Sex Differences in the Association Between Ultra-Processed Food Consumption and NAFLD: An Analysis of KNHANES 2013–2021 Data
Abstract
1. Introduction
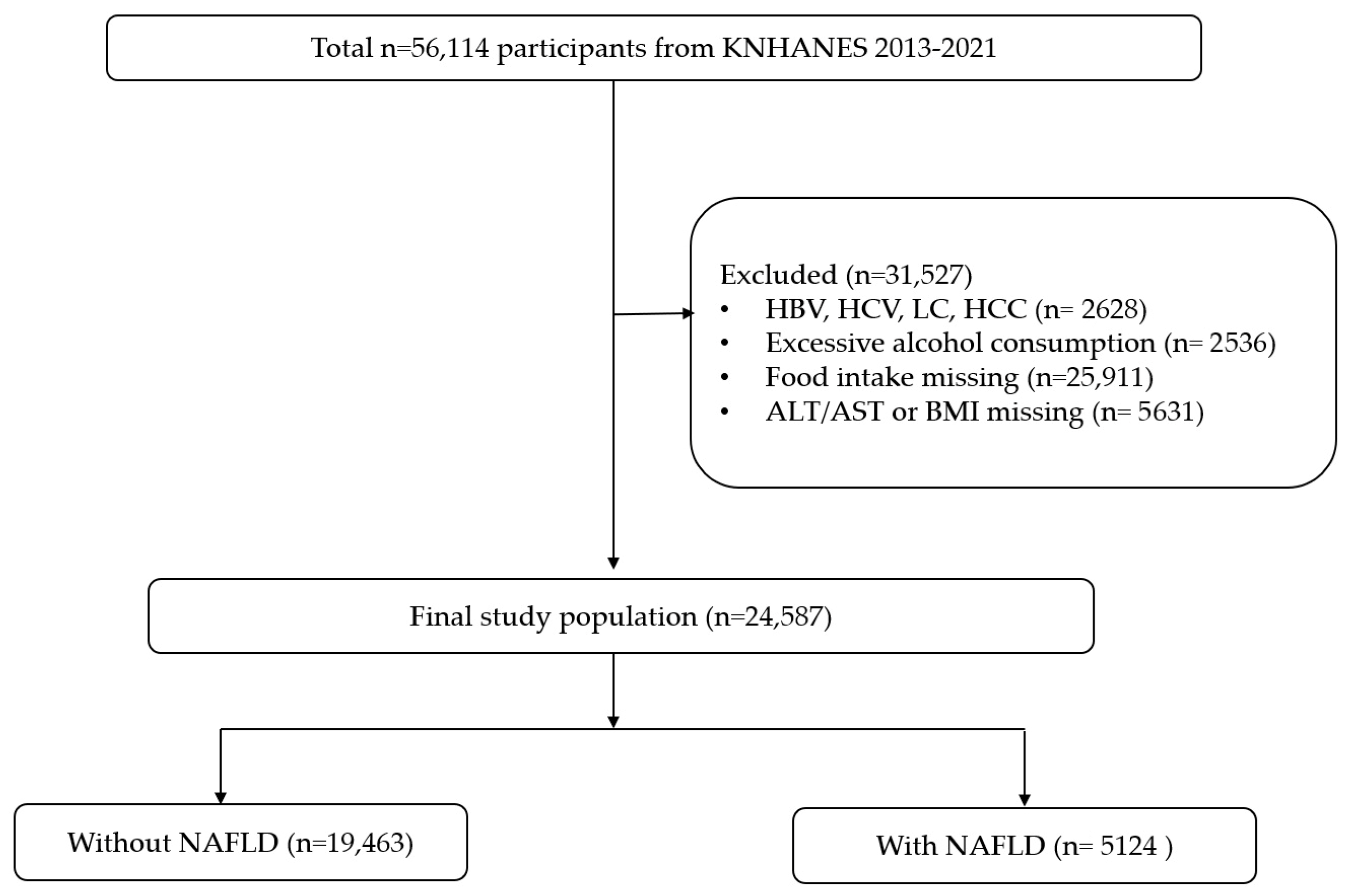
2. Materials and Methods
2.1. Study Design and Population
2.2. Exposure Assessment: Ultra-Processed Food Intake
2.3. Outcome Definition: NAFLD
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Association Between UPF Intake and NAFLD
3.3. Sex-Stratified Analyses
3.4. Continuous Trends in NAFLD Risk Across UPF Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CIs | confidence intervals |
| HSI | hepatic steatosis index |
| KNHANES | Korea National Health and Nutrition Examination Survey |
| NAFLD | non-alcoholic fatty liver disease |
| ORs | odds ratios |
| UPFs | ultra-processed foods |
References
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.-U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Hartjes, K.; Shah, U.; Khalili, H.; Arnell, H.; Ludvigsson, J.F. Non-alcoholic fatty liver disease in children and young adults is associated with increased long-term mortality. J. Hepatol. 2021, 75, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Petrus, R.R.; do Amaral Sobral, P.J.; Tadini, C.C.; Gonçalves, C.B. The NOVA classification system: A critical perspective in food science. Trends Food Sci. Technol. 2021, 116, 603–608. [Google Scholar] [CrossRef]
- Williams, A.M.; Couch, C.A.; Emmerich, S.D.; Ogburn, D.F. Ultra-processed Food Consumption in Youth and Adults: United States, August 2021–August 2023. NCHS Data Brief 2025, 536, 1–11. [Google Scholar]
- Touvier, M.; da Costa Louzada, M.L.; Mozaffarian, D.; Baker, P.; Juul, F.; Srour, B. Ultra-processed foods and cardiometabolic health: Public health policies to reduce consumption cannot wait. BMJ 2023, 383, e075294. [Google Scholar] [CrossRef] [PubMed]
- Suksatan, W.; Moradi, S.; Naeini, F.; Bagheri, R.; Mohammadi, H.; Talebi, S.; Mehrabani, S.; Hojjati Kermani, M.a.; Suzuki, K. Ultra-processed food consumption and adult mortality risk: A systematic review and dose–response meta-analysis of 207,291 participants. Nutrients 2021, 14, 174. [Google Scholar] [CrossRef]
- Formisano, A.; Dello Russo, M.; Lissner, L.; Russo, P.; Ahrens, W.; De Henauw, S.; Hebestreit, A.; Intemann, T.; Hunsberger, M.; Molnár, D.; et al. Ultra-Processed Foods Consumption and Metabolic Syndrome in European Children, Adolescents, and Adults: Results from the I.Family Study. Nutrients 2025, 17, 2252. [Google Scholar] [CrossRef]
- Canhada, S.L.; Vigo, Á.; Giatti, L.; Fonseca, M.J.; Lopes, L.J.; Cardoso, L.O.; Monteiro, C.A.; Schmidt, M.I.; Duncan, B.B. Associations of Ultra-Processed Food Intake with the Incidence of Cardiometabolic and Mental Health Outcomes Go Beyond Specific Subgroups-The Brazilian Longitudinal Study of Adult Health. Nutrients 2024, 16, 4291. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, L.; Chen, X.-P. Ultra-processed foods and non-alcoholic fatty liver disease: An updated systematic review and dose-response meta-analysis. Front. Nutr. 2025, 12, 1631975. [Google Scholar] [CrossRef]
- Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Webb, M.; Bentov, I.; Shibolet, O.; Kariv, R.; Zelber-Sagi, S. Ultra-processed food is associated with features of metabolic syndrome and non-alcoholic fatty liver disease. Liver Int. 2021, 41, 2635–2645. [Google Scholar] [CrossRef]
- Rich, N.E.; Oji, S.; Mufti, A.R.; Browning, J.D.; Parikh, N.D.; Odewole, M.; Mayo, H.; Singal, A.G. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 198–210.e192. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Wang, J.; Shi, J.; Ma, Y.; Li, Y.; Li, Q.; Hu, X.; Chen, J.; Bao, Z. Prevalence of MAFLD in the U.S. based on NHANES 2009-2018: Differences in demographic characteristics, physical indices and lifestyle conditions. BMC Gastroenterol. 2025, 25, 329. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex differences in nonalcoholic fatty liver disease: State of the art and identification of research gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, S.; Kim, J.Y. Development of Korean NOVA food classification and estimation of ultra-processed food intake among adults: Using 2018 Korea National Health and Nutrition Examination Survey. Korean J. Community Nutr. 2022, 27, 455–467. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. Available online: https://odphp.health.gov/our-work/nutrition-physical-activity/physical-activity-guidelines/current-guidelines (accessed on 18 September 2025).
- Zhang, S.; Gan, S.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Wu, H.; Gu, Y.; Wang, Y.; Zhang, T.; et al. Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease in the Tianjin Chronic Low-grade Systemic Inflammation and Health Cohort Study. Int. J. Epidemiol. 2022, 51, 237–249. [Google Scholar] [CrossRef]
- Henney, A.E.; Gillespie, C.S.; Alam, U.; Hydes, T.J.; Cuthbertson, D.J. Ultra-Processed Food Intake Is Associated with Non-Alcoholic Fatty Liver Disease in Adults: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2266. [Google Scholar] [CrossRef]
- Grinshpan, L.S.; Eilat-Adar, S.; Ivancovsky-Wajcman, D.; Kariv, R.; Gillon-Keren, M.; Zelber-Sagi, S. Ultra-processed food consumption and non-alcoholic fatty liver disease, metabolic syndrome and insulin resistance: A systematic review. JHEP Rep. 2024, 6, 100964. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e63. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef]
- Fridén, M.; Kullberg, J.; Ahlström, H.; Lind, L.; Rosqvist, F. Intake of Ultra-Processed Food and Ectopic-, Visceral- and Other Fat Depots: A Cross-Sectional Study. Front. Nutr. 2022, 9, 774718. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, K.W. Gender Differences in Ultra-Processed Food Consumption and Its Association with Obesity Among Korean Adults. Nutrients 2025, 17, 2027. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Handisurya, A. Metabolic diseases and associated complications: Sex and gender matter! Eur. J. Clin. Investig. 2009, 39, 631–648. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, J.K. NAFLD and NASH in Postmenopausal Women: Implications for Diagnosis and Treatment. Endocrinology 2020, 161, bqaa134. [Google Scholar] [CrossRef]
- Ter Horst, K.W.; Gilijamse, P.W.; De Weijer, B.A.; Kilicarslan, M.; Ackermans, M.T.; Nederveen, A.J.; Nieuwdorp, M.; Romijn, J.A.; Serlie, M.J. Sexual dimorphism in hepatic, adipose tissue, and peripheral tissue insulin sensitivity in obese humans. Front. Endocrinol. 2015, 6, 182. [Google Scholar] [CrossRef]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef]
- Tran, C.; Jacot-Descombes, D.; Lecoultre, V.; Fielding, B.A.; Carrel, G.; Lê, K.-A.; Schneiter, P.; Bortolotti, M.; Frayn, K.N.; Tappy, L. Sex differences in lipid and glucose kinetics after ingestion of an acute oral fructose load. Br. J. Nutr. 2010, 104, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.N.; O’Sullivan, A.J. Sex differences in energy metabolism need to be considered with lifestyle modifications in humans. J. Nutr. Metab. 2011, 2011, 391809. [Google Scholar] [CrossRef] [PubMed]
- Gavin, K.M.; Bessesen, D.H. Sex differences in adipose tissue function. Endocrinol. Metab. Clin. 2020, 49, 215–228. [Google Scholar] [CrossRef] [PubMed]

| Quartile of %UPF Intake in Total Foods Consumed | p-Value | |||||
|---|---|---|---|---|---|---|
| Total | Q1 | Q2 | Q3 | Q4 | ||
| n | 24,587 | 6147 | 6146 | 6148 | 6146 | |
| %UPF in total food intake, median (IQR) | 12.85 (4.96, 26.58) | 1.89 (0.91, 3.10) | 7.57 (5.88, 9.39) | 16.77 (13.91, 20.03) | 36.80 (29.52, 47.24) | - |
| Age | 43.66 ± 15.66 | 48.18 ± 16.33 | 46.74 ± 16.17 | 43.43 ± 15.60 | 37.75 ± 12.83 | <0.001 |
| Sex | <0.001 | |||||
| Men | 10,419 (48.48) | 2118 (39.11) | 2269 (41.89) | 2708 (50.16) | 3324 (59.86) | |
| Women | 14,168 (51.52) | 4029 (60.89) | 3877 (58.11) | 3440 (49.84) | 2822 (40.14) | |
| BMI | 23.81 ± 3.69 | 23.59 ± 3.64 | 23.74 ± 3.65 | 23.84 ± 3.61 | 24.01 ± 3.75 | <0.001 |
| Education | <0.001 | |||||
| Elementary school or lower | 3166 (9.10) | 976 (12.07) | 931 (10.85) | 803 (9.22) | 456 (5.16) | |
| Middle school | 1862 (6.40) | 539 (7.53) | 539 (7.87) | 459 (6.20) | 325 (4.45) | |
| High school | 8209 (38.12) | 1858 (33.67) | 1968 (35.96) | 1986 (37.58) | 2397 (43.98) | |
| College or higher | 10,055 (46.37) | 2459 (46.74) | 2423 (45.32) | 2552 (47.00) | 2621 (46.41) | |
| Household income | <0.001 | |||||
| Low | 3471 (11.40) | 976 (12.89) | 922 (12.18) | 846 (10.94) | 727 (9.97) | |
| Lower middle | 5882 (23.32) | 1434 (22.48) | 1497 (23.17) | 1474 (23.28) | 1477 (24.15) | |
| Upper middle | 7186 (31.00) | 1750 (30.11) | 1737 (29.76) | 1802 (31.50) | 1897 (32.28) | |
| High | 7960 (34.29) | 1964 (34.52) | 1969 (34.90) | 2005 (34.28) | 2022 (33.60) | |
| Alcohol consumption | <0.001 | |||||
| At least once per month | 13,559 (60.07) | 2725 (48.79) | 2962 (52.98) | 3470 (59.73) | 4402 (75.24) | |
| Less than once per month | 10,552 (39.93) | 3304 (51.21) | 3083 (47.02) | 2565 (40.27) | 1600 (24.76) | |
| Smoking status | <0.001 | |||||
| Non-smokers | 20,556 (80.84) | 5552 (88.70) | 5390 (85.91) | 5113 (80.51) | 4501 (70.70) | |
| Smokers | 4031 (19.16) | 595 (11.30) | 756 (14.09) | 1035 (19.49) | 1645 (29.30) | |
| Physical activity | <0.001 | |||||
| Low | 11,005 (49.91) | 2821 (51.72) | 2852 (51.16) | 2777 (50.59) | 2555 (46.82) | |
| High | 9768 (50.09) | 2379 (48.28) | 2409 (48.84) | 2398 (49.41) | 2582 (53.18) | |
| Total energy intake, kcal/day | 2154.04 ± 961.75 | 1940.07 ± 863.30 | 2052.91 ± 848.71 | 2179.13 ± 938.42 | 2385.35 ± 1049.60 | <0.001 |
| Men, n | 10,419 | 2118 | 2269 | 2708 | 3324 | |
| %UPF in total food intake, median (IQR) | 16.21 (6.44, 30.85) | 2.02 (0.96, 3.23) | 7.75 (5.91, 9.46) | 17.03 (14.08, 20.24) | 37.35 (29.82, 48.03) | - |
| Age | 43.14 ± 14.48 | 48.52 ± 15.73 | 47.16 ± 15.15 | 43.11 ± 14.27 | 38.05 ± 12.02 | <0.001 |
| BMI | 24.61 ± 3.29 | 24.47 ± 3.22 | 24.64 ± 3.32 | 24.54 ± 3.15 | 24.73 ± 3.36 | 0.058 |
| Education | <0.001 | |||||
| Elementary school or lower | 1016 (6.34) | 278 (9.41) | 265 (7.39) | 269 (6.68) | 204 (3.89) | |
| Middle school | 772 (5.88) | 184 (6.92) | 219 (8.22) | 204 (5.80) | 165 (4.02) | |
| High school | 3597 (39.64) | 621 (33.79) | 736 (36.02) | 914 (39.03) | 1326 (45.27) | |
| College or higher | 4402 (48.13) | 896 (49.89) | 946 (48.37) | 1148 (48.49) | 1412 (46.82) | |
| Household income | 0.128 | |||||
| Low | 1421 (10.55) | 341 (12.17) | 339 (11.54) | 338 (9.58) | 403 (9.85) | |
| Lower middle | 2438 (22.62) | 509 (22.36) | 550 (22.39) | 642 (22.45) | 737 (23.01) | |
| Upper middle | 3087 (31.68) | 584 (29.46) | 650 (30.53) | 822 (32.76) | 1031 (32.71) | |
| High | 3440 (35.15) | 677 (36.01) | 723 (35.54) | 898 (35.21) | 1141 (34.43) | |
| Alcohol consumption | <0.001 | |||||
| At least once per month | 7286 (72.68) | 1292 (64.36) | 1472 (67.18) | 1894 (70.87) | 2628 (81.56) | |
| Less than once per month | 2890 (27.32) | 776 (35.64) | 760 (32.82) | 758 (29.13) | 596 (18.44) | |
| Smoking status | <0.001 | |||||
| Non-smokers | 7040 (66.05) | 1635 (75.64) | 1644 (71.77) | 1826 (66.11) | 1935 (57.73) | |
| Smokers | 3379 (33.95) | 483 (24.36) | 625 (28.23) | 882 (33.89) | 1389 (42.27) | |
| Physical activity | 0.034 | |||||
| Low | 4291 (46.14) | 884 (47.39) | 995 (47.74) | 1123 (47.30) | 1289 (43.68) | |
| High * | 4431 (53.86) | 8883 (52.61) | 950 (52.26) | 1128 (52.70) | 1470 (56.32) | |
| Total energy intake, kcal/day | 2538.94 ± 972.17 | 2282.26 ± 916.31 | 2435.22 ± 842.66 | 2534.70 ± 945.45 | 2735.15 ± 1029.32 | <0.001 |
| Women, n | 14,168 | 4029 | 3877 | 3440 | 2822 | |
| %UPF in total food intake, median (IQR) | 10.26 (3.97, 22.14) | 1.80 (0.87, 3.00) | 7.41 (5.86, 9.29) | 16.46 (13.76, 19.79) | 35.70 (29.01, 46.26) | - |
| Age | 44.116 ± 16.72 | 47.966 ± 16.65 | 46.44 ± 16.87 | 43.75 ± 16.91 | 37.30 ± 14.00 | <0.001 |
| BMI | 23.05 ± 3.90 | 23.02 ± 3.78 | 23.10 ± 3.74 | 23.13 ± 3.93 | 22.94 ± 4.10 | 0.614 |
| Education | <0.001 | |||||
| Elementary school or lower | 2150 (11.65) | 698 (13.73) | 666 (13.35) | 534 (11.73) | 252 (7.03) | |
| Middle school | 1090 (6.89) | 355 (7.91) | 320 (7.62) | 255 (6.61) | 160 (5.07) | |
| High school | 4612 (36.72) | 1237 (33.60) | 1232 (35.92) | 1072 (36.14) | 1071 (42.10) | |
| College or higher | 5653 (44.74) | 1563 (44.77) | 1477 (43.11) | 1404 (45.52) | 1209 (45.80) | |
| Household income | 0.009 | |||||
| Low | 2050 (12.20) | 635 (13.36) | 583 (12.63) | 508 (12.31) | 324 (10.14) | |
| Lower middle | 3444 923.98) | 925 (22.55) | 947 (23.74) | 832 (24.11) | 740 (25.85) | |
| Upper middle | 4099 (30.35) | 1166 (30.53) | 1087 (29.19) | 980 (30.24) | 866 (31.64) | |
| High | 4520 (33.47) | 1287 (33.57) | 1245 (34.43) | 1107 (33.34) | 881 (32.36) | |
| Alcohol consumption | <0.001 | |||||
| At least once per month | 6273 (48.28) | 1433 (38.85) | 1490 (42.75) | 1576 (48.59) | 1774 (65.91) | |
| Less than once per month | 7662 (51.72) | 2528 (61.15) | 2323 (57.25) | 1807 (51.41) | 1004 (34.09) | |
| Smoking status | <0.001 | |||||
| Non-smokers | 13,516 (94.75) | 3917 (97.09) | 3746 (96.11) | 3287 (95.01) | 2566 (90.04) | |
| Smokers | 652 (5.25) | 112 (2.91) | 131 (3.90) | 153 (4.99) | 256 (9.96) | |
| Physical activity | 0.246 | |||||
| Low | 6714 (53.40) | 1937 (54.41) | 1857 (53.62) | 1654 (53.83) | 1266 (51.43) | |
| High | 5337 (46.60) | 1496 (45.59) | 1459 (46.38) | 1270 (46.17) | 1112 (48.57) | |
| Total energy intake, kcal/day | 1791.90 ± 753.35 | 1720.30 ± 716.02 | 1777.29 ± 711.02 | 1821.27 ± 740.58 | 1863.67 ± 827.36 | <0.001 |
| Quartile of %Total Food Intake from UPF | p for Trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| All | 6147 | 6146 | 6148 | 6146 | |
| No. of cases (%) | 1122 (19.12) | 1260 (21.44) | 1302 (22.39) | 1440 (24.11) | |
| Unadjusted | Ref | 1.15 (1.04, 1.28) | 1.22 (1.10, 1.36) | 1.34 (1.21, 1.49) | <0.001 |
| Age, sex adjusted | Ref | 1.13 (1.02, 1.26) | 1.13 (1.02, 1.26) | 1.16 (1.04, 1.30) | 0.012 |
| Multivariable adjusted ** | Ref | 1.17 (1.04, 1.31) | 1.18 (1.05, 1.33) | 1.24 (1.10, 1.41) | 0.001 |
| Men | 2118 | 2269 | 2708 | 3324 | |
| No. of cases (%) | 471 (25.06) | 593 (28.33) | 685 (27.46) | 934 (28.83) | |
| Unadjusted | Ref | 1.18 (1.01, 1.39) | 1.13 (0.97, 1.32) | 1.21 (1.05, 1.40) | 0.032 |
| Age adjusted | Ref | 1.16 (0.98, 1.36) | 1.02 (0.87, 1.19) | 0.99 (0.85, 1.15) | 0.383 |
| Multivariable adjusted * | Ref | 1.18 (0.99, 1.42) | 1.08 (0.91, 1.29) | 1.06 (0.89, 1.26) | 0.943 |
| Women | 4029 | 3877 | 3440 | 2822 | |
| No. of cases (%) | 651 (15.31) | 667 (16.47) | 617 (17.29) | 506 (17.09) | |
| Unadjusted | Ref | 1.09 (0.95, 1.25) | 1.16 (1.00, 1.34) | 1.14 (0.98, 1.32) | 0.054 |
| Age adjusted | Ref | 1.13 (0.98, 1.29) | 1.26 (1.09, 1.46) | 1.44 (1.23, 1.68) | <0.001 |
| Multivariable adjusted * | Ref | 1.15 (0.99, 1.34) | 1.29 (1.09, 1.51) | 1.52 (1.28, 1.81) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwan, B.S.; Ko, N.G.; Park, J.E. Sex Differences in the Association Between Ultra-Processed Food Consumption and NAFLD: An Analysis of KNHANES 2013–2021 Data. J. Clin. Med. 2025, 14, 7930. https://doi.org/10.3390/jcm14227930
Kwan BS, Ko NG, Park JE. Sex Differences in the Association Between Ultra-Processed Food Consumption and NAFLD: An Analysis of KNHANES 2013–2021 Data. Journal of Clinical Medicine. 2025; 14(22):7930. https://doi.org/10.3390/jcm14227930
Chicago/Turabian StyleKwan, Byung Soo, Nak Gyeong Ko, and Ji Eun Park. 2025. "Sex Differences in the Association Between Ultra-Processed Food Consumption and NAFLD: An Analysis of KNHANES 2013–2021 Data" Journal of Clinical Medicine 14, no. 22: 7930. https://doi.org/10.3390/jcm14227930
APA StyleKwan, B. S., Ko, N. G., & Park, J. E. (2025). Sex Differences in the Association Between Ultra-Processed Food Consumption and NAFLD: An Analysis of KNHANES 2013–2021 Data. Journal of Clinical Medicine, 14(22), 7930. https://doi.org/10.3390/jcm14227930







