Hands Deserve Better: A Systematic Review of Surgical Glove Indicator Systems and Identification of Glove Perforation
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Systematic reviews and meta-analysis;
- Randomized controlled clinical studies;
- Quasi-experimental studies;
- Cohort with control designs;
- Case-controlled designs;
- Observational studies;
- Healthy volunteer studies;
- In vitro studies.
- Dentistry or orthodontics;
- Local, interventional procedures performed outside of the operating theater (e.g., emergency department, etc.);
- In vitro studies or simulated surgeries;
- Veterinary surgery;
- From 1979 or older;
- No abstract available;
- “Antimicrobial glove” studies;
- Narrative reviews, commentaries, and letters to the editor;
- Quality improvement projects, case studies, or case series.
2.2. Information Sources
2.3. Search Strategy
2.4. Data Management and Selection Process
2.5. Data Items
2.6. Risk of Bias
2.7. Data Synthesis
2.8. Meta-Bias
2.9. Confidence in Cumulative Evidence
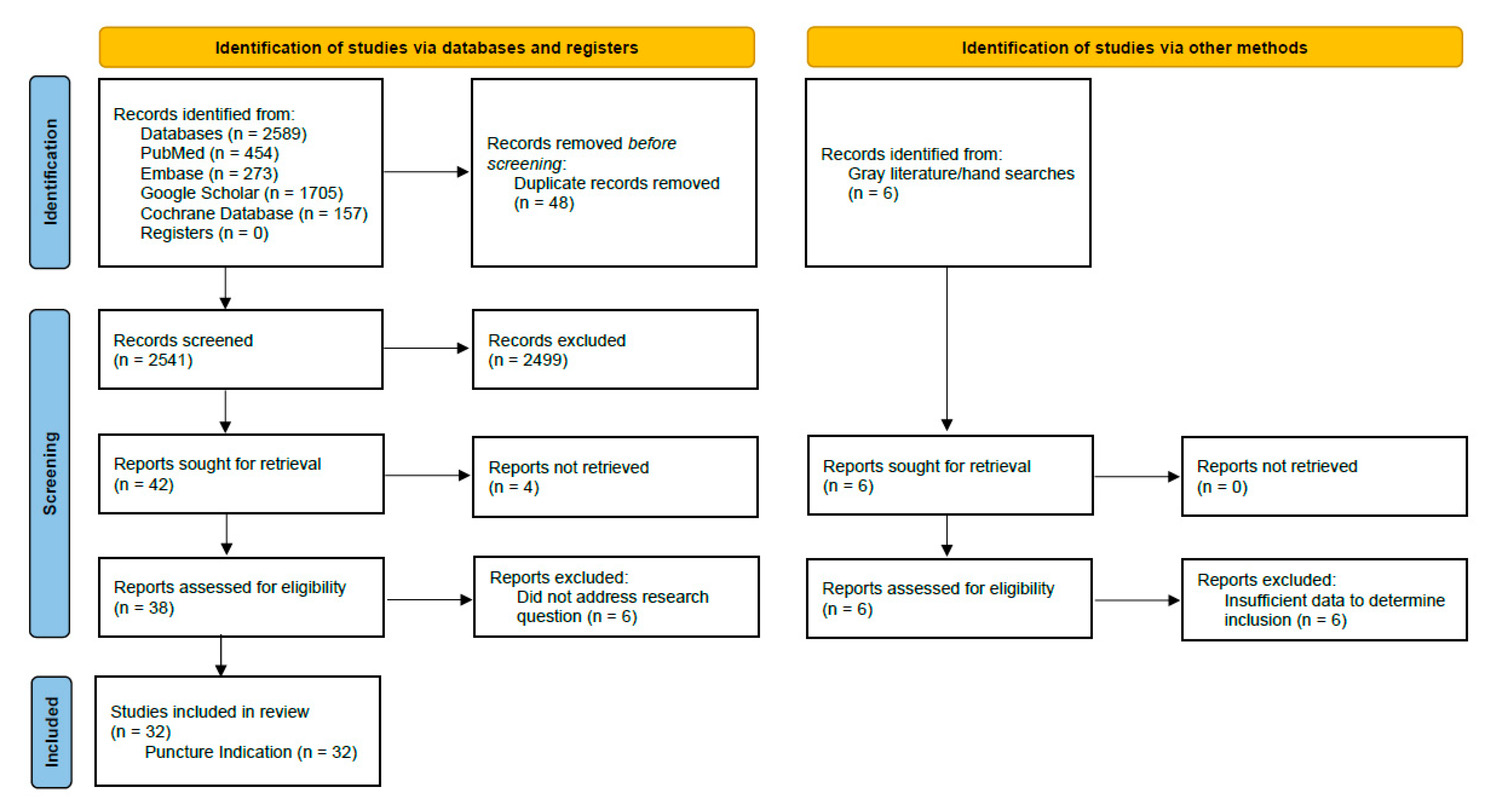
3. Results
3.1. Summary of Evidence Prior to Current Review
3.2. The Effect of Using Indicator Glove Systems on the Rate of Detection of Glove Perforations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernigou, P. The Strange History of Surgical Gloves in Orthopaedic Surgery (Part I): From No Gloves and No Hand Washing to the Introduction of Cotton Gloves in Orthopaedic Surgery. Int. Orthop. 2022, 46, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Boceno, A.; Potage, D. Rubber Gloves in Orthopaedic Surgery (Part II): Cooke and Goodyear; Halsted and Caroline’s Gloves of Love; from Cotton to Rubber after Perthes’ Experiments; Double Glove Technique with Urist. Int. Orthop. 2023, 47, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Arnau, A.M.; Pesqué, D.; Maibach, H.I. Contact Urticaria Syndrome: A Comprehensive Review. Curr. Dermatol. Rep. 2022, 11, 194–201. [Google Scholar] [CrossRef]
- Partecke, L.I.; Goerdt, A.-M.; Langner, I.; Jaeger, B.; Assadian, O.; Heidecke, C.-D.; Kramer, A.; Huebner, N.-O. Incidence of Microperforation for Surgical Gloves Depends on Duration of Wear. Infect. Control Hosp. Epidemiol. 2009, 30, 409–414. [Google Scholar] [CrossRef]
- Aarnio, P.; Laine, T. Glove Perforation Rate in Vascular Surgery—A Comparison between Single and Double Gloving. VASA Z. Gefasskrankh. 2001, 30, 122–124. [Google Scholar] [CrossRef]
- Agarwal, A.; Agarwal, R. Glove Perforation and Contamination in Primary Total Hip Arthroplasty. J. Bone Jt. Surg. Br. 2005, 87, 1585. [Google Scholar] [CrossRef]
- Al-Maiyah, M.; Bajwa, A.; Mackenney, P.; Port, A.; Gregg, P.J.; Hill, D.; Finn, P. Glove Perforation and Contamination in Primary Total Hip Arthroplasty. J. Bone Jt. Surg. Br. 2005, 87, 556–559. [Google Scholar] [CrossRef]
- Anand, S.; Pogorelić, Z.; Singh, A.; Llorente Muñoz, C.M.; Krishnan, N.; Dhua, A.K.; Goel, P.; Bajpai, M. Comparison of Unnoticed Glove Perforations during Minimally Invasive versus Open Surgeries: A Systematic Review and Meta-Analysis. Children 2022, 9, 179. [Google Scholar] [CrossRef]
- Enz, A.; Klinder, A.; Bisping, L.; Lutter, C.; Warnke, P.; Tischer, T.; Mittelmeier, W.; Lenz, R. Knot Tying in Arthroplasty and Arthroscopy Causes Lesions to Surgical Gloves: A Potential Risk of Infection. Knee Surg. Sports Traumatol. Arthrosc. 2022, 31, 1824–1832. [Google Scholar] [CrossRef]
- Enz, A.; Kamaleddine, I.; Groß, J.; Schafmayer, C.; Alwafai, E.; Sievers, L.; Mittelmeier, W.; Klinder, A. Is Single Gloving Still Acceptable? Investigation and Evaluation of Damages on Sterile Latex Gloves in General Surgery. J. Clin. Med. 2021, 10, 3887. [Google Scholar] [CrossRef] [PubMed]
- Babayan, R.K. Re: Microperforations of Surgical Gloves in Urology: Minimally Invasive versus Open Surgeries. J. Urol. 2011, 186, 2267. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.V.J.; Nahas, F.X.; Ferreira, L.M.; Farah, A.B.; Ayaviri, N.A.M.; Bariani, R.L. Risk of Glove Perforation in Minor and Major Plastic Surgery Procedures. Aesthetic Plast. Surg. 2003, 27, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Timler, D.; Kusiński, M.; Iltchev, P.; Szarpak, Ł.; Śliwczyński, A.; Kuzdak, K.; Marczak, M. Glove Failure in Elective Thyroid Surgery: A Prospective Randomized Study. Int. J. Occup. Med. Environ. Health 2015, 28, 499–505. [Google Scholar] [CrossRef]
- ReD Associates Ethnographic Research Study. 2021. Available online: https://www.redassociates.com/home (accessed on 27 October 2025).
- Stearns, K.L.; Johnson, P.; Enz, A.; Bah-Rösman, J.; Brindle, C.T. A Systematic Review of the Impact of Double Gloving Compared to Single Gloving on the Risk of Complications During Surgery. 2025; submitted. [Google Scholar]
- Enz, A.; Boermeester, M.A.; Chatterjee, A.; Coombs, N.; Dye, L.; Johnson, P.; Lingaas, E.; Mittelmeier, W.; Munakata, K.; Sawyer, R.G.; et al. Hands Deserve Better: Global Clinical Consensus Recommendations on Surgical Gloving Practice. 2025; submitted. [Google Scholar]
- Mischke, C.; Verbeek, J.H.; Saarto, A.; Lavoie, M.-C.; Pahwa, M.; Ijaz, S. Gloves, Extra Gloves or Special Types of Gloves for Preventing Percutaneous Exposure Injuries in Healthcare Personnel. Cochrane Database Syst. Rev. 2014, 2014, CD009573. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. Available online: https://web.archive.org/web/20210716121605id_/www3.med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf (accessed on 27 October 2025).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.T.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R. ROBIS: A New Tool to Assess Risk of Bias in Systematic Reviews Was Developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef]
- Dearholt, S.; Dang, D. Johns Hopkins Evidence-Based Practice Model and Guidelines, 2nd ed.; Sigma Theta Tau International: Indianapolis, IN, USA, 2012. [Google Scholar]
- Abdulkarim, A.; Moriarty, A.; Chen, Y.; Devine, D.; Sheehan, E. The Effect of Orthopaedic Surgery on the Intrinsic Properties of Surgical Gloves. Int. J. Surg. 2015, 23, S24. [Google Scholar] [CrossRef]
- Avery, C.M.; Taylor, J.; Johnson, P.A. Double Gloving and a System for Identifying Glove Perforations in Maxillofacial Trauma Surgery. Br. J. Oral Maxillofac. Surg. 1999, 37, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.J.; Bromberg, W.J.; Zura, R.D.; Foresman, P.A.; Morgan, R.G.; Edlich, R.F. New Advances in Electronic Devices for Hole Detection. J. Appl. Biomater. 1994, 5, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Laine, T.; Kaipia, A.; Santavirta, J.; Aarnio, P. Glove Perforations in Open and Laparoscopic Abdominal Surgery: The Feasibility of Double Gloving. Scand. J. Surg. 2004, 93, 73–76. [Google Scholar] [CrossRef]
- Laine, T.; Aarnio, P. How Often Does Glove Perforation Occur in Surgery? Comparison between Single Gloves and a Double-Gloving System. Am. J. Surg. 2001, 181, 564–566. [Google Scholar] [CrossRef]
- Lee, S.Y. What Role Does a Colored Under Glove Have in Detecting Glove Perforation in Foot and Ankle Procedures? Clin. Orthop. 2022, 480, 2327–2334. [Google Scholar] [CrossRef]
- Naver, L.P.; Gottrup, F. Incidence of Glove Perforations in Gastrointestinal Surgery and the Protective Effect of Double Gloves: A Prospective, Randomised Controlled Study. Eur. J. Surg. Acta Chir. 2000, 166, 293–295. [Google Scholar] [CrossRef]
- Nicolai, P.; Aldam, C.H.; Allen, P.W. Increased Awareness of Glove Perforation in Major Joint Replacement. A Prospective, Randomised Study of Regent Biogel Reveal Gloves. J. Bone Jt. Surg. Br. 1997, 79, 371–373. [Google Scholar] [CrossRef]
- Tanner, J.; Parkinson, H. Double Gloving to Reduce Surgical Cross-Infection. Cochrane Database Syst. Rev. 2006, 3, CD003087. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.; Parkinson, H. Surgical Glove Practice: The Evidence. J. Perioper. Pract. 2007, 17, 216–218, 220–222, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.N. Surgeon Protection: Early Recognition of Glove Perforation Using a Green under Glove. J. R. Coll. Surg. Edinb. 1996, 41, 395–396. [Google Scholar]
- de Oliveira, A.C.; Gama, C.S. Evaluation of Surgical Glove Integrity during Surgery in a Brazilian Teaching Hospital. Am. J. Infect. Control 2014, 42, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Edlich, R.F.; Wind, T.C.; Hill, L.G.; Thacker, J.G. Resistance of Double-Glove Hole Puncture Indication Systems to Surgical Needle Puncture. J. Long. Term. Eff. Med. Implant. 2003, 13, 85–90. [Google Scholar] [CrossRef]
- Edlich, R.F.; Wind, T.C.; Heather, C.L.; Thacker, J.G. Reliability and Performance of Innovative Surgical Double-Glove Hole Puncture Indication Systems. J. Long. Term Eff. Med. Implant. 2003, 13, 69–83. [Google Scholar] [CrossRef]
- Edlich, R.; Wind, T.C.; Heather, C.L.; Thacker, J.G. An Update on the Innovative Surgical Double-Glove Hole Puncture Indication Systems: Reliability and Performance. J. Long. Term Eff. Med. Implant. 2017, 27, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Edlich, R.F.; Wind, T.C.; Hill, L.G.; Thacker, J.G.; McGregor, W. Reducing Accidental Injuries during Surgery. J. Long. Term Eff. Med. Implant. 2003, 13, 317–326. [Google Scholar] [CrossRef]
- Florman, S.; Burgdorf, M.; Finigan, K.; Slakey, D.; Hewitt, R.; Nichols, R.L. Efficacy of Double Gloving with an Intrinsic Indicator System. Surg. Infect. 2005, 6, 385–395. [Google Scholar] [CrossRef]
- Grant, C. Biogel Super-Sensitive and Biogel Indicator Glove Systems. Br. J. Nurs. 2001, 10, 1148–1151. [Google Scholar] [CrossRef]
- Hollaus, P.H.; Lax, F.; Janakiev, D.; Wurnig, P.N.; Pridun, N.S. Glove Perforation Rate in Open Lung Surgery. Eur. J. Cardio-Thorac. Surg. 1999, 15, 461–464. [Google Scholar] [CrossRef]
- Lee, S.W.; Cho, M.-R.; Lee, H.-H.; Choi, W.-K.; Lee, J.-H. Perforation of Surgical Gloves during Lower Extremity Fracture Surgery and Hip Joint Replacement Surgery. Hip Pelvis 2015, 27, 17–22. [Google Scholar] [CrossRef]
- Macintyre, I.M.; Currie, J.S.; Smith, D.N.; Anderson, I.D.; Cadossi, R. Reducing the Risk of Viral Transmission at Operation by Electronic Monitoring of the Surgeon-Patient Barrier. Br. J. Surg. 1994, 81, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Manson, T.T.; Bromberg, W.G.; Thacker, J.G.; McGregor, W.; Morgan, R.F.; Edlich, R.F. A New Glove Puncture Detection System. J. Emerg. Med. 1995, 13, 357–364. [Google Scholar] [CrossRef]
- Martinez, A.; Han, Y.; Sardar, Z.M.; Beckman, L.; Steffen, T.; Miller, B.S.; Martineau, P.A. Risk of Glove Perforation with Arthroscopic Knot Tying Using Different Surgical Gloves and High-Tensile Strength Sutures. Arthroscopy 2013, 29, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Sarih, N.M.; Dzulkafly, N.S.; Maher, S.; Rashid, A.A. Wearable Natural Rubber Latex Gloves with Curcumin for Torn Glove Detection in Clinical Settings. Polymers 2022, 14, 3048. [Google Scholar] [CrossRef] [PubMed]
- Sayın, S.; Yılmaz, E.; Baydur, H. Rate of Glove Perforation in Open Abdominal Surgery and the Associated Risk Factors. Surg. Infect. 2019, 20, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Shek, K.M.-Y.; Chau, J.P.-C. Surgical Glove Perforation among Nurses in Ophthalmic Surgery: A Case-Control Study. Int. J. Nurs. Pract. 2014, 20, 179–186. [Google Scholar] [CrossRef]
- Shimantani, M.; Matsui, Y.; Sakakibara, K.; Iwase, T.; Yano, K. Investigation of the Rate of Glove Perforations in Orthopedic Procedures with an Indicator Underglove System (IUS) in a Japanese Hospital. Am. J. Infect. Control 2009, 37, E117–E118. [Google Scholar]
- Sohn, R.L.; Murray, M.T.; Franko, A.; Hwang, P.K.; Dulchavsky, S.A.; Grimm, M.J. Detection of Surgical Glove Integrity. Am. Surg. 2000, 66, 302–306. [Google Scholar] [CrossRef]
- Walczak, D.A.; Zakrzewski, J.; Pawelczak, D.; Grobelski, B.; Pasieka, Z. Evaluation of Surgical Glove Perforation after Laparoscopic and Open Cholecystectomy. Acta Chir. Belg. 2013, 113, 423–428. [Google Scholar] [CrossRef]
- Wigmore, S.J.; Rainey, J.B. Use of Coloured Undergloves to Detect Glove Puncture. Br. J. Surg. 1994, 81, 1480. [Google Scholar] [CrossRef]
- Wittmann, A.; Kralj, N.; Köver, J.; Gasthaus, K.; Hofmann, F. Study of Blood Contact in Simulated Surgical Needlestick Injuries with Single or Double Latex Gloving. Infect. Control Hosp. Epidemiol. 2009, 30, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, A.; Kralj, N.; Köver, J.; Gasthaus, K.; Lerch, H.; Hofmann, F. Comparison of 4 Different Types of Surgical Gloves Used for Preventing Blood Contact. Infect. Control Hosp. Epidemiol. 2010, 31, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
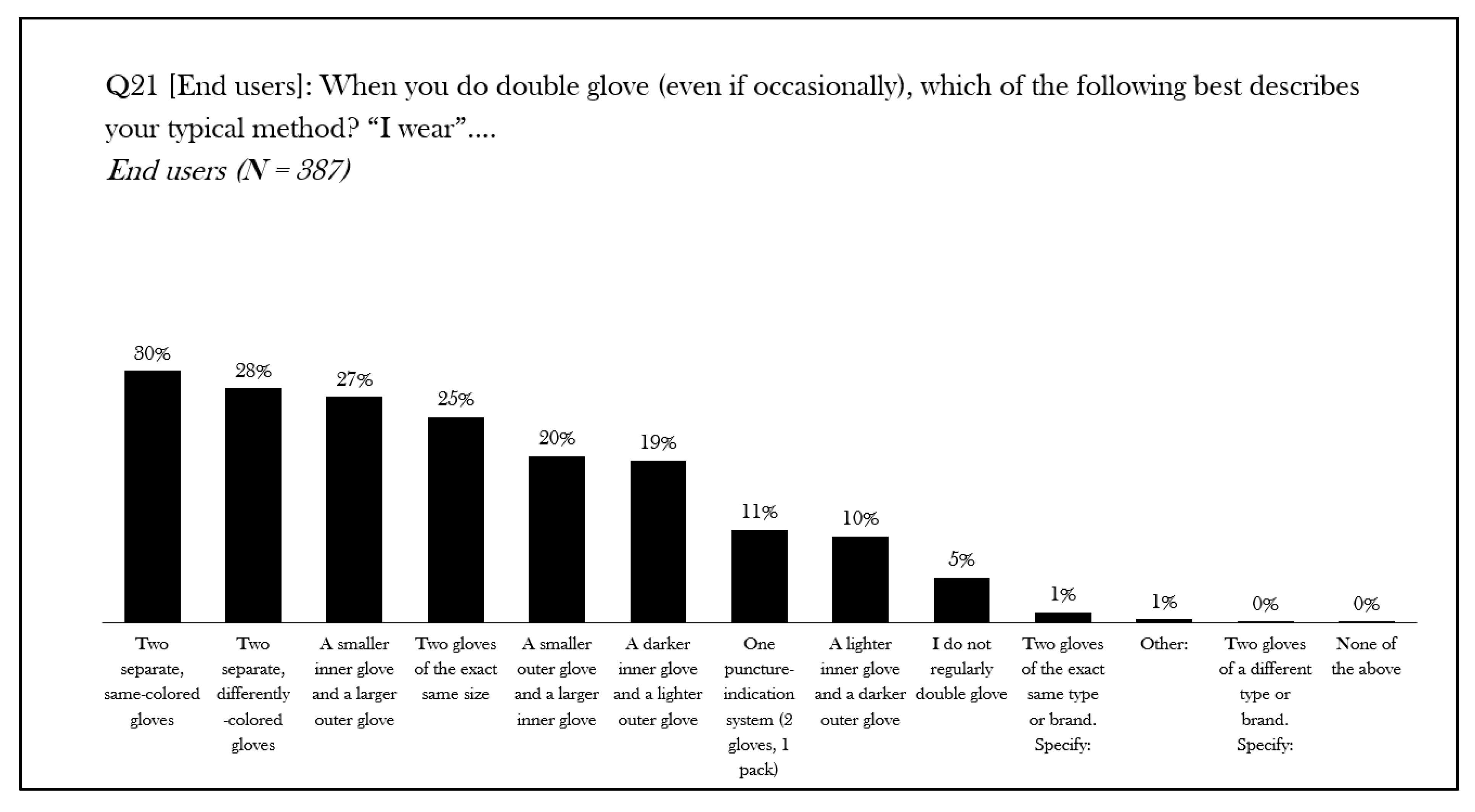

| Author, Year | Title | Aim | Key Findings |
|---|---|---|---|
| Abdulkarim A. et al., 2015 [23] | The effect of orthopedic surgery on the intrinsic properties of surgical gloves |
|
|
| Avery, C. M. E. et al., 1999 [24] | Double gloving and a system for identifying glove perforations in maxillofacial trauma surgery |
|
|
| Cox, M. J. et al., 1994 [25] | New advances in electronic devices for hole detection |
|
|
| Laine, T. et al., 2004 [26] | Glove perforations in open and laparoscopic abdominal surgery: the feasibility of double gloving |
|
|
| Laine, T. and Aarnio, P., 2001 [27] | How often does glove perforation occur in surgery? Comparison between single gloves and a double-gloving system |
|
|
| Lee, S. Y., 2022 [28] | What role does a colored under glove have in detecting glove perforation in foot and ankle procedures? |
|
|
| Naver, L. P. and Gottrup, F., 2000 [29] | Incidence of glove perforations in gastrointestinal surgery and the protective effect of double gloves: a prospective, randomized controlled study |
|
|
| Nicolai, P. et al., 1997 [30] | Increased awareness of glove perforation in major joint replacement. A prospective, randomized study of Regent Biogel Reveal gloves |
|
|
| Tanner, J. and Parkinson, H., 2006 [31] | Double gloving to reduce surgical cross-infection |
|
|
| Tanner, J. and Parkinson, H., 2007 [32] | Surgical glove practice: the evidence |
|
|
| Author, Year | Title | Key Findings |
|---|---|---|
| Brown, J. N., 1996 [33] | Surgeon protection: early recognition of glove perforation using a green under glove | A total of 40 consecutive cases/single surgeon. Green indicator gloves. A total of 26 perforations in 26 outer gloves. A percentage pf 48% of all cases. Outer glove rate 25%. Instruments 59%, exposed bone 30%. Left index finger most common. |
| de Oliveira, A. C. et al., 2014 [34] | Evaluation of surgical glove integrity during surgery in a Brazilian teaching hospital | Latex single glove study. No indicator. A percentage of 65% of cases; 12 post-op perf rate. No indicator used—suggests that is good to do based on the rate of undetected perforations. |
| Edlich, R. F. et al., 2003 [35] | Resistance of Double-Glove Hole Puncture Indication Systems to Surgical Needle Puncture | Thicker gloves are more resistant to needlestick puncture. Double gloves are more resistant to needle puncture. Latex single and double glove systems are less resistant to puncture than non-latex and latex single and double gloving. |
| Edlich, R. F. et al., 2003 [36] | Reliability and Performance of Innovative Surgical Double-Glove Hole Puncture Indication Systems | Indicator systems provide visual evidence of glove puncture. |
| Edlich, R. F. et al., 2017 [37] | An Update on the Innovative Surgical Double-Glove Hole Puncture Indication Systems: Reliability and Performance | Surgical needle puncture holes were detected by all glove indicator systems assessed. |
| Edlich, R. F. et al., 2003 [38] | Reducing Accidental Injuries During Surgery | Resistance to needle puncture is significantly greater in double-glove indicator systems than single gloves. |
| Florman, S. and Burgdorf, M., 2005 [39] | Efficacy of Double Gloving with an Intrinsic Indicator System | Latex—84% of glove perforations were detected, Latex-free—56% of glove perforations were detected. |
| Grant, C., 2013 [40] | Biogel® Super-Sensitive and Biogel® Indicator glove systems | A combination of Biogel® Super-Sensitive and Biogel® Indicator glove systems was accepted for use by staff following a clinical evaluation. |
| Hollaus, P. H. et al., 1999 [41] | Glove perforation rate in open lung surgery | Rate of outer glove perforation was 8.9% whilst perforation rate for inner gloves was only 1.13%. |
| Lee, S. W. et al., 2015 [42] | Perforation of Surgical Gloves during Lower Extremity Fracture Surgery and Hip Joint Replacement Surgery | A total of 25% of gloves were perforated during surgery. |
| MacIntyre, I. M. C. et al., 2005 [43] | Reducing the Risk of Viral Transmission at Operation by Electronic Monitoring of the Surgeon–Patient Barrier | Glove perforation rate was about 39.8%. The surgeon detected the hole in 11.1%. Only 16.9% of alarms were from glove holes, most were from wet gowns. |
| Manson, T. T. et al., 1995 [44] | A New Glove Puncture Detection System | More force was required to penetrate the double-glove Biogel Reveal system gloves with a needle than single gloves. |
| Martinez, A. et al., 2013 [45] | Risk of Glove Perforation with Arthroscopic Knot Tying Using Different Surgical Gloves and High-tensile Strength Sutures | % thin gloves perforated, 6.8% thick gloves. No perforations of both doubled gloves. Suture made no difference. |
| Sarih, N. M. et al., 2022 [46] | Wearable Natural Rubber Latex Gloves with Curcumin for Torn Glove Detection in Clinical Settings | No change frequency data. Overall glove perforation rate was 11.8%. A total of 21.7% of perforations were noted intra-operatively. |
| Sayın, S. et al., 2019 [47] | Wearable Natural Rubber Latex Gloves with Curcumin for Torn Glove Detection in Clinical Settings | Successfully added an indicator layer to natural latex gloves. Improved tear strength with coating. |
| Shek, K. M. Y. and Chau, J. P. C., 2014 [48] | Surgical Glove Perforation Among Nurses in Ophthalmic Surgery: A Case–Control Study | Change frequency undefined. A total of 8% of gloves were perforated, none were detected intra-operatively. |
| Shimantani M. et al., 2009 [49] | Investigation of the Rate of Glove Perforations in Orthopedic Procedures with an Indicator Underglove System (IUS) in a Japanese Hospital | No comment on glove changes. Outer glove perforation rate 14.1%, inner glove perforation rate 4.5% by WLT. The rate of intra-operative detection was 69.6% for surgeons and 44.4% for nurses. |
| Sohn, R. L. et al., 2000 [50] | Detection of surgical glove integrity | Electrical conductance testing was more sensitive than water leak test. |
| Walczak, D. A. et al., 2013 [51] | Evaluation of Surgical Glove Perforation after Laparoscopic and Open Cholecystectomy | No change frequency data given. Overall perforation rate was 8%; perforations more common with laparoscopic surgery. |
| Wigmore, S. J. and Rainey, J. B., 1994 [52] | Use of Colored Undergloves to Detect Glove Puncture | Outerglove perforation was detected in 29% of outergloves. Indicator gloves had a 97% accuracy of detection rate. The indicator system had a 3% false negative rate. |
| Wittmann, A. et al., 2009 [53] | Study of Blood Contact in Simulated Surgical Needlestick Injuries with Single or Double Latex Gloving | Punctures clearly identified by green layer indicator glove. |
| Wittmann, A. et al., 2010 [54] | Comparison of 4 Different Types of Surgical Gloves used for Preventing Blood Contact | Volume of blood transferred by needlestick reduced by a factor of two when using double-glove indicator system. |
| Author, Year | Level of Evidence | Quality | Risk of Bias |
|---|---|---|---|
| Abdulkarim A. et al., 2015 [23] | Level I | Good | Low |
| Avery, C. M. E. et al., 1999 [24] | Level I | High | Low |
| Brown, J. N., 1996 [33] | Level II | Good | High |
| Cox, M. J. et al., 1994 [25] | Level I | Good | Low |
| de Oliveira, A. C. et al., 2014 [34] | Level II | Good | High |
| Edlich, R. F. et al., 2003 [35] | Level II | Good | Low |
| Edlich, R. F. et al., 2003 [36] | Level II | Good | Low |
| Edlich, R. F. et al., 2017 [37] | Level II | Good | Low |
| Edlich, R. F. et al., 2003 [38] | Level II | Good | Low |
| Florman, S. and Burgdorf, M., 2005 [39] | Level II | Good | Low |
| Grant C., 2013 [40] | Level III | Low | High |
| Hollaus, P. H. et al., 1999 [41] | Level II | Good | High |
| Laine, T. et al., 2004 [26] | Level I | High | Some |
| Laine, T. and Aarnio, P., 2001 [27] | Level I | High | Some |
| Lee, S. W. et al., 2015 [42] | Level III | Good | High |
| Lee, S. Y., 2022 [28] | Level I | High | Low |
| MacIntyre, I. M. C. et al., 2005 [43] | Level III | Good | High |
| Manson, T. T. et al., 1995 [44] | Level V | Low | High |
| Martinez, A. et al., 2013 [45] | Level III | Good | High |
| Naver, L. P. and Gottrup, F., 2000 [29] | Level I | High | Low |
| Nicolai, P. et al., 1997 [30] | Level I | High | Low |
| Sarih, N. M. et al., 2022 [46] | Level V | Low | High |
| Sayın, S. et al., 2019 [47] | Level III | Good | High |
| Shek, K. M. Yand Chau, J. P. C., 2014 [48] | Level III | Good | High |
| Shimantani M. et al., 2009 [49] | Level III | Good | High |
| Sohn, R. L. et al., 2000 [50] | Level V | Low | High |
| Tanner, J. and Parkinson, H., 2007 [32] | Level I | High | Low |
| Tanner, J. et al., 2006 [31] | Level I | High | Low |
| Walczak, D. A. et al., 2013 [51] | Level III | Good | High |
| Wigmore, S. J. and Rainey, J. B., 1994 [52] | Level III | Good | High |
| Wittmann, A. et al., 2009 [53] | Level V | Low | High |
| Wittmann, A. et al., 2010 [54] | Level V | Low | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wharton, K.R.; Sawyer, R.G.; Enz, A.; Bah-Rösman, J.; Brindle, C.T. Hands Deserve Better: A Systematic Review of Surgical Glove Indicator Systems and Identification of Glove Perforation. J. Clin. Med. 2025, 14, 7977. https://doi.org/10.3390/jcm14227977
Wharton KR, Sawyer RG, Enz A, Bah-Rösman J, Brindle CT. Hands Deserve Better: A Systematic Review of Surgical Glove Indicator Systems and Identification of Glove Perforation. Journal of Clinical Medicine. 2025; 14(22):7977. https://doi.org/10.3390/jcm14227977
Chicago/Turabian StyleWharton, Kurt R., Robert G. Sawyer, Andreas Enz, Jessica Bah-Rösman, and C. Tod Brindle. 2025. "Hands Deserve Better: A Systematic Review of Surgical Glove Indicator Systems and Identification of Glove Perforation" Journal of Clinical Medicine 14, no. 22: 7977. https://doi.org/10.3390/jcm14227977
APA StyleWharton, K. R., Sawyer, R. G., Enz, A., Bah-Rösman, J., & Brindle, C. T. (2025). Hands Deserve Better: A Systematic Review of Surgical Glove Indicator Systems and Identification of Glove Perforation. Journal of Clinical Medicine, 14(22), 7977. https://doi.org/10.3390/jcm14227977







