Adipokine Profile Signature in Adolescent Girls with Menstrual Disorders and Hyperandrogenism Differs from That of Regularly Menstruating Girls
Abstract
1. Introduction
2. Material and Methods
- Anthropometric measurements: body weight, height, and waist circumference were measured using standard procedures. Body weight was recorded to the nearest 0.1 kg, and height to the nearest 0.1 cm using a Harpenden stadiometer (Holtain Ltd., Crymych, Wales, UK). Body mass index (BMI) and the BMI standard deviation score (BMI z-score) were calculated. The severity of hirsutism was assessed using the modified Ferriman–Gallwey (mFG) scoring system, with a score of ≥8 considered indicative of clinically significant hirsutism.
- Body composition was assessed by bioelectrical impedance analysis using the Tanita MC-780 device (Tanita Corporation, Tokyo, Japan). The following parameters were evaluated: body fat percentage, fat mass (kg), fat-free mass (FFM), and muscle mass (kg).
- Biochemical parameters: total cholesterol (TC), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), triglycerides (TG), and glucose concentrations were measured. Glucose levels were determined at fasting (GLU 0′) and 120 min after the oral glucose tolerance test (OGTT; GLU 120′).
- Hormonal parameters: fasting (INS 0′) and 120 min post-OGTT (INS 120′) insulin concentrations were measured. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the formula:
3. Results
4. Discussion
4.1. Relationship of Adipokine Networks to Parameters of Carbohydrate Metabolism
4.2. Relationship of Adipokine Networks to Lipid Metabolism Parameters
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| BMI z-Score | Waist Circumference | WHtR | Fat Mass on BIA (%) | |
|---|---|---|---|---|
| adiponectin | −0.404 (p = 0.013) 95% CI (−0.728; −0.080) | −0.355 (p = 0.027) 95% CI (−0.693; −0.016) | −0.372 (p = 0.022) 95% CI (−0.705; −0.038) | −0.140 (p = 0.242) 95% CI (−0.544; 0.263) |
| leptin | 0.501 (p = 0.002) 95% CI (0.211; 0.791) | 0.604 (p = 0) 95% CI (0.358; 0.850) | 0.422 (p = 0.01) 95% CI (0.104; 0.740) | 0.593 (p = 0.001) 95% CI (0.325; 0.860) |
| omentin | −0.225 (p = 0.116) 95% CI (−0.593; 0.142) | −0.124 (p = 0.256) 95% CI (−0.505; 0.257) | −0.131 (p = 0.246) 95% CI (−0.511; 0.25) | −0.198 (p = 0.161) 95% CI (−0.594; 0.197) |
| visfatin | 0.254 (p = 0.088) 95% CI (−0.108; 0.616) | 0.293 (p = 0.058) 95% CI (−0.061; 0.647) | 0.305 (p = 0.051) 95% CI (−0.047; 0.656) | 0.115 (p = 0.283) 95% CI (−0.291; 0.522) |
| L/A | 0.509 (p = 0.002) 95% CI (0.222; 0.796) | 0.638 (p < 0.001) 95% CI (0.409; 0.868) | 0.444 (p = 0.007) 95% CI (0.133; 0.755) | 0.492 (p = 0.005) 95% CI (0.179; 0.804) |
| (a) Correlations between adipokines levels and parameters of carbohydrate metabolism in the control group | |||||||
| GLU 0′ | GLU 120′ | INS 0′ | INS 120′ | ||||
| adiponectin | −0.199 (p = 0.146) 95% CI (−0.571; 0.173) | −0.220 (p = 0.135) 95% CI (−0.612; 0.172) | −0.064 (p = 0.371) 95% CI (−0.457; 0.329) | −0.187 (p = 0.176) 95% CI (−0.584; 0.211) | |||
| leptin | 0.337 (p = 0.034) 95% CI (−0.006; 0.680) | 0.465 (p = 0.007) 95% CI (0.142; 0.788) | 0.341 (p = 0.035) 95% CI (−0.008; 0.690) | 0.252 (p = 0.102) 95% CI (−0.134; 0.638) | |||
| omentin | −0.033 (p = 0.432) 95% CI (−0.419; 0.354) | −0.332 (p = 0.045) 95% CI (−0.699; 0.034) | −0.056 (p = 0.386) 95% CI (−0.45; 0.338) | −0.265 (p = 0.091) 95% CI (−0.648; 0.118) | |||
| resistin | 0.165 (p = 0.191) 95% CI (−0.211; 0.542) | 0.190 (p = 0.171) 95% CI (−0.207; 0.587) | 0.187 (p = 0.166) 95% CI (−0.194; 0.568) | 0.144 (p = 0.238) 95% CI (−0.260; 0.547) | |||
| visfatin | 0.06 (p = 0.375) 95% CI (−0.325; 0.446) | −0.045 (p = 0.413) 95% CI (−0.456; 0.367) | −0.062 (p = 0.375) 95% CI (−0.455; 0.331) | −0.079 (p = 0.347) 95% CI (−0.489; 0.33) | |||
| L/A | 0.417 (p = 0.011) 95% CI (0.098; 0.737) | 0.549 (p = 0.002) 95% CI (0.261; 0.837) | 0.451 (p = 0.007) 95% CI (0.137; 0.766) | 0.366 (p = 0.030) 95% CI (0.009; 0.723) | |||
| (b) Correlations between adipokines levels and parameters of insulin resistance/sensitivity in the control group | |||||||
| HOMA-IR | QUICKI | TyG index | |||||
| adiponectin | −0.099 (p = 0.304) 95% CI (−0.49; 0.291) | −0.071 (p = 0.358) 95% CI (−0.464; 0.322) | −0.050 (p = 0.4) 95% CI (−0.452; 0.352) | ||||
| leptin | 0.352 (p = 0.031) 95% CI (0.005; 0.698) | −0.333 (p = 0.039) 95% CI (−0.684; 0.018) | −0.11 (p = 0.289) 95% CI (−0.508; 0.288) | ||||
| omentin | −0.063 (p = 0.372) 95% CI (−0.457; 0.330) | 0.028 (p = 0.442) 95% CI (−0.366; 0.423) | 0.331 (p = 0.043) 95% CI (−0.028; 0.690) | ||||
| resistin | 0.216 (p = 0.131) 95% CI (−0.161; 0.592) | −0.122 (p = 0.263) 95% CI (−0.511; 0.266) | −0.006 (p = 0.488) 95% CI (−0.409; 0.397) | ||||
| visfatin | −0.047 (p = 0.404) 95% CI (−0.441; 0.347) | 0.271 (p = 0.078) 95% CI (−0.095; 0.637) | 0.297 (p = 0.063) 95% CI (−0.071; 0.664) | ||||
| L/A | 0.472 (p = 0.005) 95% CI (0.166; 0.779) | −0.364 (p = 0.026) 95% CI (−0.706; −0.021) | 0.005 (p = 0.49) 95% CI (−0.398; 0.408) | ||||
| Total Cholesterol | LDL Cholesterol | HDL Cholesterol | Triglycerides | |
|---|---|---|---|---|
| Adiponectin | −0.201 (p = 0.153) 95% CI (−0.587; 0.186) | −0.345 (p = 0.036) 95% CI (−0.7; 0.010) | 0.309 (p = 0.055) 95% CI (−0.055; 0.674) | 0.029 (p = 0.442) 95% CI (−0.374; 0.432) |
| Apelin | 0.254 (p = 0.096) 95% CI (−0.123; 0.631) | 0.309 (p = 0.055) 95% CI (−0.056; 0.673) | −0.112 (p = 0.285) 95% CI (−0.510; 0.286) | 0.165 (p = 0.201) 95% CI (−0.227; 0.557) |
| Leptin | 0.232 (p = 0.117) 95% CI (−0.149; 0.614) | 0.338 (p = 0.039) 95% CI (−0.019; 0.695) | −0.115 (p = 0.280) 95% CI (−0.513; 0.283) | −0.194 (p = 0.161) 95% CI (−0.582; 0.194) |
| Omentin | −0.213 (p = 0.139) 95% CI (−0.597; 0.172) | −0.138 (p = 0.242) 95% CI (−0.533; 0.258) | −0.464 (p = 0.006) 95% CI (−0.78; −0.147) | 0.379 (p = 0.023) 95% CI (0.034; 0.724) |
| Resistin | 0.068 (p = 0.365) 95% CI (−0.333; 0.47) | 0.057 (p = 0.386) 95% CI (−0.345; 0.459) | 0.098 (p = 0.310) 95% CI (−0.301; 0.497) | −0.085 (p = 0.334) 95% CI (−0.485; 0.316) |
| Vaspine | 0.163 (p = 0.203) 95% CI (−0.229; 0.555) | 0.182 (p = 0.177) 95% CI (−0.208; 0.572) | −0.038 (p = 0.425) 95% CI (−0.440; 0.365) | 0.126 (p = 0.261) 95% CI (−0.270; 0.523) |
| Visfatin | 0.466 (p = 0.006) 95% CI (0.15; 0.781) | 0.456 (p = 0.007) 95% CI (0.137; 0.776) | 0.09 (p = 0.325) 95% CI (−0.31; 0.49) | 0.316 (p = 0.051) 95% CI (−0.047; 0.679) |
| L/A | 0.262 (p = 0.089) 95% CI (−0.114; 0.637) | 0.420 (p = 0.013) 95% CI (0.088; 0.752) | −0.279 (p = 0.075) 95% CI (−0.651; 0.092) | −0.120 (p = 0.272) 95% CI (−0.517; 0.277) |
References
- Guo, F.; Gong, Z.; Fernando, T.; Zhang, L.; Zhu, X.; Shi, Y. The Lipid Profiles in Different Characteristics of Women with PCOS and the Interaction Between Dyslipidemia and Metabolic Disorder States: A Retrospective Study in Chinese Population. Front. Endocrinol. 2022, 13, 892125. [Google Scholar] [CrossRef]
- Kim, J.Y.; Tfayli, H.; Michaliszyn, S.F.; Arslanian, S. Impaired Lipolysis, Diminished Fat Oxidation, and Metabolic Inflexibility in Obese Girls With Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2018, 103, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Verduci, E.; Cena, H.; Magenes, V.C.; Todisco, C.F.; Tenuta, E.; Gregorio, C.; De Giuseppe, R.; Bosetti, A.; Di Profio, E.; et al. Polycystic Ovary Syndrome in Insulin-Resistant Adolescents with Obesity: The Role of Nutrition Therapy and Food Supplements as a Strategy to Protect Fertility. Nutrients 2021, 13, 1848. [Google Scholar] [CrossRef] [PubMed]
- Tay, C.T.; Hart, R.J.; Hickey, M.; Moran, L.J.; Earnest, A.; Doherty, D.A.; Teede, H.J.; Joham, A.E. Updated adolescent diagnostic criteria for polycystic ovary syndrome: Impact on prevalence and longitudinal body mass index trajectories from birth to adulthood. BMC Med. 2020, 18, 389. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Ciołek, M.; Stachowiak, J.J.; Krok, K.; Sokal, J.; Malczyk, Ż.; Skrzyńska, K.; Zachurzok, A. Adolescent PCOS and long-term metabolic risk: Insights from triglycerides to high-density lipoprotein cholesterol ratio and high-density lipoprotein cholesterol profiles. Front. Endocrinol. 2025, 16, 1579217. [Google Scholar] [CrossRef]
- Murawska-Ciałowicz, E. Adipose tissue—Morphological and biochemical characteristic of different depots. Postepy Hig. Med. Dosw. 2017, 71, 466–484. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Eur. J. Endocrinol. 2023, 189, G43–G64. [Google Scholar] [CrossRef]
- Benrick, A.; Chanclón, B.; Micallef, P.; Wu, Y.; Hadi, L.; Shelton, J.M.; Stener-Victorin, E.; Asterholm, I.W. Adiponectin protects against development of metabolic disturbances in a PCOS mouse model. Proc. Natl. Acad. Sci. USA 2017, 114, E7187–E7196. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef]
- Olszanecka-Glinianowicz, M.; Kuglin, D.; Dąbkowska-Huć, A.; Skałba, P. Serum adiponectin and resistin in relation to insulin resistance and markers of hyperandrogenism in lean and obese women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 154, 51–56. [Google Scholar] [CrossRef]
- Mirza, S.S.; Shafique, K.; Shaikh, A.R.; Khan, N.A.; Anwar Qureshi, M. Association between circulating adiponectin levels and polycystic ovarian syndrome. J. Ovarian Res. 2014, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Majerczyk, M.; Olszanecka-Glinianowicz, M.; Puzianowska-Kuźnicka, M.; Chudek, J. Retinol-binding protein 4 (RBP4) as the causative factor and marker of vascular injury related to insulin resistance. Postepy Hig. Med. Dosw. 2016, 70, 1267–1275. [Google Scholar]
- Steinhoff, J.S.; Lass, A.; Schupp, M. Biological Functions of RBP4 and Its Relevance for Human Diseases. Front. Physiol. 2021, 12, 659977. [Google Scholar] [CrossRef] [PubMed]
- Alkharfy, K.M.; Al-Daghri, N.M.; Vanhoutte, P.M.; Krishnaswamy, S.; Xu, A. Serum retinol-binding protein 4 as a marker for cardiovascular disease in women. PLoS ONE 2012, 7, e48612. [Google Scholar] [CrossRef]
- Jia, J.; Bai, J.; Liu, Y.; Yin, J.; Yang, P.; Yu, S.; Ye, J.; Wang, D.; Yuan, G. Association between retinol-binding protein 4 and polycystic ovary syndrome: A meta-analysis. Endocr. J. 2014, 61, 995–1002. [Google Scholar] [CrossRef]
- Lingaiah, S.; Morin-Papunen, L.; Piltonen, T.; Sundström-Poromaa, I.; Stener-Victorin, E.; Tapanainen, J.S. Serum retinol-binding protein 4 levels in polycystic ovary syndrome. Endocr. Connect. 2019, 8, 709–717. [Google Scholar] [CrossRef]
- Cakal, E.; Ustun, Y.; Engin-Ustun, Y.; Ozkaya, M.; Kilinç, M. Serum vaspin and C-reactive protein levels in women with polycystic ovaries and polycystic ovary syndrome. Gynecol. Endocrinol. 2011, 27, 491–495. [Google Scholar] [CrossRef]
- Dogan, K.; Helvacioglu, C.; Baghaki, S.; Ekin, M. Comparison of body mass index and metabolic parameters with serum vaspin levels in women with polycystic ovary syndrome. Diabetes Metab. Syndr. 2020, 14, 137–139. [Google Scholar] [CrossRef]
- Pinhas-Hamiel, O.; Singer, S.; Pilpel, N.; Koren, I.; Boyko, V.; Hemi, R.; Pariente, C.; Kanety, H. Adiponectin levels in adolescent girls with polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2009, 71, 823–827. [Google Scholar] [CrossRef]
- Kale-Gurbuz, T.; Akhan, S.E.; Bastu, E.; Telci, A.; Iyibozkurt, A.C.; Topuz, S. Adiponectin, leptin and ghrelin levels in obese adolescent girls with polycystic ovary syndrome. J. Pediatr. Adolesc. Gynecol. 2013, 26, 27–30. [Google Scholar] [CrossRef]
- Özgen, I.T.; Oruçlu, Ş.; Selek, S.; Kutlu, E.; Guzel, G.; Cesur, Y. Omentin-1 level in adolescents with polycystic ovarian syndrome. Pediatr. Int. 2019, 61, 147–151. [Google Scholar] [CrossRef]
- Yang, H.Y.; Ma, Y.; Lu, X.H.; Liang, X.H.; Suo, Y.J.; Huang, Z.X.; Lu, D.C.; Qin, Y.F.; Luo, Z.J. The correlation of plasma omentin-1 with insulin resistance in non-obese polycystic ovary syndrome. Ann. Endocrinol. 2015, 76, 620–627. [Google Scholar] [CrossRef]
- Tan, B.K.; Adya, R.; Farhatullah, S.; Lewandowski, K.C.; O’Hare, P.; Lehnert, H.; Randeva, H.S. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: Ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes 2008, 57, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, H.; Song, J.; Feng, D.; Na, Z.; Jiang, H.; Meng, Y.; Shi, B.; Li, D. Elevated Serum Leptin Levels as a Predictive Marker for Polycystic Ovary Syndrome. Front. Endocrinol. 2022, 13, 845165. [Google Scholar] [CrossRef] [PubMed]
- Zaletel, J.; Barlovic, D.P.; Prezelj, J. Adiponectin-leptin ratio: A useful estimate of insulin resistance in patients with Type 2 diabetes. J. Endocrinol. Investig. 2010, 33, 514–518. [Google Scholar] [CrossRef]
- Inoue, M.; Yano, M.; Yamakado, M.; Maehata, E.; Suzuki, S. Relationship between the adiponectin-leptin ratio and parameters of insulin resistance in subjects without hyperglycemia. Metabolism 2006, 55, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Borsuk, A.; Biernat, W.; Zięba, D. Multidirectional action of resistin in the organism. Postepy Hig. Med. Dosw. Polska Akademia Nauk. 2018, 72, 327–338. [Google Scholar] [CrossRef]
- Rodríguez, M.; Moltó, E.; Aguado, L.; Gallardo, N.; Andrés, A.; Arribas, C. S-resistin, a non secretable resistin isoform, impairs the insulin signalling pathway in 3T3-L1 adipocytes. J. Physiol. Biochem. 2015, 71, 381–390. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Altinkaya, S.Ö.; Nergiz, S.; Küçük, M.; Yüksel, H. Apelin levels in relation with hormonal and metabolic profile in patients with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 176, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Goren, K.; Sagsoz, N.; Noyan, V.; Yucel, A.; Caglayan, O.; Bostancİ, M.S. Plasma apelin levels in patients with polycystic ovary syndrome. J. Turk. Ger. Gynecol. Assoc. 2012, 13, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Daghestani, M.H.; Daghestani, M.; Daghistani, M.; El-Mazny, A.; Bjørklund, G.; Chirumbolo, S.; Al Saggaf, S.H.; Warsy, A. A study of ghrelin and leptin levels and their relationship to metabolic profiles in obese and lean Saudi women with polycystic ovary syndrome (PCOS). Lipids. Health Dis. 2018, 17, 195. [Google Scholar] [CrossRef] [PubMed]
- Tsouma, I.; Kouskouni, E.; Demeridou, S.; Boutsikou, M.; Hassiakos, D.; Chasiakou, A.; Hassiakou, S.; Baka, S. Correlation of visfatin levels and lipoprotein lipid profiles in women with polycystic ovary syndrome undergoing ovarian stimulation. Gynecol. Endocrinol. 2014, 30, 516–519. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Portincasa, P.; Colina, I.; Gómez-Ambrosi, J. Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Sci. Rep. 2017, 7, 6619. [Google Scholar] [CrossRef]
- Carbone, F.; Burger, F.; Roversi, G.; Tamborino, C.; Casetta, I.; Seraceni, S.; Trentini, A.; Padroni, M.; Bertolotto, M.; Dallegri, F.; et al. Leptin/adiponectin ratio predicts poststroke neurological outcome. Eur. J. Clin. Investig. 2015, 45, 1184–1191. [Google Scholar] [CrossRef]
- Gupta, V.; Mishra, S.; Mishra, S.; Gupta, V. L:A ratio, Insulin resistance and metabolic risk in women with polycystic ovarian syndrome. Diabetes Metab. Syndr. 2017, 11 (Suppl. 2), S697–S701. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, L.; Zhang, Z.; Xu, J.; Wan, Z.; Qin, L. Resistin induces lipolysis and suppresses adiponectin secretion in cultured human visceral adipose tissue. Regul. Pept. 2014, 194–195, 49–54. [Google Scholar] [CrossRef]
- Yilmaz, M.; Bukan, N.; Demırcı, H.; Öztürk, Ç.; Kan, E.; Ayvaz, G.; Arslan, M. Serum resistin and adiponectin levels in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2009, 25, 246–252. [Google Scholar] [CrossRef]
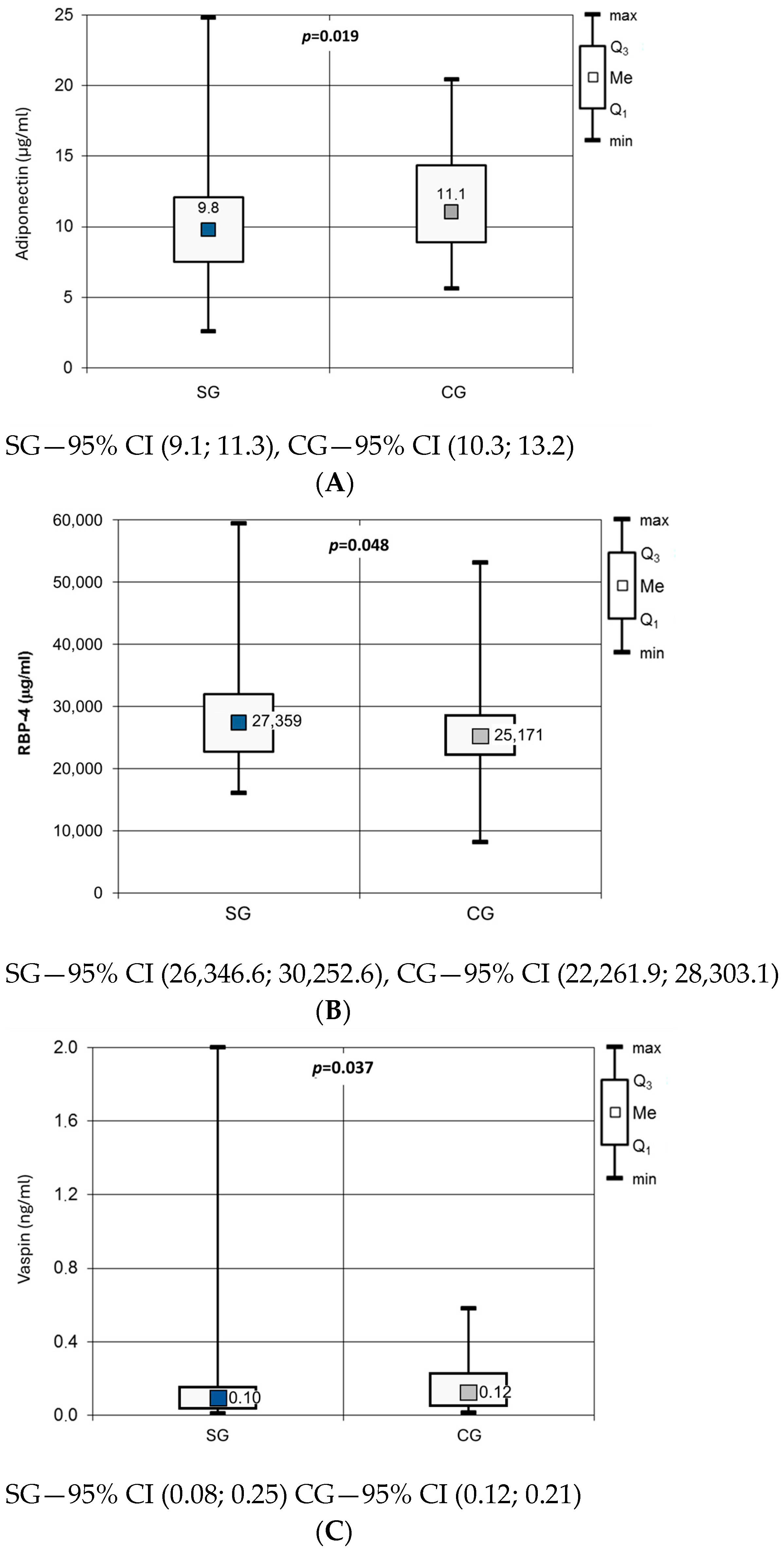
| Group | |||
|---|---|---|---|
| SG (n = 66) | CG (n = 30) | ||
| Type of menstrual disorder | Oligomenorrhea | 39 (59.1%) | 0 |
| Primary amenorrhea | 1 (1.5%) | 0 | |
| Secondary amenorrhea | 26 (39.4%) | 0 | |
| Regular menstrual cycles | 0 | 30 (100%) | |
| Hirsutism score ≥ 8 | 30 (45.5%) | 0 | |
| Testosterone > 42 ng/dL | 57 (86.4%) | 10 (33.3%) | |
| Study Group (n = 66) | Control Group (n = 30) | p | |
|---|---|---|---|
| Chronological age (years) | Me = 16.8; IQR (15.5; 17.5) 95% CI (16.3; 16.8) | Me = 16.6; IQR (15.6; 17.1) 95% CI (15.6; 16.7) | 0.129 * |
| Gynaecological age (years) | 4.2 ± 1.6 95% CI (3.8; 4.6) | 4.3 ± 1.4 95% CI (3.8; 4.8) | 0.396 |
| Age of first menstruation (years) | Me = 12.0; IQR (12.0; 13.0) 95% CI (12.0; 12.7) | 11.87 ± 1.68 95% CI (11.2; 12.5) | 0.049 * |
| BMI (kg/m2) | Me = 26.1; IQR (21.1; 32.7) 95% CI (25.8; 29.3) | Me = 22.9; IQR (21.3; 27.4) 95% CI (22.4; 26.1) | 0.043 * |
| BMI z-score | Me = 1.20; IQR (0.06; 1.99) 95% CI (0.77; 1.29) | 0.68 ± 0.94 95% CI (0.33; 1.03) | 0.052 * |
| Waist circumference (m) | Me = 0.83; IQR (0.70; 0.97) 95% CI (0.80; 0.89) | Me = 0.72; IQR (0.69; 0.83) 95% CI (0.72; 0.79) | 0.007 * |
| WHtR | Me = 0.51; IQR (0.43; 0.58) 95% CI (0.49; 0.54) | Me = 0.44; IQR (0.42; 0.50) 95% CI (0.44; 0.48) | 0.011 * |
| Fat mass on BIA (kg) | Me = 25.4; IQR (15.2; 42.4) 95% CI (25.2; 33.9) | Me = 17.8; IQR (14.9; 26.9) 95% CI (17.6; 25.5) | 0.024 * |
| Fat mass on BIA (%) | Me = 37.1; IQR (28.3; 44.8) 95% CI (33.8; 39.3) | Me = 29.2; IQR (26.1; 37.1) 95% CI (28.7; 34.3) | 0.019 * |
| Study Group (n = 66) | Control Group (n = 30) | p | |
|---|---|---|---|
| Total cholesterol (mg/dL) | Me = 166.5; IQR (153; 182.3) 95% CI (159.7; 175.6) | 166.0 ± 33.1 95% CI (153.1; 178.8) | 0.329 * |
| LDL cholesterol (mg/dL) | 96.7 ± 26.4 95% CI (89.8; 103.6) | 94.6 ± 28.7 95% CI (83.5; 105.7) | 0.366 |
| HDL cholesterol (mg/dL) | Me = 46.8; IQR (40.7; 56.2) 95% CI (45.9; 51.5) | 55.0 ± 10.2 95% CI (51.0; 59.0) | 0.002 * |
| Triglycerides (mg/dL) | Me = 101.0; IQR (79.5; 134.8) 95% CI (104.4; 131.7) | Me = 74.5; IQR (69.8; 92.3) 95% CI (73.9; 91.0) | <0.001 * |
| GLU 0′ (mg/dL) | Me = 88.0; IQR (85.0; 93.0) 95% CI (87.1; 90.5) | 88.5 ± 6.7 95% CI (86.0; 91.0) | 0.352 * |
| GLU 120′ (mg/dL) | Me = 106.5; IQR (94.0; 122.5) 95% CI (104.6; 119.9) | 108.2 ± 27.4 95% CI (97.3; 119.0) | 0.330 * |
| INS 0′ (uIU/mL) | Me = 13.3; IQR (7.7; 19.3) 95% CI (12.8; 18.0) | Me = 11.1; IQR (8.3; 18.1) 95% CI (11.0; 17.3) | 0.448 * |
| INS 120′ (uIU/mL) | Me = 67.4; IQR (48.0; 122.0) 95% CI (75.6; 110.0) | Me = 68.7; IQR (55.6; 99.4) 95% CI (66.8; 122.2) | 0.384 * |
| HOMA-IR | Me = 2.93; IQR (1.54; 4.05) 95% CI (2.82; 4.04) | Me = 2.42; IQR (1.69; 3.82) 95% CI (2.38; 3.93) | 0.438 * |
| QUICKI | Me = 0.33; IQR (0.31; 0.36) 95% CI (0.32; 0.34) | Me = 0.33; IQR (0.31; 0.35) 95% CI (0.32; 0.35) | 0.438 * |
| TyG index | 8.47 ± 0.44 95% CI (8.36; 8.58) | 8.17 ± 0.27 95% CI (8.06; 8.27) | <0.001 |
| Study Group (n = 66) | Control Group (n = 30) | p | |
|---|---|---|---|
| apelin (ng/mL) | Me = 0.124; IQR (0.088; 0.186) 95% CI (0.123; 0.161) | 0.155 ± 0.078 95% CI (0.125; 0.184) | 0.177 * |
| leptin (ng/mL) | Me = 38,5; IQR (17.4; 68.4) 95% CI (42.4; 63.8) | Me = 34.1; IQR (20.3; 49.6) 95% CI (29.3; 50.3) | 0.161 * |
| omentin-1 (ng/mL) | 413.3 ± 153.9 95% CI (375.4; 451.1) | Me = 401.3; IQR (335.8; 565.6) 95% CI (394.4; 565.7) | 0.213 * |
| resistin (ng/mL) | Me = 6.62; IQR (5.66; 9.85) 95% CI (6.85; 9.70) | 7.70 ± 2.82 95% CI (6.65; 8.76) | 0.495 * |
| visfatin (ng/mL) | Me = 1.32; IQR (0.77; 2.88) 95% CI (1.63; 2.55) | Me = 1.26; IQR (0.66; 2.34) 95% CI (1.17; 2.78) | 0.247 * |
| L/A | 3.97; IQR (1.71; 9.63) 95% CI (4.90; 8.23) | Me = 3.14; IQR (1.54; 4.51) 95% CI (2.52; 5.12) | 0.059 * |
| BMI z-Score | Waist Circumference | WHtR | Fat Mass on BIA (%) | |
|---|---|---|---|---|
| adiponectin | −0.253 (p = 0.020) 95% CI (−0.487; −0.020) | −0.274 (p = 0.025) 95% CI (−0.537; −0.012) | −0.331 (p = 0.008) 95% CI (−0.584; −0.078 | −0.327 (p = 0.01) 95% CI (−0.584; −0.071) |
| leptin | 0.652 (p < 0.001) 95% CI (0.508; 0.795) | 0.736 (p < 0.001) 95% CI (0.606; 0.866) | 0.737 (p < 0.001) 95% CI (0.607; 0.867) | 0.804 (p < 0.001) 95% CI (0.702; 0.905) |
| omentin | −0.391 (p = 0.001) 95% CI (−0.603; −0.180) | −0.293 (p = 0.018) 95% CI (−0.552; −0.033) | −0.335 (p = 0.008) 95% CI (−0.587; −0.083) | −0.316 (p = 0.010) 95% CI (−0.575; −0.058) |
| visfatin | 0.370 (p = 0.001) 95% CI (0.155; 0.586) | 0.415 (p = 0.001) 95% CI (0.180; 0.650) | 0.446 (p < 0.001) 95% CI (0.219; 0.674) | 0.424 (p = 0.001) 95% CI (0.189; 0.660) |
| L/A | 0.633 (p < 0.001) 95% CI (0.483; 0.783) | 0.715 (p < 0.001) 95% CI (0.576; 0.854) | 0.750 (p < 0.001) 95% CI (0.625; 0.874) | 0.782 (p < 0.001) 95% CI (0.671; 0.894) |
| (a) Correlations between adipokines levels and parameters of carbohydrate metabolism in the study group | |||||||
| GLU 0′ | GLU 120′ | INS 0′ | INS 120′ | ||||
| adiponectin | 0.003 (p = 0.490) 95% CI (−0.248; 0.255) | −0.077 (p = 0.279) 95% CI (−0.339; 0.184) | −0.237 (p = 0.029) 95% CI (−0.475; 0.001) | −0.215 (p = 0.048) 95% CI (−0.463; 0.034) | |||
| leptin | 0.092 (p = 0.233) 95% CI (−0.157; 0.342) | 0.028 (p = 0.416) 95% CI (−0.235; 0.291) | 0.481 (p < 0.001) 95% CI (0.287; 0.674) | 0.332 (p = 0.004) 95% CI (0.100; 0.564) | |||
| omentin | −0.198 (p = 0.057) 95% CI (−0.440; 0.044) | −0.018 (p = 0.445) 95% CI (−0.281; 0.244) | −0.274 (p = 0.013) 95% CI (−0.507; −0.042) | −0.322 (p = 0.006) 95% CI (−0.556; −0.089) | |||
| resistin | 0.384 (p < 0.001) 95% CI (0.169; 0.599) | 0.041 (p = 0.379) 95% CI (−0.222; 0.303) | −0.011 (p = 0.467) 95% CI (−0.262; 0.241) | −0.068 (p = 0.300) 95% CI (−0.328; 0.191) | |||
| visfatin | 0.114 (p = 0.183) 95% CI (−0.134; 0.363) | 0.068 (p = 0.303) 95% CI (−0.194; 0.33) | 0.108 (p = 0.196) 95% CI (−0.141; 0.357) | 0.055 (p = 0.337) 95% CI (−0.205; 0.315) | |||
| L/A | 0.040 (p = 0.374) 95% CI (−0.211; 0.292) | 0.082 (p = 0.267) 95% CI (−0.179; 0.343) | 0.461 (p < 0.001) 95% CI (0.263; 0.659) | 0.435 (p < 0.001) 95% CI (0.224; 0.646) | |||
| (b) Correlations between adipokines levels and parameters of insulin resistance/sensitivity in the study group | |||||||
| HOMA-IR | QUICKI | TyG index | |||||
| adiponectin | −0.223 (p = 0.037) 95% CI (−0.463; 0.016) | 0.239 (p = 0.028) 95% CI (0.002; 0.476) | −0.051 (p = 0.345) 95% CI (−0.304; 0.202) | ||||
| leptin | 0.451 (p < 0.001) 95% CI (0.250; 0.652) | −0.493 (p < 0.001) 95% CI (−0.683; −0.302) | 0.259 (p = 0.019) 95% CI (0.022; 0.496) | ||||
| omentin | −0.283 (p = 0.011) 95% CI (−0.515; −0.051) | 0.331 (p = 0.004) 95% CI (0.107; 0.556) | −0.219 (p = 0.041) 95% CI (−0.460; 0.023) | ||||
| resistin | 0.025 (p = 0.421) 95% CI (−0.226; 0.277) | −0.108 (p = 0.195) 95% CI (−0.357; 0.14) | 0.080 (p = 0.265) 95% CI (−0.172; 0.332) | ||||
| visfatin | 0.091 (p = 0.235) 95% CI (−0.158; 0.341) | −0.223 (p = 0.037) 95% CI (−0.462; 0.016) | −0.057 (p = 0.238) 95% CI (−0.310; 0.196) | ||||
| L/A | 0.427 (p < 0.001) 95% CI (0.221; 0.633) | −0.473 (p < 0.001) 95% CI (−0.668; −0.277) | 0.313 (p = 0.006) 95% CI (0.084; 0.542) | ||||
| Total Cholesterol | LDL Cholesterol | HDL Cholesterol | Triglycerides | |
|---|---|---|---|---|
| Adiponectin | −0.048 (p = 0.352) 95% CI (−0.302; 0.205) | −0.203 (p = 0.061) 95% CI (−0.458; 0.051) | 0.267 (p = 0.017) 95% CI (0.031; 0.503) | −0.093 (p = 0.233) 95% CI (−0.345; 0.159) |
| Apelin | 0.122 (p = 0.168) 95% CI (−0.128; 0.372) | 0.020 (p = 0.439) 95% CI (−0.245; 0.286) | 0.213 (p = 0.046) 95% CI (−0.030; 0.455) | 0.067 (p = 0.299) 95% CI (−0.186; 0.320) |
| Leptin | 0.160 (p = 0.104) 95% CI (−0.088; 0.407) | 0.213 (p = 0.053) 95% CI (−0.041; 0.466) | −0.309 (p = 0.006) 95% CI (−0.539; −0.08) | 0.225 (p = 0.037) 95% CI (−0.016; 0.466) |
| Omentin | −0.019 (p = 0.441) 95% CI (−0.273; 0.235) | −0.078 (p = 0.280) 95% CI (−0.341; 0.186) | 0.270 (p = 0.015) 95% CI (0.035; 0.506) | −0.222 (p = 0.039) 95% CI (−0.464; 0.019) |
| Resistin | −0.341 (p = 0.003) 95% CI (−0.565; −0.116) | −0.296 (p = 0.011) 95% CI (−0.538; −0.054) | −0.200 (p = 0.057) 95% CI (−0.444; 0.044) | −0.022 (p = 0.433) 95% CI (−0.275; 0.232) |
| Vaspine | −0.021 (p = 0.436) 95% CI (−0.274; 0.233) | −0.020 (p = 0.440) 95% CI (−0.285; 0.245) | −0.053 (p = 0.338) 95% CI (−0.306; 0.200) | 0.016 (p = 0.451) 95% CI (−0.238; 0.270) |
| Visfatin | −0.231 (p = 0.033) 95% CI (−0.472; 0.009) | −0.197 (p = 0.067) 95% CI (−0.452; 0.058) | −0.236 (p = 0.030) 95% CI (−0.476; 0.004) | −0.054 (p = 0.337) 95% CI (−0.307; 0.199) |
| L/A | 0.153 (p = 0.113) 95% CI (−0.095; 0.401) | 0.210 (p = 0.056) 95% CI (−0.044; 0.463) | −0.361 (p = 0.002) 95% CI (−0.582; −0.14) | 0.300 (p = 0.008) 95% CI (0.069; 0.531) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foryś, E.; Drosdzol-Cop, A.; Małecka-Tendera, E.; Gawlik-Starzyk, A.M.; Skrzyńska, K.; Olszanecka-Glinianowicz, M.; Zachurzok, A. Adipokine Profile Signature in Adolescent Girls with Menstrual Disorders and Hyperandrogenism Differs from That of Regularly Menstruating Girls. J. Clin. Med. 2025, 14, 7987. https://doi.org/10.3390/jcm14227987
Foryś E, Drosdzol-Cop A, Małecka-Tendera E, Gawlik-Starzyk AM, Skrzyńska K, Olszanecka-Glinianowicz M, Zachurzok A. Adipokine Profile Signature in Adolescent Girls with Menstrual Disorders and Hyperandrogenism Differs from That of Regularly Menstruating Girls. Journal of Clinical Medicine. 2025; 14(22):7987. https://doi.org/10.3390/jcm14227987
Chicago/Turabian StyleForyś, Elżbieta, Agnieszka Drosdzol-Cop, Ewa Małecka-Tendera, Aneta Monika Gawlik-Starzyk, Karolina Skrzyńska, Magdalena Olszanecka-Glinianowicz, and Agnieszka Zachurzok. 2025. "Adipokine Profile Signature in Adolescent Girls with Menstrual Disorders and Hyperandrogenism Differs from That of Regularly Menstruating Girls" Journal of Clinical Medicine 14, no. 22: 7987. https://doi.org/10.3390/jcm14227987
APA StyleForyś, E., Drosdzol-Cop, A., Małecka-Tendera, E., Gawlik-Starzyk, A. M., Skrzyńska, K., Olszanecka-Glinianowicz, M., & Zachurzok, A. (2025). Adipokine Profile Signature in Adolescent Girls with Menstrual Disorders and Hyperandrogenism Differs from That of Regularly Menstruating Girls. Journal of Clinical Medicine, 14(22), 7987. https://doi.org/10.3390/jcm14227987








