Metabolic and Endocrine ADRs of Atypical Antipsychotics (AAPs) in Paediatric Patients with Autism Spectrum Disorder (ASD): A Review of Prevalence, Risk Factors, and Implications for Clinical Monitoring
Abstract
1. Introduction
2. Adverse Drug Reactions (ADRs)
2.1. ADRs Associated with AAPs
2.1.1. Metabolic ADRs
2.1.2. Endocrine ADRs
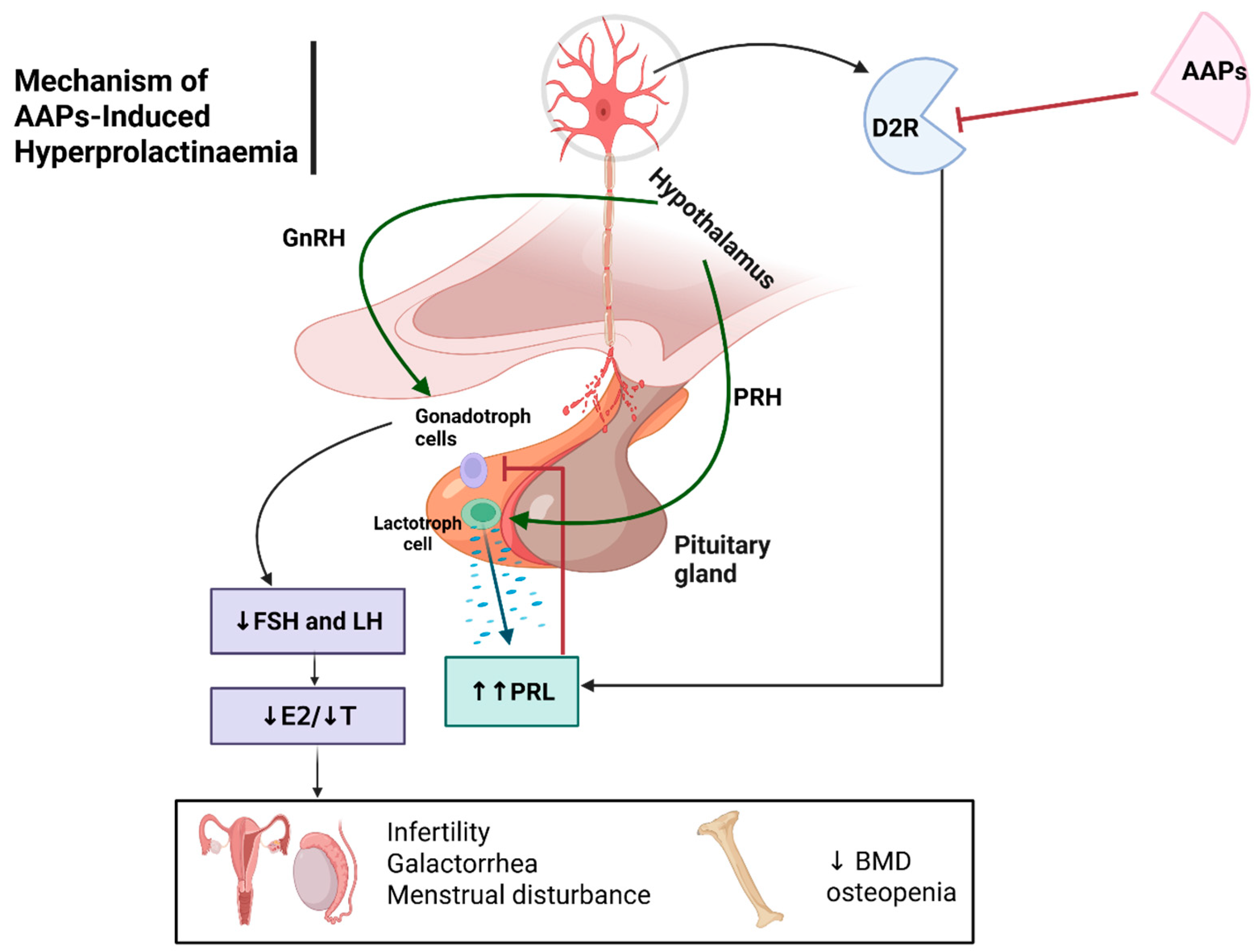
Hyperprolactinaemia ADRs
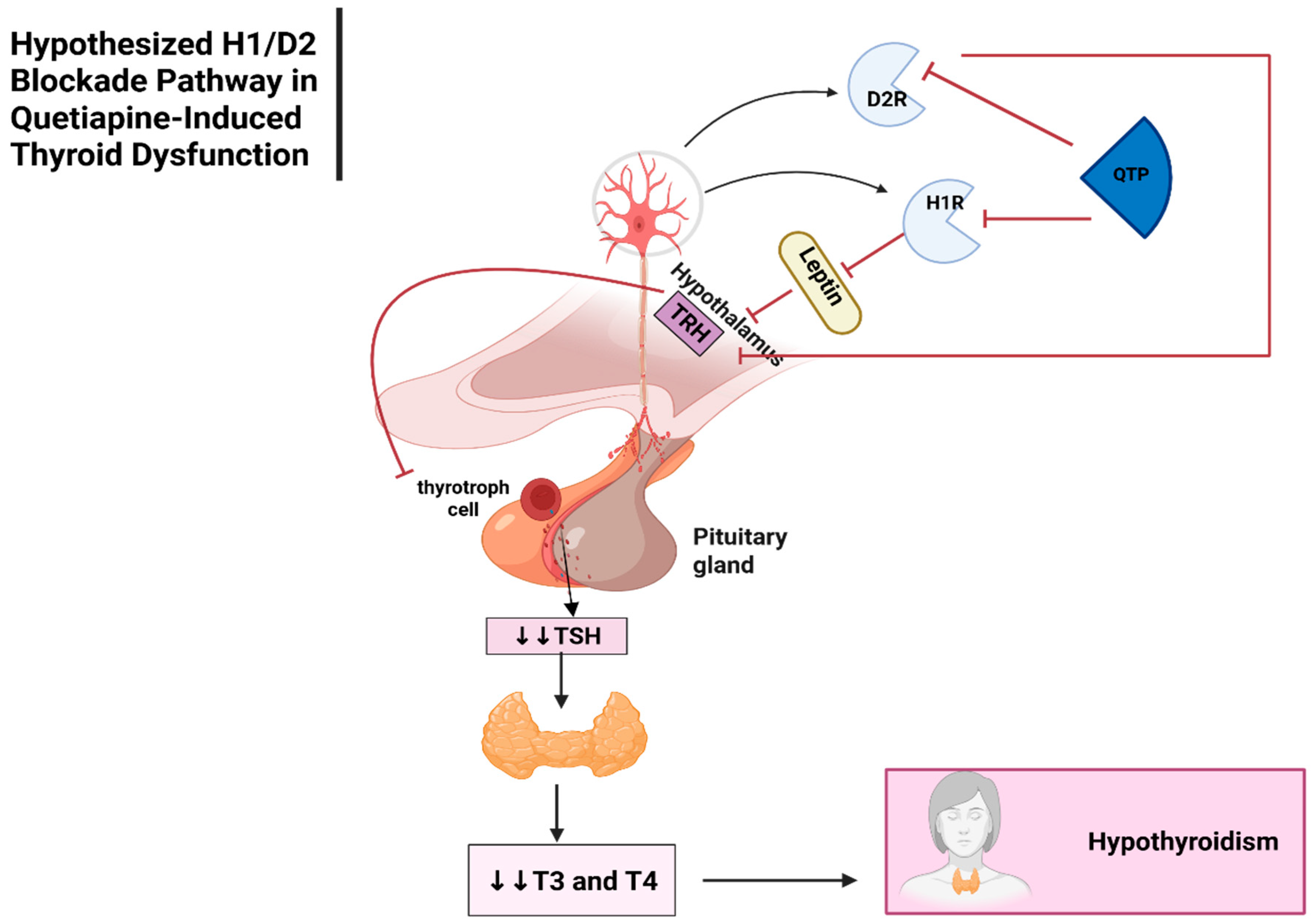
Thyroid Dysfunction ADRs
3. Risk Factors Influencing the Prevalence of Metabolic and Endocrine ADRs
3.1. Medication-Related Factors
3.1.1. Mechanism of Metabolic ADRs
Weight Gain and Hyperglycaemia
Hyperlipidaemia
3.1.2. Mechanism of Endocrine ADRs
Hyperprolactinaemia
Thyroid Dysfunction
3.1.3. Pharmacokinetic Factors
Risperidone
Aripiprazole
Olanzapine
Quetiapine
Lurasidone
Ziprasidone
3.2. Patient-Specific Factors
3.2.1. Age
3.2.2. Gender
3.2.3. Ethnicity
3.3. Healthcare System Factors
3.4. Disease-Related Factors
4. Research Gap and Future Directions
5. Clinical Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAPs | Atypical antipsychotics |
| ADRs | Adverse drug reactions |
| AgRP | Agouti-related protein |
| AMPK | AMP-activated protein kinase |
| ASD | Autism spectrum disorder |
| AUC | Area under the curve |
| BMI | Body mass index |
| CAMESA | Canadian Alliance for Monitoring Effectiveness and Safety of Antipsychotics |
| Cmax | Maximum plasma concentration |
| CNS | Central nervous system |
| CI | Confidence interval |
| CPIC | Clinical Pharmacogenetics Implementation Consortium |
| C/D | Concentration-to-dosage |
| D2 | Dopamine D2 |
| D2R | Dopamine receptor D2 |
| DDIs | Drug–drug interactions |
| DPWG | Dutch Pharmacogenetics Working Group |
| DSM-IV | Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition |
| EMA | European Medicines Agency and the Medicines |
| EMs | Extensive metabolizers |
| ERK | Extracellular signal-regulated kinase |
| FDA | Food and Drug Administration |
| FSH | Follicle-stimulating hormone |
| G6Pase | Glucose-6-Phosphatase |
| GCGR | Glucagon receptor |
| GFR | Glomerular filtration rate |
| GLUT4 | Glucose transporter type 4 |
| GnRH | Gonadotrophin-releasing hormone |
| H1, H1R | Histamine 1 Receptor |
| HCPs | Healthcare professionals |
| HPT | Hypothalamic-pituitary-thyroid |
| ICD-10 | International Classification of Diseases, 10th edition |
| IL-6/2 | Interleukin-6/2 |
| IRS | Insulin receptor substrate |
| LH | Luteinizing hormone |
| M1 | Muscarinic acetylcholine |
| MD | Mean difference |
| medianD | Median difference |
| MHRA | Healthcare Products Regulatory Agency |
| MoA | Mechanism of Action |
| mTOR | Mammalian target of rapamycin |
| NPY | Neuropeptide Y |
| OR | Odds ratio |
| P-gp | P-glycoprotein |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PI3K | Phosphoinositide 3-kinase proteins |
| PMs | Poor metabolizers |
| POMC | Pro-opiomelanocortin |
| PPARγ | Peroxisome proliferator-activated receptor-γ |
| PPB | Plasma protein binding |
| SFDA | Saudi Food and Drug Authority |
| SMD | Standardised mean difference |
| SREBP1/2 | Sterol regulatory element-binding protein 1/2 |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| t1/2 | Half-life |
| TNF-α | Tumour necrosis factor-alpha |
| TRH | Thyrotropin-releasing hormone |
| TSH | Thyroid-stimulating hormone |
| UGT | Uridine diphosphate glucuronosyltransferase |
| Vd | Volume distribution |
| WHO | World Health Organization |
| α-MSH | α-melanocyte stimulating hormone |
| α1/α2 | alpha-1/2 adrenergic |
| 5-HT1A/2A | 5-Hydroxytryptamine (Serotonin) 1A/2A subtype |
| 5-HT2C | 5-Hydroxytryptamine (Serotonin) 2C subtype |
References
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9 (Suppl. 1), S55. [Google Scholar] [CrossRef]
- O’Nions, E.; Petersen, I.; Buckman, J.E.; Charlton, R.; Cooper, C.; Corbett, A.; Happé, F.; Manthorpe, J.; Richards, M.; Saunders, R. Autism in England: Assessing underdiagnosis in a population-based cohort study of prospectively collected primary care data. Lancet Reg. Health Eur. 2023, 29, 100626. [Google Scholar] [CrossRef]
- AlBatti, T.H.; Alsaghan, L.B.; Alsharif, M.F.; Alharbi, J.S.; BinOmair, A.I.; Alghurair, H.A.; Aleissa, G.A.; Bashiri, F.A. Prevalence of autism spectrum disorder among Saudi children between 2 and 4 years old in Riyadh. Asian J. Psychiatry 2022, 71, 103054. [Google Scholar] [CrossRef]
- Maenner, M.J. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef]
- Hassan, A. Arab views on autism. In Encyclopedia of Autism Spectrum Disorders; Springer: Berlin/Heidelberg, Germany, 2021; pp. 302–305. [Google Scholar]
- Davico, C.; Secci, I.; Vendrametto, V.; Vitiello, B. Pharmacological treatments in autism spectrum disorder: A narrative review. J. Psychopathol. 2023, 29, 38–52. [Google Scholar]
- Zhou, M.S.; Nasir, M.; Farhat, L.C.; Kook, M.; Artukoglu, B.B.; Bloch, M.H. Meta-analysis: Pharmacologic treatment of restricted and repetitive behaviors in autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 35–45. [Google Scholar] [CrossRef]
- de Pablo, G.S.; Jorda, C.P.; Vaquerizo-Serrano, J.; Moreno, C.; Cabras, A.; Arango, C.; Hernández, P.; Veenstra-VanderWeele, J.; Simonoff, E.; Fusar-Poli, P. Systematic review and meta-analysis: Efficacy of pharmacological interventions for irritability and emotional dysregulation in autism spectrum disorder and predictors of response. J. Am. Acad. Child Adolesc. Psychiatry 2023, 62, 151–168. [Google Scholar] [CrossRef]
- Vita, G.; Nöhles, V.B.; Ostuzzi, G.; Barbui, C.; Tedeschi, F.; Heuer, F.H.; Keller, A.; DelBello, M.P.; Welge, J.A.; Blom, T.J.; et al. Systematic Review and Network Meta-Analysis: Efficacy and Safety of Antipsychotics vs. Antiepileptics or Lithium for Acute Mania in Children and Adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2025, 64, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Persico, A.M.; Ricciardello, A.; Lamberti, M.; Turriziani, L.; Cucinotta, F.; Brogna, C.; Vitiello, B.; Arango, C. The pediatric psychopharmacology of autism spectrum disorder: A systematic review-Part I: The past and the present. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110, 110326. [Google Scholar] [CrossRef]
- Alsayouf, H.A. Growing evidence of pharmacotherapy effectiveness in managing attention-deficit/hyperactivity disorder in young children with or without autism spectrum disorder: A minireview. Front. Psychiatry 2024, 15, 1408876. [Google Scholar] [CrossRef]
- D’Alò, G.L.; De Crescenzo, F.; Amato, L.; Cruciani, F.; Davoli, M.; Fulceri, F.; Minozzi, S.; Mitrova, Z.; Morgano, G.P.; Nardocci, F. Impact of antipsychotics in children and adolescents with autism spectrum disorder: A systematic review and meta-analysis. Health Qual. Life Outcomes. 2021, 19, 33. [Google Scholar] [CrossRef]
- Fonseca, M.; Carmo, F.; Martel, F. Metabolic effects of atypical antipsychotics: Molecular targets. J. Neuroendocrinol. 2023, 35, e13347. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.G. Severe hyperprolactinemia during lurasidone treatment in a 16-year old girl with schizophrenia–a case report. Scand. J. Child Adolesc. Psychiatry Psychol. 2022, 10, 87. [Google Scholar]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fasciani, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical antipsychotics and metabolic syndrome: From molecular mechanisms to clinical differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef]
- Jiang, Y.; Ni, W. Estimating the impact of adherence to and persistence with atypical antipsychotic therapy on health care costs and risk of hospitalization. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 813–822. [Google Scholar] [CrossRef]
- Panagioti, M.H.A.; Planner, C.; Dhingra, N.; Gupta, N. Global Burden of Preventable Medication-Related Harm in Health Care: A Systematic Review; World Health Organization (WHO): Geneva, Switzerland, 2024; p. 43. [Google Scholar]
- Robinson, M.; Amare, M. Adverse drug reactions. Anaesth. Intensive Care Med. 2023, 24, 210–214. [Google Scholar] [CrossRef]
- Lee, L.M.; Carias, D.C.; Gosser, R.; Hannah, A.; Stephens, S.; Templeman, W.A. ASHP guidelines on adverse drug reaction monitoring and reporting. Am. J. Health Syst. Pharm. 2022, 79, e83–e89. [Google Scholar] [CrossRef]
- Alenzi, K.A.; Alanazi, N.S.; Almalki, M.; Alatawi, F.O. The evaluation of adverse drug reactions in Saudi Arabia: A retrospective observational study. Saudi Pharm. J. 2022, 30, 735–741. [Google Scholar] [CrossRef]
- Aronson, J.K. When I use a word… Medical definitions: Adverse events, effects, and reactions. Br. Med. J. 2023, 381, 917. [Google Scholar] [CrossRef]
- World Health Organization. Safety of Medicines: A Guide to Detecting and Reporting Adverse Drug Reactions: Why Health Professionals Need to Take Action; Pharmacy Board of Sierra Leone: Freetown, Sierra Leone, 2002. [Google Scholar]
- Uitvlugt, E.B.; Janssen, M.J.; Siegert, C.E.; Kneepkens, E.L.; van den Bemt, B.J.; van den Bemt, P.M.; Karapinar-Çarkit, F. Medication-related hospital readmissions within 30 days of discharge: Prevalence, preventability, type of medication errors and risk factors. Front. Pharmacol. 2021, 12, 567424. [Google Scholar] [CrossRef]
- Khan, M.A.A.; Hamid, S.; Babar, Z.-U.-D. Pharmacovigilance in high-income countries: Current developments and a review of literature. Pharmacy 2023, 11, 10. [Google Scholar] [CrossRef]
- Osanlou, R.; Walker, L.; Hughes, D.A.; Burnside, G.; Pirmohamed, M. Adverse drug reactions, multimorbidity and polypharmacy: A prospective analysis of 1 month of medical admissions. BMJ Open 2022, 12, e055551. [Google Scholar] [CrossRef] [PubMed]
- Abu Esba, L.C.; Al Mardawi, G.; AlJasser, M.I.; Aljohani, B.; Abu Alburak, A. Adverse drug reactions spontaneously reported at a tertiary care hospital and preventable measures implemented. J. Clin. Pharm. Ther. 2021, 46, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Syahfitri, H. Adverse Drug Reactions of Atypical Antipsychotics: A Review. IOSR J. Pharm. 2021, 11, 37–45. [Google Scholar]
- Matijević, B.; Delalić, Đ.; Meštrović, D.; Petrinović, M.; Jug, J.; Prkačin, I. Side-effects of Medications in Emergency Medicine. Cardiol. Croat. 2022, 17, 96–101. [Google Scholar] [CrossRef]
- Srisuriyachanchai, W.; Cox, A.R.; Kampichit, S.; Jarernsiripornkul, N. Severity and management of adverse drug reactions reported by patients and healthcare professionals: A cross-sectional survey. Int. J. Environ. Res. Public Health 2023, 20, 3725. [Google Scholar] [CrossRef]
- Palanivel, M.; Suresh, A.; Palaniappan, D.R.; Srinivasan, D. A Step towards Patient Safety by Comparing a Trigger Tool with Pre-Existing Tools in the Pharmacovigilance. Indian. J. Pharm. Pract. 2024, 17, 167–180. [Google Scholar] [CrossRef]
- Biswas, M.; Vanwong, N.; Sukasem, C. Pharmacogenomics in clinical practice to prevent risperidone-induced hyperprolactinemia in autism spectrum disorder. Pharmacogenomics 2022, 23, 493–503. [Google Scholar] [CrossRef]
- Xiao, T.; Hu, J.Q.; Liu, S.J.; Lu, H.Q.; Li, X.L.; Kong, W.; Huang, S.Q.; Zhu, X.Q.; Zhang, M.; Lu, H.Y. Population pharmacokinetics and dosing optimization of olanzapine in Chinese paediatric patients: Based on the impact of sex and concomitant valproate on clearance. J. Clin. Pharm. Ther. 2022, 47, 1811–1819. [Google Scholar] [CrossRef]
- Chieh, A.Y.; Bryant, B.M.; Kim, J.W.; Li, L. Systematic review investigating the relationship between autism spectrum disorder and metabolic dysfunction. Res. Autism Spectr. Disord. 2021, 86, 101821. [Google Scholar] [CrossRef]
- Goltz, J.; Ivanov, I.; Rice, T.R. Second generation antipsychotic-induced weight gain in youth with autism spectrum disorders: A brief review of mechanisms, monitoring practices, and indicated treatments. Int. J. Dev. Disabil. 2021, 67, 159–167. [Google Scholar] [CrossRef]
- Alsabhan, J.F.; Al Backer, N.B.; Hassan, F.M.; Albaker, A.B.; Assiry, G. Metabolic Side Effects of Risperidone in Pediatric Patients with Neurological Disorders: A Prospective Cohort Study. J. Clin. Med. 2024, 13, 5565. [Google Scholar] [CrossRef]
- Carnovale, C.; Battini, V.; Santoro, C.; Riccio, M.P.; Carucci, S.; Nobile, M.; Formisano, P.; Bravaccio, C.; Zuddas, A.; Clementi, E. Umbrella review: Association between antipsychotic drugs and metabolic syndrome hallmarks in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2024, 63, 313–335. [Google Scholar] [CrossRef]
- Arango, C.; Ng-Mak, D.; Finn, E.; Byrne, A.; Loebel, A. Lurasidone compared to other atypical antipsychotic monotherapies for adolescent schizophrenia: A systematic literature review and network meta-analysis. Eur. Child Adolesc. Psychiatry 2020, 29, 1195–1205. [Google Scholar] [CrossRef]
- Pagsberg, A.K.; Tarp, S.; Glintborg, D.; Stenstrøm, A.D.; Fink-Jensen, A.; Correll, C.U.; Christensen, R. Acute antipsychotic treatment of children and adolescents with schizophrenia-spectrum disorders: A systematic review and network meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 191–202. [Google Scholar] [CrossRef]
- DelBello, M.P.; Kadakia, A.; Heller, V.; Singh, R.; Hagi, K.; Nosaka, T.; Loebel, A. Systematic review and network meta-analysis: Efficacy and safety of second-generation antipsychotics in youths with bipolar depression. J. Am. Acad. Child Adolesc. Psychiatry 2022, 61, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, Y.J.; Choe, Y.; Shin, C.H.; Lee, Y.A. Predictors for thyroid dysfunction after discontinuation of levothyroxine in children and adolescents with Hashimoto thyroiditis. Ann. Pediatr. Endocrinol. Metab. 2024, 29, 337. [Google Scholar] [CrossRef]
- Koch, M.T.; Carlson, H.E.; Kazimi, M.M.; Correll, C.U. Antipsychotic-related prolactin levels and sexual dysfunction in mentally ill youth: A 3-month cohort study. J. Am. Acad. Child Adolesc. Psychiatry 2023, 62, 1021–1050. [Google Scholar] [CrossRef]
- Krøigaard, S.M.; Clemmensen, L.; Tarp, S.; Pagsberg, A.K. A meta-analysis of antipsychotic-induced hypo-and hyperprolactinemia in children and adolescents. J. Child Adolesc. Psychopharmacol. 2022, 32, 374–389. [Google Scholar] [CrossRef]
- Khan, A.A.; Sharma, R.; Ata, F.; Khalil, S.K.; Aldien, A.S.; Hasnain, M.; Sadiq, A.; Bilal, A.B.I.; Mirza, W. Systematic review of the association between thyroid disorders and hyperprolactinemia. Thyroid. Res. 2025, 18, 1. [Google Scholar] [CrossRef]
- Menard, M.-L.; Thümmler, S.; Giannitelli, M.; Cruzel, C.; Bonnot, O.; Cohen, D.; Askenazy, F.; Boublil, M.; Chambry, J.; Charvet, D.; et al. Incidence of adverse events in antipsychotic-naïve children and adolescents treated with antipsychotic drugs: Results of a multicenter naturalistic study (ETAPE). Eur. Neuropsychopharmacol. 2019, 29, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Fraguas, D.; Merchán-Naranjo, J.; Laita, P.; Parellada, M.; Moreno, D.; Ruiz-Sancho, A.; Cifuentes, A.; Giráldez, M.; Arango, C. Metabolic and hormonal side effects in children and adolescents treated with second-generation antipsychotics. J. Clin. Psychiatry 2008, 69, 1166–1175. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Li, X. Changes in serum thyroid hormone levels in psychiatric patients treated with second-generation antipsychotics. Endokrynol. Pol. 2020, 71, 292–298. [Google Scholar] [CrossRef]
- Kelly, D.L.; Conley, R.R. Thyroid function in treatment-resistant schizophrenia patients treated with quetiapine, risperidone, or fluphenazine. J. Clin. Psychiatry 2005, 66, 20986. [Google Scholar] [CrossRef]
- Santos, N.C.; Costa, P.; Ruano, D.; Macedo, A.; Soares, M.J.; Valente, J.; Pereira, A.T.; Azevedo, M.H.; Palha, J.A. Revisiting thyroid hormones in schizophrenia. J. Thyroid. Res. 2012, 2012, 569147. [Google Scholar] [CrossRef]
- Jazi, S.; Ben-Amor, L.; Abadie, P.; Menard, M.-L.; Choquette, R.; Berthiaume, C.; Mottron, L.; Ilies, D. Long-term metabolic monitoring of youths treated with second-generation antipsychotics 5 years after publication of the CAMESA guidelines are we making Progress? Surveillance Métabolique à long Terme des Jeunes Traités par Antipsychotiques de Deuxième Génération, Cinq ans Après la publication des Lignes Directrices Camesa: Faisons-nous des Progrès? Can. J. Psychiatry 2021, 66, 645–656. [Google Scholar]
- Khoodoruth, M.A.S.; Abdo, A.K.A.; Ouanes, S. Quetiapine-induced thyroid dysfunction: A systematic review. J. Clin. Pharmacol. 2022, 62, 20–35. [Google Scholar] [CrossRef]
- Alvarez-Herrera, S.; Escamilla, R.; Medina-Contreras, O.; Saracco, R.; Flores, Y.; Hurtado-Alvarado, G.; Maldonado-García, J.L.; Becerril-Villanueva, E.; Pérez-Sánchez, G.; Pavón, L. Immunoendocrine peripheral effects induced by atypical antipsychotics. Front. Endocrinol. 2020, 11, 195. [Google Scholar] [CrossRef]
- Findling, R.L.; Pathak, S.; Earley, W.R.; Liu, S.; DelBello, M.P. Efficacy and safety of extended-release quetiapine fumarate in youth with bipolar depression: An 8 week, double-blind, placebo-controlled trial. J. Child Adolesc. Psychopharmacol. 2014, 24, 325–335. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef]
- Chien, H.-Y.; Chen, S.-M.; Li, W.-C. Dopamine receptor agonists mechanism of actions on glucose lowering and their connections with prolactin actions. Front. Clin. Diabetes Healthc. 2023, 4, 935872. [Google Scholar] [CrossRef]
- Libowitz, M.R.; Nurmi, E.L. The burden of antipsychotic-induced weight gain and metabolic syndrome in children. Front. Psychiatry 2021, 12, 623681. [Google Scholar] [CrossRef]
- Soria-Chacartegui, P.; Villapalos-García, G.; Zubiaur, P.; Abad-Santos, F.; Koller, D. Genetic polymorphisms associated with the pharmacokinetics, pharmacodynamics and adverse effects of olanzapine, aripiprazole and risperidone. Front. Pharmacol. 2021, 12, 711940. [Google Scholar] [CrossRef]
- Al-Zoairy, R.; Pedrini, M.T.; Khan, M.I.; Engl, J.; Tschoner, A.; Ebenbichler, C.; Gstraunthaler, G.; Salzmann, K.; Bakry, R.; Niederwanger, A. Serotonin improves glucose metabolism by Serotonylation of the small GTPase Rab4 in L6 skeletal muscle cells. Diabetol. Metab. Syndr. 2017, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Grajales, D.; Valverde, Á.M. Adipose tissue as a target for second-generation (atypical) antipsychotics: A molecular view. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158534. [Google Scholar] [CrossRef]
- Wang, K.-Y.; Tanimoto, A.; Yamada, S.; Guo, X.; Ding, Y.; Watanabe, T.; Watanabe, T.; Kohno, K.; Hirano, K.-I.; Tsukada, H. Histamine regulation in glucose and lipid metabolism via histamine receptors: Model for nonalcoholic steatohepatitis in mice. Am. J. Pathol. 2010, 177, 713–723. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, X.; Huang, X.; Wang, C.; Li, Y. The IRS/PI3K/Akt signaling pathway mediates olanzapine-induced hepatic insulin resistance in male rats. Life Sci. 2019, 217, 229–236. [Google Scholar] [CrossRef]
- Nagata, M.; Yokooji, T.; Nakai, T.; Miura, Y.; Tomita, T.; Taogoshi, T.; Sugimoto, Y.; Matsuo, H. Blockade of multiple monoamines receptors reduce insulin secretion from pancreatic β-cells. Sci. Rep. 2019, 9, 16438. [Google Scholar] [CrossRef]
- Zhao, S.; Lin, Q.; Xiong, W.; Li, L.; Straub, L.; Zhang, D.; Zapata, R.; Zhu, Q.; Sun, X.-N.; Zhang, Z. Hyperleptinemia contributes to antipsychotic drug–associated obesity and metabolic disorders. Sci. Transl. Med. 2023, 15, eade8460. [Google Scholar] [CrossRef]
- Khan, M.M.; Khan, Z.A.; Khan, M.A. Metabolic complications of psychotropic medications in psychiatric disorders: Emerging role of de novo lipogenesis and therapeutic consideration. World J. Psychiatry 2024, 14, 767. [Google Scholar] [CrossRef]
- Anjum, F.; Ali, M.M.; Jakoby, M.; Williams, V. Abstract# 1401109: Quetiapine-induced Hypoglycemia in an Elderly Non-diabetic Patient with Dementia. Endocr. Pract. 2023, 29, S9–S10. [Google Scholar]
- Vasiliu, O. Therapeutic management of atypical antipsychotic-related metabolic dysfunctions using GLP-1 receptor agonists: A systematic review. Exp. Ther. Med. 2023, 26, 355. [Google Scholar] [CrossRef]
- Ebrahimian, Z.; Razavi, B.M.; Mousavi Shaegh, S.A.; Hosseinzadeh, H. Exploring the therapeutic potential of chlorogenic acid in alleviating olanzapine-induced metabolic syndrome in rats: A key role of hypothalamic satiety proteins. Nutr. Neurosci. 2025, 28, 1055–1074. [Google Scholar] [CrossRef]
- Ballon, J.S.; Pajvani, U.; Freyberg, Z.; Leibel, R.L.; Lieberman, J.A. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol. Metab. 2014, 25, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Xu, Y.; Hou, W.; Chen, J.; Li, Q.; Liu, Z.; Dou, G.; Sun, Y.; Li, R.; Ma, X. Mechanistic/mammalian target of rapamycin and side effects of antipsychotics: Insights into mechanisms and implications for therapy. Transl. Psychiatry 2022, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Sobiś, J.; Kunert, Ł.; Rykaczewska-Czerwińska, M.; Świętochowska, E.; Gorczyca, P. The effect of aripiprazole on leptin levels of patients with chronic schizophrenia and a comparison of leptin, acute phase protein, and cytokine levels with regard to body mass and body composition indexes. Endokrynol. Pol. 2022, 73, 35–42. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, W.; Bu, Y.; Li, J.; Hao, Y.; Bi, Y. Effects of 6-week olanzapine treatment on serum IL-2, IL-4, IL-8, IL-10, and TNF-α levels in drug-naive individuals with first-episode schizophrenia. BMC Psychiatry 2024, 24, 703. [Google Scholar] [CrossRef]
- Pillinger, T.; McCutcheon, R.A.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef]
- Spina, E.; Barbieri, M.A.; Cicala, G.; de Leon, J. Clinically relevant interactions between atypical antipsychotics and anti-infective agents. Pharmaceuticals 2020, 13, 439. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Silverblatt, N.S.; Vervaeke, H.E.; Horton, C.C.; Girma, E.; Kaye, A.D.; Kaye, A.; Kaye, J.S.; Garcia, A.J.; Neuchat, E.E. Hyperprolactinemia, clinical considerations, and infertility in women on antipsychotic medications. Psychopharmacol. Bull. 2021, 51, 131. [Google Scholar] [CrossRef]
- Jumaili, W.A.; Muzwagi, A. Review of the Long-Term Effect of the Atypical Antipsychotic Medication on the Bone Mineral Density of the Pediatric Patient with Consideration of Autism Spectrum Disorder. J. Pharmacol. Pharmacother. 2022, 13, 24–30. [Google Scholar] [CrossRef]
- Stojkovic, M.; Radmanovic, B.; Jovanovic, M.; Janjic, V.; Muric, N.; Ristic, D.I. Risperidone induced hyperprolactinemia: From basic to clinical studies. Front. Psychiatry 2022, 13, 874705. [Google Scholar] [CrossRef]
- Baykara, H.B.; Güney, S.A.; Avcil, S.; Buran, B.Ş.; Cıray, R.O.; Ermis, C.; Inal, N. Safety of Atypical Antipsychotics in a Child and Adolescent Inpatient Setting: A Naturalistic Study. Psychiatry Clin. Psychopharmacol. 2024, 34, 109. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Chowdhury, S.; Saha, P.K.; Chowdhury, J. Hyperprolactinemia vs. Thyroid Autoimmunity: Which is a Greater Concern with Atypical Antipsychotics? Res. Squar 2024. [Google Scholar] [CrossRef]
- Pei, X.; Du, X.; Lu, H.; Liu, D. Successful Rescue of Hypotension Shock Induced by Quetiapine: A Case Report. Ann. Clin. Case Rep. 2024, 9, 2653. [Google Scholar]
- Theilade, S.; Christensen, M.B.; Vilsbøll, T.; Knop, F.K. An overview of obesity mechanisms in humans: Endocrine regulation of food intake, eating behaviour and common determinants of body weight. Diabetes Obes. Metab. 2021, 23, 17–35. [Google Scholar] [CrossRef]
- Kong, L.; Shen, Y.; Hu, S.; Lai, J. The impact of quetiapine monotherapy or in combination with lithium on the thyroid function in patients with bipolar depression: A retrospective study. CNS Neurosci. Ther. 2024, 30, e14342. [Google Scholar] [CrossRef]
- Liang, J.; Ringeling, L.T.; Hermans, R.A.; Bayraktar, I.; Bosch, T.M.; Egberts, K.M.; Kloosterboer, S.M.; de Winter, B.; Dierckx, B.; Koch, B.C. Clinical pharmacokinetics of antipsychotics in pediatric populations: A scoping review focusing on dosing regimen. Expert. Opin. Drug Metab. Toxicol. 2023, 19, 501–509. [Google Scholar] [CrossRef]
- Chen, F.; Liu, H.; Wang, B.; Yang, L.; Cai, W.; Jiao, Z.; Yang, Z.; Chen, Y.; Quan, Y.; Xiang, X. Physiologically based pharmacokinetic modeling to understand the absorption of risperidone orodispersible film. Front. Pharmacol. 2020, 10, 1692. [Google Scholar] [CrossRef]
- Townsend, R.W. Low Dose Risperidone Every 3.8 Hours: Superior Efficacy In Treatment of Bipolar Disorders. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.-q.; Jia, J.-y.; Liu, Y.-m.; Liu, G.-y.; Li, S.-j.; Wang, W.; Weng, L.-p.; Yu, C. Bioequivalence and pharmacokinetic evaluation of two formulations of risperidone 2 mg: An open-label, single-dose, fasting, randomized-sequence, two-way crossover study in healthy male Chinese volunteers. Drugs R D 2013, 13, 29–36. [Google Scholar] [CrossRef] [PubMed]
- de Leon, J. Personalizing dosing of risperidone, paliperidone and clozapine using therapeutic drug monitoring and pharmacogenetics. Neuropharmacology 2020, 168, 107656. [Google Scholar] [CrossRef]
- Caccia, S. Safety and pharmacokinetics of atypical antipsychotics in children and adolescents. Pediatr. Drugs. 2013, 15, 217–233. [Google Scholar] [CrossRef]
- Stahl, S.M.; Strawn, J.R. Prescriber’s Guide-Children and Adolescents: Stahl’s Essential Psychopharmacology; Cambridge University Press: Singapore, 2024. [Google Scholar]
- de Leon, J. Precision psychiatry: The complexity of personalizing antipsychotic dosing. Eur. Neuropsychopharmacol. 2022, 58, 80–85. [Google Scholar] [CrossRef]
- Lu, J.; Yang, Y.; Lu, J.; Wang, Z.; He, Y.; Yan, Y.; Fu, K.; Jiang, W.; Xu, Y.; Wu, R. Effect of CYP2D6 polymorphisms on plasma concentration and therapeutic effect of risperidone. BMC Psychiatry 2021, 21, 70. [Google Scholar] [CrossRef]
- Xiang, J.; Xu, N.; Wang, X.; Li, S.; Yu, Q.; Liang, M.; Nan, F.; Shu, S.; Yan, R.; Zhu, Y. Bioequivalence of 2 aripiprazole orally disintegrating tablets in healthy Chinese volunteers under fasting and fed conditions. Clin. Pharmacol. Drug Dev. 2021, 10, 840–849. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, H.; Mishra, A.; Mishra, A.K. Aripiprazole: An FDA approved bioactive compound to treat schizophrenia-A mini review. Curr. Drug Discov. Technol. 2020, 17, 23–29. [Google Scholar] [CrossRef]
- Kirino, E. Profile of aripiprazole in the treatment of bipolar disorder in children and adolescents. Adolesc. Health Med. Ther. 2014, 5, 211–221. [Google Scholar] [CrossRef]
- Biswas, M.; Vanwong, N.; Sukasem, C. Pharmacogenomics and non-genetic factors affecting drug response in autism spectrum disorder in Thai and other populations: Current evidence and future implications. Front. Pharmacol. 2024, 14, 1285967. [Google Scholar] [CrossRef]
- Egberts, K.; Reuter-Dang, S.-Y.; Fekete, S.; Kulpok, C.; Mehler-Wex, C.; Wewetzer, C.; Karwautz, A.; Mitterer, M.; Holtkamp, K.; Boege, I. Therapeutic drug monitoring of children and adolescents treated with aripiprazole: Observational results from routine patient care. J. Neural Transm. 2020, 127, 1663–1674. [Google Scholar] [CrossRef]
- Findling, R.L.; Kauffman, R.E.; Sallee, F.R.; Carson, W.H.; Nyilas, M.; Mallikaarjun, S.; Shoaf, S.E.; Forbes, R.A.; Boulton, D.W.; Pikalov, A. Tolerability and pharmacokinetics of aripiprazole in children and adolescents with psychiatric disorders: An open-label, dose-escalation study. J. Clin. Psychopharmacol. 2008, 28, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Jukić, M.M.; Smith, R.L.; Molden, E.; Ingelman-Sundberg, M. Evaluation of the CYP2D6 haplotype activity scores based on metabolic ratios of 4,700 patients treated with three different CYP2D6 substrates. Clin. Pharmacol. Ther. 2021, 110, 750–758. [Google Scholar] [CrossRef]
- Shan, Y.; Cheung, L.; Zhou, Y.; Huang, Y.; Huang, R.S. A systematic review on sex differences in adverse drug reactions related to psychotropic, cardiovascular, and analgesic medications. Front. Pharmacol. 2023, 14, 1096366. [Google Scholar] [CrossRef]
- Xin, Y.; Gao, L.; Tuo, Y.; Nie, G.; Mei, Y.; Chen, C.; Wang, J.; Li, S.; Sun, D.; Qian, Q. Understanding inter-individual variability in pharmacokinetics/pharmacodynamics of aripiprazole in children with tic disorders: Individualized administration based on physiological development and CYP2D6 genotypes. Front. Pharmacol. 2022, 13, 1048498. [Google Scholar] [CrossRef]
- Rudå, D.; Jensen, K.G.; Decara, M.S.; Klauber, D.G.; Fagerlund, B.; Møllegaard, J.R.; Linnet, K.; Werge, T.; Correll, C.U.; Fink-Jensen, A. CYP2D6 genotyping and antipsychotic-associated extrapyramidal adverse effects in a randomized trial of aripiprazole versus quetiapine extended release in children and adolescents, aged 12–17 years, with first episode psychosis. J. Clin. Psychopharmacol. 2021, 41, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Zubiaur, P.; Soria-Chacartegui, P.; Koller, D.; Navares-Gómez, M.; Ochoa, D.; Almenara, S.; Saiz-Rodríguez, M.; Mejía-Abril, G.; Villapalos-García, G.; Román, M. Impact of polymorphisms in transporter and metabolizing enzyme genes on olanzapine pharmacokinetics and safety in healthy volunteers. Biomed. Pharmacother. 2021, 133, 111087. [Google Scholar] [CrossRef]
- Maharaj, A.R.; Wu, H.; Zimmerman, K.O.; Autmizguine, J.; Kalra, R.; Al-Uzri, A.; Sherwin, C.M.; Goldstein, S.L.; Watt, K.; Erinjeri, J. Population pharmacokinetics of olanzapine in children. Br. J. Clin. Pharmacol. 2021, 87, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Penzak, S.R.; Hon, Y.Y.; Lawhorn, W.D.; Shirley, K.L.; Spratlin, V.; Jann, M.W. Influence of ritonavir on olanzapine pharmacokinetics in healthy volunteers. J. Clin. Psychopharmacol. 2002, 22, 366–370. [Google Scholar] [CrossRef]
- Kolli, P.; Kelley, G.; Rosales, M.; Faden, J.; Serdenes, R. Olanzapine pharmacokinetics: A clinical review of current insights and remaining questions. Pharmgenomics Pers. Med. 2023, 16, 1097–1108. [Google Scholar] [CrossRef]
- Erickson-Ridout, K.K.; Zhu, J.; Lazarus, P. Olanzapine metabolism and the significance of UGT1A448V and UGT2B1067Y variants. Pharmacogenet Genom. 2011, 21, 539–551. [Google Scholar] [CrossRef]
- Mao, J.-H.; Han, L.; Liu, X.-Q.; Jiao, Z. Significant predictors for olanzapine pharmacokinetics: A systematic review of population pharmacokinetic studies. Expert. Rev. Clin. Pharmacol. 2023, 16, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Fekete, S.; Wewetzer, C.; Mehler-Wex, C.; Holtkamp, K.; Burger, R.; Reichert, S.; Taurines, R.; Romanos, M.; Gerlach, M.; Egberts, K. Therapeutic drug monitoring in children and adolescents under pharmacotherapy with olanzapine in daily clinical practice. Ther. Drug Monit. 2017, 39, 273–281. [Google Scholar] [CrossRef]
- Grothe, D.R.; Calis, K.A.; Jacobsen, L.; Kumra, S.; DeVane, C.L.; Rapoport, J.L.; Bergstrom, R.F.; Kurtz, D.L. Olanzapine pharmacokinetics in pediatric and adolescent inpatients with childhood-onset schizophrenia. J. Clin. Psychopharmacol. 2000, 20, 220–225. [Google Scholar] [CrossRef]
- Aichhorn, W.; Marksteiner, J.; Walch, T.; Zernig, G.; Hinterhuber, H.; Stuppaeck, C.; Kemmler, G. Age and gender effects on olanzapine and risperidone plasma concentrations in children and adolescents. J. Child Adolesc. Psychopharmacol. 2007, 17, 665–673. [Google Scholar] [CrossRef]
- Keepers, G.A.; Fochtmann, L.J.; Anzia, J.M.; Benjamin, S.; Lyness, J.M.; Mojtabai, R.; Servis, M.; Walaszek, A.; Buckley, P.; Lenzenweger, M.F. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am. J. Psychiatry 2020, 177, 868–872. [Google Scholar] [CrossRef]
- Blacker, C.J. Clinical issues to consider for clozapine patients who vape: A case illustration. Focus 2020, 18, 55–57. [Google Scholar] [CrossRef]
- Yükselmiş, U.; Akçay, M.; Alomari, O.; Yılmaz, M.K. A case report of combined hemoperfusion and hemodiafiltration utilization in pediatric severe Quetiapine poisoning. J. Med. Surg. Public Health 2024, 4, 100147. [Google Scholar] [CrossRef]
- Ortega-Ruiz, M.; Soria-Chacartegui, P.; Villapalos-García, G.; Abad-Santos, F.; Zubiaur, P. The pharmacogenetics of treatment with quetiapine. Future Pharmacol. 2022, 2, 276–286. [Google Scholar] [CrossRef]
- Joshi, K.; Rao, S.; Mehta, S. A Review of Pharmacokinetic and Pharmacodynamic Properties of Quetiapine IR and XR: Insights and Clinical Practice Implications. Cureus 2025, 17, e86258. [Google Scholar] [CrossRef] [PubMed]
- Bertol, E.; Vaiano, F.; Argo, A.; Zerbo, S.; Trignano, C.; Protani, S.; Favretto, D. Overdose of quetiapine—A case report with QT prolongation. Toxics 2021, 9, 339. [Google Scholar] [CrossRef]
- Shnayder, N.A.; Abdyrakhmanova, A.K.; Nasyrova, R.F. Oxidation of antipsychotics. Encyclopedia 2022, 2, 974–989. [Google Scholar] [CrossRef]
- Castberg, I.; Skogvoll, E.; Spigset, O. Quetiapine and drug interactions: Evidence from a routine therapeutic drug monitoring service. J. Clin. Psychiatry 2007, 68, 1540–1545. [Google Scholar] [CrossRef]
- Fekete, S.; Hiemke, C.; Gerlach, M. Dose-related concentrations of neuroactive/psychoactive drugs expected in blood of children and adolescents. Ther. Drug Monit. 2020, 42, 315–324. [Google Scholar] [CrossRef]
- McConville, B.J.; Arvanitis, L.A.; Thyrum, P.T.; Yeh, C.; Wilkinson, L.A.; Chaney, R.O.; Foster, K.D.; Sorter, M.T.; Friedman, L.M.; Brown, K.L. Pharmacokinetics, tolerability, and clinical effectiveness of quetiapine fumarate: An open-label trial in adolescents with psychotic disorders. J. Clin. Psychiatry 2000, 61, 252–260. [Google Scholar] [CrossRef]
- Zubiaur, P.; Fernández-Campos, P.; Navares-Gómez, M.; Soria-Chacartegui, P.; Villapalos-García, G.; Román, M.; Mejía-Abril, G.; Ochoa, D.; Abad-Santos, F. Variants in COMT, CYP3A5, CYP2B6, and ABCG2 alter quetiapine pharmacokinetics. Pharmaceutics 2021, 13, 1573. [Google Scholar] [CrossRef]
- Yau, K.; McArthur, E.; Jeyakumar, N.; Tsobo Muanda, F.; Kim, R.B.; Clemens, K.K.; Wald, R.; Garg, A.X. Adverse events with quetiapine and clarithromycin coprescription: A population-based retrospective cohort study. Health Sci. Rep. 2023, 6, e1375. [Google Scholar] [CrossRef]
- Jang, J.-H.; Jeong, S.-H. Pharmacokinetic Prediction of Immediate-and Extended-Release Tablets for Patients with Liver Disease Using Whole Body Physiologically-Based Pharmacokinetic Modeling for the Antipsychotic Drug Quetiapine. AAPS PharmSciTech 2025, 26, 8. [Google Scholar] [CrossRef]
- Li, K.-Y.; Li, X.; Cheng, Z.-N.; Zhang, B.-K.; Peng, W.-X.; Li, H.-D. Effect of erythromycin on metabolism of quetiapine in Chinese suffering from schizophrenia. Eur. J. Clin. Pharmacol. 2005, 60, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Jang, J.-H.; Jeong, S.-H. Exploring gender differences in pharmacokinetics of central nervous system related medicines based on a systematic review approach. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 8311–8347. [Google Scholar] [CrossRef]
- McGrane, I.R.; Salyers, L.A.; Molinaro, J.R.; Munjal, R.C. Roux-en-Y gastric bypass and antipsychotic therapeutic drug monitoring: Two cases. J. Pharm. Pract. 2021, 34, 503–506. [Google Scholar] [CrossRef]
- Amerio, A.; Giacomini, C.; Fusar-Poli, L.; Aguglia, A.; Costanza, A.; Serafini, G.; Aguglia, E.; Amore, M. Efficacy and safety of lurasidone in children and adolescents: Recommendations for clinical management and future research. Curr. Pharm. Des. 2021, 27, 4062–4069. [Google Scholar] [CrossRef]
- Findling, R.L.; Goldman, R.; Chiu, Y.-Y.; Silva, R.; Jin, F.; Pikalov, A.; Loebel, A. Pharmacokinetics and tolerability of lurasidone in children and adolescents with psychiatric disorders. Clin. Ther. 2015, 37, 2788–2797. [Google Scholar] [CrossRef]
- Siwek, M.; Krupa, A.; Wasik, A. Lurasidone–pharmacodynamic and pharmacokinetic properties, clinical potential and interaction risk. Pharmacother. Psychiatry Neurol. 2020, 36, 117–134. [Google Scholar] [CrossRef]
- Mostafa, Y.E.; Metwally, M.E.S.; Elsebaei, F. Prominently selective fluorescence approach with distinctive biopharmaceutical utility for analysis of lurasidone in human plasma and urine: Application to in vitro dissolution and content uniformity testing. Luminescence 2024, 39, e4887. [Google Scholar] [CrossRef]
- Lin, S.-K. Racial/ethnic differences in the pharmacokinetics of antipsychotics: Focusing on East Asians. J. Pers. Med. 2022, 12, 1362. [Google Scholar] [CrossRef]
- Shirley, M. Lurasidone in schizophrenia in adolescents: A profile of its use. Drugs Ther. Perspect. 2021, 37, 347–353. [Google Scholar] [CrossRef]
- Mole, T.B.; Furlong, Y.; Clarke, R.J.; Rao, P.; Moore, J.K.; Pace, G.; Van Odyck, H.; Chen, W. Lurasidone for adolescents with complex mental disorders: A case series. J. Pharm. Pract. 2022, 35, 800–804. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Xiao, T.; Ni, X.; Song, E.; Dai, L.; Chen, Y.; Lu, H.; Shang, D.; Wen, Y. Preliminary Determination of the Therapeutic Reference Range of Lurasidone in Chinese Patients and Analysis of the Factors Influencing Lurasidone Dose-Corrected Concentrations. Ther. Drug Monit. 2022, 47, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-Y.; Ereshefsky, L.; Preskorn, S.H.; Poola, N.; Loebel, A. Lurasidone drug-drug interaction studies: A comprehensive review. Drug Metab. Drug Interact. 2014, 29, 191–202. [Google Scholar] [CrossRef]
- Paun, J.S.; Tank, H.M.; Savalia, V.B.; Chaitanya, J.K. Ziprasidone Hydrochloride Nanosuspension for Bioavailability Enhancement: Design, Development and Evaluation. E3S Web Conf. 2025, 619, 05004. [Google Scholar] [CrossRef]
- Khan, A.A.; Strawn, J.R.; Croarkin, P.E. Emerging treatment options in bipolar disorder in adolescents: Focus on ziprasidone. Adolesc. Health Med. Ther. 2010, 1, 137–143. [Google Scholar]
- Bao, S.; Yang, S.; Hua, Z.; Li, J.; Zang, Y.; Li, X. Ziprasidone population pharmacokinetics and co-medication effects in Chinese patients. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 9811–9821. [Google Scholar] [CrossRef]
- Simon, N.; Torrents, R.; Azorin, J.-M. Comorbidities and the right dose: Antipsychotics. Expert. Opin. Drug Metab. Toxicol. 2022, 18, 507–518. [Google Scholar] [CrossRef]
- Sallee, F.R.; Miceli, J.J.; Tensfeldt, T.; Robarge, L.; Wilner, K.; Patel, N.C. Single-dose pharmacokinetics and safety of ziprasidone in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 720–728. [Google Scholar] [CrossRef]
- Miceli, J.; Smith, M.; Robarge, L.; Morse, T.; Laurent, A. The effects of ketoconazole on ziprasidone pharmacokinetics—A placebo-controlled crossover study in healthy volunteers. Br. J. Clin. Pharmacol. 2000, 49, 71–76. [Google Scholar] [CrossRef]
- Cicala, G.; Barbieri, M.A.; Santoro, V.; Tata, C.; Colucci, P.V.; Vanadia, F.; Drago, F.; Russo, C.; Cutroneo, P.M.; Gagliano, A. Safety and tolerability of antipsychotic drugs in pediatric patients: Data from a 1-year naturalistic study. Front. Psychiatry 2020, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Schoretsanitis, G.; de Leon, J.; Correll, C.U. How can we better address the pharmacokinetics of antipsychotics in children and adolescents? Expert. Opin. Drug Metab. Toxicol. 2024, 20, 719–726. [Google Scholar] [CrossRef]
- Chapron, B.D.; Chapron, A.; Leeder, J.S. Recent advances in the ontogeny of drug disposition. Br. J. Clin. Pharmacol. 2022, 88, 4267–4284. [Google Scholar] [CrossRef]
- O’hara, K. Paediatric pharmacokinetics and drug doses. Aust. Prescr. 2016, 39, 208. [Google Scholar] [CrossRef]
- Sanyal, S.; Calarge, C.A.; Rowan, P.J.; Aparasu, R.R.; Abughosh, S.; Chen, H. Impact of the AACAP practice parameters on the metabolic adverse event monitoring for second-generation antipsychotics (SGAs) in children and adolescents. J. Psychiatr. Res. 2023, 165, 170–173. [Google Scholar] [CrossRef]
- Hoekstra, S.; Bartz-Johannessen, C.; Sinkeviciute, I.; Reitan, S.K.; Kroken, R.A.; Løberg, E.-M.; Larsen, T.K.; Rettenbacher, M.; Johnsen, E.; Sommer, I.E. Sex differences in antipsychotic efficacy and side effects in schizophrenia spectrum disorder: Results from the BeSt InTro study. npj Schizophr. 2021, 7, 39. [Google Scholar] [CrossRef]
- Johansen, I.T.; Steen, N.E.; Rødevand, L.; Werner, M.C.; Lunding, S.H.; Hjell, G.; Ormerod, M.B.; Agartz, I.; Melle, I.; Lagerberg, T.V. Sex-specific associations between metabolic hormones, severe mental disorders and antipsychotic treatment. Psychoneuroendocrinology 2022, 146, 105927. [Google Scholar] [CrossRef]
- Zucker, I.; Prendergast, B.J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex. Differ. 2020, 11, 32. [Google Scholar] [CrossRef]
- Klein, S.L.; Morgan, R. The impact of sex and gender on immunotherapy outcomes. Biol. Sex. Differ. 2020, 11, 24. [Google Scholar] [CrossRef]
- Jovanović, M.; Vučićević, K.; Miljković, B. Understanding variability in the pharmacokinetics of atypical antipsychotics–focus on clozapine, olanzapine and aripiprazole population models. Drug Metab. Rev. 2020, 52, 1–18. [Google Scholar] [CrossRef]
- Brand, B.A.; Haveman, Y.R.; De Beer, F.; De Boer, J.N.; Dazzan, P.; Sommer, I.E. Antipsychotic medication for women with schizophrenia spectrum disorders. Psychol. Med. 2022, 52, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Elmeliegy, M.; Vourvahis, M.; Guo, C.; Wang, D.D. Effect of P-glycoprotein (P-gp) inducers on exposure of P-gp substrates: Review of clinical drug–drug interaction studies. Clin. Pharmacokinet. 2020, 59, 699–714. [Google Scholar] [CrossRef]
- Watson, S.; Caster, O.; Rochon, P.A.; Den Ruijter, H. Reported adverse drug reactions in women and men: Aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine 2019, 17, 100188. [Google Scholar] [CrossRef] [PubMed]
- Shilbayeh, S.A.R.; Adeen, I.S.; Alhazmi, A.S.; Aldilaijan, K.E.; Aloyouni, S.Y. Risperidone pharmacogenetics: The impact of star alleles’ predicted phenotypes on global safety in autistic children. Int. J. Pharmacol. 2023, 19, 485–504. [Google Scholar] [CrossRef]
- Beunk, L.; Nijenhuis, M.; Soree, B.; de Boer-Veger, N.J.; Buunk, A.-M.; Guchelaar, H.J.; Houwink, E.J.; Risselada, A.; Rongen, G.A.; van Schaik, R.H. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2D6, CYP3A4 and CYP1A2 and antipsychotics. Eur. J. Hum. Genet. 2024, 32, 278–285. [Google Scholar] [CrossRef]
- Tveito, M.; Molden, E.; Høiseth, G.; Correll, C.U.; Smith, R.L. Impact of age and CYP2D6 genetics on exposure of aripiprazole and dehydroaripiprazole in patients using long-acting injectable versus oral formulation: Relevance of poor and intermediate metabolizer status. Eur. J. Clin. Pharmacol. 2020, 76, 41–49. [Google Scholar] [CrossRef]
- Jukic, M.M.; Smith, R.L.; Haslemo, T.; Molden, E.; Ingelman-Sundberg, M. Effect of CYP2D6 genotype on exposure and efficacy of risperidone and aripiprazole: A retrospective, cohort study. Lancet Psychiatry 2019, 6, 418–426. [Google Scholar] [CrossRef]
- Ahmed, Z.; Hao, S.; Williamson, T.; McMorris, C.A.; Bousman, C.A. Psychotropic prescribing rates and pharmacogenomic testing implications for autism in the Canadian primary care sentinel surveillance network. Pharmacogenet Genom. 2022, 32, 94–100. [Google Scholar] [CrossRef]
- Cui, Y.; Yan, H.; Su, Y.; Wang, L.; Lu, T.; Zhang, D.; Yue, W. CYP2D6 genotype-based dose recommendations for risperidone in Asian people. Front. Pharmacol. 2020, 11, 936. [Google Scholar] [CrossRef]
- Chamnanphon, M.; Vanwong, N.; Prommas, S.; Koomdee, N.; Sukprasong, R.; Rachanakul, J.; Nuntharadthanaphong, N.; Hongkaew, Y.; John, S.; Ngamsamut, N. Risperidone plasma concentrations are associated with hyperprolactinemia in autism spectrum disorder children: The impact of CYP2D6 polymorphisms. Res. Autism Spectr. Disord. 2022, 96, 102002. [Google Scholar] [CrossRef]
- Merino, D.; Fernandez, A.; Gerard, A.O.; Ben Othman, N.; Rocher, F.; Askenazy, F.; Verstuyft, C.; Drici, M.-D.; Thümmler, S. Adverse Drug Reactions of Olanzapine, Clozapine and Loxapine in Children and Youth: A Systematic Pharmacogenetic Review. Pharmaceuticals 2022, 15, 749. [Google Scholar] [CrossRef]
- Baldacci, A.; Saguin, E.; Balcerac, A.; Mouchabac, S.; Ferreri, F.; Gaillard, R.; Colas, M.-D.; Delacour, H.; Bourla, A. Pharmacogenetic guidelines for psychotropic drugs: Optimizing prescriptions in clinical practice. Pharmaceutics 2023, 15, 2540. [Google Scholar] [CrossRef] [PubMed]
- Dubale, A.T.; Tareke, A.A.; Butta, F.W.; Shibabaw, A.A.; Eniyew, E.B.; Ahmed, M.H.; Kassie, S.Y.; Demsash, A.W.; Chereka, A.A.; Dube, G.N. Healthcare professionals’ willingness to utilize a mobile health application for adverse drug reaction reporting in a limited resource setting: An input for digital health, 2023. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2024, 23, 100324. [Google Scholar] [CrossRef]
- Worakunphanich, W.; Youngkong, S.; Suwankesawong, W.; Anderson, C.; Thavorncharoensap, M. Comparison of patient adverse drug reaction reporting systems in nine selected countries. Int. J. Environ. Res. Public Health 2022, 19, 4447. [Google Scholar] [CrossRef]
- Alshammari, T.M. Pharmacovigilance and outcomes: Experience from Saudi Arabia narrative review. Expert. Rev. Pharmacoecon. Outcomes Res. 2025, 25, 7–15. [Google Scholar] [CrossRef]
- Costa, C.; Abeijon, P.; Rodrigues, D.A.; Figueiras, A.; Herdeiro, M.T.; Torre, C. Factors associated with underreporting of adverse drug reactions by patients: A systematic review. Int. J. Clin. Pharm. 2023, 45, 1349–1358. [Google Scholar] [CrossRef]
- Gauci, R. Digitalisation of Adverse Drug Reactions Information Source; University of Malta: Msida, Malta, 2024. [Google Scholar]
- Al-Worafi, Y.M. Adverse Drug Reactions (ADRs) in Developing Countries. In Handbook of Medical and Health Sciences in Developing Countries: Education, Practice, and Research; Springer: Cham, Switzerland, 2023; pp. 1–18. [Google Scholar]
- Beversdorf, D.Q.; Anagnostou, E.; Hardan, A.; Wang, P.; Erickson, C.A.; Frazier, T.W.; Veenstra-VanderWeele, J. Precision medicine approaches for heterogeneous conditions such as autism spectrum disorders (The need for a biomarker exploration phase in clinical trials-Phase 2m). Front. Psychiatry 2023, 13, 1079006. [Google Scholar] [CrossRef]
- Lacivita, E.; Niso, M.; Mastromarino, M.; Garcia Silva, A.; Resch, C.; Zeug, A.; Loza, M.I.; Castro, M.; Ponimaskin, E.; Leopoldo, M. Knowledge-based design of long-chain arylpiperazine derivatives targeting multiple serotonin receptors as potential candidates for treatment of autism spectrum disorder. ACS Chem. Neurosci. 2021, 12, 1313–1327. [Google Scholar] [CrossRef]
- Clavenna, A.; Cartabia, M.; Fortino, I.; Bonati, M. Drug prescription profile in children with autism spectrum disorders. Eur. J. Clin. Pharmacol. 2024, 80, 297–299. [Google Scholar] [CrossRef]
- Ritter, C.; Hewitt, K.; McMorris, C.A. Psychotropic polypharmacy among children and youth with autism: A systematic review. J. Child Adolesc. Psychopharmacol. 2021, 31, 244–258. [Google Scholar] [CrossRef]
- Dargenio, V.N.; Dargenio, C.; Castellaneta, S.; De Giacomo, A.; Laguardia, M.; Schettini, F.; Francavilla, R.; Cristofori, F. Intestinal barrier dysfunction and microbiota–gut–brain axis: Possible implications in the pathogenesis and treatment of autism spectrum disorder. Nutrients 2023, 15, 1620. [Google Scholar] [CrossRef]
- Długosz, A.; Wróblewski, M.; Błaszak, B.; Szulc, J. The Role of Nutrition, Oxidative Stress, and Trace Elements in the Pathophysiology of Autism Spectrum Disorders. Int. J. Mol. Sci. 2025, 26, 808. [Google Scholar] [CrossRef] [PubMed]
- De Luca, F. Endocrinological abnormalities in autism. Semin. Pediatr. Neurol. 2020, 35, 100582. [Google Scholar] [CrossRef]
- Blake, K.V.; Zaccaria, C.; Domergue, F.; La Mache, E.; Saint-Raymond, A.; Hidalgo-Simon, A. Comparison between paediatric and adult suspected adverse drug reactions reported to the European medicines agency: Implications for pharmacovigilance. Pediatr. Drugs. 2014, 16, 309–319. [Google Scholar] [CrossRef]
- Dubrall, D.; Leitzen, S.; Toni, I.; Stingl, J.; Schulz, M.; Schmid, M.; Neubert, A.; Sachs, B. Descriptive analysis of adverse drug reaction reports in children and adolescents from Germany: Frequently reported reactions and suspected drugs. BMC Pharmacol. Toxicol. 2021, 22, 56. [Google Scholar] [CrossRef]
- Braykova, R.; Toneva, A. Aspects of Insulin Resistance in Children with Autism. J. IMAB. 2025, 31, 6111–6115. [Google Scholar] [CrossRef]
- Aljead, M.; Qashta, A.; Jalal, Z.; Jones, A.M. Review of Autism Spectrum Disorder (ASD): Epidemiology, Aetiology, Pathology, and Pharmacological Treatment. Pharmaceuticals 2025, 18, 1644. [Google Scholar] [CrossRef]
- Al-Huseini, S.; Al-Barhoumi, A.; Al-Balushi, M.; Al-Hosni, A.; Al-Mahrouqi, T.; Al-Mahrizi, B.; Jaju, S.; Mirza, H. Effectiveness and adverse effects of risperidone in children with autism spectrum disorder in a naturalistic clinical setting at a university hospital in Oman. Autism Res. Treat. 2022, 2022, 2313851. [Google Scholar] [CrossRef]
- Rahim Shilbayeh, S.A.; Adeen, I.S. Management of autism spectrum disorder: A pilot study in saudi paediatrics. Mil. Med. Sci. Lett. 2024, 93, 120–130. [Google Scholar] [CrossRef]
- Makary, S.; Abd El Moez, K.; Elsayed, M.; Hassan, H. Second-generation antipsychotic medications and metabolic disturbance in children and adolescents. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 14. [Google Scholar] [CrossRef]
- Man, K.K.; Shao, S.-C.; Chang, Y.-C.; Chi, M.-H.; Jeong, H.E.; Lin, S.-J.; Su, C.-C.; Shin, J.-Y.; Wong, K.H.; Wong, I.C. Cardiovascular and metabolic risk of antipsychotics in children and young adults: A multinational self-controlled case series study. Epidemiol. Psychiatr. Sci. 2021, 30, e65. [Google Scholar] [CrossRef]
- Iasevoli, F.; Barone, A.; Buonaguro, E.F.; Vellucci, L.; de Bartolomeis, A. Safety and tolerability of antipsychotic agents in neurodevelopmental disorders: A systematic review. Expert. Opin. Drug Saf. 2020, 19, 1419–1444. [Google Scholar] [CrossRef]
- Hernandez, M.; Cullell, N.; Cendros, M.; Serra-Llovich, A.; Arranz, M.J. Clinical utility and implementation of pharmacogenomics for the personalisation of antipsychotic treatments. Pharmaceutics 2024, 16, 244. [Google Scholar] [CrossRef]
- Mead, L.; Ayres, A.; Blake, J.A.; Scott, J.G. Monitoring of metabolic side-effects in children and adolescents prescribed antipsychotic medication: A systematic review. Aust. N. Z. J. Psychiatry 2021, 55, 763–771. [Google Scholar] [CrossRef]
- Fekete, S.; Güntzel, T.; Egberts, K.; Geissler, J.; Neubert, A.; Gerlach, M.; Romanos, M.; Taurines, R. Serious adverse drug reactions to antipsychotics in minors with multiple disabilities: Preventability and potential cost savings by therapeutic drug monitoring. Pharmacopsychiatry 2023, 56, 32–39. [Google Scholar] [CrossRef]

| Level | Severity | Definition | Example |
|---|---|---|---|
| 1 | Mild | ADR is detected without requiring discontinuation of medication or intervention. | Nausea, vomiting, constipation, dizziness, and headache |
| 2 | Mild | ADR detected, leading to discontinuation of medication but without need for intervention. | Sedation, extrapyramidal side effects |
| 3 | Moderate | ADR detected, requiring medication discontinuation or modification but no need for prolonged hospitalisation. | Weight gain |
| 4 | Moderate | ADR is detected and results in prolonged hospitalisation. | Hyperglycaemia |
| 5 | Severe | ADR is detected and leads to life-threatening situations or temporary disability. | Hyperlipidaemia |
| 6 | Severe | ADR contributes to permanent disability. | Hyperprolactinemia |
| 7 | Severe | ADR results in death. | Myocarditis |
| Drug/PK | Absorption | Distribution | Metabolism | Excretion | ||||
|---|---|---|---|---|---|---|---|---|
| cmax (nM) | Bioavailability (%) | Food Effect | Vd (L/kg) | PPB (%) | Enzyme(s) | t1/2 (Hour) | Route | |
| Risperidone | 36.5 | 70 | X | 1–2 | 90 | CYP2D6 -With CYP2D6-inhibitors: ↓ by 55% | Risperidone and 9-OH-risperidone: 20 -PM: 20 -EM: 3 | Urine: 70% Faeces: 14% |
| Aripiprazole | 240.8 -F: ↑ by 11% | 87 | X | ~5 | 99 | CYP2D6 | Aripiprazole: 75 Dehydroaripiprazole: 94 -PM: 150 | Urine: 30% Faeces: 55% |
| Olanzapine | 48 | ≥65 | X | 21 | 93 | CYP1A2 CYP2D6 UGT1 -F: ↓ by 30% -With CYP1A2 inducer (smoker): ↑ by 40% | 30–52 b | Urine: 57% Faeces:30% |
| Quetiapine | 1291.4 | 10 | X a | 6–14 | 83 | CYP3A4 -Hepatic impairment: dose ↓ by 0.1–0.5-fold | Quetiapine: 6 -N-desalkyl quetiapine: 12 -CYP3A4 inhibitor: ↑ by 92% | Urine: 73% Faeces: 20% |
| Lurasidone | 60.9 -CYP3A4 inhibitor: ↑ by 7 times | 19 September | ↑ by 2–3 times | 2.4–20 | 99 | CYP3A4 -CYP3A4 inhibitor: contraindication | lurasidone: 29 ID-14283: ↓ by 2.5 times | Urine: 9% Faeces: 80% |
| Ziprasidone | 121 -F: ↑ by 25% -CYP3A4 inhibitor: ↑ by 34% | 60 | Increased | 1.5 | 99 | glutathione and aldehyde oxidase CYP3A4 (lesser extent) -CYP3A4 inhibitor: monitoring therapy | 5 | Urine: 20% Faeces:66% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljead, M.; Qashta, A.; Jalal, Z.; Jones, A.M. Metabolic and Endocrine ADRs of Atypical Antipsychotics (AAPs) in Paediatric Patients with Autism Spectrum Disorder (ASD): A Review of Prevalence, Risk Factors, and Implications for Clinical Monitoring. J. Clin. Med. 2025, 14, 7942. https://doi.org/10.3390/jcm14227942
Aljead M, Qashta A, Jalal Z, Jones AM. Metabolic and Endocrine ADRs of Atypical Antipsychotics (AAPs) in Paediatric Patients with Autism Spectrum Disorder (ASD): A Review of Prevalence, Risk Factors, and Implications for Clinical Monitoring. Journal of Clinical Medicine. 2025; 14(22):7942. https://doi.org/10.3390/jcm14227942
Chicago/Turabian StyleAljead, Mashal, Aya Qashta, Zahraa Jalal, and Alan M. Jones. 2025. "Metabolic and Endocrine ADRs of Atypical Antipsychotics (AAPs) in Paediatric Patients with Autism Spectrum Disorder (ASD): A Review of Prevalence, Risk Factors, and Implications for Clinical Monitoring" Journal of Clinical Medicine 14, no. 22: 7942. https://doi.org/10.3390/jcm14227942
APA StyleAljead, M., Qashta, A., Jalal, Z., & Jones, A. M. (2025). Metabolic and Endocrine ADRs of Atypical Antipsychotics (AAPs) in Paediatric Patients with Autism Spectrum Disorder (ASD): A Review of Prevalence, Risk Factors, and Implications for Clinical Monitoring. Journal of Clinical Medicine, 14(22), 7942. https://doi.org/10.3390/jcm14227942







