Post-Traumatic Stress Disorder (PTSD) and Cardiovascular Diseases: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection Process and Data Collection Process
2.4. Data Items
2.5. Risk of Bias Assessment
2.6. Meta-Analytic Approach
3. Results
3.1. Study Selection
3.2. Quality Assessment Results
3.3. Risk Ratio for CVD in PTSD
3.3.1. Characteristics of the Included Studies
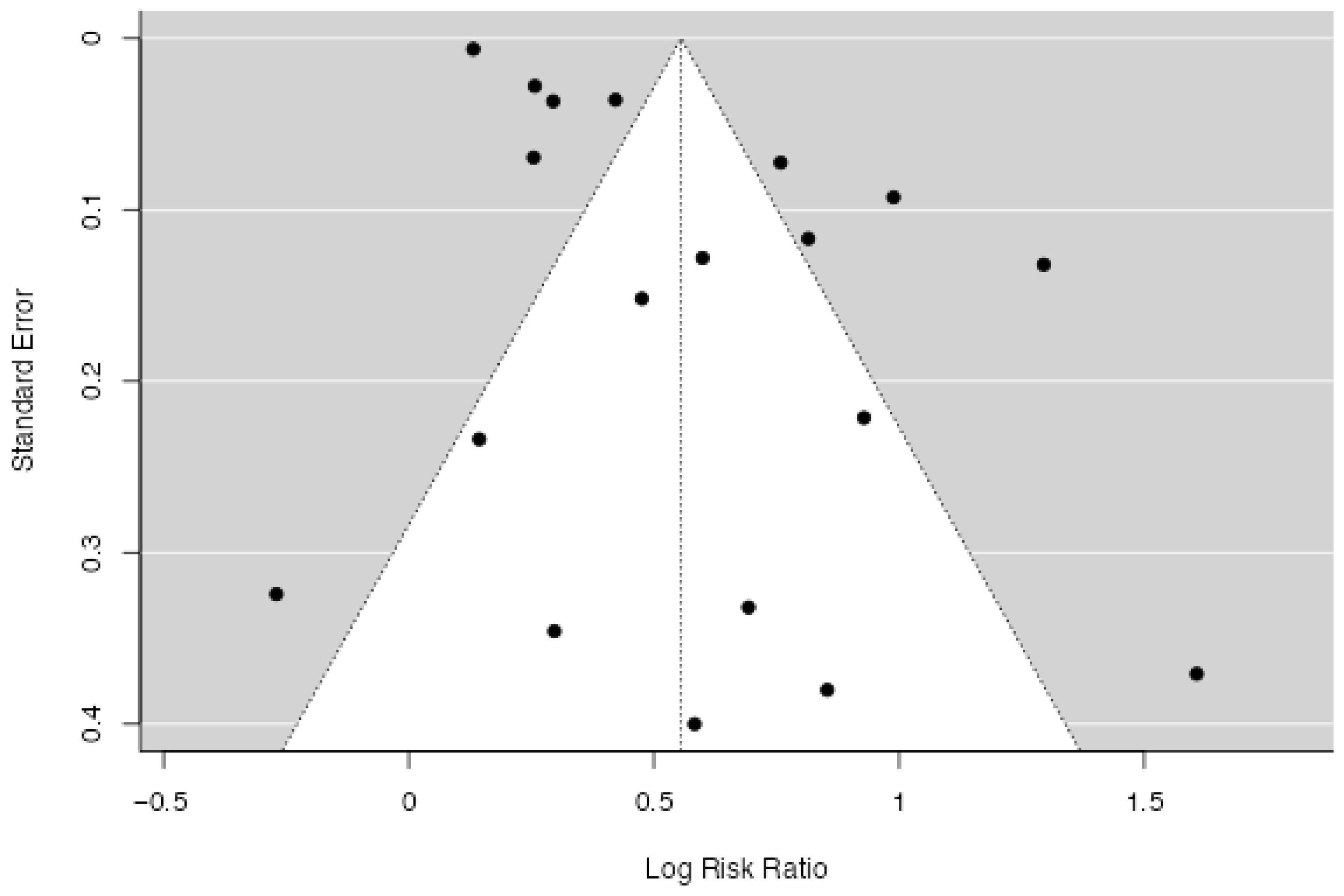
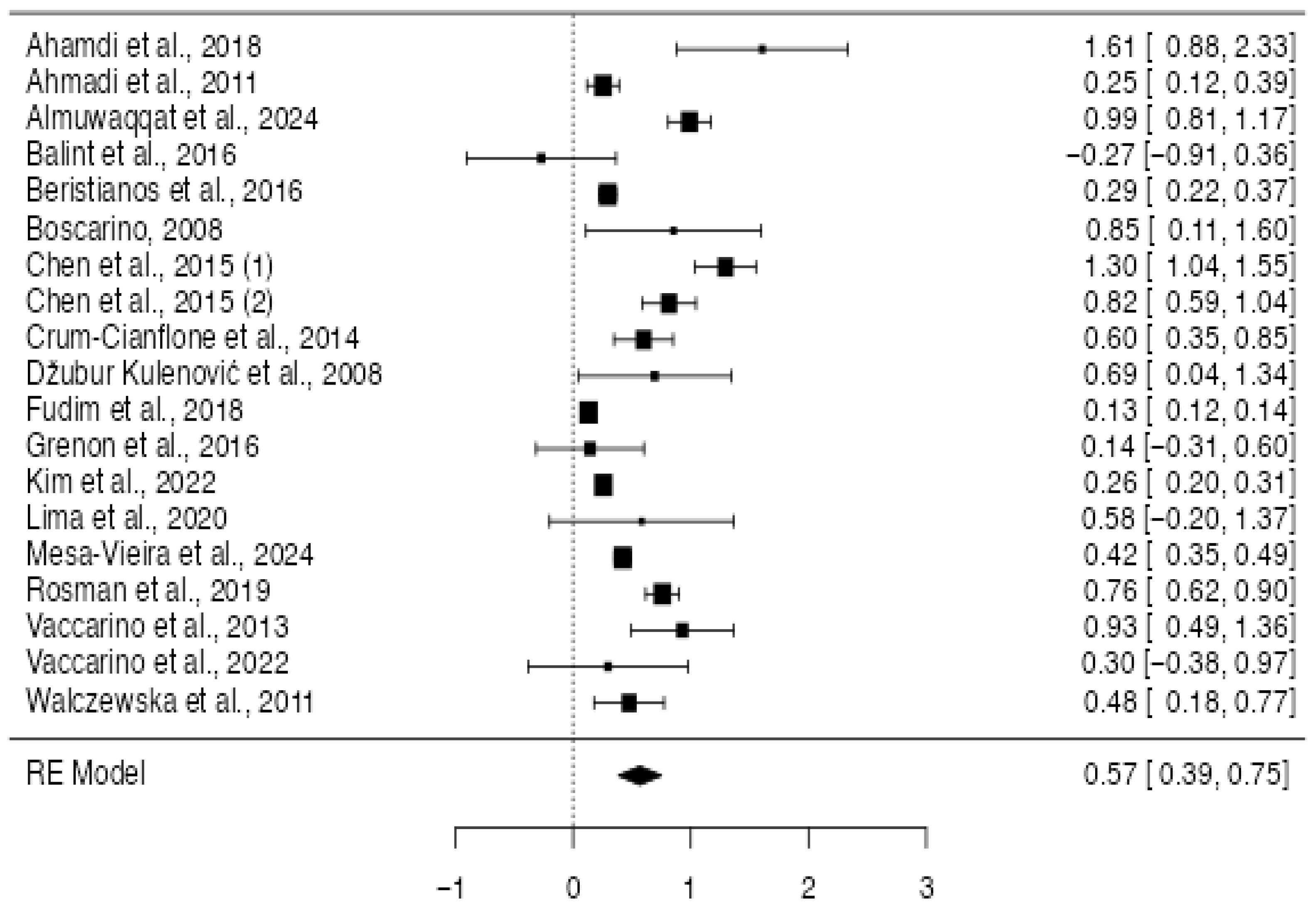
3.3.2. Quantitative Synthesis
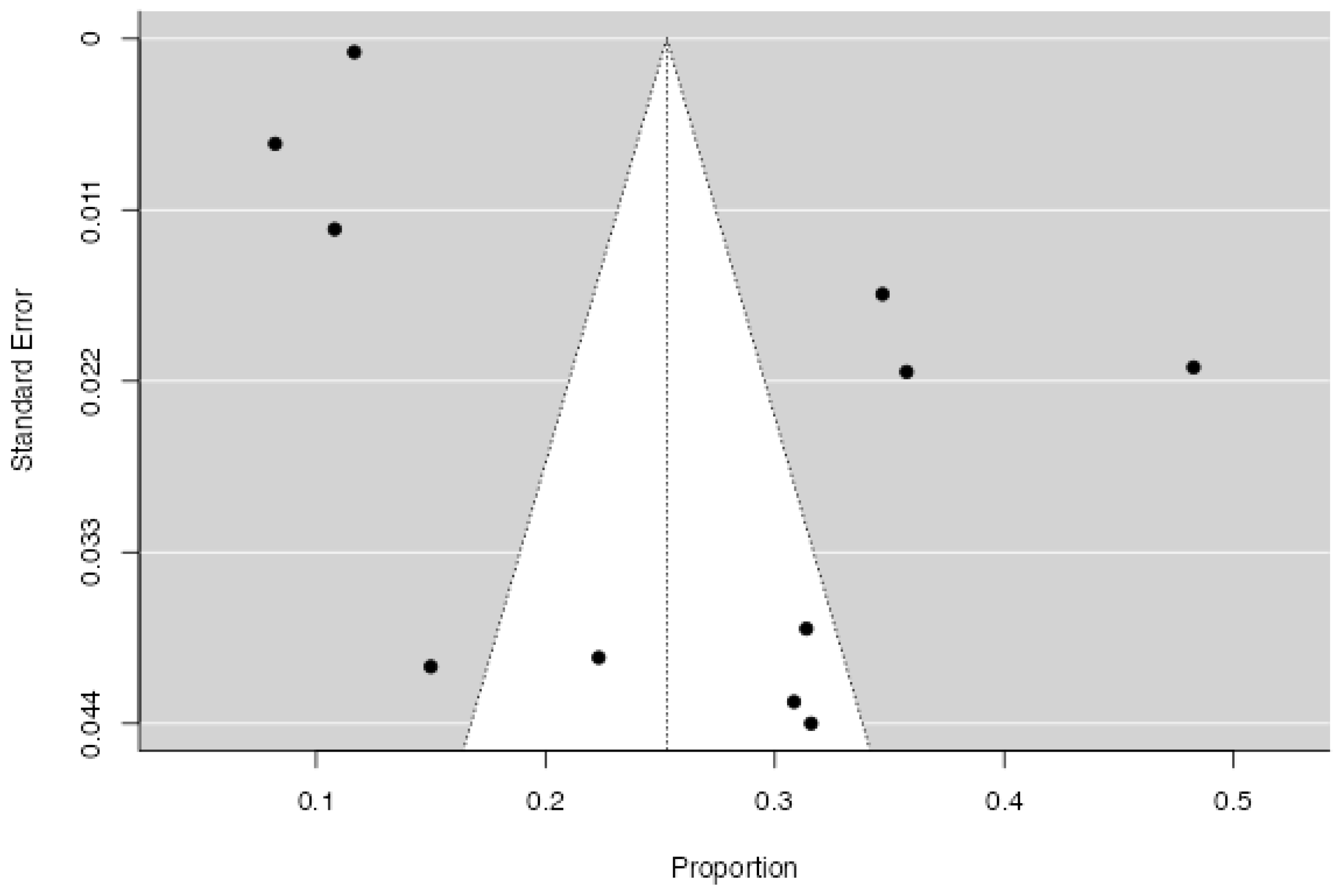
3.4. Proportion of PTSD in CVD
3.4.1. Characteristics of the Included Studies
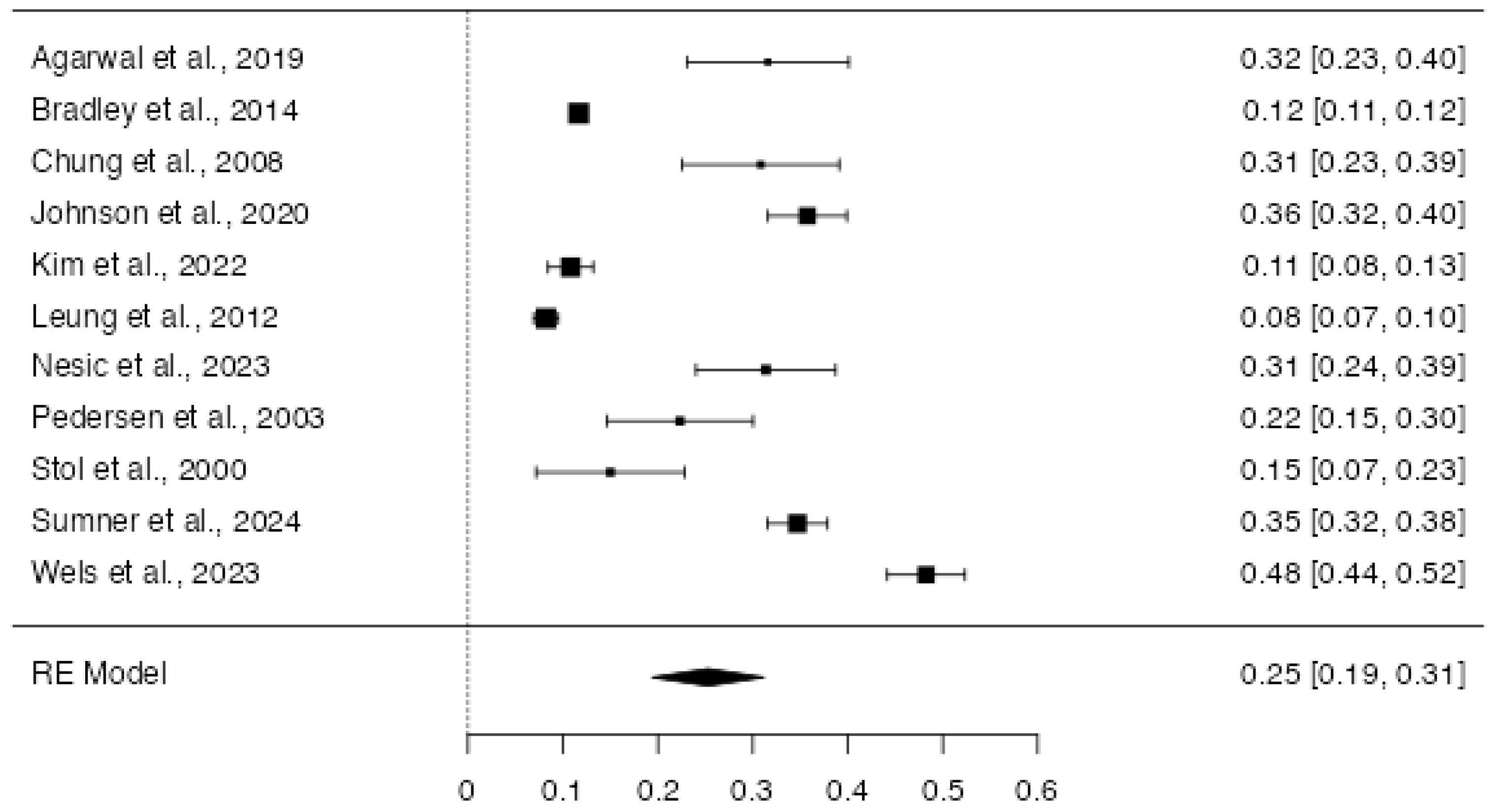
3.4.2. Quantitative Synthesis
4. Discussion
Limitations and Further Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association, DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Watson, P. PTSD as a Public Mental Health Priority. Curr. Psychiatry Rep. 2019, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.B.; Smith, S.M.; Chou, S.P.; Saha, T.D.; Jung, J.; Zhang, H.; Pickering, R.P.; Ruan, W.J.; Huang, B.; Grant, B.F. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc. Psychiatry Psychiatr. Epidemiol. 2016, 51, 1137–1148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, D.J.; Bovin, M.J.; Weathers, F.W.; Palmieri, P.A.; Schnurr, P.P.; Sloan, D.M.; Keane, T.M.; Marx, B.P. Latent factor structure of DSM-5 posttraumatic stress disorder: Evaluation of method variance and construct validity of novel symptom clusters. Psychol. Assess. 2019, 31, 46–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kubzansky, L.D.; Koenen, K.C. Is posttraumatic stress disorder related to development of heart disease? An update. Cleve. Clin. J. Med. 2009, 76 (Suppl. S2), S60–S65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Donnell, C.J.; Schwartz Longacre, L.; Cohen, B.E.; Fayad, Z.A.; Gillespie, C.F.; Liberzon, I.; Pathak, G.A.; Polimanti, R.; Risbrough, V.; Ursano, R.J.; et al. Posttraumatic Stress Disorder and Cardiovascular Disease: State of the Science, Knowledge Gaps, and Research Opportunities. JAMA Cardiol. 2021, 6, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.; Richardson, S.; Falzon, L.; Davidson, K.W.; Mills, M.A.; Neria, Y. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: A meta-analytic review. PLoS ONE 2012, 7, e38915, Erratum in PLoS ONE 2019, 14, e0213635. https://doi.org/10.1371/journal.pone.0213635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Padhi, B.K.; Khatib, M.N.; Serhan, H.A.; Gaidhane, A.M.; Rustagi, S.; Zahiruddin, Q.S.; Sharma, R.K.; Satapathy, P. Cardiovascular impact of post-traumatic stress disorder: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102632. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Hajsadeghi, F.; Mirshkarlo, H.B.; Budoff, M.; Yehuda, R.; Ebrahimi, R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am. J. Cardiol. 2011, 108, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.C.; Kaloupek, D.G. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom. Med. 2001, 63, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Brudey, C.; Park, J.; Wiaderkiewicz, J.; Kobayashi, I.; Mellman, T.A.; Marvar, P.J. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R315–R321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thayer, J.F.; Ahs, F.; Fredrikson, M.; Sollers, J.J., III; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Seligowski, A.V.; Webber, T.K.; Marvar, P.J.; Ressler, K.J.; Philip, N.S. Involvement of the brain-heart axis in the link between PTSD and cardiovascular disease. Depress. Anxiety 2022, 39, 663–674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seligowski, A.V.; Ressler, K.J. Sex Differences in the Co-Occurrence of PTSD and Cardiovascular Disease. Psychiatr. Ann. 2022, 52, 26–30. [Google Scholar] [CrossRef]
- Morris, M.C.; Compas, B.E.; Garber, J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clin. Psychol. Rev. 2012, 32, 301–315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, Q. Autonomic dysfunction and cardiovascular risk in post-traumatic stress disorder. Auton. Neurosci. 2022, 237, 102923. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Agarwal, S.; Presciutti, A.; Cornelius, T.; Birk, J.; Roh, D.J.; Park, S.; Claassen, J.; Elkind, M.S.V.; Edmondson, D. Cardiac Arrest and Subsequent Hospitalization–Induced Posttraumatic Stress Is Associated With 1-Year Risk of Major Adverse Cardiovascular Events and All-Cause Mortality. Crit. Care Med. 2019, 47, e502–e505. [Google Scholar] [CrossRef]
- Ahmadi, N.; Hajsadeghi, F.; Nabavi, V.; Olango, G.; Molla, M.; Budoff, M.; Vaidya, N.; Quintana, J.; Pynoos, R.; Hauser, P.; et al. The Long-Term Clinical Outcome of Posttraumatic Stress Disorder with Impaired Coronary Distensibility. Psychosom. Med. 2018, 80, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Almuwaqqat, Z.; Liu, C.; Ko, Y.A.; Elon, L.; Moazzami, K.; Wang, M.; Murrah, N.; Shallenberger, L.; Lewis, T.T.; Shah, A.J.; et al. Posttraumatic Stress Disorder and the Risk of Heart Failure Hospitalizations Among Individuals With Coronary Artery Disease. Circ. Cardiovasc. Qual. Outcomes 2024, 17, e011040. [Google Scholar] [CrossRef]
- Balint, E.M.; Boseva, P.; Schury, K.; Guendel, H.; Rottbauer, W.; Waller, C. High prevalence of posttraumatic stress in patients with primary hypertension. Gen. Hosp. Psychiatry 2016, 38, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Beristianos, M.H.; Yaffe, K.; Cohen, B.; Byers, A.L. PTSD and Risk of Incident Cardiovascular Disease in Aging Veterans. Am. J. Geriatr. Psychiatry 2016, 24, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Boscarino, J.A. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: Implications for surveillance and prevention. Psychosom. Med. 2008, 70, 668–676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bradley, S.M.; Stanislawski, M.A.; Bekelman, D.B.; Monteith, L.L.; Cohen, B.E.; Schilling, J.H.; Hunt, S.C.; Milek, D.; Maddox, T.M.; Ho, P.M.; et al. Invasive coronary procedure use and outcomes among veterans with posttraumatic stress disorder: Insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Am. Heart J. 2014, 168, 381–390.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Pan, T.L.; Li, C.T.; Lin, W.C.; Chen, Y.S.; Lee, Y.C.; Tsai, S.J.; Hsu, J.W.; Huang, K.L.; Tsai, C.F.; et al. Risk of stroke among patients with post-traumatic stress disorder: Nationwide longitudinal study. Br. J. Psychiatry 2015, 206, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.C.; Berger, Z.; Jones, R.; Rudd, H. Posttraumatic stress and co-morbidity following myocardial infarction among older patients: The role of coping. Aging Ment. Health 2008, 12, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Crum-Cianflone, N.F.; Jacobson, I. Gender differences of postdeployment post-traumatic stress disorder among service members and veterans of the Iraq and Afghanistan conflicts. Epidemiol. Rev. 2014, 36, 5–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dzubur Kulenović, A.; Kucukalić, A.; Malec, D. Changes in plasma lipid concentrations and risk of coronary artery disease in army veterans suffering from chronic posttraumatic stress disorder. Croat. Med. J. 2008, 49, 506–514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fudim, M.; Cerbin, L.P.; Devaraj, S.; Ajam, T.; Rao, S.V.; Kamalesh, M. Post-Traumatic Stress Disorder and Heart Failure in Men Within the Veteran Affairs Health System. Am. J. Cardiol. 2018, 122, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Grenon, S.M.; Owens, C.D.; Alley, H.; Perez, S.; Whooley, M.A.; Neylan, T.C.; Aschbacher, K.; Gasper, W.J.; Hilton, J.F.; Cohen, B.E. Posttraumatic Stress Disorder Is Associated With Worse Endothelial Function Among Veterans. J. Am. Heart Assoc. 2016, 5, e003010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, A.K.; Hayes, S.N.; Sawchuk, C.; Johnson, M.P.; Best, P.J.; Gulati, R.; Tweet, M.S. Analysis of Posttraumatic Stress Disorder, Depression, Anxiety, and Resiliency Within the Unique Population of Spontaneous Coronary Artery Dissection Survivors. J. Am. Heart Assoc. 2020, 9, e014372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, K.; Tsai, A.C.; Sumner, J.A.; Jung, S.J. Posttraumatic stress disorder, cardiovascular disease outcomes and the modifying role of socioeconomic status. J. Affect. Disord. 2022, 319, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Levantsevych, O.M.; Elon, L.; Lewis, T.T.; Suglia, S.F.; Bremner, J.D.; Quyyumi, A.A.; Pearce, B.; Raggi, P.; Vaccarino, V.; et al. Early life stress and autonomic response to acute mental stress in individuals with coronary heart disease. J. Trauma. Stress 2022, 35, 521–532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leung, Y.W.; Alter, D.A.; Prior, P.L.; Stewart, D.E.; Irvine, J.; Grace, S.L. Posttraumatic growth in coronary artery disease outpatients: Relationship to degree of trauma and health service use. J. Psychosom. Res. 2012, 72, 293–299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lima, B.B.; Hammadah, M.; Pearce, B.D.; Shah, A.; Moazzami, K.; Kim, J.H.; Sullivan, S.; Levantsevych, O.; Lewis, T.T.; Weng, L.; et al. Association of Posttraumatic Stress Disorder With Mental Stress-Induced Myocardial Ischemia in Adults After Myocardial Infarction. JAMA Netw. Open 2020, 3, e202734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mesa-Vieira, C.; Didden, C.; Schomaker, M.; Mouton, J.P.; Folb, N.; van den Heuvel, L.L.; Gastaldon, C.; Cornell, M.; Tlali, M.; Kassanjee, R.; et al. Post-traumatic stress disorder as a risk factor for major adverse cardiovascular events: A cohort study of a South African medical insurance scheme. Epidemiol. Psychiatr. Sci. 2024, 33, e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nesic, M.; Vogel, J.; Krüger, J.P.; Wenzel, W.; Sahebi, A.; Rassaf, T.; Siebermair, J.; Wesemann, U. Association between different dimensions of anger and symptoms of post-traumatic stress disorder in at-risk cardiovascular patients during the COVID-19 pandemic. Front. Psychiatry 2023, 14, 1228192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pedersen, S.S.; Middel, B.; Larsen, M.L. Posttraumatic stress disorder in first-time myocardial infarction patients. Heart Lung 2003, 32, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Rosman, L.; Sico, J.J.; Lampert, R.; Gaffey, A.E.; Ramsey, C.M.; Dziura, J.; Chui, P.W.; Cavanagh, C.E.; Brandt, C.; Haskell, S.; et al. Posttraumatic Stress Disorder and Risk for Stroke in Young and Middle-Aged Adults: A 13-Year Cohort Study. Stroke 2019, 50, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; Schelling, G.; Goetz, A.E.; Kilger, E.; Bayer, A.; Kapfhammer, H.P.; Rothenhäusler, H.B.; Kreuzer, E.; Reichart, B.; Peter, K. Health-related quality of life and post-traumatic stress disorder in patients after cardiac surgery and intensive care treatment. J. Thorac. Cardiovasc. Surg. 2000, 120, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.A.; Kim, E.S.H.; Wood, M.J.; Chi, G.; Nolen, J.; Grodzinsky, A.; Gornik, H.L.; Kadian-Dodov, D.; Wells, B.J.; Hess, C.N.; et al. Posttraumatic Stress Disorder After Spontaneous Coronary Artery Dissection: A Report of the International Spontaneous Coronary Artery Dissection Registry. J. Am. Heart Assoc. 2024, 13, e032819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vaccarino, V.; Goldberg, J.; Rooks, C.; Shah, A.J.; Veledar, E.; Faber, T.L.; Votaw, J.R.; Forsberg, C.W.; Bremner, J.D. Post-traumatic stress disorder and incidence of coronary heart disease: A twin study. J. Am. Coll. Cardiol. 2013, 62, 970–978. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vaccarino, V.; Shah, A.J.; Moncayo, V.; Nye, J.; Piccinelli, M.; Ko, Y.A.; Ma, X.; Murrah, N.; Shallenberger, L.; Driggers, E.; et al. Posttraumatic Stress Disorder, Myocardial Perfusion, and Myocardial Blood Flow: A Longitudinal Twin Study. Biol. Psychiatry 2022, 91, 615–625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walczewska, J.; Rutkowski, K.; Wizner, B.; Cwynar, M.; Grodzicki, T. Stiffness of large arteries and cardiovascular risk in patients with post-traumatic stress disorder. Eur. Heart J. 2011, 32, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.; Heal, C.; Reeves, D.; Capobianco, L. Prevalence of post-traumatic stress and tests of metacognition as a PTSD risk marker in patients with coronary heart disease and elevated HADS scores: Analysis of data from the PATHWAY RCT’s in UK cardiac rehabilitation. Front. Psychiatry 2023, 14, 1198202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akosile, W.; Colquhoun, D.; Young, R.; Lawford, B.; Voisey, J. The association between post-traumatic stress disorder and coronary artery disease: A meta-analysis. Australas. Psychiatry 2018, 26, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.J.; Lampert, R.; Goldberg, J.; Veledar, E.; Bremner, J.D.; Vaccarino, V. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol. Psychiatry 2013, 73, 1103–1110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demirci, Ö.; Erbaş, O. Neurobiological Insights into PostTraumatic Stress Disorder. JEB Med. Sci. 2024, 5, 214–221. [Google Scholar]
- Dennis, P.A.; Watkins, L.L.; Calhoun, P.S.; Oddone, A.; Sherwood, A.; Dennis, M.F.; Rissling, M.B.; Beckham, J.C. Posttraumatic stress, heart rate variability, and the mediating role of behavioral health risks. Psychosom. Med. 2014, 76, 629–637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.P.; Cheng, H.L.; Ding, K.; Zhang, Y.; Gao, F.; Zhu, G.; Zhang, Z. New recognition of the heart-brain axis and its implication in the pathogenesis and treatment of PTSD. Eur. J. Neurosci. 2024, 60, 4661–4683. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Zhang, H.; Hill, M.A.; Zhang, C.; Park, Y. Interaction of IL-6 and TNF-α contributes to endothelial dysfunction in type 2 diabetic mouse hearts. PLoS ONE 2017, 12, e0187189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, Z.; Messiri, N.E.; Iqbal, E.; Hassan, H.; Tanweer, M.S.; Sadia, S.R.; Taj, M.; Zaidi, U.; Yusuf, K.; Syed, N.I.; et al. On the role of epigenetic modifications of HPA axis in posttraumatic stress disorder and resilience. J. Neurophysiol. 2025, 133, 742–759. [Google Scholar] [CrossRef] [PubMed]
- van den Berk-Clark, C.; Secrest, S.; Walls, J.; Hallberg, E.; Lustman, P.J.; Schneider, F.D.; Scherrer, J.F. Association between posttraumatic stress disorder and lack of exercise, poor diet, obesity, and co-occuring smoking: A systematic review and meta-analysis. Health Psychol. 2018, 37, 407–416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whitworth, J.W.; Scioli, E.R.; Keane, T.M.; Marx, B.P. Physical inactivity, cigarette smoking, and psychiatric comorbidity among veterans with posttraumatic stress disorder. Health Psychol. 2022, 41, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Schein, J.; Houle, C.; Urganus, A.; Cloutier, M.; Patterson-Lomba, O.; Wang, Y.; King, S.; Levinson, W.; Guérin, A.; Lefebvre, P.; et al. Prevalence of post-traumatic stress disorder in the United States: A systematic literature review. Curr. Med. Res. Opin. 2021, 37, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Janiri, D.; Kotzalidis, G.D.; Sani, G. Improving the Assessment of COVID-19-Associated Posttraumatic Stress Disorder-Reply. JAMA Psychiatry 2021, 78, 795–796. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.; von Känel, R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry 2017, 4, 320–329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rauch, S.L.; Shin, L.M.; Phelps, E.A. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research--past, present, and future. Biol. Psychiatry 2006, 60, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Tawakol, A.; Ishai, A.; Takx, R.A.; Figueroa, A.L.; Ali, A.; Kaiser, Y.; Truong, Q.A.; Solomon, C.J.; Calcagno, C.; Mani, V.; et al. Relation between resting amygdalar activity and cardiovascular events: A longitudinal and cohort study. Lancet 2017, 389, 834–845, Erratum in Lancet 2017, 389, 804. https://doi.org/10.1016/S0140-6736(17)30082-X. Erratum in Lancet 2017, 389, 804. https://doi.org/10.1016/S0140-6736(17)30344-6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rozanski, A.; Bavishi, C.; Kubzansky, L.D.; Cohen, R. Association of Optimism With Cardiovascular Events and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1912200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Princip, M.; Ledermann, K.; von Känel, R. Posttraumatic Stress Disorder as a Consequence of Acute Cardiovascular Disease. Curr. Cardiol. Rep. 2023, 25, 455–465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kilpatrick, D.G.; Resnick, H.S.; Milanak, M.E.; Miller, M.W.; Keyes, K.M.; Friedman, M.J. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J. Trauma. Stress 2013, 26, 537–547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenbaum, S.; Vancampfort, D.; Steel, Z.; Newby, J.; Ward, P.B.; Stubbs, B. Physical activity in the treatment of Post-traumatic stress disorder: A systematic review and meta-analysis. Psychiatry Res. 2015, 230, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Lane, R.D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 2007, 74, 224–242. [Google Scholar] [CrossRef] [PubMed]
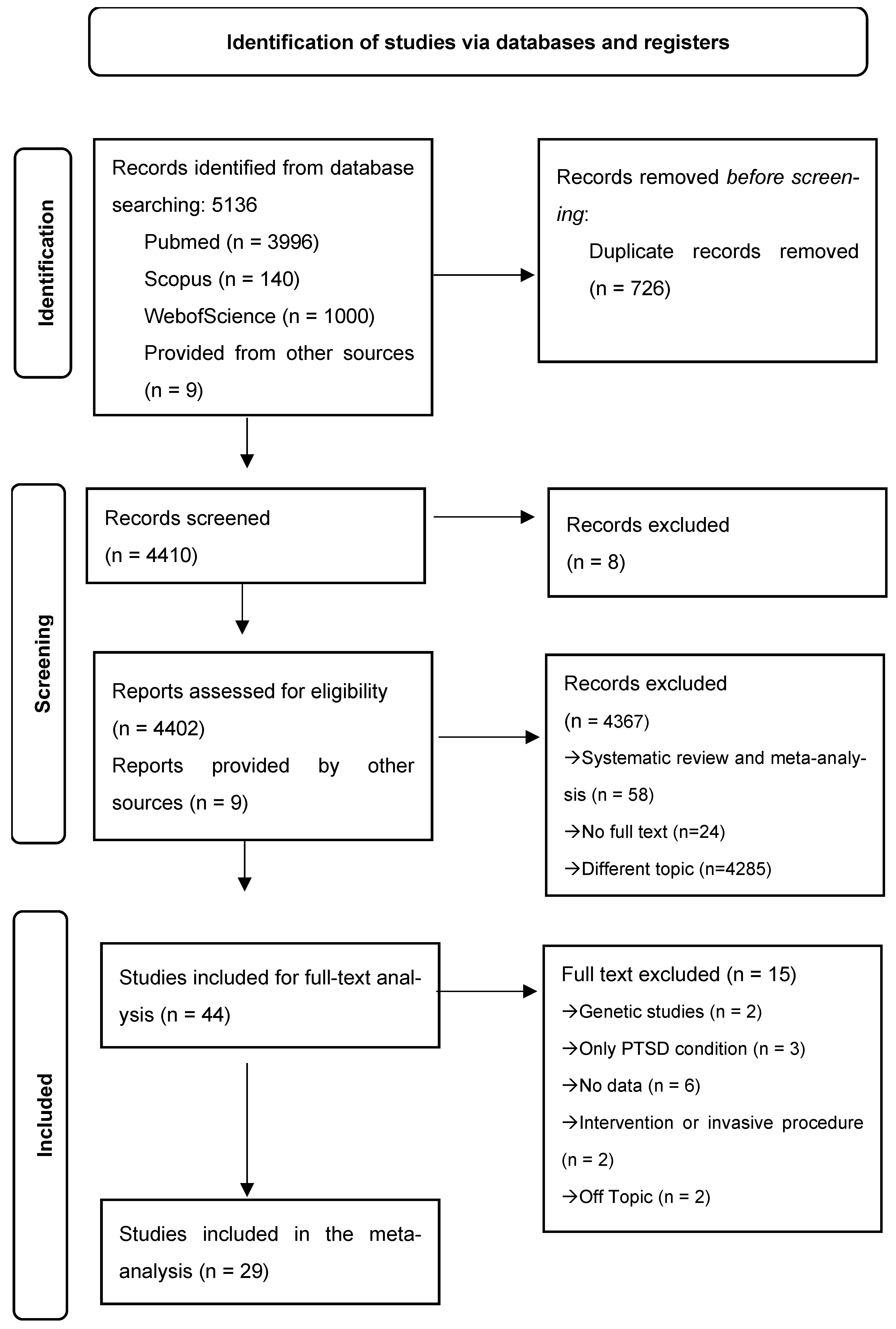
| Database | Search Terms | Limits/Filters | Results Retrieved | Date of Search |
|---|---|---|---|---|
| PubMed | (“Post-Traumatic Stress Disorder” OR “PTSD” OR “trauma”) AND “Coronary Artery Disease” | English language only | 3996 | May 2025 |
| Scopus | TITLE-ABS-KEY (“PTSD” OR “Post-Traumatic Stress Disorder” OR “trauma”) AND “Coronary Artery Disease” | English language only | 140 | May 2025 |
| Web of Science | (“PTSD” OR “Post-Traumatic Stress Disorder” OR “trauma”) AND “Coronary Artery Disease” | English; article/review types | 1000 | May 2025 |
| Study | Selection (Max 4 Stars) | Comparability (Max 2 Stars) | Outcome (Max 3 Stars) | Risk of Bias Assessment | Risk of Bias |
|---|---|---|---|---|---|
| Agarwal et al./2019 [21] | ★,★,★, N/A | ★★ | ★,★,★ | High Quality | L |
| Ahmadi et al./2011 [9] | ★,★,★,★ | ★★ | ★,★,★ | High Quality | L |
| Ahmadi et al./2018 [22] | ★,★,★,★ | ★★ | ★,★,★ | High Quality | L |
| Almuwaqqat et al./2024 [23] | ★,★,★,★ | ★★ | ★,★,★ | High Quality | L |
| Balint et al./2016 [24] | ★, N/A, ★, N/A | ★ | ★, N/A, N/A | Moderate Quality | M |
| Beristianos et al., 2016 [25] | ★,★,★,★ | ★★ | ★,★,★ | High Quality | L |
| Boscarino/2008 [26] | ★,★,★,★ | ★★ | ★,★,★ | High Quality | L |
| Bradley et al./2014 [27] | ★,★,★, N/A | ★★ | ★, ★, ★ | High Quality | L |
| Chen et al., 2015 [28] | ★,★,★,★ | ★★ | ★,★,★ | High Quality | L |
| Chung et al./2008 [29] | ★,★,★, N/A | ★ | ★,★,★ | High Quality | L |
| Crum-Cianflone and Jacobson, 2014 [30] | ★,★,★,★ | ★★ | ★,★,★ | High Quality | L |
| Dzubur Kulenović et al./2008 [31] | ★,★,★, N/A | ★ | ★, N/A, N/A | Moderate Quality | M |
| Fudim et al./2018 [32] | ★,★,★,★ | ★★ | ★,★,★ | High Quality | L |
| Grenon et al./2016 [33] | ★,★,★, N/A | ★★ | ★,★,★ | High Quality | L |
| Johnson et al./2020 [34] | ★, N/A, ★, N/A | ★ | ★, N/A, N/A | Moderate Quality | M |
| Kim et al./2022 [35] | ★, N/A, ★, N/A | ★★ | ★, N/A, N/A | Moderate Quality | M |
| Kim et al./2022 [36] | ★,★,★,★ | ★★ | ★,★,★ | High Quality | L |
| Leung et al./2012 [37] | ★, N/A, ★, ★ | ★ | ★,★,★ | High Quality | L |
| Lima et al./2020 [38] | ★,★,★, N/A | ★★ | ★,★,★ | High Quality | L |
| Mesa-Vieira et al., 2024 [39] | ★,★,★,★ | ★★ | ★,★,★ | High Quality | L |
| Nesic et al./2023 [40] | ★, N/A, ★, N/A | ★ | ★, N/A, N/A | Moderate Quality | M |
| Pedersen et al./2003 [41] | ★,★, N/A, N/A | ★ | ★,★,★ | Moderate Quality | M |
| Rosman et al., 2019 [42] | ★,★,★,★ | ★★ | ★,★,★ | High Quality | L |
| Stoll et al./2000 [43] | ★, ★, ★, N/A | ★ | ★, ★, ★ | Moderate Quality | L |
| Sumner et al./2024 [44] | ★, N/A, ★, N/A | N/A | ★, N/A | Low Quality | H |
| Vaccarino et al./2013 [45] | ★,★,★,★ | ★★ | ★,★,★ | High Quality | L |
| Vaccarino et al./2022 [46] | ★,★,★,★ | ★★ | ★,★,★ | High Quality | L |
| Walczewska et al./2011 [47] | ★,★,★,★ | ★★ | ★, N/A, N/A | High Quality | L |
| Wells et al./2023 [48] | ★, N/A, ★, N/A | ★ | ★, N/A, N/A | Moderate Quality | M |
| Study | Country | Sample Size | Type of Sample | Male Percentage | Mean Age of the Sample | PTSD-Diagnosis | Cardiovascular Condition | Main Results |
|---|---|---|---|---|---|---|---|---|
| Ahmadi et al., 2011 [9] | USA | 637 | Veterans | 100 | 58.5 | DSM-IV | CAC; Framingam Risk Score | PTSD associated with presence and severity of CAC PTSD independent predictor of mortality |
| Ahmadi et al., 2018 [22] | USA | 246 | General Population | 72 | 61.5 | DSM-IV | MACE | PTSD independent predictors of MACE |
| Almuwaqqat et al., 2024 [23] | USA | 736 | General Population | 65 | 59 | DSM-IV | Incidence of CVD | Higher level of current PTSD symptoms independently associated with increased risk of first and recurrent Heart Failure hospitalizations. |
| Balint et al., 2016 [24] | Germany | 56 | General Population | 65 | 62.5 | DSM-IV | CAD | No differences were reported in prevalence |
| Beristianos et al., 2016 [25] | USA | 138,341 | Veterans | 95.9 | 65.9 | ICD-9 | CVD | PTSD higher rate of CVD |
| Boscarino, 2008 [26] | USA | 4328 | Veterans | 100 | - | DSM-III-R | CVD | PTSD prospectively associated with HD mortality |
| Chen et al., 2015 (1) [28] | Taiwan | 26,085 | General Population | 20.9 | 36.6 | ICD-9 | Stroke | Higher incidence of stroke in PTSD condition |
| Chen et al., 2015 (2) [28] | Taiwan | 26,085 | General Population | 20.9 | 36.6 | ICD-9 | IHD | Higher incidence of IHD in PTSD condition |
| Crum-Cianflone et al., 2014 [30] | USA | 60,025 | Veterans | 60 | 34.4 | Self-report | CHD | Higher incidence of CHD in PTSD condition |
| Dzubur Kulenović et al., 2008 [31] | Bosnia-Erzegovina | 100 | Veterans | 100 | 40–50 | Self-report | CAD | Chronic PTSD associated with dyslipidemia and increased risk of CAD |
| Fudim et al., 2018 [32] | USA | 111,970 | Veterans | 100 | 66.5 | ICD-9 | Not Specified HC | Higher rate of CAD in PTDS condition |
| Grenon et al., 2016 [33] | USA | 214 | Veterans | 99 | 69 | DSM-IV | CAD | No differences were reported in prevalence |
| Kim et al., 2022 [35] | Korea | 214,996 | General Population | 35 | 50 | ICDI-10 | CAD; hemorrhagic stroke, and cardiovascular mortality | Associations between PTSD and CVD outcomes |
| Lima et al., 2020 [38] | USA | 303 | General Population | 52 | 50 | DSM-IV | HF | No differences were reported in prevalence |
| Mesa-Vieira et al., 2024 [39] | USA | 1,009,113 | General Population | 40 | 38.6 | ICD-10 | MACE | Higher incidence of MACE in PTSD condition |
| Rosman et al., 2019 [42] | IRAQ | 987,855 | General Population | - | 30 | ICD-9 | TIA | Higher incidence of TIA in PTSD condition |
| Vaccarino et al., 2013 [45] | USA | 562 | Veteran Twins | 100 | 42.6 | DSM-III-R | AMI; CHD; ACS | PTSD was associated with greater than twice the risk of CHD over a median follow-up of 13 years |
| Vaccarino et al., 2022 [46] | USA | 275 | Veteran Twins | 100 | 68 | DSM-IV | CHD | PTSD higher rate of CHD |
| Walczewska et al., 2011 [47] | Polonia | 150 | Former Deportees (Siberia) vs. Control with no PTSD | 50 | 70 | DSM-IV | CHD | Former deportees with PTSD had higher prevalence of CHD |
| Study | Country | Sample Size | Male Percentage | Mean Age of the Sample | PTSD-Diagnosis | Cardiovascular Condition | Percentage of PTSD in Clinical Sample |
|---|---|---|---|---|---|---|---|
| Agarwal et al., 2019 [21] | USA | 114 | - | - | PCL-5 | Cardiac Arrest | 32 |
| Bradley et al., 2014 [27] | USA | 142,954 | - | - | ICD-9 (Interview) | CAD | 12 |
| Chung et al., 2008 [29] | UK | 120 | 78 | 67 | DSM-IV (Interview) | AMI | 31 |
| Johnson et al., 2020 [34] | USA | 512 | 2.5 | 52 | PDS | Coronary Artery Dissection | 36 |
| Kim et al., 2022 [36] | USA | 657 | 65 | 58.4 | DSM-IV (Interview) | CHD | 11 |
| Leung et al., 2012 [37] | Toronto | 1692 | 75 | 65.5 | PTGI | MACE (HF; stroke; AMI) | 8 |
| Nesic et al., 2023 [40] | Germany | 153 | 50 | 61 | PCL-5 | CAD | 31 |
| Pedersen et al., 2003 [41] | Denmark | 112 | 70 | 60 | PDS | AMI | 22 |
| Stoll et al., 2000 [43] | Germany | 80 | 51 | 63.5 | PTSS-10 | Cardiac Surgery | 15 |
| Sumner et al., 2024 [44] | USA | 859 | 6 | 52.3 | DSM-IV Checklist | Coronary Artery Dissection | 35 |
| Wells et al., 2023 [48] | UK | 572 | 63 | 60.5 | IES-R | CHD | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Nakhebi, O.A.S.; Albu-Kalinovic, R.; Neda-Stepan, O.; Giurgi-Oncu, C.; Crișan, C.-A.; Enatescu, V.-R.; Marinescu, I. Post-Traumatic Stress Disorder (PTSD) and Cardiovascular Diseases: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 7979. https://doi.org/10.3390/jcm14227979
Al Nakhebi OAS, Albu-Kalinovic R, Neda-Stepan O, Giurgi-Oncu C, Crișan C-A, Enatescu V-R, Marinescu I. Post-Traumatic Stress Disorder (PTSD) and Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(22):7979. https://doi.org/10.3390/jcm14227979
Chicago/Turabian StyleAl Nakhebi, Omar Anwar Saleh, Raluka Albu-Kalinovic, Oana Neda-Stepan, Catalina Giurgi-Oncu, Cătălina-Angela Crișan, Virgil-Radu Enatescu, and Ileana Marinescu. 2025. "Post-Traumatic Stress Disorder (PTSD) and Cardiovascular Diseases: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 22: 7979. https://doi.org/10.3390/jcm14227979
APA StyleAl Nakhebi, O. A. S., Albu-Kalinovic, R., Neda-Stepan, O., Giurgi-Oncu, C., Crișan, C.-A., Enatescu, V.-R., & Marinescu, I. (2025). Post-Traumatic Stress Disorder (PTSD) and Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(22), 7979. https://doi.org/10.3390/jcm14227979






