An Updated Review of Topical Tretinoin in Dermatology: From Acne and Photoaging to Skin Cancer
Abstract
1. Introduction
2. Methods
- Primary inclusion: Randomized controlled trials (RCTs) and prospective clinical studies.
- Secondary inclusion: Retrospective studies with >20 patients when prospective data were unavailable.
- Exclusion: Case reports, case series ≤20 patients, animal/in vitro studies, conference abstracts without full text, and studies on systemic retinoids only.
3. Results: Dermatological Applications of Topical Tretinoin
3.1. FDA-Approved Indications
3.1.1. Acne Vulgaris (Table 2)
| Study | Design | Population | Intervention | Duration | Main Outcomes |
|---|---|---|---|---|---|
| Berger et al. [10], 2007 | Double-blind, multicenter RCT | 178 subjects (≥12 years) with acne vulgaris | Tretinoin microsphere 0.04% vs. vehicle | 12 weeks | Significant reduction in total inflammatory and non-inflammatory lesions (35.5%, 38.2%, and 33.6% vs. 20.9%, 19.2%, and 20.4%; all p < 0.05). |
| Berger et al. [11], 2007 | Randomized comparative study | 156 subjects with mild-to-moderate acne | Tretinoin microsphere 0.04% vs. tretinoin 0.1% | 12 weeks | Both effective; tretinoin 0.04% showed better tolerability. |
| Cook-Bolden et al. [12], 2019 | Post hoc analysis of RCTs | 766 Hispanic subjects (11–50 years) with moderate-to-severe acne | Tretinoin 0.05% lotion vs. vehicle | 12 weeks | Reduction in inflammatory (60.1% vs. 51.1%) and non-inflammatory (53% vs. 38.7%) lesions; treatment success (≥2-grade improvement in EGSS) in 19.6% vs. 12.7% with vehicle (p = 0.015). |
| Dogra et al. [13], 2021 | Randomized, comparative study | 750 patients with mild-to-moderate acne | Tretinoin 0.04% + clindamycin 1% vs. clindamycin 1% vs. tretinoin 0.04% | 12 weeks | Mean reduction in lesion count: inflammatory, −77%; non-inflammatory, −71%; total lesions, −73%. Proportion of patients achieving ≥2-grade ISGA improvement and rated as “clear” or “almost clear”: 46% with combination vs. 31% with tretinoin monotherapy and 33% with clindamycin monotherapy (p < 0.02). |
| Draelos et al. [14], 2012 | Randomized, double-blind study | 66 subjects (≥18 years) with moderate acne | BPO 5.5% + tretinoin 0.025% vs. clindamycin 1% + BPO 5% + tretinoin 0.025% | 12 weeks | Both regimens showed significant reductions in inflammatory and non-inflammatory lesions, with comparable tolerability. Mean reduction in total lesion count: 66.7% vs. 73.9%. Statistically significant improvement from baseline in skin tone, brightness, pores, and overall appearance. Mild, transient irritation observed in both groups resolved by week 8. |
| Eichenfield et al. [15], 2008 | Phase IV, open-label trial | 544 subjects (≥12 years) with mild-to-moderate acne | Tretinoin gel microsphere (TGM) 0.04% (n = 361) or 0.1% (n = 183), applied once daily via pump | 12 weeks | Significant improvement in mGAGS score from baseline to week 12: −4.2 (0.04%) and −4.7 (0.1%) (both p < 0.0001). At week 12, 72% of patients showed ≥ moderate improvement (IGE), and 25% were rated as clear or almost clear. High treatment compliance (≈95%) and low rate of adverse events (13.6%, mostly mild). Both concentrations well tolerated and effective. |
| Eichenfield et al. [16], 2012 | Randomized, double-blind trial | 110 preadolescents (9–11 years) with acne vulgaris | Tretinoin microsphere 0.04% pump vs. vehicle | 12 weeks | Significant reduction in non-inflammatory lesions with tretinoin vs. vehicle (–19.9 vs. −9.7; p = 0.04). IGA improvement (≥2-point) achieved in 25.5% with tretinoin vs. 13.0% with vehicle (p = 0.02). No significant differences in static IGA or inflammatory lesions. Cutaneous irritation was mild and comparable between groups. |
| Han et al. [17], 2019 | Post hoc analysis of RCTs | 69 Asian patients (12–48 years, 61% female) with moderate-to-severe acne | Tretinoin 0.05% lotion vs. vehicle | 12 weeks | Reduction in inflammatory (58.6% vs. 41.5%) and non-inflammatory (51.4% vs. 23.9%) lesions (p = 0.012 for non-inflammatory lesions); QoL improved; well tolerated. |
| Harper et al. [18], 2019 | Post hoc analysis of RCTs | 606 adult females (≥18 years) with moderate-to-severe acne (EGSS 3–4) | Tretinoin 0.05% lotion vs. vehicle, once daily | 12 weeks | In moderate acne, tretinoin achieved greater lesion reductions (inflammatory 58.5% vs. 50.3%; non-inflammatory 55.5% vs. 39.8%; both p < 0.05) and higher treatment success (25.4% vs. 15.4%; p = 0.006). QoL and patient satisfaction significantly improved. In severe acne, lesion reductions were numerically greater but not significant. Most AEs were mild; application site pain (2.9%) and dryness (5.0%) were most frequent. |
| Tyring et al. [19], 2018 | Double-blind RCT | 1640 patients with moderate-to-severe acne | Tretinoin 0.05% lotion vs. vehicle | 12 weeks | Reduction in inflammatory (52%) and non-inflammatory (46%) lesions; 18% treatment success; favorable safety profile. |
| Tyring et al. [20], 2020 | Pooled post hoc analysis of 2 RCTs | 1640 patients with moderate-to-severe acne (EGSS 3–4) | Tretinoin 0.05% lotion vs. vehicle, once daily | 12 weeks | Tretinoin showed statistically significant improvement over vehicle in all Acne-QoL domains: self-perception (7.4 vs. 6.7), role—emotional (6.8 vs. 6.0), role—social (4.8 vs. 4.6), and acne symptoms (6.5 vs. 5.6), all p < 0.05. Strong correlation between symptom improvement and QoL gain (r = 0.66–0.69). EGSS success (≥2-point improvement + clear/almost clear) achieved in 25.4% vs. 15.4% (p = 0.006). Safety profile favorable. |
| Babayeva et al. [21], 2011 | Single-blind, RCT, comparative | 46 adults (18–35 y) with mild-to-moderate facial AV; 23 per group | Tretinoin 0.05% + clindamycin vs. salicylic acid 3% + clindamycin | 12 weeks | Both treatments significantly reduced total, inflammatory, and non-inflammatory lesion counts. Tretinoin + CDP led to faster reduction in total lesions (50% reduction at week 2 in 21.7% vs. 0%, p = 0.049) and greater improvement in inflammatory lesions at week 4 (p = 0.005). No differences at week 12. Mild-to-moderate side effects in both groups, mostly transient. Quality of life improved similarly in both arms. |
| Jarratt et al. [22], 2012 | Randomized, double-blind, vehicle-controlled trial | 1649 participants with acne vulgaris | Clindamycin 1.2% + tretinoin 0.025% (CT gel) vs. clindamycin alone, tretinoin alone, or vehicle | 12 weeks | CT gel was significantly more effective than monotherapies and vehicle in reducing total lesions (vs. all), non-inflammatory lesions (vs. clindamycin), and inflammatory lesions (vs. tretinoin). More patients achieved ≥2-grade improvement in ISGA. Tolerability was comparable to tretinoin alone; most irritation occurred at week 2. No increase in adverse events with CT gel. |
| Kircik [23], 2009 | Two RCTs (12-week, multicenter, double-blind) | 147 patients with moderate-to-severe acne (12–46 years; 59.9% female) | BPO 5% + clindamycin 1% + RAM 0.04% (Group 1) vs. BPO 5% wash (AM) + CPT gel (clindamycin 1.2% + tretinoin 0.025%) (PM) (Group 2), both once daily | 12 weeks | Mean reduction in inflammatory lesions: 48% (Group 1) vs. 42% (Group 2). At week 12, 80.8% in Group 1 and 87.8% in Group 2 were rated “clear” or “almost clear”. Greater % of patients achieved ≥75% reduction in inflammatory lesions with Group 1 (52.8% vs. 43.7%). Group 1 showed significantly faster improvement in inflammatory lesions at weeks 4 and 8. Group 2 had significantly less dryness and peeling at week 8. Pruritus absent in 91.7% vs. 79.4% (Group 2 vs. Group 1) at week 12. Both regimens were well tolerated. |
| Nilfroushzadeh et al. [24], 2009 | Single-blind RCT | 42 females (15–25 y) with mild-to-moderate acne (randomized 1:1:1) | Group A: 1% clindamycin lotion BID (C lotion) Group B: 1% clindamycin + 0.025% tretinoin QHS (CT lotion) Group C: 1% clindamycin + 2% salicylic acid lotion BID (CS lotion) | 12 weeks | CS lotion showed significantly greater efficacy than clindamycin alone in reducing total lesion count (TLC, −81.80%) and acne severity index (ASI, −73.73%). CT lotion achieved −72.20% TLC and −55.95% ASI. Differences between CT and CS were not statistically significant. CS lotion also had best results for CCN (−87.05%) and PPN (−84.5%). No significant differences found for open comedones or pustules. Adverse events were mild; slight irritation in 21.4% (CT), burning in 50% (CS), none in clindamycin. |
| Pariser et al. [25], 2010 | Randomized, investigator-blinded, phase 4 trial | 247 patients (mean age: 18.5 years) with moderate facial acne (≥12 years) | Tretinoin 0.04% gel microsphere + BPO 5% wash, morning/morning vs. morning/evening regimen | 12 weeks | Lesion count reduction similar between morning/morning and morning/evening regimens; no significant difference in tolerability. |
| Rosso et al. [26], 2023 | Two phase 3, double-blind, vehicle-controlled RCTs | Study 1: 424 subjects (E-BPO/T n = 281; vehicle n = 143) Study 2: 434 subjects (E-BPO/T n = 290; vehicle n = 144) Subjects ≥9 years with moderate-to-severe facial acne (IGA 3–4) | E-BPO/T: 3% encapsulated benzoyl peroxide + 0.1% encapsulated tretinoin cream, QHS vs. vehicle cream QHS, both after cleansing | 12 weeks | IGA success (clear/almost clear + ≥2-grade improvement): Study 1: 38.5% (E-BPO/T) vs. 11.5% (vehicle); Study 2: 25.4% vs. 14.7% (p < 0.05). Inflammatory lesion count: Study 1: −21.6 (E-BPO/T) vs. −14.8; Study 2: −16.2 vs. −14.1 (p < 0.05). Noninflammatory lesion count: Study 1: −28.7 vs. −19.8; Study 2: −24.2 vs. −17.4 (p < 0.001). Improvements seen from week 2. AEs mainly mild to moderate and cutaneous. Local tolerability scores comparable to vehicle. |
| Trifu et al. [27], 2011 | RCT, double-blind, 3 arms | 77 patients (age 18–45) with mild-to-moderate facial acne | CB-03-01 cream 1% QHS (n = 30) Tretinoin cream 0.05% QHS (n = 32) Placebo cream (vehicle) QHS (n = 15)—all applied once daily to affected areas for 8 weeks | 8 weeks | CB-03-01 vs. placebo (week 8): TLC: −28.3% (p = 0.0017) ILC: −27.9% (p = 0.0134) ASI: −23.4% (p = 0.009) IGA success (score ≤1): 22% vs. 7% (no p-value reported) CB-03-01 vs. tretinoin: no statistically significant differences for any variable (TLC, ILC, ASI, or IGA). Irritation score significantly lower with CB-03-01 than tretinoin at week 1 (p = 0.0412); no serious AEs reported. |
| Zaenglein et al. [28], 2013 | Phase IV, open-label single-arm study | 97 patients (12–30 y) with moderate to severe acne (IGA 3–4, mean age 18.3 ± 4.2 y) | Oral minocycline ER 1 mg/kg QD + topical clindamycin phosphate 1.2%/tretinoin 0.025% QD + 6% BP foaming cloths QD | 12 weeks | At week 12, 89% showed ≥1-grade IGA improvement and 55% ≥2-grade. Lesion counts reduced by 56.5% (total), 61.8% (inflammatory), 48.8% (non-inflammatory). AEs mild/moderate in 19%. |
| Zeichner et al. [29], 2013 | Single-blind, randomized, controlled study | 40 patients aged 12–53 years with mild to severe facial acne (80% female) | Tretinoin 0.025% + clindamycin 1.2% gel (TCP) once daily vs. TCP + BPO 6% cleansing cloths (evening) | 12 weeks | PGA success (clear/almost clear) at week 12: 68.4% in TCP + BPO vs. 60.0% in TCP alone (p = NS). SSA success (clear/almost clear) at week 12: 26.3% vs. 10.0% (p = NS). No statistically significant difference in efficacy, but trend favoring TCP + BPO. |
3.1.2. Photoaging (Table 3)
| Study | Design | Population | Tretinoin Formulation | Comparator | Main Outcomes |
|---|---|---|---|---|---|
| Bagatin et al. [30], 2018 | RCT, 24 weeks | 86 women with facial photoaging | 0.05% cream | 0.3% adapalene gel | Comparable efficacy; improvement in wrinkles and pigmentation; similar tolerability. |
| McDaniel et al. [31], 2017 | Split-face, 12 weeks | 48 patients with photodamage | 0.025% cream | Double-conjugated retinoid cream | Similar improvement; tretinoin associated with more irritation. |
| Sumita et al. [8], 2018 | Evaluator-blinded RCT, 24 weeks | 24 postmenopausal women (forearms) | 0.05% cream (3×/week) | 5% tretinoin peel (biweekly) | Both effective; cream improved dermal echogenicity and Ki67; peel more effective for field cancerization. |
| Chien et al. [32], 2022 | Mechanistic study, 24 weeks | 24 patients with moderate-to-severe photoaging | 0.02% cream | Retinol + retinyl esters | Similar clinical improvement; tretinoin increased erythema; MMP2 suppression correlated with improvement in fine wrinkles. |
| Siddiqui et al. [33], 2024 | Systematic review | Multiple RCTs reviewed | Various (0.02–0.1%) | Retinol, glycolic acid, others | Tretinoin superior in efficacy; AEs (irritation, erythema) more frequent at higher concentrations. |
| Kligman and Draelos [34], 2004 | Open-label trial | 32 patients with facial photoaging | 0.25% cream | None (titration protocol) | High-strength tretinoin tolerated with gradual escalation and moisturizers; early improvement observed. |
3.2. Off-Label Use
3.2.1. Melasma and Post-Inflammatory Hyperpigmentation
3.2.2. Acanthosis Nigricans
3.2.3. Rosacea
3.2.4. Striae Distensae (Stretch Marks)
3.2.5. Flat Warts (Verruca Plana)
3.2.6. Disorders of Keratinization
3.2.7. Prevention of Hypertrophic Scars and Keloids
3.2.8. Treatment of Hypertrophic Scars/Post-Burn Scars
3.2.9. Androgenetic Alopecia
3.2.10. Alopecia Areata
3.2.11. Wound Healing
3.2.12. Prevention and Treatment of Skin Cancer
Treatment of Actinic Keratosis
Prevention of Keratinocyte Carcinoma
Treatment of Non-Melanoma Skin Cancer
3.2.13. Tretinoin Prior to Energy-Based Therapy
3.2.14. Tretinoin Prior to Botulinum Toxin
4. Safety and Tolerability of Topical Tretinoin
5. Discussion
6. Limitations
7. Conclusions
Funding
Conflicts of Interest
References
- Sitohang, I.B.S.; Makes, W.I.; Sandora, N.; Suryanegara, J. Topical tretinoin for treating photoaging: A systematic review of randomized controlled trials. Int. J. Women’s Dermatol. 2022, 8, e003. [Google Scholar] [CrossRef]
- Szymański, Ł.; Skopek, R.; Palusińska, M.; Schenk, T.; Stengel, S.; Lewicki, S.; Kraj, L.; Kamiński, P.; Zelent, A. Retinoic Acid and Its Derivatives in Skin. Cells 2020, 9, 2660. [Google Scholar] [CrossRef]
- Bikowski, J.B. Mechanisms of the comedolytic and anti-inflammatory properties of topical retinoids. J. Drugs Dermatol. JDD 2005, 4, 41–47. [Google Scholar] [PubMed]
- Kwon, S.Y.; Park, S.D.; Park, K. Comparative effect of topical silicone gel and topical tretinoin cream for the prevention of hypertrophic scar and keloid formation and the improvement of scars. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.A.; Abu-Raghif, A.R.; Ridha-Salman, H. Evaluation of common topical therapeutic agents of plane warts. Arch. Dermatol. Res. 2025, 317, 246. [Google Scholar] [CrossRef] [PubMed]
- Issa, N.; Alexis, A.; Baldwin, H.; Hamzavi, I.; Hebert, A.; Kwong, P.; Lain, E.; Moore, A.; Noor, O.; Schlesinger, T.; et al. Recommendations to Improve Outcomes in Acne and Acne Sequelae: A Focus on Trifarotene and Other Retinoids. Dermatol. Ther. 2025, 15, 563–577. [Google Scholar] [CrossRef]
- Bitterman, D.; Patel, P.; Zafar, K.; Wang, J.; Kabakova, M.; Gollogly, J.M.; Cohen, M.; Austin, E.; Jagdeo, J. Systematic review of topical, laser, and oral treatments in acanthosis nigricans clinical trials. Arch. Dermatol. Res. 2024, 316, 424. [Google Scholar] [CrossRef]
- Sumita, J.M.; Miot, H.A.; Bagatin, E.; Soares, J.L.M.; Raminelli, A.C.P.; Pereira, S.M.; Ogawa, M.M.; Picosse, F.R.; Guadanhim, L.R.S.; Enokihara, M.M.S.S.; et al. Tretinoin (0.05% cream vs. 5% peel) for photoaging and field cancerization of the forearms: Randomized, evaluator-blinded, clinical trial. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1819–1826. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Berger, R.; Barba, A.; Fleischer, A.; Leyden, J.J.; Lucky, A.; Pariser, D.; Rafal, E.; Thiboutot, D.; Wilson, D.; Grossman, R.; et al. A double-blinded, randomized, vehicle-controlled, multicenter, parallel-group study to assess the safety and efficacy of tretinoin gel microsphere 0.04% in the treatment of acne vulgaris in adults. Cutis 2007, 80, 152–157. [Google Scholar]
- Berger, R.; Rizer, R.; Barba, A.; Wilson, D.; Stewart, D.; Grossman, R.; Nighland, M.; Weiss, J. Tretinoin gel microspheres 0.04% versus 0.1% in adolescents and adults with mild to moderate acne vulgaris: A 12-week, multicenter, randomized, double-blind, parallel-group, phase IV trial. Clin. Ther. 2007, 29, 1086–1097. [Google Scholar] [CrossRef]
- Cook-Bolden, F.E.; Weinkle, S.H.; Guenin, E.; Bhatt, V. Novel Tretinoin 0.05% Lotion for Once-Daily Treatment of Moderate-to-Severe Acne Vulgaris in a Hispanic Population. J. Drugs Dermatol. JDD 2019, 18, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Sumathy, T.K.; Nayak, C.; Ravichandran, G.; Vaidya, P.P.; Mehta, S.; Mittal, R.; Mane, A.; Charugulla, S.N. Efficacy and safety comparison of combination of 0.04% tretinoin microspheres plus 1% clindamycin versus their monotherapy in patients with acne vulgaris: A phase 3, randomized, double-blind study. J. Dermatol. Treat. 2021, 32, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D.; Shalita, A.R.; Thiboutot, D.; Oresajo, C.; Yatskayer, M.; Raab, S. A multicenter, double-blind study to evaluate the efficacy and safety of 2 treatments in participants with mild to moderate acne vulgaris. Cutis 2012, 89, 287–293. [Google Scholar] [PubMed]
- Eichenfield, L.F.; Nighland, M.; Rossi, A.B.; Cook-Bolden, F.; Grimes, P.; Fried, R.; Levy, S.; PUMP Study Group. Phase 4 study to assess tretinoin pump for the treatment of facial acne. J. Drugs Dermatol. JDD 2008, 7, 1129–1136. [Google Scholar]
- Eichenfield, L.F.; Hebert, A.A.; Schachner, L.; Paller, A.S.; Rossi, A.B.; Lucky, A.W. Tretinoin Microsphere Gel 0.04% Pump for Treating Acne Vulgaris in Preadolescents: A Randomized, Controlled Study. Pediatr. Dermatol. 2012, 29, 598–604. [Google Scholar] [CrossRef]
- Han, G.; Armstrong, A.W.; Desai, S.R.; Guenin, E. Novel Tretinoin 0.05% Lotion for the Once-Daily Treatment of Moderate-to-Severe Acne Vulgaris in an Asian Population. J. Drugs Dermatol. JDD 2019, 18, 910–916. [Google Scholar] [CrossRef]
- Harper, J.C.; Baldwin, H.; Stein Gold, L.; Guenin, E. Efficacy and Tolerability of a Novel Tretinoin 0.05% Lotion for the Once-Daily Treatment of Moderate or Severe Acne Vulgaris in Adult Females. J. Drugs Dermatol. JDD 2019, 18, 1147–1154. [Google Scholar]
- Tyring, S.K.; Kircik, L.H.; Pariser, D.M.; Guenin, E.; Bhatt, V.; Pillai, R. Novel Tretinoin 0.05% Lotion for the Once-Daily Treatment of Moderate-to-Severe Acne Vulgaris: Assessment of Efficacy and Safety in Patients Aged 9 Years and Older. J. Drugs Dermatol. JDD 2018, 17, 1084–1091. [Google Scholar]
- Tyring, S.K.; Kircik, L.; Pariser, D.M.; Woolery-Lloyd, H.C.; Harper, J.C.; Bhatt, V.; Pillai, R.; Guenin, E. The Effects of Once-Daily Tretinoin 0.05% Lotion on Quality of Life in Patients with Moderate-to-Severe Acne Vulgaris. Am. J. Clin. Dermatol. 2020, 21, 891–899. [Google Scholar] [CrossRef]
- Babayeva, L.; Akarsu, S.; Fetil, E.; Güneş, A. Comparison of tretinoin 0.05% cream and 3% alcohol-based salicylic acid preparation in the treatment of acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 328–333. [Google Scholar] [CrossRef]
- Jarratt, M.T.; Brundage, T. Efficacy and safety of clindamycin-tretinoin gel versus clindamycin or tretinoin alone in acne vulgaris: A randomized, double-blind, vehicle-controlled study. J. Drugs Dermatol. JDD 2012, 11, 318–326. [Google Scholar] [PubMed]
- Kircik, L.H. Comparative efficacy and safety results of two topical combination acne regimens. J. Drugs Dermatol. JDD 2009, 8, 624–630. [Google Scholar] [PubMed]
- NilFroushzadeh, M.A.; Siadat, A.H.; Baradaran, E.H.; Moradi, S. Clindamycin lotion alone versus combination lotion of clindamycin phosphate plus tretinoin versus combination lotion of clindamycin phosphate plus salicylic acid in the topical treatment of mild to moderate acne vulgaris: A randomized control trial. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Pariser, D.; Bucko, A.; Fried, R.; Jarratt, M.T.; Kempers, S.; Kircik, L.; Lucky, A.W.; Rafal, E.; Rendon, M.; Weiss, J.; et al. Tretinoin gel microsphere pump 0.04% plus 5% benzoyl peroxide wash for treatment of acne vulgaris: Morning/morning regimen is as effective and safe as morning/evening regimen. J. Drugs Dermatol. JDD 2010, 9, 805–813. [Google Scholar]
- Rosso, J.D.; Sugarman, J.; Green, L.; Lain, T.; Levy-Hacham, O.; Mizrahi, R.; Gold, L.S. Efficacy and safety of microencapsulated benzoyl peroxide and microencapsulated tretinoin for the treatment of acne vulgaris: Results from two phase 3 double-blind, randomized, vehicle-controlled studies. J. Am. Acad. Dermatol. 2023, 89, 719–727. [Google Scholar] [CrossRef]
- Trifu, V.; Tiplica, G.-S.; Naumescu, E.; Zalupca, L.; Moro, L.; Celasco, G. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. A pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br. J. Dermatol. 2011, 165, 177–183. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Shamban, A.; Webster, G.; Del Rosso, J.; Dover, J.S.; Swinyer, L.; Stein, L.; Lin, X.; Draelos, Z.; Gold, M.; et al. A phase IV, open-label study evaluating the use of triple-combination therapy with minocycline HCl extended-release tablets, a topical antibiotic/retinoid preparation and benzoyl peroxide in patients with moderate to severe acne vulgaris. J. Drugs Dermatol. JDD 2013, 12, 619–625. [Google Scholar]
- Zeichner, J.A.; Wong, V.; Linkner, R.V.; Haddican, M. Efficacy and safety of tretinoin 0.025%/clindamycin phosphate 1.2% gel in combination with benzoyl peroxide 6% cleansing cloths for the treatment of facial acne vulgaris. J. Drugs Dermatol. JDD 2013, 12, 277–282. [Google Scholar]
- Bagatin, E.; Gonçalves Hde, S.; Sato, M.; Almeida, L.M.C.; Miot, H.A. Comparable efficacy of adapalene 0.3% gel and tretinoin 0.05% cream as treatment for cutaneous photoaging. Eur. J. Dermatol. EJD 2018, 28, 343–350. [Google Scholar] [CrossRef]
- McDaniel, D.H.; Mazur, C.; Wortzman, M.S.; Nelson, D.B. Efficacy and tolerability of a double-conjugated retinoid cream vs. 1.0% retinol cream or 0.025% tretinoin cream in subjects with mild to severe photoaging. J. Cosmet. Dermatol. 2017, 16, 542–548. [Google Scholar] [CrossRef]
- Chien, A.L.; Kim, D.J.; Cheng, N.; Shin, J.; Leung, S.G.; Nelson, A.M.; Zang, J.; Suh, H.; Rainer, B.; Wallis, L.; et al. Biomarkers of Tretinoin Precursors and Tretinoin Efficacy in Patients With Moderate to Severe Facial Photodamage: A Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 879–886. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Zufall, A.; Nash, M.; Rao, D.; Hirani, R.; Russo, M. Comparing Tretinoin to Other Topical Therapies in the Treatment of Skin Photoaging: A Systematic Review. Am. J. Clin. Dermatol. 2024, 25, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Kligman, D.E.; Draelos, Z.D. High-Strength Tretinoin for Rapid Retinization of Photoaged Facial Skin. Dermatol. Surg. 2004, 30, 864–866. [Google Scholar] [PubMed]
- Faghihi, G.; Shahingohar, A.; Siadat, A.H. Comparison between 1% tretinoin peeling versus 70% glycolic acid peeling in the treatment of female patients with melasma. J. Drugs Dermatol. JDD 2011, 10, 1439–1442. [Google Scholar] [PubMed]
- Chan, R.; Park, K.C.; Lee, M.H.; Lee, E.-S.; Chang, S.; Leow, Y.; Tay, Y.-K.; Legarda-Montinola, F.; Tsai, R.-Y.; Tsai, T.-H.; et al. A randomized controlled trial of the efficacy and safety of a fixed triple combination (fluocinolone acetonide 0.01%, hydroquinone 4%, tretinoin 0.05%) compared with hydroquinone 4% cream in Asian patients with moderate to severe melasma. Br. J. Dermatol. 2008, 159, 697–703. [Google Scholar]
- Gong, Z.; Lai, W.; Zhao, G.; Wang, X.; Zheng, M.; Li, L.; Yang, Q.; Dang, Y.; Liu, L.; Zou, Y. Efficacy and Safety of Fluocinolone Acetonide, Hydroquinone, and Tretinoin Cream in Chinese Patients with Melasma: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Parallel-Group Study. Clin. Drug Investig. 2015, 35, 385–395. [Google Scholar] [CrossRef]
- Grimes, P.; Watson, J. Treating epidermal melasma with a 4% hydroquinone skin care system plus tretinoin cream 0.025%. Cutis 2013, 91, 47–54. [Google Scholar]
- Hu, H.; Zhou, P.; Yao, H.; Zhu, C.; Shen, B.; Feng, B.; Yu, X.; Liu, L. Efficacy and Safety of Generic Fluocinolone Acetonide, Hydroquinone, and Tretinoin Cream Compared With TRI-LUMA for the Treatment of Moderate-To-Severe Melasma in Chinese Patients: A Randomized, Single-Center, Placebo-Controlled Trial. J. Cosmet. Dermatol. 2025, 24, e70205. [Google Scholar] [CrossRef]
- Pennitz, A.; Kinberger, M.; Avila Valle, G.; Passeron, T.; Nast, A.; Werner, R.N. Self-applied topical interventions for melasma: A systematic review and meta-analysis of data from randomized, investigator-blinded clinical trials. Br. J. Dermatol. 2022, 187, 309–317. [Google Scholar] [CrossRef]
- Callender, V.D.; Baldwin, H.; Cook-Bolden, F.E.; Alexis, A.F.; Gold, L.S.; Guenin, E. Effects of Topical Retinoids on Acne and Post-inflammatory Hyperpigmentation in Patients with Skin of Color: A Clinical Review and Implications for Practice. Am. J. Clin. Dermatol. 2022, 23, 69–81. [Google Scholar] [CrossRef]
- Kroon, M.W.; Wind, B.S.; Beek, J.F.; van der Veen, J.W.; Nieuweboer-Krobotová, L.; Bos, J.D.; Wolkerstorfer, A. Nonablative 1550-nm fractional laser therapy versus triple topical therapy for the treatment of melasma: A randomized controlled pilot study. J. Am. Acad. Dermatol. 2011, 64, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Leerapongnan, P.; Jurairattanaporn, N.; Kanokrungsee, S.; Udompataikul, M. Comparison of the effectiveness of fractional 1550-nm erbium fiber laser and 0.05% tretinoin cream in the treatment of acanthosis nigricans: A prospective, randomized, controlled trial. Lasers Med. Sci. 2020, 35, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Woodhall, K.E.; Goldman, M.P.; Gold, M.H.; Biron, J. Benefits of using a hydroquinone/tretinoin skin care system in patients undergoing intense pulsed light therapy for photorejuvenation: A placebo-controlled study. J. Drugs Dermatol. JDD 2009, 8, 862–867. [Google Scholar] [PubMed]
- Schlessinger, J.; Kenkel, J.; Werschler, P. Further Enhancement of Facial Appearance With a Hydroquinone Skin Care System Plus Tretinoin in Patients Previously Treated With Botulinum Toxin Type A. Aesthet. Surg. J. 2011, 31, 529–539. [Google Scholar] [CrossRef]
- Kritsanaviparkporn, C.; Treesirichod, A. Comparing the efficacy and safety profiles of 0.025% and 0.05% tretinoin creams in treating acanthosis nigricans: A randomized double-blinded study. Arch. Dermatol. Res. 2023, 315, 963–970. [Google Scholar] [CrossRef]
- Treesirichod, A.; Chaithirayanon, S.; Chaikul, T.; Chansakulporn, S. The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans. J. Dermatol. Treat. 2021, 32, 837–842. [Google Scholar] [CrossRef]
- Rajegowda, H.M.; Kalegowda, D.; Madegowda, S.B.; Manjunath, K. To compare the efficacy and safety of trichloroacetic acid peel with topical tretinoin in the treatment of acanthosis nigricans: A randomized controlled study. J. Pak. Assoc. Dermatol. 2019, 29, 170–175. [Google Scholar]
- Treesirichod, A.; Chaithirayanon, S.; Wongjitrat, N. Comparison of the efficacy and safety of 0.1% adapalene gel and 0.025% tretinoin cream in the treatment of childhood acanthosis nigricans. Pediatr. Dermatol. 2019, 36, 330–334. [Google Scholar] [CrossRef]
- Ghiasi, M.; Samii, R.; Tootoonchi, N.; Balighi, K.; Heidari, S. Comparison of efficacy and safety of tretinoin 0.05% and glycolic acid peeling 70% in axillary and neck lesions of acanthosis nigricans: A single-blinded, randomized trial. J. Cosmet. Dermatol. 2024, 23, 2090–2096. [Google Scholar] [CrossRef]
- Chang, A.L.S.; Alora-Palli, M.; Lima, X.T.; Chang, T.C.; Cheng, C.; Chung, C.M.; Amir, O.; Kimball, A.B. A randomized, double-blind, placebo-controlled, pilot study to assess the efficacy and safety of clindamycin 1.2% and tretinoin 0.025% combination gel for the treatment of acne rosacea over 12 weeks. J. Drugs Dermatol. JDD 2012, 11, 333–339. [Google Scholar] [PubMed]
- Freeman, S.A.; Moon, S.D.; Spencer, J.M. Clindamycin phosphate 1.2% and tretinoin 0.025% gel for rosacea: Summary of a placebo-controlled, double-blind trial. J. Drugs Dermatol. JDD 2012, 11, 1410–1414. [Google Scholar] [PubMed]
- Lu, H.; Guo, J.; Hong, X.; Chen, A.; Zhang, X.; Shen, S. Comparative effectiveness of different therapies for treating striae distensae: A systematic review and network meta-analysis. Medicine 2020, 99, e22256. [Google Scholar] [CrossRef]
- Ud-Din, S.; McGeorge, D.; Bayat, A. Topical management of striae distensae (stretch marks): Prevention and therapy of striae rubrae and albae. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Gamil, H.D.; Ibrahim, S.A.; Ebrahim, H.M.; Albalat, W. Platelet-Rich Plasma Versus Tretinoin in Treatment of Striae Distensae: A Comparative Study. Dermatol. Surg. 2018, 44, 697–704. [Google Scholar] [CrossRef]
- Hexsel, D.; Soirefmann, M.; Porto, M.D.; Schilling-Souza, J.; Siega, C.; Dal’FOrno, T. Superficial Dermabrasion Versus Topical Tretinoin on Early Striae Distensae: A Randomized, Pilot Study. Dermatol. Surg. 2014, 40, 537. [Google Scholar] [CrossRef]
- Yousaf, A.; Gul, S.; Zahra, A.; Aslam, A.; Khan, A.N.; Hafeez, L.; Tahir, R. Comparison of efficacy of 30% solution of trichloroacetic acid (TCA) with 0.025% tretinoin in the treatment of flat warts at a tertiary care hospital. J. Pak. Assoc. Dermatol. 2022, 31, 600–604. [Google Scholar]
- David, P.; James, S.; Brian, B.; Suzanne, B.; Lisa, P.; Kenneth, G. Using a Hydroquinone/Tretinoin-based Skin Care System Before and After Electrodesiccation and Curettage of Superficial Truncal Basal Cell Carcinoma. J. Clin. Aesthetic Dermatol. 2009, 2, 38–43. [Google Scholar]
- Dematte, M.F.; Gemperli, R.; Salles, A.G.; Dolhnikoff, M.; Lanças, T.; Saldiva, P.H.N.; Ferreira, M.C. Mechanical evaluation of the resistance and elastance of post-burn scars after topical treatment with tretinoin. Clinics 2011, 66, 1949–1954. [Google Scholar] [CrossRef]
- Salles, A.G.; Gemperli, R.; Toledo, P.N.; Ferreira, M.C. Combined Tretinoin and Glycolic Acid Treatment Improves Mouth Opening for Postburn Patients. Aesthetic Plast. Surg. 2006, 30, 356–362. [Google Scholar] [CrossRef]
- Shin, H.S.; Won, C.H.; Lee, S.H.; Kwon, O.S.; Kim, K.H.; Eun, H.C. Efficacy of 5% Minoxidil versus Combined 5% Minoxidil and 0.01% Tretinoin for Male Pattern Hair Loss. Am. J. Clin. Dermatol. 2007, 8, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Goren, A.; Shapiro, J.; Roberts, J.; McCoy, J.; Desai, N.; Zarrab, Z.; Pietrzak, A.; Lotti, T. Clinical utility and validity of minoxidil response testing in androgenetic alopecia. Dermatol. Ther. 2015, 28, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Goren, A.; Dhurat, R.; Agrawal, S.; Sinclair, R.; Trüeb, R.M.; Vañó-Galván, S.; Chen, G.; Tan, Y.; Kovacevic, M.; et al. Tretinoin enhances minoxidil response in androgenetic alopecia patients by upregulating follicular sulfotransferase enzymes. Dermatol. Ther. 2019, 32, e12915. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ghorami, R.C.; Chatterjee, T.; Banerjee, G. Comparative Assessment of Topical Steroids, Topical Tretenoin (0.05%) and Dithranol Paste in Alopecia Areata. Indian J. Dermatol. 2010, 55, 148. [Google Scholar] [CrossRef]
- Tom, W.L.; Peng, D.H.; Allaei, A.; Hsu, D.; Hata, T.R. The Effect of Short-Contact Topical Tretinoin Therapy for Foot Ulcers in Patients With Diabetes. Arch. Dermatol. 2005, 141, 1373–1377. [Google Scholar] [CrossRef]
- Ianhez, M.; Pinto, S.A.; Miot, H.A.; Bagatin, E. A randomized, open, controlled trial of tretinoin 0.05% cream vs. low-dose oral isotretinoin for the treatment of field cancerization. Int. J. Dermatol. 2019, 58, 365–373. [Google Scholar] [CrossRef]
- Weinstock, M.A.; Bingham, S.F.; DiGiovanna, J.J.; Rizzo, A.E.; Marcolivio, K.; Hall, R.; Eilers, D.; Naylor, M.; Kirsner, R.; Kalivas, J.; et al. Tretinoin and the Prevention of Keratinocyte Carcinoma (Basal and Squamous Cell Carcinoma of the Skin): A Veterans Affairs Randomized Chemoprevention Trial. J. Investig. Dermatol. 2012, 132, 1583–1590. [Google Scholar] [CrossRef]
- Nahm, W.; Nichols, A.; Rapoport, E.; Kirsner, R.; Badiavas, E.; Wyant, W.; Arthur, A.; Shen, J. Keratinocyte Carcinoma Chemoprevention With a Combination of Imiquimod, 5-Fluorouracil, and Tretinoin. J. Drugs Dermatol. JDD 2023, 22, 486–490. [Google Scholar] [CrossRef]
- Nahm, W.J.; Shen, J.; Zito, P.M.; Gonzalez, A.; Nagrani, N.; Moore, K.; Badiavas, E.; Kirsner, R.; Nichols, A. A Non-Surgical and Cost-Effective Treatment Approach Employing Topical Imiquimod, 5-Fluorouracil, and Tretinoin for Primary Non-Melanoma Skin Cancers. J. Drugs Dermatol. JDD 2021, 20, 260–267. [Google Scholar] [CrossRef]
- Nahm, W.J.; Badiavas, E.V.; Kirsner, R.S.; Nichols, A.J.; Harris, Z.C.; Phillips, A.R.; Shen, J. Treating keratinocyte carcinomas with a combination of imiquimod, 5-fluorouracil, and tretinoin using store-and-forward telemedicine in the age of coronavirus disease 2019 to promote social distancing. JAAD Case Rep. 2020, 6, 931–934. [Google Scholar] [CrossRef]
- Momosawa, A.; Kurita, M.; Ozaki, M.; Miyamoto, S.; Kobayashi, Y.; Ban, I.; Harii, K. Combined Therapy Using Q-Switched Ruby Laser and Bleaching Treatment with Tretinoin and Hydroquinone for Periorbital Skin Hyperpigmentation in Asians. Plast. Reconstr. Surg. 2008, 121, 282. [Google Scholar] [CrossRef] [PubMed]
- Momosawa, A.; Yoshimura, K.; Uchida, G.; Sato, K.; Aiba, E.; Matsumoto, D.; Yamaoka, H.; Mihara, S.; Tsukamoto, K.; Harii, K.; et al. Combined Therapy Using Q-Switched Ruby Laser and Bleaching Treatment With Tretinoin and Hydroquinone for Acquired Dermal Melanocytosis. Dermatol. Surg. 2003, 29, 1001. [Google Scholar] [PubMed]
- Buchanan, P.J.; Gilman, R.H. Retinoids: Literature Review and Suggested Algorithm for Use Prior to Facial Resurfacing Procedures. J. Cutan. Aesthetic Surg. 2016, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Latriano, L.; Tzimas, G.; Wong, F.; Wills, R.J. The percutaneous absorption of topically applied tretinoin and its effect on endogenous concentrations of tretinoin and its metabolites after single doses or long-term use. J. Am. Acad. Dermatol. 1997, 36, S37–S46. [Google Scholar] [CrossRef]
- Shapiro, S.; Heremans, A.; Mays, D.A.; Martin, A.L.; Hernandez-Medina, M.; Lanes, S. Use of topical tretinoin and the development of noncutaneous adverse events: Evidence from a systematic review of the literature. J. Am. Acad. Dermatol. 2011, 65, 1194–1201. [Google Scholar] [CrossRef]
- Loureiro, K.D.; Kao, K.K.; Jones, K.L.; Alvarado, S.; Chavez, C.; Dick, L.; Felix, R.; Johnson, D.; Chambers, C.D. Minor malformations characteristic of the retinoic acid embryopathy and other birth outcomes in children of women exposed to topical tretinoin during early pregnancy. Am. J. Med. Genet. A 2005, 136, 117–121. [Google Scholar] [CrossRef]
- Panchaud, A.; Csajka, C.; Merlob, P.; Schaefer, C.; Berlin, M.; De Santis, M.; Vial, T.; Ieri, A.; Malm, H.; Eleftheriou, G.; et al. Pregnancy Outcome Following Exposure to Topical Retinoids: A Multicenter Prospective Study. J. Clin. Pharmacol. 2012, 52, 1844–1851. [Google Scholar] [CrossRef]
- Shapiro, L.; Pastuszak, A.; Curto, G.; Koren, G. Safety of first-trimester exposure to topical tretinoin: Prospective cohort study. Lancet 1997, 350, 1143–1144. [Google Scholar] [CrossRef]
- Jick, S.S.; Terris, B.Z.; Jick, H. First trimester topical tretinoin and congenital disorders. Lancet 1993, 341, 1181–1182. [Google Scholar] [CrossRef]
- Reynolds, R.V.; Yeung, H.; Cheng, C.E.; Cook-Bolden, F.; Desai, S.R.; Druby, K.M.; Freeman, E.E.; Keri, J.E.; Gold, L.F.S.; Tan, J.K.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2024, 90, 1006.e1–1006.e30. [Google Scholar] [CrossRef]
- Asai, Y.; Baibergenova, A.; Dutil, M.; Humphrey, S.; Hull, P.; Lynde, C.; Poulin, Y.; Shear, N.H.; Tan, J.; Toole, J.; et al. Management of acne: Canadian clinical practice guideline. CMAJ 2016, 188, 118–126. [Google Scholar] [CrossRef]
- Thielitz, A.; Gollnick, H. Topical retinoids in acne vulgaris: Update on efficacy and safety. Am. J. Clin. Dermatol. 2008, 9, 369–381. [Google Scholar] [CrossRef]
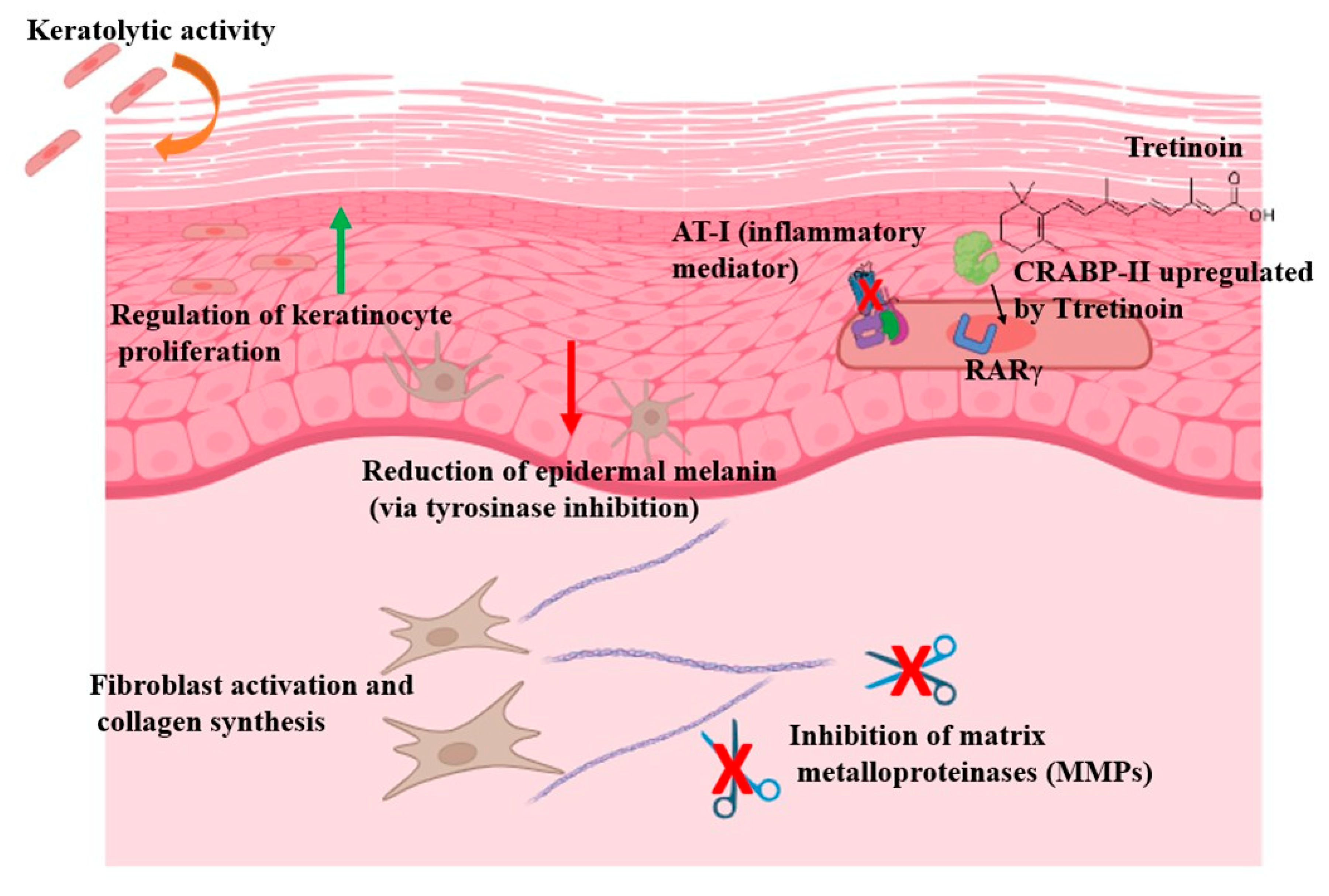
| Mechanism | Associated Clinical Effect |
|---|---|
| Keratolytic activity | Promotes desquamation and prevents microcomedone formation |
| Regulation of keratinocyte proliferation and differentiation | Normalizes epidermal turnover; beneficial in acne, psoriasis and keratinization disorders |
| Fibroblast activation | Stimulates dermal repair and collagen production |
| Induction of collagen synthesis and recycling | Improves skin texture and elasticity; useful in photoaging and scarring |
| Inhibition of matrix metalloproteinases (MMP-1 and MMP-8) | Prevents collagen degradation |
| Reduction of epidermal melanin (via tyrosinase inhibition) | Helps lighten hyperpigmentation such as melasma and post-inflammatory hyperpigmentation |
| Activation of nuclear retinoic acid receptors (RARα, RARβ, RARγ) | Regulates gene transcription related to differentiation, inflammation and tissue remodeling |
| Preferential interaction with RARγ | Highly expressed in epidermis; key in therapeutic response and cutaneous irritation |
| Suppression of inflammatory mediators (e.g., AP-1 inhibition) | Contributes to anti-inflammatory effects in conditions like acne and rosacea |
| Upregulation of CRABP-II expression in the skin | Enhances intracellular transport and bioavailability; modulates treatment intensity |
| Skin Aging | Photoaging [33] |
|---|---|
| Inflammatory disorders | Acne vulgaris [15] |
| Pigmentary disorders | Melasma [37] Post-inflammatory hyperpigmentation [41] Acanthosis nigricans [7] |
| Scarring | Striae distensae [53] Prevention of hypertrophic scars/keloids [4] Treatment of post-burn scars [60] |
| Infections | Flat/plane warts [5] |
| Disorders of keratinization | Granular parakeratosis Darier’s disease Keratosis pilaris |
| Alopecia | Alopecia areata [64] Androgenetic alopecia [61] |
| Skin neoplasms | Treatment of AK and field cancerization [66] Prevention and treatment of keratinocyte carcinoma * [67] |
| Start Topical Tretinoin Therapy with Lower Concentrations (0.025% or 0.05%) |
|---|
| Apply the product two or three nights/week initially, and gradually increase the frequency according to tolerability. |
| Avoid excessive skin cleansing. |
| Wait 20–30 min after cleansing to ensure skin is completely dry before application. |
| Avoid aggressive exfoliation of the skin. |
| Intensive use of emollients and moisturizers. |
| Recommend photoprotection, including broad-spectrum sunscreens (SPF 30 or higher). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balado-Simó, P.; Morgado-Carrasco, D.; Gómez-Armayones, S.; López-Ferrer, A.; Barco, D.; Ferrándiz-Pulido, C.; Podlipnik, S. An Updated Review of Topical Tretinoin in Dermatology: From Acne and Photoaging to Skin Cancer. J. Clin. Med. 2025, 14, 7958. https://doi.org/10.3390/jcm14227958
Balado-Simó P, Morgado-Carrasco D, Gómez-Armayones S, López-Ferrer A, Barco D, Ferrándiz-Pulido C, Podlipnik S. An Updated Review of Topical Tretinoin in Dermatology: From Acne and Photoaging to Skin Cancer. Journal of Clinical Medicine. 2025; 14(22):7958. https://doi.org/10.3390/jcm14227958
Chicago/Turabian StyleBalado-Simó, Pablo, Daniel Morgado-Carrasco, Sara Gómez-Armayones, Anna López-Ferrer, Didac Barco, Carla Ferrándiz-Pulido, and Sebastian Podlipnik. 2025. "An Updated Review of Topical Tretinoin in Dermatology: From Acne and Photoaging to Skin Cancer" Journal of Clinical Medicine 14, no. 22: 7958. https://doi.org/10.3390/jcm14227958
APA StyleBalado-Simó, P., Morgado-Carrasco, D., Gómez-Armayones, S., López-Ferrer, A., Barco, D., Ferrándiz-Pulido, C., & Podlipnik, S. (2025). An Updated Review of Topical Tretinoin in Dermatology: From Acne and Photoaging to Skin Cancer. Journal of Clinical Medicine, 14(22), 7958. https://doi.org/10.3390/jcm14227958







