The Etiopathogenesis of Preeclampsia: Where Do We Stand Now?
Abstract
1. Introduction
2. Preeclampsia
2.1. Definition and Diagnostic Criteria, Clinical Presentation
2.2. Subtypes of Preeclampsia
3. Shallow Trophoblast Invasion in the Etiopathogenesis of Preeclampsia
3.1. Stage 1: Abnormal Placental Development and Trophoblast Invasion
3.2. Stage 2 Involves an Imbalance in Circulating Angiogenic Factors and Underlies the Development of Maternal Syndrome
3.3. Cytokines and Changes in Immune Cells
3.4. Renin–Angiotensin–Aldosterone System
3.5. Homocysteine
3.6. Nitric Oxide and ADMA
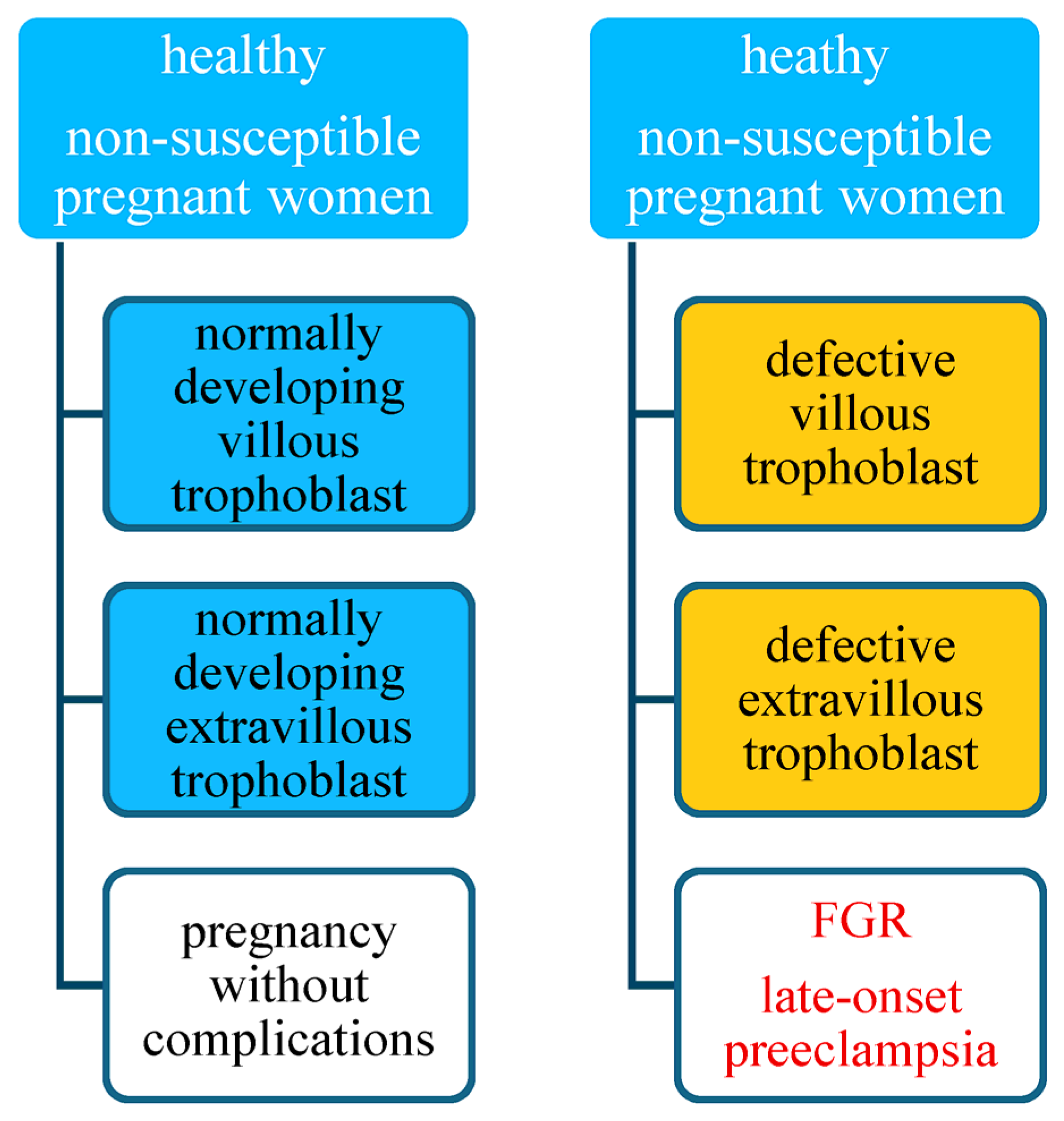
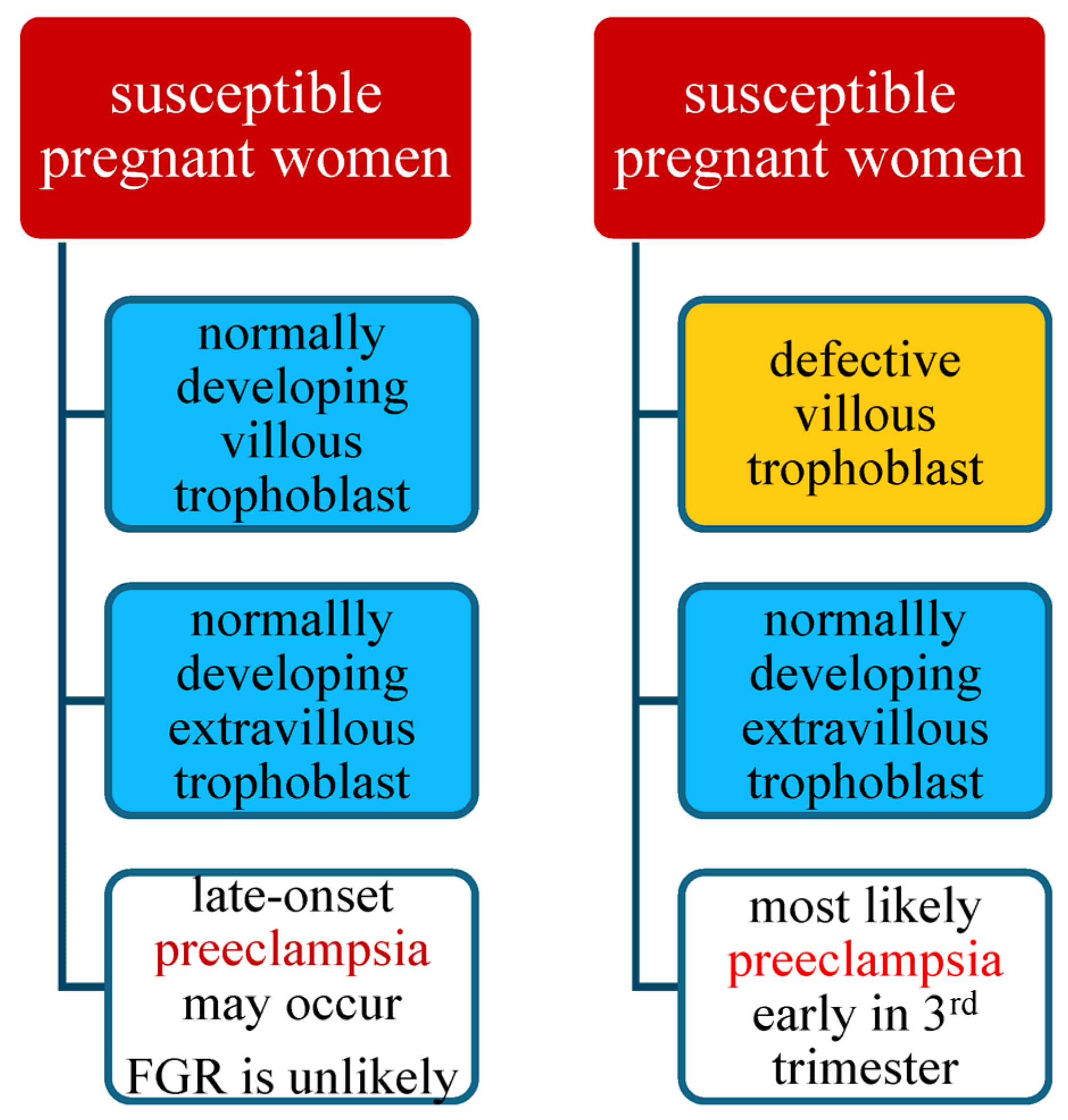
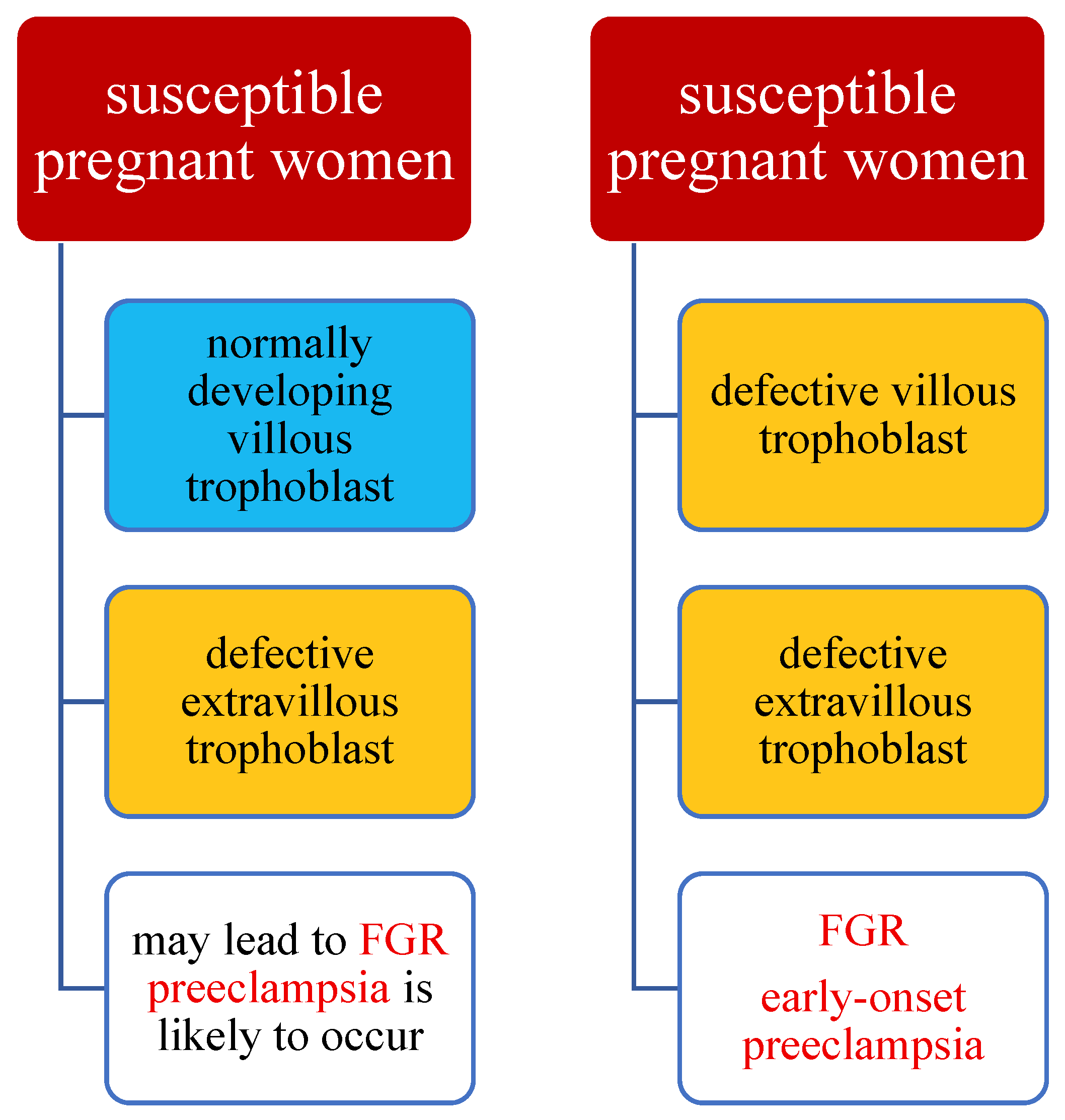
4. A Hypothesis of the Preeclampsia Etiology Takes into Account Maternal Susceptibility, Development of the Villous Trophoblast, and of the Extravillous Trophoblast
5. Clinical Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poon, L.C.; Shennan, A.; Hyett, J.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019, 145 (Suppl. S1), 1–33. [Google Scholar] [CrossRef]
- Huppertz, B. The Critical Role of Abnormal Trophoblast Development in the Etiology of Preeclampsia. Curr. Pharm. Biotechnol. 2018, 19, 771–780. [Google Scholar] [CrossRef]
- Staff, A.C. The two-stage placental model of preeclampsia: An update. J. Reprod. Immunol. 2019, 134–135, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-Eclampsia: Pathogenesis, Novel Diagnostics and Therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Latest advances in understanding preeclampsia. Science 2005, 308, 1592–1594. [Google Scholar] [CrossRef]
- Romero, R.; Chaiworapongsa, T. Preeclampsia: A link between trophoblast dysregulation and an antiangiogenic state. J. Clin. Investig. 2013, 123, 2775–2777. [Google Scholar] [CrossRef] [PubMed]
- Palei, A.C.; Spradley, F.T.; Warrington, J.P.; George, E.M.; Granger, J.P. Pathophysiology of hypertension in pre-eclampsia: A lesson in integrative physiology. Acta Physiol. 2013, 208, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Annesi, L.; Tossetta, G.; Borghi, C.; Piani, F. The Role of Xanthine Oxidase in Pregnancy Complications: A Systematic Review. Antioxidants 2024, 13, 1234. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Patel, K.; Lala-Trindade, A.; Feltovich, H.; Vieira, L.; Kontorovich, A.; Anath, C.; Taqueti, V.R.; Mitrani, L.; Stern, T.; et al. Pathophysiology of Preeclampsia-Induced Vascular Dysfunction and Implications for Subclinical Myocardial Damage and Heart Failure. JACC Adv. 2024, 3, 100980. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Brown, M.A.; Zeeman, G.G.; Dekker, G.; Sibai, B.M. The Definition of Severe and Early-Onset Preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens. 2013, 3, 44–47. [Google Scholar] [CrossRef]
- Newman, C.; Petruzzi, V.; Ramirez, P.T.; Hobday, C. Hypertensive Disorders of Pregnancy. Methodist Debakey Cardiovasc. J. 2024, 20, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.; Adovi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Fox, R.; Kitt, J.; Leeson, P.; Aye, C.Y.L.; Lewandowski, A.J. Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. J. Clin. Med. 2019, 8, 1625. [Google Scholar] [CrossRef]
- Bokslag, A.; van Wessenbruch, M.; Mol, B.W.; de Groot, C.J.M. Preeclampsia: Short and long-term consequences for mother and neonate. Early Hum. Dev. 2016, 102, 47–50. [Google Scholar] [CrossRef]
- Harmon, Q.E.; Huang, L.; Umbach, D.M.; Klungsøyr, K.; Engel, S.M.; Magnus, P.; Skjærven, R.; Zhang, J.; Wilcox, A.J. Risk of fetal death with preeclampsia. Obstet. Gynecol. 2015, 125, 628–635. [Google Scholar] [CrossRef]
- Groenhof, T.K.; Zoet, G.; Franx, A.; Gansevoort, R.T.; Bots, M.L.; Groen, H.; Lely, A.T.; PREVEND Group. Trajectory of Cardiovascular Risk Factors After Hypertensive Disorders of Pregnancy. Hypertension 2019, 73, 171–178. [Google Scholar] [CrossRef]
- Huppertz, B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 2008, 51, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Mongraw-Chaffin, M.L.; Cirillo, P.M.; Cohn, B.A. Preeclampsia and cardiovascular disease death: Prospective evidence from the child health and development studies cohort. Hypertension 2010, 56, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.; Peterson, E. A critical review of early-onset and late-onset preeclampsia. Obstet. Gynecol. Surv. 2011, 66, 497–506. [Google Scholar] [CrossRef]
- Theilen, L.H.; Meeks, H.; Fraser, A.; Esplin, M.S.; Smith, K.R.; Varner, M.W. Long-term mortality risk and life expectancy following recurrent hypertensive disease of pregnancy. Am. J. Obstet. Gynecol. 2018, 219, e1–e107. [Google Scholar] [CrossRef]
- Von Dadelszen, P.; Magee, L.A.; Roberts, J.M. Subclassification of preeclampsia. Hypertens. Pregnancy 2003, 22, 143–148. [Google Scholar] [CrossRef]
- Lisonkova, S.; Joseph, K.S. Incidence of preeclampsia: Risk factors and outcomes associated with early- versus late-onset disease. Am. J. Obstet. Gynecol. 2013, 209, e1–e544. [Google Scholar] [CrossRef]
- Verlohren, S.; Melchiorre, K.; Khalil, A.; Thilaganathan, B. Uterine artery Doppler, birth weight and timing of onset of pre-eclampsia: Providing insights into the dual etiology of late-onset pre-eclampsia. Ultrasound Obstet. Gynecol. 2014, 44, 293–298. [Google Scholar] [CrossRef]
- Xiong, X.; Demianczuk, N.N.; Saunders, L.D.; Wang, F.L.; Fraser, W.D. Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am. J. Epidemiol. 2002, 155, 203–209. [Google Scholar] [CrossRef]
- Correa, P.J.; Palmeiro, Y.; Soto, M.J.; Ugarte, C.; Illanes, S.E. Etiopathogenesis, prediction, and prevention of preeclampsia. Hypertens. Pregnancy 2016, 35, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Umapathy, A.; Chamley, L.W.; James, J.L. Reconciling the distinct roles of angiogenic/antiangiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies. Angiogenesis 2020, 23, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Brownbill, P.; McKeeman, G.C.; Brockelsby, J.C.; Crocker, I.P.; Sibley, C.P. Vasoactive and permeability effects of vascular endothelial growth factor-165 in the term in vitro dually perfused human placental lobule. Endocrinology 2007, 148, 4734–4744. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, P.W.; Stillman, I.E.; Epstein, F.K.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2022, 226, S1019–S1034. [Google Scholar] [CrossRef]
- Roberts, J.M.; Taylor, R.N.; Musci, T.J.; Rodgers, G.M.; Hubel, C.A.; McLaughlin, M.K. Preeclampsia: An endothelial cell disorder. Am. J. Obstet. Gynecol. 1989, 161, 1200–1204. [Google Scholar] [CrossRef]
- Brosens, I.; Robertson, W.B.; Dixon, H.G. The physiological response of the vessels of the placental bed to normal pregnancy. J. Pathol. Bacteriol. 1967, 93, 569–579. [Google Scholar] [CrossRef]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef]
- Zhou, Y.; Damsky, C.H.; Fisher, S.J. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J. Clin. Investig. 1997, 99, 2152–2164. [Google Scholar] [CrossRef]
- Brosens, I.; Renaer, M. On the pathogenesis of placental infarcts in preeclampsia. J. Obstet. Gynaecol. Br. Commonw. 1972, 79, 794–799. [Google Scholar] [CrossRef]
- De Wolf, F.; Robertson, W.B.; Brosens, I. The ultrastructure of acute atherosis in hypertensive pregnancy. Am. J. Obstet. Gynecol. 1975, 123, 164–174. [Google Scholar] [CrossRef]
- Lin, S.; Shimizu, I.; Suehara, N.; Nkayama, M.; Aono, T. Uterine artery Doppler velocimetry in relation to trophoblast migration into the myometrium of the placental bed. Obstet. Gynecol. 1995, 85, 760–765. [Google Scholar] [CrossRef] [PubMed]
- North, R.A.; Ferrier, C.; Long, D.; Townend, K.; Kincaid-Smith, P. Uterine artery Doppler flow velocity waveforms in the second trimester for the prediction of preeclampsia and fetal growth retardation. Obstet. Gynecol. 1994, 83, 378–386. [Google Scholar] [PubMed]
- Hecht, J.L.; Zsengeller, Z.K.; Spiel, M.; Karumanchi, S.A.; Rosen, S. Revisiting decidual vasculopathy. Placenta 2016, 42, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Stanek, J. Histological features of shallow placental implantation unify early-onset and late-onset preeclampsia. Pediatr. Dev. Pathol. 2018, 22, 112–122. [Google Scholar] [CrossRef]
- Stevens, D.U.; Al-Nasiry, S.; Bulten, J.; Spaanderman, M.E. Decidual vasculopathy in preeclampsia: Lesion characteristics relate to disease severity and perinatal outcome. Placenta 2013, 34, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Rabaglino, M.B.; Post Uiterweer, E.D.; Jeyabalan, A.; Hogge, W.; Conrad, K.P. Bioinformatics approach reveals evidence for impaired endometrial maturation before and during early pregnancy in women who developed preeclampsia. Hypertension 2015, 65, 421–429. [Google Scholar] [CrossRef]
- Garrido-Gomez, T.; Dominguez, F.; Quiñonero, A.; Diaz-Gimeno, P.; Kapidzic, M.; Gormley, M.; Ona, K.; Padilla-Iserte, P.; McMaster, M.; Genbacev, O.; et al. Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc. Natl. Acad. Sci. USA 2017, 114, E8468–E8477. [Google Scholar] [CrossRef] [PubMed]
- Soleymanlou, N.; Jurisica, I.; Nevo, O.; Ietta, F.; Zhang, X.; Zamudio, S.; Post, M.; Caniggia, I. Molecular evidence of placental hypoxia in preeclampsia. J. Clin. Endocrinol. Metab. 2005, 90, 4299–4308. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.P.; Skepper, J.N.; Burton, G.J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Caniggia, I.; Mostachfi, H.; Winter, J.; Gassmann, M.; Lye, S.J.; Kuliszewski, M.; Post, M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ(3). J. Clin. Investig. 2000, 105, 577–587. [Google Scholar] [CrossRef]
- Kanasaki, K.; Palmsten, K.; Sugimoto, H.; Ahmad, S.; Hamano, Y.; Xie, L.; Parry, S.; Augustin, H.G.; Gattone, V.H.; Folkman, J.; et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 2008, 453, 1117–1121. [Google Scholar] [CrossRef]
- Huang, Q.T.; Wang, S.S.; Zhang, M.; Huang, L.P.; Tian, J.W.; Yu, Y.H.; Wang, Z.J.; Zhong, M. Advanced oxidation protein products enhances soluble Fms-like tyrosine kinase 1 expression in trophoblasts: A possible link between oxidative stress and preeclampsia. Placenta 2013, 34, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Genbacev, O.; Zhou, Y.; Ludlow, J.W.; Fisher, S.J. Regulation of human placental development by oxygen tension. Science 1997, 277, 1669–1672. [Google Scholar] [CrossRef]
- Hu, X.Q.; Zhang, L. Hypoxia and Mitochondrial Dysfunction in Pregnancy Complications. Antioxidants 2021, 10, 405. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Polito, L.; Bortolott, M.; Bolognesi, A. Xanthine Oxidoreductase-Derived Reactive Species: Physiological and Pathological Effects. Oxidative Med. Cell. Longev. 2016, 2016, 3527579. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Veith, A.; Moorthy, B. Role of Cytochrome P450S in the generation and metabolism of reactive oxygen species. Curr. Opin. Toxicol. 2018, 7, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, T.; Nagamatsu, T.; Morita, K.; Mimura, N.; Iriyama, T.; Fujii, T.; Shibuya, M. HIF-2α, but not HIF-1α, mediates hypoxia-induced up-regulation of Flt-1 gene expression in placental trophoblasts. Sci. Rep. 2018, 8, 17375. [Google Scholar] [CrossRef] [PubMed]
- Nevo, O.; Soleymanlou, N.; Wu, Y.; Xu, J.; Kingdom, J.; Many, A.; Zamudio, S.; Caniggia, I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1085-93. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.L.; Skepper, J.N.; Jauniaux, E.; Burton, G.J. Susceptibility of human placental syncytiotrophoblastic mitochondria to oxygen-mediated damage in relation to gestational age. J. Clin. Endocrinol. Metab. 1998, 83, 1697–1705. [Google Scholar] [CrossRef]
- Wei, T.; Chen, C.; Hou, J.; Xin, W.; Mori, A. Nitric oxide induces oxidative stress and apoptosis in neuronal cells. Biochim. Biophys. Acta 2000, 1498, 72–79. [Google Scholar] [CrossRef]
- San Juan-Reyes, S.; Gómez-Oliván, L.M.; Islas-Flores, H.; Dublán-García, O. Oxidative stress in pregnancy complicated by preeclampsia. Arch. Biochem. Biophys. 2020, 681, 108255. [Google Scholar] [CrossRef]
- Sánchez-Aranguren, L.C.; Prada, C.E.; Carlos ERiaño-Medina, C.E.; Lopez, M. Endothelial dysfunction and preeclampsia: Role of oxidative stress. Front. Physiol. 2014, 5, 372. [Google Scholar] [CrossRef]
- Hung, T.H.; Burton, G.J. Hypoxia and reoxygenation: A possible mechanism for placental oxidative stress in preeclampsia. Taiwan. J. Obstet. Gynecol. 2006, 45, 189–200. [Google Scholar] [CrossRef]
- Scifres, C.M.; Nelson, D.M. Intrauterine growth restriction, human placental development and trophoblast cell death. J. Physiol. 2009, 587 Pt 14, 3453–3458. [Google Scholar] [CrossRef]
- Jauniaux, E.; Hempstock, J.; Greenwold, N.; Burton, G.J. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am. J. Pathol. 2003, 162, 115–125. [Google Scholar] [CrossRef]
- Jardim, L.L.; Rios, D.R.A.; Perucci, L.O.; de Sousa, L.P.; Gomes, K.B.; Dusse, L.M. Is the imbalance between pro-angiogenic and anti-angiogenic factors associated with preeclampsia? Clin. Chim. Acta 2015, 447, 34–38. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef]
- Caillon, H.; Tardif, C.; Dumontet, E.; Winer, N.; Masson, D. Evaluation of sFlt-1/PlGF Ratio for Predicting and Improving Clinical Management of Pre-eclampsia: Experience in a Specialized Perinatal Care Center. Ann. Lab. Med. 2018, 38, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, M.; Laskowska, K.; Oleszczuk, J. The relation of maternal serum eNOS, NOSTRIN and ADMA levels with aetiopathogenesis of preeclampsia and/or intrauterine fetal growth restriction. J. Matern. Fetal Neonatal Med. 2015, 28, 26–32. [Google Scholar] [CrossRef]
- Laskowska, M.; Laskowska, K.; Oleszczuk, J. Endoglin in pregnancy complicated by fetal intrauterine growth restriction in normotensive and preeclamptic pregnant women: A comparison between preeclamptic patients with appropriate-for-gestational-age weight infants and healthy pregnant women. J. Matern. Fetal Neonatal Med. 2012, 25, 806–811. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Dunk, C.; Ahmad, S.; Khaliq, A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen--a review. Placenta 2000, 21 (Suppl. A), S16–S24. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Geusens, N.; Morton, J.; Verhaegen, I.; Hering, L.; Herse, F.; Dudenhausen, J.W.; Muller, D.N.; Luft, F.C.; Cartwright, J.E.; et al. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension 2010, 56, 304–310. [Google Scholar] [CrossRef]
- Elliot, M.G.; Crespi, B.J. Genetic recapitulation of human pre-eclampsia risk during convergent evolution of reduced placental invasiveness in eutherian mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140069. [Google Scholar] [CrossRef]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Horke, S.; Förstermann, U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 2014, 237, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Higaki, T.; Matsubara, Y.; Nawa, A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. Int. J. Mol. Sci. 2015, 16, 4600–4614. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Zsengellér, Z.K.; Rajakumar, A.; Hunter, J.T.; Salahuddin, S.; Rana, S.; Stillman, I.E.; Karumanchi, S.A. Trophoblast mitochondrial function is impaired in preeclampsia and correlates negatively with the expression of soluble fms-like tyrosine kinase 1. Pregnancy Hypertens. 2016, 6, 313–319. [Google Scholar] [CrossRef]
- Yung, H.W.; Calabrese, S.; Hynx, D.; Hemmings, B.A.; Cetin, I.; Charnock-Jones, D.S.; Burton, G.J. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am. J. Pathol. 2008, 173, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Yung, H.W.; Korolchuk, S.; Tolkovsky, A.M.; Charnock-Jones, D.S.; Burton, G.J. Endoplasmic reticulum stress exacerbates ischemia-reperfusion-induced apoptosis through attenuation of Akt protein synthesis in human choriocarcinoma cells. FASEB J. 2007, 21, 872–884. [Google Scholar] [CrossRef]
- Lian, I.A.; Løset, M.; Mundal, S.B.; Fenstad, M.H.; Johnson, M.P.; Eide, I.P.; Bjørge, L.; Freed, K.A.; Moses, E.K.; Austgulen, R. Increased endoplasmic reticulum stress in decidual tissue from pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Placenta 2011, 32, 823–829. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, L.; Wang, L.; Zhu, X. Expression of markers of endoplasmic reticulum stress-induced apoptosis in the placenta of women with early and late onset severe pre-eclampsia. Taiwan. J. Obstet. Gynecol. 2015, 54, 19–23. [Google Scholar] [CrossRef]
- Damsky, C.H.; Fitzgerald, M.L.; Fisher, S.J. Distribution Patterns of Extracellular Matrix Components and Adhesion Receptors Are Intricately Modulated during First Trimester Cytotrophoblast Differentiation along the Invasive Pathway, in Vivo. J. Clin. Investig. 1992, 89, 210–222. [Google Scholar] [CrossRef]
- Zhou, Y.; Genbacev, O.; Fisher, S.J. The Human Placenta Remodels the Uterus by Using a Combination of Molecules That Govern Vasculogenesis or Leukocyte Extravasation. Ann. N. Y. Acad. Sci. 2003, 995, 73–83. [Google Scholar] [CrossRef]
- Meekins, J.W.; Pijnenborg, R.; Hanssens, M.; McFadyen, I.R.; van Asshe, A. A Study of Placental Bed Spiral Arteries and Trophoblast Invasion in Normal and Severe Pre-Eclamptic Pregnancies. Br. J. Obstet. Gynaecol. 1994, 101, 669–674. [Google Scholar] [CrossRef]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C.P. Rheological and Physiological Consequences of Conversion of the Maternal Spiral Arteries for Uteroplacental Blood Flow during Human Pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Bujold, E.; Romero, R.; Chaiworapongsa, T.; Kim, Y.M.; Kim, G.J.; Kim, M.R.; Espinoza, J.; Gonçalves, L.F.; Edwin, S.; Mazor, M. Evidence Supporting That the Excess of the SVEGFR-1 Concentration in Maternal Plasma in Preeclampsia Has a Uterine Origin. J. Matern.-Fetal Neonatal Med. 2005, 18, 9–16. [Google Scholar] [CrossRef]
- Clark, D.E.; Smith, S.K.; He, Y.; Day, K.A.; Licence, D.R.; Corps, A.N.; Lammoglia, R.; Charnock-Jones, D.S. A Vascular Endothelial Growth Factor Antagonist Is Produced by the Human Placenta and Released into the Maternal Circulation1. Biol. Reprod. 1998, 59, 1540–1548. [Google Scholar] [CrossRef]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.-H.; Yuan, H.-T.; Libermann, T.A.; et al. Soluble Endoglin Contributes to the Pathogenesis of Preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Weel, I.C.; Baergen, R.N.; Romão-Veiga, M.; Borges, V.T.; Ribeiro, V.R.; Witkin, S.S.; Bannwart-Castro, C.; Peraçoli, J.C.; De Oliveira, L.; Peraçoli, M.T. Association between Placental Lesions, Cytokines and Angiogenic Factors in Pregnant Women with Preeclampsia. PLoS ONE 2016, 11, e0157584. [Google Scholar] [CrossRef] [PubMed]
- De Falco, S. The discovery of placenta growth factor and its biological activity. Exp. Mol. Med. 2012, 44, 1–9. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmed, A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ. Res. 2004, 95, 884–891. [Google Scholar] [CrossRef]
- Bergmann, A.; Ahmad, S.; Cudmore, M.; Gruber, A.D.; Wittschen, P.; Lindenmaier, W.; Christofori, G.; Gross, V.; Gonzalves ACh Gröne, H.J.; Ahmed, A.; et al. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J. Cell Mol. Med. 2010, 14, 1857–1867. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Ying Ma, J.; Kapoun, A.M.; Shao, Q.; Kerr, A.; O’Young, G.; Sannajust, F.; Stathis, P.; Schreiner, G.; et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension 2007, 50, 686–692. [Google Scholar] [CrossRef]
- Hladunewich, M.A.; Steinberg, G.; Karumanchi, S.A.; Levine, R.J.; Keating, S.; Kingdom, J.; Keunen, J. Angiogenic factor abnormalities and fetal demise in a twin pregnancy. Nat. Rev. Nephrol. 2009, 5, 658–662. [Google Scholar] [CrossRef]
- Stepan, H.; Faber, R. Elevated sFlt1 level and preeclampsia with parvovirus-induced hydrops. N. Engl. J. Med. 2006, 354, 1857–1858. [Google Scholar] [CrossRef]
- Powe, C.E.; Levine, R.J.; Karumanchi, S.A. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011, 123, 2856–2869. [Google Scholar] [CrossRef]
- Maharaj, A.S.; Walshe, T.E.; Saint-Geniez, M.; Venkatesha, S.; Maldonado, A.E.; Himes, N.C.; Matharu, K.S.; Karumanchi, S.A.; D’Amor, P.A. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J. Exp. Med. 2008, 205, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.; Bean, C.; Bowles, T.; Spencer, S.K.; Randle, W.; Kyle, P.B.; Shaffery, J. Hypertension, anxiety, and blood-brain barrier permeability are increased in postpartum severe preeclampsia/hemolysis, elevated liver enzymes, and low platelet count syndrome rats. Hypertensions 2018, 72, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Guller, S.; Tang, Z.; Ma, Y.Y.; Di Santo, S.; Sager, R.; Schneider, H. Protein composition of microparticles shed from human placenta during placental perfusion: Potential role in angiogenesis and fibrinolysis in preeclampsia. Placenta 2011, 32, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Yao, J.; He, Q.; Liu, M.; Duan, T.; Wang, K. Exosomes from women with preeclampsia induced vascular dysfunction by delivering sFlt (Soluble Fms-Like Tyrosine Kinase)-1 and sEng (Soluble Endoglin) to endothelial cells. Hypertension 2018, 72, 1381–1390. [Google Scholar] [CrossRef]
- Saito, S.; Sakai, M. Th1/Th2 balance in preeclampsia. J. Reprod. Immunol. 2003, 59, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, T.G.; Lin, H.; Guilbert, L.; Mosmann, T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a TH2 phenomenon? Immunol Today. 1993, 14, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, S.; Sri Manjari, K.; Ramaiah, A.; Sunitha, T.; Nallari, P.; Jyothy, A.; Venkateshwari, A. Interleukin 10 gene promoter polymorphisms in women with early-onset pre-eclampsia. Clin. Exp. Immunol. 2014, 178, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qian, L.; Wu, F.; Li, M.; Wang, H. Significance of toll-like receptor 4 signaling in peripheral blood monocytes of preeclamptic patients. Hypertens. Pregnancy 2015, 34, 486–494. [Google Scholar] [CrossRef]
- Medeiros, L.T.; Peraçoli, J.C.; Bannwart-Castro, C.F.; Romão, M.; Weel, I.C.; Golim, M.A.; de Oliveira, L.G.; Kurokawa, C.S.; Borges, V.T.M.; Peraçoli, M.T.S. Monocytes from pregnant women with pre-eclampsia are polarized to a M1 phenotype. Am. J. Reprod. Immunol. 2014, 72, 5–13. [Google Scholar] [CrossRef]
- Campos-Cañas, J.; Romo-Palafox, I.; Albani-Campanario, M.; Hernández-Guerrero, C. An imbalance in the production of proinflammatory and anti-inflammatory cytokines is observed in whole blood cultures of preeclamptic women in comparison with healthy pregnant women. Hypertens. Pregnancy 2014, 33, 236–249. [Google Scholar] [CrossRef]
- Cristofalo, R.; Bannwart-Castro, C.F.; Magalhães, C.G.; Borges, V.T.M.; Peraçoli, J.C.; Witkin, S.S.; Peraçoli, M.T. Silibinin attenuates oxidative metabolism and cytokine production by monocytes from preeclamptic women. Free Radic. Res. 2013, 47, 268–275. [Google Scholar] [CrossRef]
- Gelber, S.E.; Brent, E.; Redecha, P.; Perino, G.; Tomlinson, S.; Davisson, R.L.; Salmon, J.E. Prevention of defective placentation and pregnancy loss by blocking innate immune pathways in a syngeneic model of placental insufficiency. J. Immunol. 2015, 195, 1129–1138. [Google Scholar] [CrossRef]
- Qing, X.; Redecha, P.B.; Burmeister, M.A.; Tomlinson, S.; D’Agati, V.D.; Davisson, R.L.; Salmon, J.E. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney Int. 2011, 79, 331–339. [Google Scholar] [CrossRef]
- Yang, X.; Wang, F.; Lau, W.B.; Zhang, S.; Zhang, S.; Liu, H.; Ma, X.-L. Autoantibodies isolated from preeclamptic patients induce endothelial dysfunction via interaction with the angiotensin II AT1 receptor. Cardiovasc. Toxicol. 2014, 14, 21–29. [Google Scholar] [CrossRef]
- Dechend, R.; Homuth, V.; Wallukat, G.; Park, J.K.; Theuer, J.; Juepner, A.; Gulba, D.C.; Mackman, N.; Halle, H.; Luft, F.C. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation 2000, 101, 2382–2387. [Google Scholar] [CrossRef] [PubMed]
- LaMarca, B.; Wallukat, G.; Llinas, M.; Herse, F.; Dechend, R.; Granger, J.P. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 2008, 52, 1168–1172. [Google Scholar] [CrossRef]
- Parrish, M.R.; Murphy, S.R.; Rutland, S.; Wallace, K.; Wenzel, K.; Wallukat, G.; Keiser, S.; Ray, F.L.; Dechend, R.; Martin, J.N.; et al. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am. J. Hypertens. 2010, 23, 911–916. [Google Scholar] [CrossRef]
- Herse, F.; LaMarca, B. Angiotensin II type 1 receptor autoantibody (AT1-AA)-mediated pregnancy hypertension. Am. J. Reprod. Immunol. 2013, 69, 413–418. [Google Scholar] [CrossRef]
- Dymara-Konopka, W.; Laskowska, M. The Role of Nitric Oxide, ADMA, and Homocysteine in The Etiopathogenesis of Preeclampsia-Review. Int. J. Mol. Sci. 2019, 20, 2757. [Google Scholar] [CrossRef]
- Dymara-Konopka, W.; Laskowska, M.; Błażewicz, A. Angiogenic Imbalance as a Contributor of Preeclampsia. Curr. Pharm. Biotechnol. 2018, 19, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Borzychowski, A.M.; Sargent, I.L.; Redman, C.W.G. Inflammation and pre-eclampsia. Semin. Fetal Neonatal Med. 2006, 11, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Luppi, P.; Deloia, J.A. Monocytes of preeclamptic women spontaneously synthesize proinflammatory cytokines. Clin. Immunol. 2006, 118, 268–275. [Google Scholar] [CrossRef]
- Huppertz, B.; Schleußner, E. The Placenta Basics and Clinical Significance; Springer: Berlin, Germany, 2023; pp. 243–280. [Google Scholar]
- Sacks, G.P.; Studena, K.; Sargent, I.L.; Redman, C.W.G. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am. J. Obstet. Gynecol. 1998, 179, 80–86. [Google Scholar] [CrossRef]
- Huppertz, B.; Frank, H.G.; Kingdom, J.C.; Reister, F.; Kaufmann, P. Villous cytotrophoblast regulation of the syncytial apoptotic cascade in the human placenta. Histochem. Cell Biol. 1998, 110, 495–508. [Google Scholar] [CrossRef]
- Huppertz, B. IFPA Award in Placentology Lecture: Biology of the placental syncytiotrophoblast—Myths and facts. Placenta 2010, 31 (Suppl.), S75–S81. [Google Scholar] [CrossRef]
- Goswami, D.; Tannetta, D.S.; Magee, L.A.; Fuchisawa, A.; Redman, C.W.G.; Sargen, I.L.; von Dadelszen, P. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta 2006, 27, 56–61. [Google Scholar] [CrossRef]
- Moser, G.; Huppertz, B. Implantation and extravillous trophoblast invasion: From rare archival specimens to modern biobanking. Placenta 2017, 56, 19–26. [Google Scholar] [CrossRef]
- Huppertz, B. Trophoblast differentiation, fetal growth restriction and preeclampsia. Pregnancy Hypertens. 2011, 1, 79–86. [Google Scholar] [CrossRef]
- Stepan, H.; Herraiz, I.; Schlembach, D.; Verlohren, S.; Brennecke, S.; Chantraibe, F.; Klein, E.; Lapaire, O.; Llurba, E.; Ramoni, A.; et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: Implications for clinical practice. Ultrasound Obstet. Gynecol. 2015, 45, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, I.; Llurba, E.; Verlohren, S.; Galindo, A.; Spanish Group for the Study of Angiogenic Markers in Preeclampsia. Update on the Diagnosis and Prognosis of Preeclampsia with the Aid of the sFlt-1/PlGF Ratio in Singleton Pregnancies. Fetal Diagn. Ther. 2018, 43, 81–89. [Google Scholar] [CrossRef]
- Winkler, K.; Wetzka, B.; Hoffmann, M.M.; Friedrich, I.; Kinner, M.; Baumstark, M.W.; Wieland, H.; März, W.; Zahradnik, H.P. Low density lipoprotein (LDL) subfractions during pregnancy: Accumulation of buoyant LDL with advancing gestation. J. Clin. Endocrinol. Metab. 2000, 85, 4543–4550. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Pütz, G.; Pecks, U.; Winkler, K. Apheresis as an emerging treatment option in severe early-onset preeclampsia. Atheroscler. Suppl. 2019, 40, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Gallos, I.D.; Sivakumar, K.; Kilby, M.D.; Coomarasamy, A.; Thangaratinam, S.; Vatish, M. Pre-eclampsia is associated with, and preceded by, hypertriglyceridaemia: A meta-analysis. BJOG 2013, 120, 1321–1332. [Google Scholar] [CrossRef]
- Contini, C.; Jansen, M.; König, B. Lipoprotein turnover and possible remnant accumulation in preeclampsia: Insights from the Freiburg Preeclampsia H.E.L.P.-apheresis study. Lipids Health Dis. 2018, 17, 49. [Google Scholar] [CrossRef]
- Wang, Y.; Walli, A.K.; Schulze, A.; Blessing, F.; Frauenberger, P.; Thaler, C.; Seidel, D.; Hasbargen, U. Heparin-mediated extracorporeal low density lipoprotein precipitation as a possible therapeutic approach in preeclampsia. Transfus. Apher. Sci. 2006, 35, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Winkler, K.; Hoffmann, M.M.; Pütz, G. Letter by Winkler et al Regarding Article, “Pilot Study of Extracorporeal Removal of Soluble Fms-Like Tyrosine Kinase 1 in Preeclampsia. Circulation 2012, 125, 1161e2. [Google Scholar] [CrossRef][Green Version]
- Pecks, U.; Rath, W.; Kleine-Eggebrecht, N.; Maass, N.; Voigt, F.; Goecke, T.W.; Mohaupt, M.G.; Escher, G. Maternal Serum Lipid, Estradiol, and Progesterone Levels in Pregnancy, and the Impact of Placental and Hepatic Pathologies. Geburtshilfe Frauenheilkd. 2016, 76, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Diamond, P.; Singh, G.; Bell, C.M. Brief overview of maternal triglycerides as a risk factor for pre-eclampsia. BJOG 2006, 113, 379–386. [Google Scholar] [CrossRef]
- Winkler, K.; Wetzka, B.; Hoffmann, M.M. Triglyceride-rich lipoproteins are associated with hypertension in preeclampsia. J. Clin. Endocrinol. Metab. 2003, 88, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Thadhani, R.; Hagmann, H.; Schaarschmidt, W.; Roth, B.; Cingoez, T.; Karumanchi, S.A.; Wenger, J.; Lucchesi, K.J.; Tamez, H.; Lindner, T.; et al. Removal of Soluble Fms-Like Tyrosine Kinase-1 by Dextran Sulfate Apheresis in Preeclampsia. J. Am. Soc. Nephrol. 2015, 27, 903–913. [Google Scholar] [CrossRef]
- Haddad, B.; Lefèvre, G.; Rousseau, A.; Robert, T.; Saheb, S.; Rafat, S.; Bornes, M.; Petit-Hoang, C.; Richard, F.; Lecarpenter, E.; et al. LDL-apheresis to decrease sFlt-1 during early severe preeclampsia: Report of two cases from a discontinued phase II trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 70–74. [Google Scholar] [CrossRef]
- Palmiero, P.; Caretto, P.; Ciccone, M.M.; Maiello, M.; On Behalf Of The I C I S C U Italian Chapter Of International Society Cardiovascular Ultrasoun. Long-Term Cardiovascular Risk and Maternal History of Pre-Eclampsia. J. Clin. Med. 2025, 14, 3121. [Google Scholar] [CrossRef]
- Garovic, V.D.; Dechend, R.; Easterling, T.; Karumanchi, S.A.; Mc Murtry Baird, S.; Magee, L.A.; Rana, S.; Vermunt, J.V.; August, P. Hypertension in Pregnancy: Diagnosis, Blood Pressure Goals, and Pharmacotherapy: A Scientific Statement From the American Heart Association. Hypertension 2022, 79, e21–e41. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.Y.; Syngelaki, A.; O’Gorman, N.; de Paco Matallama, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. ASPRE trial: Performance of screening for preterm pre-eclampsia. Ultrasound Obstet. Gynecol. 2017, 50, 492–495. [Google Scholar] [CrossRef]
- Basaran, A. Pregnancy-induced hyperlipoproteinemia: Review of the literature. Reprod. Sci. 2009, 16, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Winkler, K.; Lorey, C.; Contini, C.; Agustinski, V.; Pütz, G.; Röthele, E.; Brenner, A.; Fuchs, H.; Pecks, U.; Markfeld-Erol, F.; et al. Comparison of double-filtration plasmapheresis (DFPP) versus heparin-mediated extracorporeal LDL-precipitation (HELP)-apheresis in early-onset preeclampsia. Pregnancy Hypertens. 2024, 36, 101128. [Google Scholar] [CrossRef] [PubMed]
- Makris, A.; Yeung, K.R.; Lim, S.M.; Sunderland, N.; Heffernan, S.; Thompson, J.F.; Iliopoulos, J.; Killingsworth, M.C.; Yong, J.; Xu, B.; et al. Placental growth factor reduces blood pressure in a uteroplacental ischemia model of preeclampsia in nonhuman primates. Hypertension 2016, 67, 1263–1272. [Google Scholar] [CrossRef]
- Spradley, F.T.; Tan, A.Y.; Joo, W.S.; Danioels, G.; Kusse, P.; Karumanchi, S.A.; Granger, J.P. Placental growth factor administration abolishes placental ischemia-induced hypertension. Hypertension 2016, 67, 740–747. [Google Scholar] [CrossRef]
- Sibai, B.M. Etiology and management of postpartum hypertension-preeclampsia. Am. J. Obstet. Gynecol. 2012, 206, 470–475. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskowska, M.; Bednarek, A.; Stworowski, M. The Etiopathogenesis of Preeclampsia: Where Do We Stand Now? J. Clin. Med. 2025, 14, 7992. https://doi.org/10.3390/jcm14227992
Laskowska M, Bednarek A, Stworowski M. The Etiopathogenesis of Preeclampsia: Where Do We Stand Now? Journal of Clinical Medicine. 2025; 14(22):7992. https://doi.org/10.3390/jcm14227992
Chicago/Turabian StyleLaskowska, Marzena, Anna Bednarek, and Maciej Stworowski. 2025. "The Etiopathogenesis of Preeclampsia: Where Do We Stand Now?" Journal of Clinical Medicine 14, no. 22: 7992. https://doi.org/10.3390/jcm14227992
APA StyleLaskowska, M., Bednarek, A., & Stworowski, M. (2025). The Etiopathogenesis of Preeclampsia: Where Do We Stand Now? Journal of Clinical Medicine, 14(22), 7992. https://doi.org/10.3390/jcm14227992






