Relative Efficacy of Alirocumab, Evolocumab, Inclisiran, and Bempedoic Acid on Lipids in Patients with Cardiovascular Disease or Familial Hypercholesterolaemia
Abstract
1. Introduction
2. Methods
2.1. Study Design, Setting, and Participants
2.2. Lipid Assessments
2.3. Time Points and Follow-Up
2.4. Ethics
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
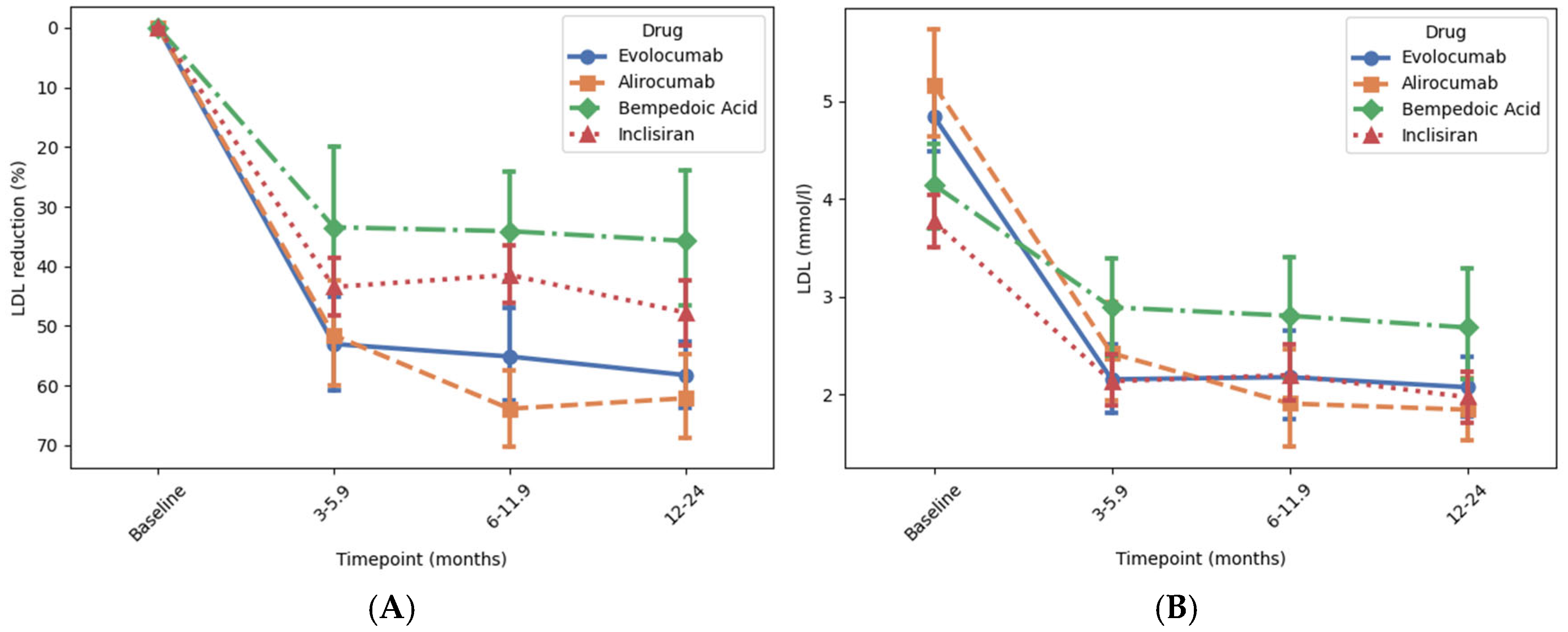
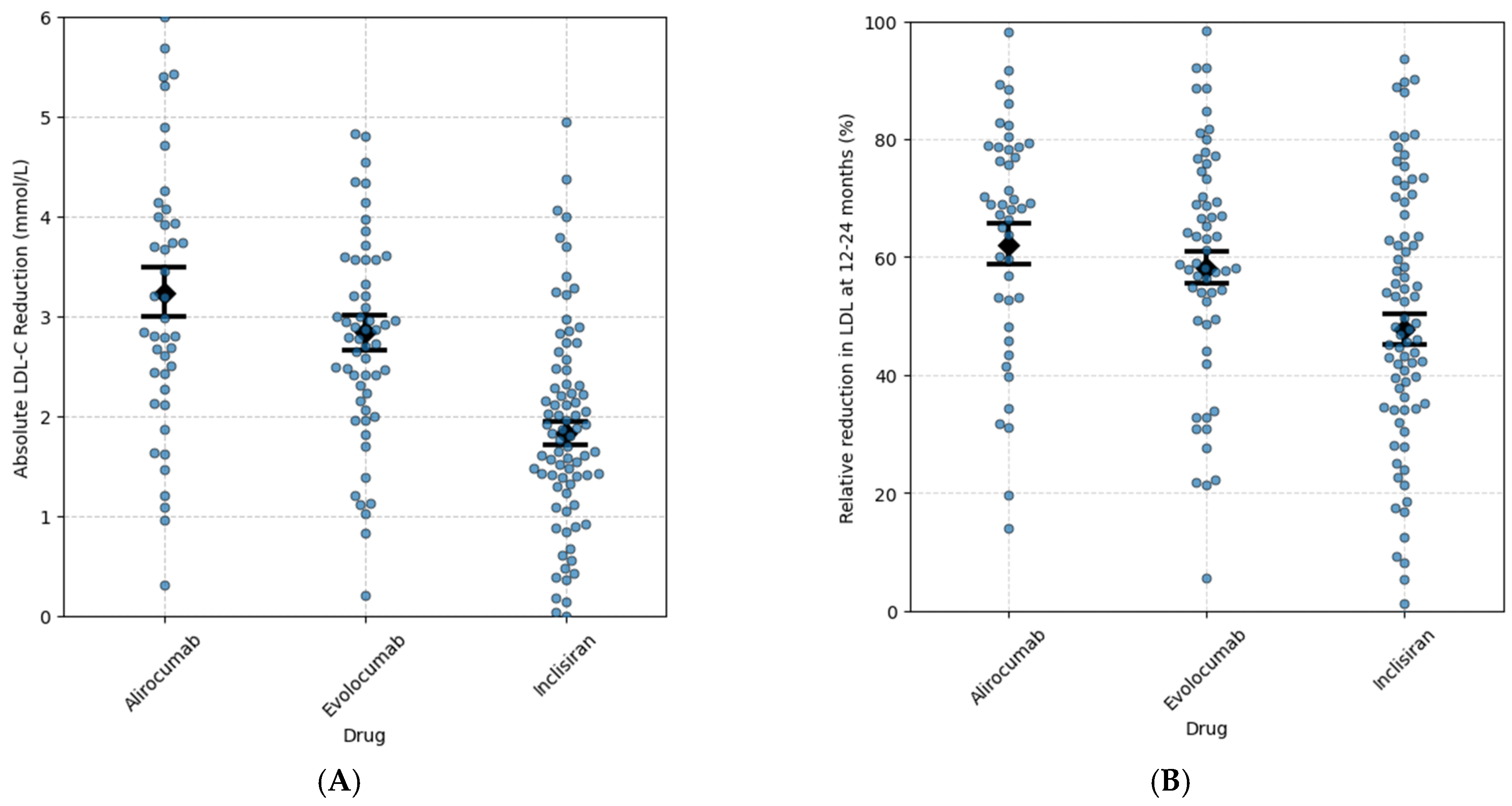
3.2. Reduction in LDL-C
3.3. Reduction in Total Cholesterol
3.4. Reduction in Triglycerides and CRP
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H.; Hobbs, H.H. Sequence Variations in PCSK9, Low LDL, and Protection against Coronary Heart Disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef]
- Mourikis, P.; Zako, S.; Dannenberg, L.; Nia, A.M.; Heinen, Y.; Busch, L.; Richter, H.; Hohlfeld, T.; Zeus, T.; Kelm, M.; et al. Lipid lowering therapy in cardiovascular disease: From myth to molecular reality. Pharmacol. Ther. 2020, 213, 107592. [Google Scholar] [CrossRef]
- Orringer, C.E.; Jacobson, T.A.; Saseen, J.J.; Brown, A.S.; Gotto, A.M.; Ross, J.L.; Underberg, J.A. Update on the use of PCSK9 inhibitors in adults: Recommendations from an Expert Panel of the National Lipid Association. J. Clin. Lipidol. 2017, 11, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, T.M.; Warden, B.A.; Minnier, J.; Miles, J.R.; Duell, P.B.; Purnell, J.Q.; Wojcik, C.; Fazio, S.; Shapiro, M.D. Application of PCSK9 Inhibitors in Practice. Circ. Res. 2019, 124, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Ji, E.; Lee, S. Antibody-based therapeutics for atherosclerosis and cardiovascular diseases. Int. J. Mol. Sci. 2021, 22, 5770. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.P.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV randomized clinical trial. JAMA—J. Am. Med. Assoc. 2016, 316, 2373–2384. [Google Scholar] [CrossRef]
- Giordano, S.; Polimeni, A.; Esposito, G.; Indolfi, C.; Spaccarotella, C. Inclisiran: Present and future perspectives of a new effective LDL cholesterol-lowering agent. Curr. Opin. Lipidol. 2023, 34, 133–140. [Google Scholar] [CrossRef]
- Ray, K.K.; Troquay, R.P.T.; Visseren, F.L.J.; Leiter, L.A.; Scott Wright, R.; Vikarunnessa, S.; Talloczy, Z.; Zang, X.; Maheux, P.; Lesogor, A.; et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): Results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. 2023, 11, 109–119. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Laufs, U.; Ray, K.K.; Leiter, L.A.; Bays, H.E.; Goldberg, A.C.; Stroes, E.S.; MacDougall, D.; Zhao, X.; Catapano, A.L. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur. J. Prev. Cardiol. 2020, 27, 593–603. [Google Scholar] [CrossRef]
- Ray, K.K.; Bays, H.E.; Catapano, A.L.; Lalwani, N.D.; Bloedon, L.T.; Sterling, L.R.; Robinson, P.L.; Ballantyne, C.M. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. N. Engl. J. Med. 2019, 380, 1022–1032. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification. NICE Guideline 181. 2016;1 (July 2014). Available online: https://www.nice.org.uk/guidance/ng238 (accessed on 1 June 2025).
- Cannon, C.P. Statin intolerance: How common is it and how do we work with patients to overcome it? Eur. Heart J. 2022, 43, 3224–3226. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Evolocumab for Treating Primary Hypercholesterolaemia and Mixed Dyslipidaemia. Vol. TA394, National Institute for Health and Care Excellence. 2016. Available online: https://www.nice.org.uk/guidance/ta394 (accessed on 31 January 2025).
- National Institute for Health and Care Excellence. Alirocumab for Treating Primary Hypercholesterolaemia and Mixed Dyslipidaemia. 22 June 2016. Available online: https://www.nice.org.uk/guidance/ta393 (accessed on 1 June 2025).
- Hartgers, M.L.; Defesche, J.C.; Langslet, G.; Hopkins, P.N.; Kastelein, J.J.P.; Baccara-Dinet, M.T.; Seiz, W.; Hamon, S.; Banerjee, P.; Stefanutti, C. Alirocumab efficacy in patients with double heterozygous, compound heterozygous, or homozygous familial hypercholesterolemia. J. Clin. Lipidol. 2018, 12, 390–396.e8. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, J.J.P.; Ginsberg, H.N.; Langslet, G.; Kees Hovingh, G.; Ceska, R.; Dufour, R.; Blom, D.; Civeira, F.; Krempf, M.; Lorenzato, C.; et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur. Heart J. 2015, 36, 2996–3003. [Google Scholar] [CrossRef]
- Roth, E.M.; Taskinen, M.R.; Ginsberg, H.N.; Kastelein, J.J.P.; Colhoun, H.M.; Robinson, J.G.; Merlet, L.; Pordy, R.; Baccara-Dinet, M.T. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: Results of a 24 week, double-blind, randomized Phase 3 trial. Int. J. Cardiol. 2014, 176, 55–61. [Google Scholar] [CrossRef]
- Ray, K.K.; Molemans, B.; Marieke Schoonen, W.; Giovas, P.; Bray, S.; Kiru, G.; Murphy, J.; Banach, M.; De Servi, S.; Gaita, D.; et al. EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: The DAVINCI study. Eur. J. Prev. Cardiol. 2021, 28, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Tokgözoğlu, L.; Norata, G.D. ORION-8: One step closer to understanding the safety and efficacy of inclisiran. Cardiovasc. Res. 2024, 120, 1365–1366. [Google Scholar] [CrossRef]
- Bernelot Moens, S.J.; Neele, A.E.; Kroon, J.; Van Der Valk, F.M.; Van Den Bossche, J.; Hoeksema, M.A.; Hoogeveen, R.M.; Schnitzler, J.G.; Baccara-Dinet, M.T.; Manvelian, G.; et al. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur. Heart J. 2017, 38, 1584–1593. [Google Scholar] [CrossRef]
- Suur, B.E.; Chemaly, M.; Lindquist Liljeqvist, M.; Djordjevic, D.; Stenemo, M.; Bergman, O.; Karlöf, E.; Lengquist, M.; Odeberg, J.; Hurt-Camejo, E.; et al. Therapeutic potential of the Proprotein Convertase Subtilisin/Kexin family in vascular disease. Front. Pharmacol. 2022, 13, 988561. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.; Leiter, L.; Stroes, E.; Baum, S.; Hanselman, J.; Bloedon, L.; Lalwani, N.D.; Patel, P.M.; Zhao, X.; Duell, P.B. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: The CLEAR Wisdom Randomized Clinical Trial. JAMA—J. Am. Med. Assoc. 2019, 322, 1780–1788. [Google Scholar] [CrossRef]
- Warden, B.A.; Cardiology, B.C.P.S.A.Q.; Purnell, J.Q.; Duell, P.B.; Fazio, S. Real-world utilization of bempedoic acid in an academic preventive cardiology practice. J. Clin. Lipidol. 2022, 16, 94–103. [Google Scholar] [CrossRef]
- Goldenberg, J.R.; Wang, X.; Lewandowski, E.D. Acyl CoA synthetase-1 links facilitated long chain fatty acid uptake to intracellular metabolic trafficking differently in hearts of male versus female mice. J. Mol. Cell. Cardiol. 2016, 94, 1–9. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Wiviott, S.D.; Raal, F.J.; Blom, D.J.; Robinson, J.; Ballantyne, C.M.; Somaratne, R.; Legg, J.; Wasserman, S.M.; et al. Efficacy and Safety of Evolocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Tokgözoğlu, L.; Corsini, A.; Sirtori, C.R. PCSK9 inhibition and inflammation: A narrative review. Atherosclerosis 2019, 288, 146–155. [Google Scholar] [CrossRef]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgözoğlu, L.; Nordestgaard, B.G.; et al. Statin-associated muscle symptoms: Impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef] [PubMed]


| Total | Alirocumab | Evolocumab | Inclisiran | Bempedoic Acid | p-Value | |
|---|---|---|---|---|---|---|
| Number of patients (% of total) | 256 | 50 (19.5) | 68 (26.5) | 108 (42.2) | 30 (11.7) | |
| Sex, N (%) | F | 18 (36.0) | 34 (50.0) | 36 (33.3) | 24 (80.0) | <0.001 |
| M | 32 (64) | 34 (50.0) | 72 (66.7) | 6 (20.0) | ||
| Age, median (Q1, Q3) | 65 [56.0, 73.0] | 60.5 [50.5, 67.2] | 67.0 [58.2, 72.2] | 67.5 [59.2, 74.8] | 0.03 | |
| Weight, median (Q1, Q3) | 79.8 [72.1, 92.2] | 83.8 [69.4, 93.0] | 78.2 [69.0, 92.1] | 71 (62.4, 83.7) | 0.028 | |
| BMI | 28.3 [26.4, 31.4] | 30.0 [25.1, 33.9] | 28.1 [24.6, 31.8] | 27.2 [25.3, 31.5] | 0.318 | |
| Ethnicity, N (%) | Asian | 8 (19.5) | 14 (23.3) | 20 (20.4) | 7 (25.9) | 0.650 |
| White | 30 (73.2) | 41 (68.3) | 69 (70.4) | 18 (66.7) | ||
| Black | 3 (7.3) | 4 (6.7) | 8 (8.2) | 1 (3.7) | ||
| Chinese | 0 | 0 | 0 | 1 (3.7) | ||
| Other | 0 | 1 (1.7) | 1 (1) | 0 | ||
| T1DM, N (%) | 1 (2.0) | 2 (3.0) | 0 | 0 | 0.279 | |
| T2DM, N (%) | 12 (24.0) | 16 (24.2) | 24 (22.4) | 5 (16.7) | 0.858 | |
| HTN, N (%) | 26 (52.0) | 23 (35.9) | 51 (47.7) | 9 (30.0) | 0.115 | |
| Smoking, N (%) | Ex-smoker | 6 (75.0) | 4 (44.4) | 18 (52.9) | 2 (66.7) | 0.738 |
| Current smoker | 1 (12.5) | 1 (11.1) | 7 (20.6) | 0 | ||
| CKD, N (%) | 6 (12.0) | 4 (6.1) | 3 (2.8) | 2 (6.7) | 0.157 | |
| PVD, N (%) | 1 (2.90) | 3 (4.5) | 2 (1.9) | 0 | 0.528 | |
| Stroke, N (%) | 3 (6.0) | 1 (1.5) | 6 (5.6) | 0 | 0.309 | |
| CABG, N (%) | 8 (16) | 6 (9.1) | 18 (16.8) | 1 (3.3) | 0.162 | |
| FH, N (%) | 18 (34) | 23 (32.9) | 16 (14.7) | 10 (31.2) | 0.005 | |
| ACS, N (%) | 19 (38) | 25 (36.8) | 40 (37) | 1 (3.3) | 0.003 | |
| ACStype (%) | Stemi | 9 (47.4) | 6 (23.1) | 15 (35.7) | 1 (100) | 0.175 |
| Nstemi | 11 (55.0) | 18 (69.2) | 27 (64.3) | 0 | ||
| UA | 0 | 2 (7.7) | 0 | 0 | ||
| High intensty statin, N (%) | 21 (77.8) | 27 (69.2) | 55 (75.3) | 5 (22.7) | 0.002 | |
| Ezetimibe, N (%) | 11 (40.7) | 21 (52.5) | 25 (34.2) | 16 (72.7) | 0.010 | |
| Baseline LDL-C (SD) | 5.2 (2.0) | 4.8 (1.6) | 3.8 (1.4) | 4.1 (1.2) | <0.001 | |
| Baseline TG (SD) | 2.1 (1.0) | 2.6 (1.9) | 2.0 (1.7) | 2.2 (1.3) | 0.157 | |
| Baseline CRP (SD) | 3.9 (2.1) | 6.5 (4.3) | 3.0 (3.5) | 2.3 (1.9) | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khattak, S.; Ochoa-Ferraro, A.; Khan, N.; George, S.; Khan, S.Q.; Townend, J.N.; Dawson, C.; Thomas, M.R. Relative Efficacy of Alirocumab, Evolocumab, Inclisiran, and Bempedoic Acid on Lipids in Patients with Cardiovascular Disease or Familial Hypercholesterolaemia. J. Clin. Med. 2025, 14, 7946. https://doi.org/10.3390/jcm14227946
Khattak S, Ochoa-Ferraro A, Khan N, George S, Khan SQ, Townend JN, Dawson C, Thomas MR. Relative Efficacy of Alirocumab, Evolocumab, Inclisiran, and Bempedoic Acid on Lipids in Patients with Cardiovascular Disease or Familial Hypercholesterolaemia. Journal of Clinical Medicine. 2025; 14(22):7946. https://doi.org/10.3390/jcm14227946
Chicago/Turabian StyleKhattak, Sophia, Antonio Ochoa-Ferraro, Nazish Khan, Sudhakar George, Sohail Q. Khan, Jonathan N. Townend, Charlotte Dawson, and Mark R. Thomas. 2025. "Relative Efficacy of Alirocumab, Evolocumab, Inclisiran, and Bempedoic Acid on Lipids in Patients with Cardiovascular Disease or Familial Hypercholesterolaemia" Journal of Clinical Medicine 14, no. 22: 7946. https://doi.org/10.3390/jcm14227946
APA StyleKhattak, S., Ochoa-Ferraro, A., Khan, N., George, S., Khan, S. Q., Townend, J. N., Dawson, C., & Thomas, M. R. (2025). Relative Efficacy of Alirocumab, Evolocumab, Inclisiran, and Bempedoic Acid on Lipids in Patients with Cardiovascular Disease or Familial Hypercholesterolaemia. Journal of Clinical Medicine, 14(22), 7946. https://doi.org/10.3390/jcm14227946





