275, 332–342. [Google Scholar] [CrossRef]
- Ha, H.K.; Kim, J.S.; Lee, M.S.; Lee, H.J.; Jeong, Y.K.; Kim, P.N.; Lee, M.G.; Kim, K.W.; Kim, M.Y.; Auh, Y.H. Differentiation of simple and strangulated small-bowel obstructions: Usefulness of known CT criteria. Radiology 1997, 204, 507–512. [Google Scholar] [CrossRef]
- Nielsen, L.B.J.; Ærenlund, M.P.; Alouda, M.; Azzam, M.; Bjerke, T.; Burcharth, J.; Dibbern, C.B.; Jensen, T.K.; Jordhøj, J.Q.; Lolle, I.; et al. Real-world accuracy of computed tomography in patients admitted with small bowel obstruction: A multicentre prospective cohort study. Langenbecks Arch. Surg. 2023, 408, 341. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Z.; Liu, C.; Li, S.; Bi, W.; Ji, Q. Diagnostic nomogram for closed-loop small bowel obstruction requiring emergency surgery. Am. J. Emerg. Med. 2023, 63, 5–11. [Google Scholar] [CrossRef]
- Sheedy, S.P.; Earnest, F.t.; Fletcher, J.G.; Fidler, J.L.; Hoskin, T.L. CT of small-bowel ischemia associated with obstruction in emergency department patients: Diagnostic performance evaluation. Radiology 2006, 241, 729–736. [Google Scholar] [CrossRef]
- Zins, M.; Millet, I.; Taourel, P. Adhesive Small Bowel Obstruction: Predictive Radiology to Improve Patient Management. Radiology 2020, 296, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Mizunuma, K.; Sugiyama, M.; Sugawara, S.; Suzuki, T.; Tomabechi, M.; Kariyasu, T.; Fukuda, T. Interobserver agreement on the diagnosis of bowel ischemia: Assessment using dynamic computed tomography of small bowel obstruction. Jpn. J. Radiol. 2010, 28, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, E.J.; Birnbaum, B.A.; Megibow, A.J.; Gordon, R.B.; Whelan, C.A.; Hulnick, D.H. Closed-loop and strangulating intestinal obstruction: CT signs. Radiology 1992, 185, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Hara, K.; Goto, K.; Okamoto, A.; Kitagawa, T.; Marukuchi, R.; Ito, R.; Nakabayashi, Y. Fluorescence angiography vs. direct palpation for bowel viability evaluation with strangulated bowel obstruction. Langenbecks Arch. Surg. 2022, 407, 797–803. [Google Scholar] [CrossRef]
- De Simone, B.; Abu-Zidan, F.M.; Boni, L.; Castillo, A.M.G.; Cassinotti, E.; Corradi, F.; Di Maggio, F.; Ashraf, H.; Baiocchi, G.L.; Tarasconi, A.; et al. Indocyanine green fluorescence-guided surgery in the emergency setting: The WSES international consensus position paper. World J. Emerg. Surg. 2025, 20, 13. [Google Scholar] [CrossRef]
- Jancelewicz, T.; Vu, L.T.; Shawo, A.E.; Yeh, B.; Gasper, W.J.; Harris, H.W. Predicting strangulated small bowel obstruction: An old problem revisited. J. Gastrointest. Surg. 2009, 13, 93–99. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, M.; Wu, M.; Cheng, Z.; Wu, X.; Zhu, R. Unenhanced CT-based predictive model to identify small bowel necrosis in patients with mechanical small bowel obstruction. BMC Med. Imaging 2023, 23, 80. [Google Scholar] [CrossRef]
- Elsayes, K.M.; Menias, C.O.; Smullen, T.L.; Platt, J.F. Closed-loop small-bowel obstruction: Diagnostic patterns by multidetector computed tomography. J. Comput. Assist. Tomogr. 2007, 31, 697–701. [Google Scholar] [CrossRef]
- Jha, A.K.; Tang, W.H.; Bai, Z.B.; Xiao, J.Q. Sensitivity and Specificity of CT and Its signs for Diagnosis of Strangulation in Patients with Acute Small Bowel Obstruction. JNMA J. Nepal. Med. Assoc. 2014, 52, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, W.; Mortele, K. Small bowel ischemia caused by strangulation in complicated small bowel obstruction. CT findings in 20 cases with histopathological correlation. JBR-BTR 2011, 94, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, A.H.; Ghamari Khameneh, A.; Khosravi, B.; Mir, A.; Saffar, H.; Radmard, A.R. Many faces of acute bowel ischemia: Overview of radiologic staging. Insights Imaging 2021, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.C.; Bach, C.R.; Wells, M.L.; Andrews, J.C.; Khandelwal, A.; Welle, C.L.; Fidler, J.L. Imaging of Bowel Ischemia: An Update, From the AJR Special Series on Emergency Radiology. AJR Am. J. Roentgenol. 2023, 220, 173–185. [Google Scholar] [CrossRef]
- Chand, J.T.; Rakesh, R.; Ganesh, M.S. Adult intussusception: A systematic review of current literature. Langenbecks Arch. Surg. 2024, 409, 235. [Google Scholar] [CrossRef]
- Marinis, A.; Yiallourou, A.; Samanides, L.; Dafnios, N.; Anastasopoulos, G.; Vassiliou, I.; Theodosopoulos, T. Intussusception of the bowel in adults: A review. World J. Gastroenterol. 2009, 15, 407–411. [Google Scholar] [CrossRef]


| Characteristics | Resection Group (n = 16) | Non-Resection Group (n = 36) | p-Value |
|---|---|---|---|
| Male | 6 (37.5) | 12 (33.3) | 0.77 |
| Age (years) | 77.5 (49–96) | 75 (20–92) | 0.36 |
| BMI (kg/m2) | 19.1 (15.8–31.4) | 20.7 (15.3–32.5) | 0.41 |
| ASA-PS: III and IV | 7 (43.8) | 8 (22.2) | 0.11 |
| History of abdominal surgery | 12 (75.0) | 26 (72.2) | 0.83 |
| * Cardiac disease | 5 (31.3) | 10 (27.8) | 0.80 |
| Hypertension | 9 (56.3) | 17 (47.2) | 0.55 |
| ** Cerebrovascular disease | 1 (6.2) | 4 (11.1) | 0.58 |
| Diabetes mellitus | 2 (12.5) | 6 (16.7) | 0.70 |
| Etiologies | Resection Group (n = 16) | Non-Resection Group (n = 36) |
|---|---|---|
| Adhesion | 11 (68.8) | 28 (77.8) |
| Incarcerated ventral hernia | 1 (6.3) | 1 (2.8) |
| Intussusception (due to cecal cancer) | 1 (6.3) | 0 |
| Incarcerated inguinal hernia | 2 (12.5) | 3 (8.3) |
| Incarcerated femoral hernia | 1 (6.3) | 2 (5.6) |
| Intestinal volvulus | 0 | 1 (2.8) |
| Internal hernia | 0 | 1 (2.8) |
| CT Findings | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|
| Closed loop | 68.8 (44.4–85.8) | 61.1 (44.9–75.2) |
| Beak sign | 87.5 (64.0–96.5) | 13.9 (6.1–28.7) |
| Whirl sign | 12.5 (3.5–36.0) | 83.3 (68.1–92.1) |
| Small bowel feces sign | 12.5 (3.5–36.0) | 83.3 (68.1–92.1) |
| Mesenteric edema | 75.0 (50.5–89.8) | 30.6 (18.0–46.9) |
| Mesenteric vessel engorgement | 25.0 (10.2–49.5) | 94.4 (81.9–98.5) |
| Absent bowel wall enhancement | 75.0 (50.5–89.8) | 86.1 (71.3–93.9) |
| Blurred Kerckring folds | 25.0 (10.2–49.5) | 83.3 (68.1–92.1) |
| Ascites | 87.5 (64.0–96.5) | 16.7 (7.9–31.9) |
| CT Findings | All Cases (n = 52) | Resection Group (n = 16) | Non-Resection Group (n = 36) | p-Value |
|---|---|---|---|---|
| Closed loop | 25 | 11 (68.8) | 14 (38.9) | 0.047 |
| Beak sign | 45 | 14 (87.5) | 31 (86.1) | 1.0 |
| Whirl sign | 8 | 2 (12.5) | 6 (16.7) | 1.0 |
| Small bowel feces sign | 8 | 2 (12.5) | 6 (16.7) | 1.0 |
| Mesenteric edema | 37 | 12 (75.0) | 25 (69.4) | 0.75 |
| Mesenteric vessel engorgement | 6 | 4 (25.0) | 2 (5.6) | 0.064 |
| Absent bowel wall enhancement | 17 | 12 (75.0) | 5 (13.9) | <0.001 |
| Blurred Kerckring folds | 10 | 4 (25.0) | 6 (16.7) | 0.48 |
| Ascites | 44 | 14 (87.5) | 30 (83.3) | 1.0 |
| B | S.E. | Wald | OR | 95%CI | p-Value | |
|---|---|---|---|---|---|---|
| Closed loop | −0.111 | 0.900 | 0.015 | 0.895 | 0.153–5.22 | 0.902 |
| Absent bowel wall enhancement | 2.981 | 0.893 | 11.152 | 19.71 | 3.426–113.377 | 0.001 |
| n | Median (h) | Range (h) | p-Value | |
|---|---|---|---|---|
| All cases | 52 | 3.4 | 2.0–23.3 | |
| Non-resection | 36 | 4.1 | 2.0–23.3 | 0.004 |
| Resection | 16 | 2.9 | 2.0–5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okumura, T.; Tsujinaka, S.; Satani, N.; Yamamoto, K.; Nakano, T.; Yamada, T.; Katayose, Y.; Shibata, C. Diagnostic Utility of CT Findings as Indicators for Bowel Resection in Strangulated Small Bowel Obstruction. J. Clin. Med. 2025, 14, 8027. https://doi.org/10.3390/jcm14228027
Okumura T, Tsujinaka S, Satani N, Yamamoto K, Nakano T, Yamada T, Katayose Y, Shibata C. Diagnostic Utility of CT Findings as Indicators for Bowel Resection in Strangulated Small Bowel Obstruction. Journal of Clinical Medicine. 2025; 14(22):8027. https://doi.org/10.3390/jcm14228027
Chicago/Turabian StyleOkumura, Takashi, Shingo Tsujinaka, Nozomi Satani, Kuniharu Yamamoto, Toru Nakano, Takayuki Yamada, Yu Katayose, and Chikashi Shibata. 2025. "Diagnostic Utility of CT Findings as Indicators for Bowel Resection in Strangulated Small Bowel Obstruction" Journal of Clinical Medicine 14, no. 22: 8027. https://doi.org/10.3390/jcm14228027
APA StyleOkumura, T., Tsujinaka, S., Satani, N., Yamamoto, K., Nakano, T., Yamada, T., Katayose, Y., & Shibata, C. (2025). Diagnostic Utility of CT Findings as Indicators for Bowel Resection in Strangulated Small Bowel Obstruction. Journal of Clinical Medicine, 14(22), 8027. https://doi.org/10.3390/jcm14228027






