Assessment of Health-Related Quality of Life and Biomarkers in Long COVID: A 12-Month Longitudinal Feasibility Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Approval
2.2. Study Population
2.3. Data Collection and Assessments
2.4. Blood Tests
2.4.1. RNA Extraction
2.4.2. cDNA Synthesis
2.4.3. qPCR Analysis
2.5. Quality of Life and Joint Pain Assessment
2.6. Statistical Analysis
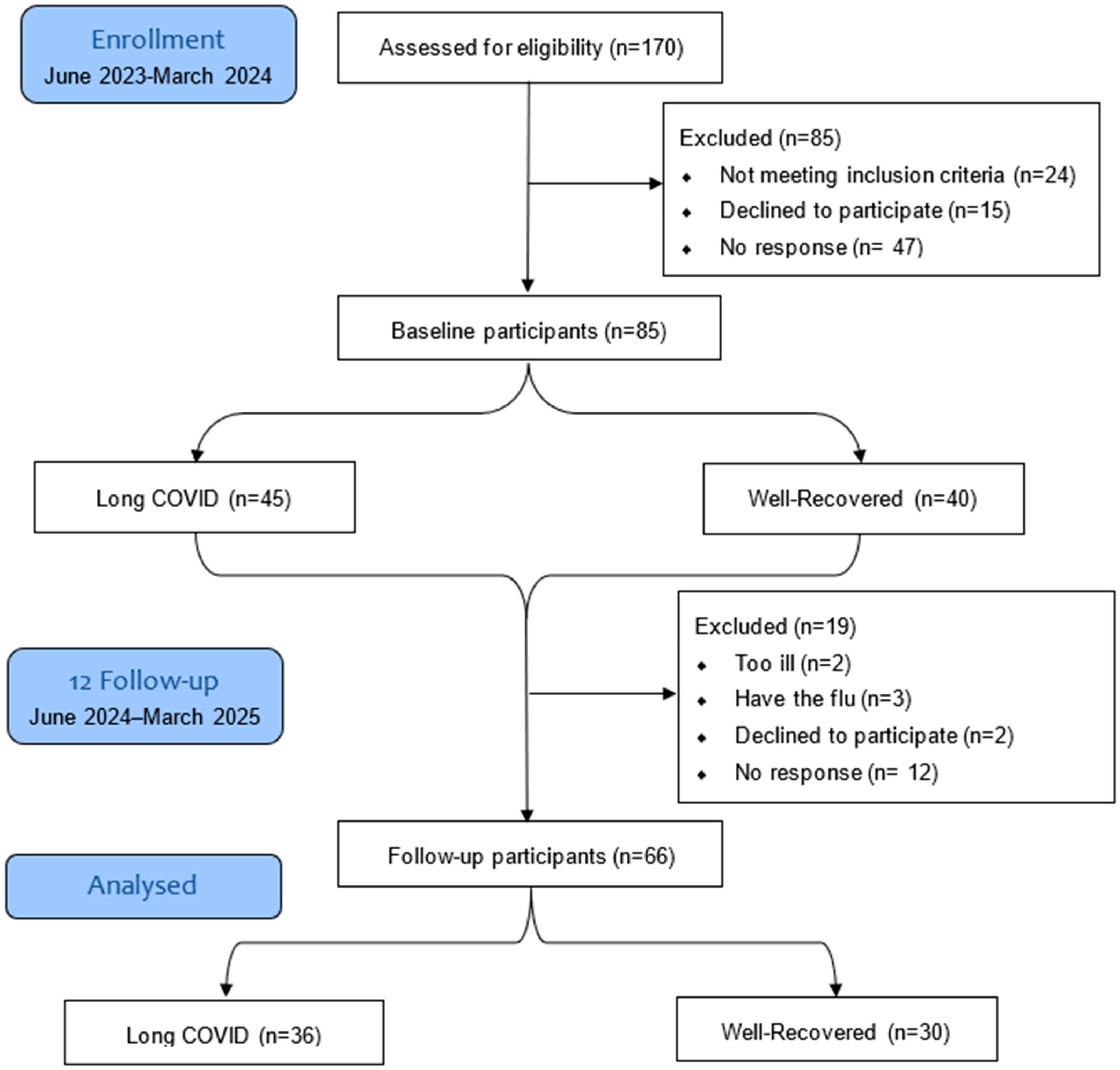
3. Results
3.1. Demographics and Characteristics
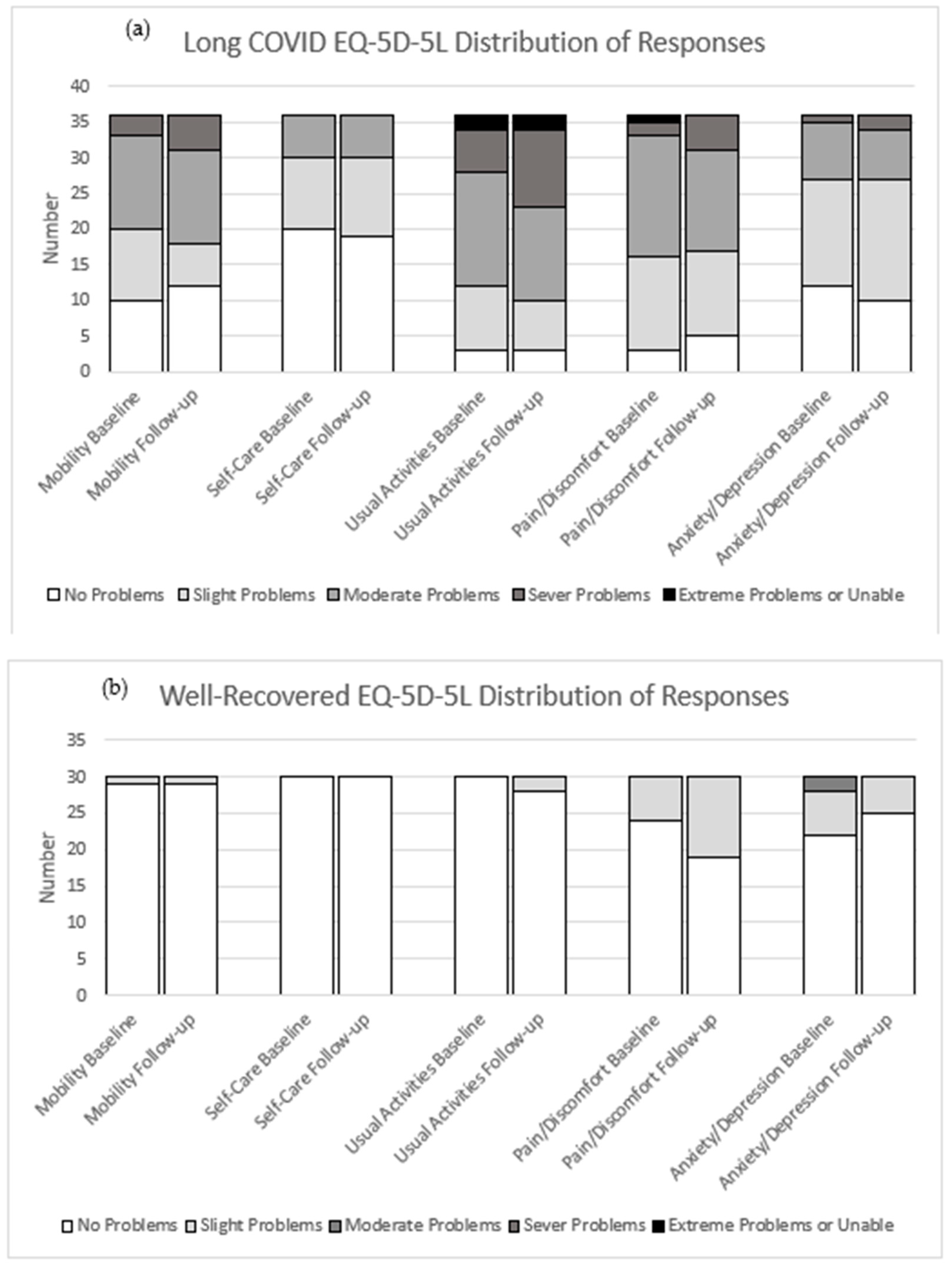
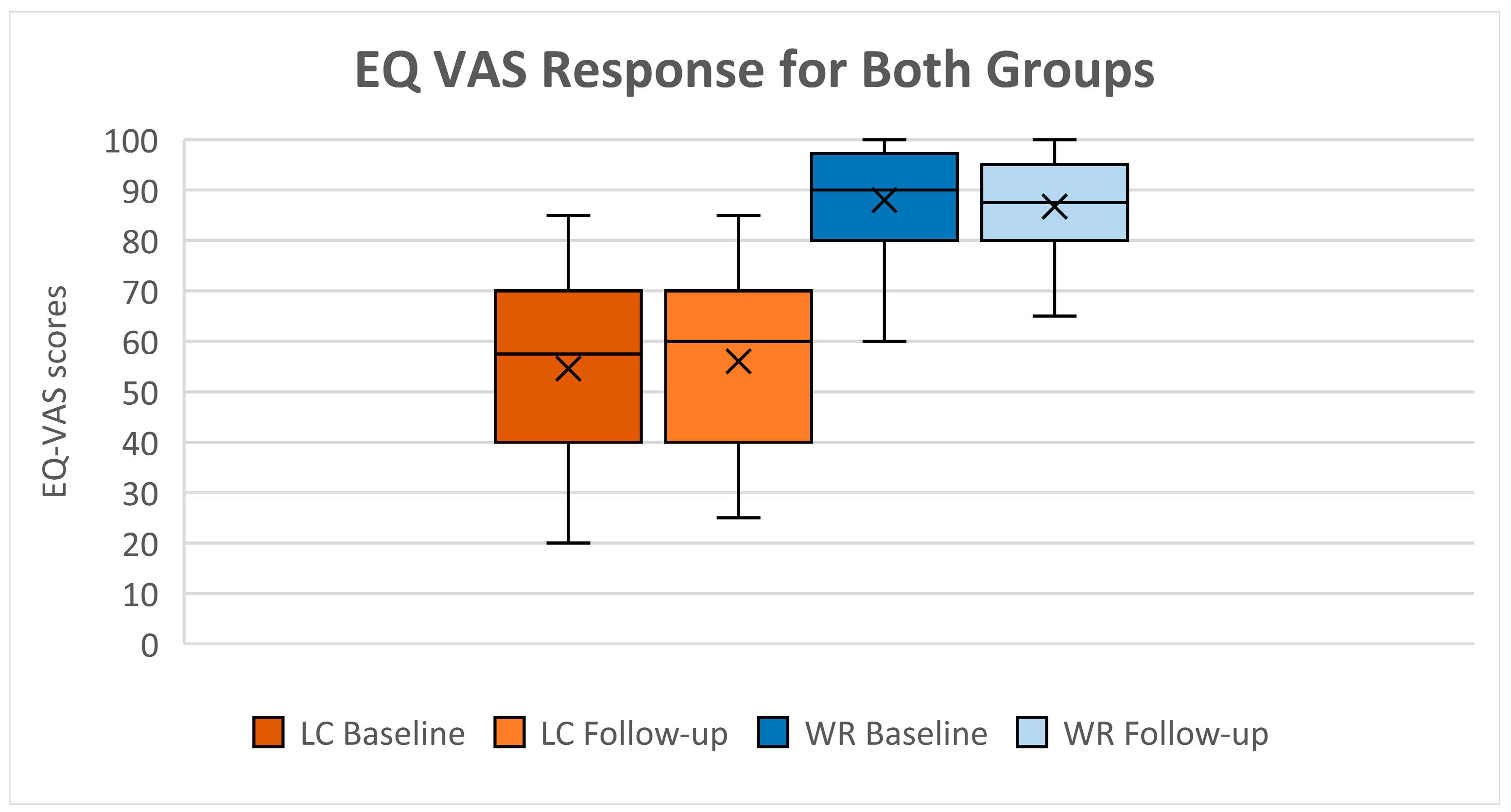
3.2. Health-Related Quality of Life (HRQoL)
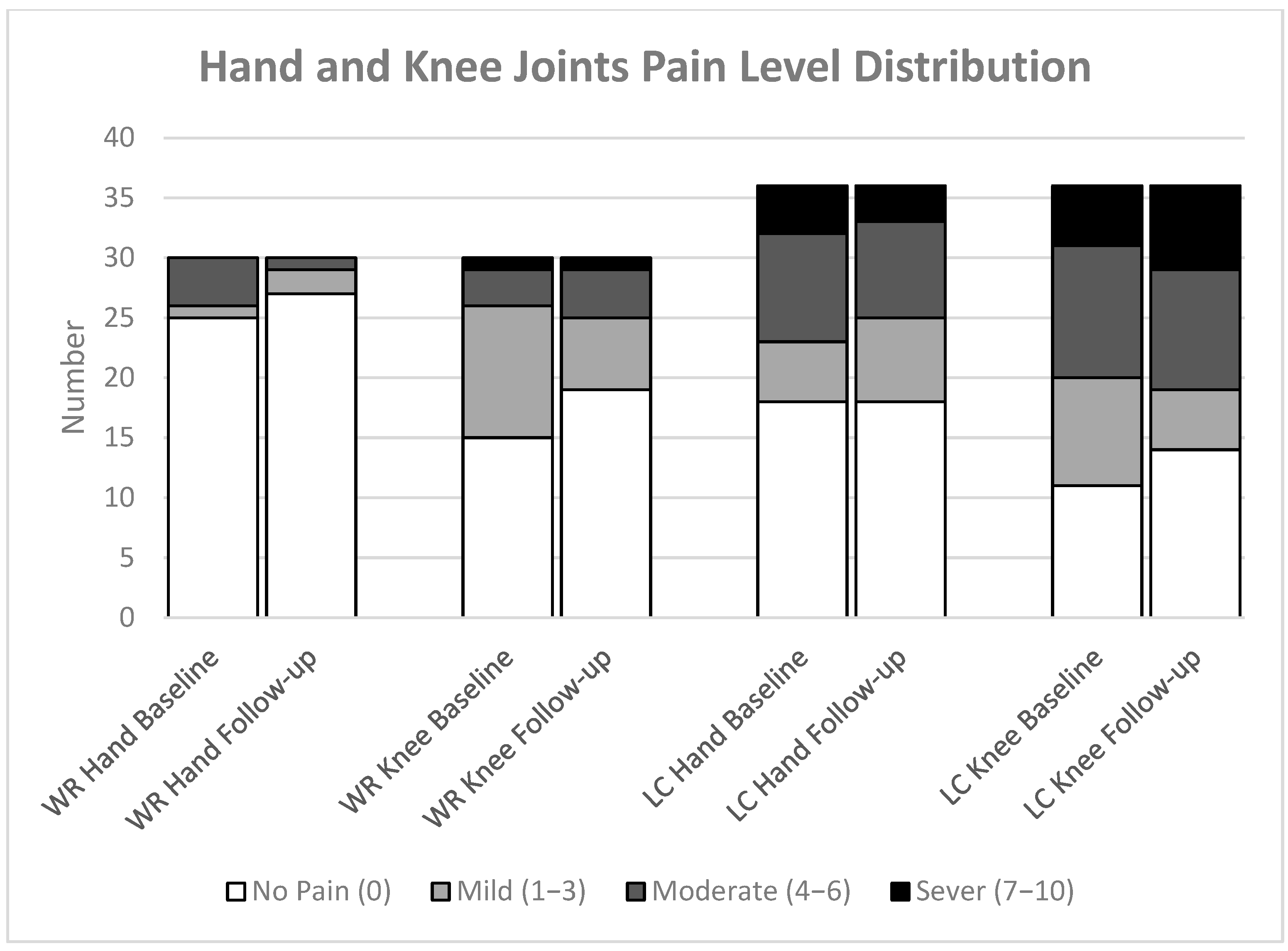
3.3. Joint Pain
3.4. Blood Analysis
4. Discussion
4.1. Long COVID Associated with Deterioration of Health-Related Quality of Life
4.2. No Association of Bone Turnover Markers in Long COVID
4.3. No Association of Inflammatory Markers in Long COVID
4.4. Vitamin D Level Initially High in Long COVID and Improved in Both Groups During 12 Months
4.5. Long COVID Linked to Persistent Joint Pain Within 12 Months
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BTM | Bone turnover markers |
| CTX | C-Terminal Telopeptide of Type 1 Collagen |
| EQ-5D-5L | EuroQol 5-Dimension 5-Level |
| HRQoL | Health-related quality of life |
| HRT | Hormone replacement therapy |
| IL | Interleukins |
| INF-γ | Interferon-Gamma |
| MSK | Musculoskeletal |
| PINP | Procollagen Type I N Propeptide |
| TNF-α | Tumour Necrosis Factor-Alpha |
| VAS | Visual analogue scale |
References
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic strategies for COVID-19: Progress and lessons learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Coronavirus Disease (COVID-19). Available online: https://www.who.int/health-topics/coronavirus#tab=tab_1 (accessed on 27 October 2023).
- Mahase, E. COVID-19: What do we know about “long covid”? BMJ 2020, 370, m2815. [Google Scholar] [CrossRef] [PubMed]
- Callard, F.; Perego, E. How and why patients made Long COVID. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Signs and Symptoms of Long COVID. Available online: https://www.cdc.gov/covid/long-term-effects/long-covid-signs-symptoms.html (accessed on 2 December 2024).
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; dos Santos Freitas, A.; Ribeiro da Silveira, P.; Tiwari, S.; Alzahrani, K.J.; Góes-Neto, A. Long-COVID and post-COVID health complications: An up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses 2021, 13, 700. [Google Scholar] [CrossRef]
- Picone, P.; Sanfilippo, T.; Guggino, R.; Scalisi, L.; Monastero, R.; Baschi, R.; Mandalà, V.; San Biagio, L.; Rizzo, M.; Giacomazza, D. Neurological consequences, mental health, physical care, and appropriate nutrition in long-COVID-19. Cell. Mol. Neurobiol. 2023, 43, 1685–1695. [Google Scholar] [CrossRef]
- Natarajan, A.; Shetty, A.; Delanerolle, G.; Zeng, Y.; Zhang, Y.; Raymont, V.; Rathod, S.; Halabi, S.; Elliot, K.; Shi, J.Q. A systematic review and meta-analysis of long COVID symptoms. Syst. Rev. 2023, 12, 88. [Google Scholar] [CrossRef]
- Nabavi, N. Long covid: How to define it and how to manage it. BMJ 2020, 370, m3489. [Google Scholar] [CrossRef]
- Bakılan, F.; Gökmen, İ.G.; Ortanca, B.; Uçan, A.; Eker Güvenç, Ş.; Şahin Mutlu, F.; Gökmen, H.M.; Ekim, A. Musculoskeletal symptoms and related factors in postacute COVID-19 patients. Int. J. Clin. Pract. 2021, 75, e14734. [Google Scholar] [CrossRef]
- Karaarslan, F.; Demircioğlu Güneri, F.; Kardeş, S. Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: Prospective follow-up by phone interviews. Rheumatol. Int. 2021, 41, 1263–1271. [Google Scholar] [CrossRef]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef]
- Karaarslan, F.; Guneri, F.D.; Kardes, S. Long COVID: Rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin. Rheumatol. 2022, 41, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Peghin, M.; Palese, A.; Venturini, M.; De Martino, M.; Gerussi, V.; Graziano, E.; Bontempo, G.; Marrella, F.; Tommasini, A.; Fabris, M. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin. Microbiol. Infect. 2021, 27, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, J.; Piroth, L.; Epaulard, O.; Le Turnier, P.; Mentré, F.; Bachelet, D.; Laouénan, C. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: Results from a large prospective cohort. Clin. Microbiol. Infect. 2021, 27, 1041.e1–1041.e4. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Jain, V.K.; Iyengar, K.P. Musculoskeletal manifestations of COVID-19. J. Clin. Orthop. Trauma 2021, 17, 280–281. [Google Scholar] [CrossRef]
- di Filippo, L.; Frara, S.; Nannipieri, F.; Cotellessa, A.; Locatelli, M.; Rovere Querini, P.; Giustina, A. Low Vitamin D Levels Are Associated with Long COVID Syndrome in COVID-19 Survivors. J. Clin. Endocrinol. Metab. 2023, 108, e1106–e1116. [Google Scholar] [CrossRef]
- Charoenporn, V.; Charernboon, T. Prevalence of vitamin D deficiency in patients with fatigue and neuropsychiatric symptoms of long COVID and its correlation with symptom severity. Curr. Nutr. Food Sci. 2025, 21, 501–507. [Google Scholar] [CrossRef]
- Matangkha, K.; Punyahotara, V.; Rintra, J.; Sittiprapaporn, P. Association Between Vitamin D Levels and Long COVID Signs and Symptoms. Med. Sci. 2025, 13, 199. [Google Scholar] [CrossRef]
- Gulzar, R.; Rasheed, A.; Riaz, S.; Adnan, W.A.; Hafeez, U.; Malik, A.M. Musculoskeletal Symptoms in Patients Recovering from COVID-19. Muscles Ligaments Tendons J. 2022, 12, 9–16. [Google Scholar] [CrossRef]
- Lai, Y.-J.; Liu, S.-H.; Manachevakul, S.; Lee, T.-A.; Kuo, C.-T.; Bello, D. Biomarkers in long COVID-19: A systematic review. Front. Med. 2023, 10, 1085988. [Google Scholar] [CrossRef]
- Espín, E.; Yang, C.; Shannon, C.P.; Assadian, S.; He, D.; Tebbutt, S.J. Cellular and molecular biomarkers of long COVID: A scoping review. Ebiomedicine 2023, 91, 104552. [Google Scholar] [CrossRef]
- Ciaffi, J.; Vanni, E.; Mancarella, L.; Brusi, V.; Lisi, L.; Pignatti, F.; Naldi, S.; Assirelli, E.; Neri, S.; Reta, M. Post-acute COVID-19 joint pain and new onset of rheumatic musculoskeletal diseases: A systematic review. Diagnostics 2023, 13, 1850. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Long-Term Effects of Coronavirus (Long COVID): What Is It? Available online: https://cks.nice.org.uk/topics/long-term-effects-of-coronavirus-long-covid/background-information/definition/ (accessed on 9 September).
- Arostegui, D.; Castro, K.; Schwarz, S.; Vaidy, K.; Rabinowitz, S.; Wallach, T. Persistent SARS-CoV-2 Nucleocapsid Protein Presence in the Intestinal Epithelium of a Pediatric Patient 3 Months After Acute Infection. JPGN Rep. 2022, 3, e152. [Google Scholar] [CrossRef]
- Lin, S. Using Genomic Approaches to Characterise the Immune Responses to Biologicals. Ph.D. Thesis, University of Exeter, Exeter, UK, 2023. [Google Scholar]
- Yang, C.; Zhao, H.; Espín, E.; Tebbutt, S.J. Association of SARS-CoV-2 infection and persistence with long COVID. Lancet Respir. Med. 2023, 11, 504–506. [Google Scholar] [CrossRef]
- Beck, G.R., Jr.; Ha, S.-W.; Camalier, C.E.; Yamaguchi, M.; Li, Y.; Lee, J.-K.; Weitzmann, M.N. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Guidarelli, G.; Ostan, R.; Giampieri, E.; Fabbri, C.; Bertarelli, C.; Nicoletti, C.; Kadi, F.; De Groot, L.C.; Feskens, E. Gender-specific association of body composition with inflammatory and adipose-related markers in healthy elderly Europeans from the NU-AGE study. Eur. Radiol. 2019, 29, 4968–4979. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, Y. The role of the immune microenvironment in bone regeneration. Int. J. Med. Sci. 2021, 18, 3697. [Google Scholar] [CrossRef]
- Vasikaran, S.; Cooper, C.; Eastell, R.; Griesmacher, A.; Morris, H.A.; Trenti, T.; Kanis, J.A. International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clin. Chem. Lab. Med. 2011, 49, 1271–1274. [Google Scholar] [CrossRef]
- Eastell, R.; Szulc, P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017, 5, 908–923. [Google Scholar] [CrossRef]
- Kruger, M.C.; Schollum, L.M.; Kuhn-Sherlock, B.; Hestiantoro, A.; Wijanto, P.; Li-Yu, J.; Agdeppa, I.; Todd, J.M.; Eastell, R. The effect of a fortified milk drink on vitamin D status and bone turnover in post-menopausal women from South East Asia. Bone 2010, 46, 759–767. [Google Scholar] [CrossRef]
- Ferrar, L.; Van Der Hee, R.; Berry, M.; Watson, C.; Miret, S.; Wilkinson, J.; Bradburn, M.; Eastell, R. Effects of calcium-fortified ice cream on markers of bone health. Osteoporos. Int. 2011, 22, 2721–2731. [Google Scholar] [CrossRef] [PubMed]
- Thermo Fisher Scientific Inc. Blood Plasma and Serum Preparation. Available online: https://www.thermofisher.com/uk/en/home/references/protocols/cell-and-tissue-analysis/elisa-protocol/elisa-sample-preparation-protocols/blood-plasma-serum-preparation.html (accessed on 5 November 2025).
- Fisher, A.; Srikusalanukul, W.; Fisher, L.; Smith, P.N. Lower serum P1NP/βCTX ratio and hypoalbuminemia are independently associated with osteoporotic nonvertebral fractures in older adults. Clin. Interv. Aging 2017, 2017, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.; Fisher, L.; Srikusalanukul, W.; Smith, P.N. Bone turnover status: Classification model and clinical implications. Int. J. Med. Sci. 2018, 15, 323. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Bramwell, L.R.; Jeffery, N.; Bunce, B.; Lee, B.P.; Knight, B.; Auckland, C.; Masoli, J.A.; Harries, L.W. Persistence of clinically relevant levels of SARS-CoV2 envelope gene subgenomic RNAs in non-immunocompromised individuals. Int. J. Infect. Dis. 2022, 116, 418–425. [Google Scholar] [CrossRef]
- Foundation, E.R. EQ-5D-3L User Guide. 2018. Available online: https://euroqol.org (accessed on 18 December 2018).
- Devlin, N.J.; Shah, K.K.; Feng, Y.; Mulhern, B.; Van Hout, B. Valuing health-related quality of life: An EQ-5 D-5 L value set for England. Health Econ. 2018, 27, 7–22. [Google Scholar] [CrossRef]
- EuroQol. Generic Health-Related Quality of Life (HRQOL). Available online: https://euroqol.org/information-and-support/resources/value-sets/ (accessed on 19 May 2025).
- National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Managing the Longterm Effects of COVID-19. Evidence Tables. Available online: https://www.nice.org.uk/guidance/ng188/evidence/evidence-review-a-risk-factors-pdf-13307838589 (accessed on 15 March 2022).
- Lam, C.L.K.; Tse, E.T.Y.; Wong, C.K.H.; Lam, J.S.M.; Chen, S.S.; Bedford, L.E.; Cheung, J.P.Y.; Or, C.K.; Kind, P. A pilot study on the validity and psychometric properties of the electronic EQ-5D-5L in routine clinical practice. Health Qual. Life Outcomes 2021, 19, 266. [Google Scholar] [CrossRef]
- Tran, B.X.; Ohinmaa, A.; Nguyen, L.T. Quality of life profile and psychometric properties of the EQ-5D-5L in HIV/AIDS patients. Health Qual. Life Outcomes 2012, 10, 132. [Google Scholar] [CrossRef]
- Feng, Y.-S.; Kohlmann, T.; Janssen, M.F.; Buchholz, I. Psychometric properties of the EQ-5D-5L: A systematic review of the literature. Qual. Life Res. 2021, 30, 647–673. [Google Scholar] [CrossRef]
- Long, D.; Polinder, S.; Bonsel, G.J.; Haagsma, J.A. Test-retest reliability of the EQ-5D-5L and the reworded QOLIBRI-OS in the general population of Italy, the Netherlands, and the United Kingdom. Qual. Life Res. 2021, 30, 2961–2971. [Google Scholar] [CrossRef]
- The British Pain Society. Outcome Measures. Available online: https://www.britishpainsociety.org/static/uploads/resources/files/Outcome_Measures_January_2019.pdf (accessed on 25 October 2025).
- Alghadir, A.H.; Anwer, S.; Iqbal, A.; Iqbal, Z.A. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J. Pain. Res. 2018, 11, 851–856. [Google Scholar] [CrossRef]
- Leung, J.L.; Twohig, H.; Muller, S.; Maxwell, L.; Mackie, S.L.; Neill, L.M.; Owen, C.E. Test-retest reliability of pain VAS/NRS, stiffness VAS/NRS, HAQ-DI and mHAQ in polymyalgia rheumatica: An OMERACT study. Semin. Arthritis Rheum. 2023, 62, 152239. [Google Scholar] [CrossRef]
- Armitage, P.; Berry, G.; Matthews, J. Statistical Methods in Medical Research; John Wiley & Sons, Inc.: New York, NY, USA, 1971; pp. 362–365. [Google Scholar]
- McHugh, M.L. The chi-square test of independence. Biochem. Medica 2013, 23, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor. Dent. Endod. 2017, 42, 152. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, F.; Mokbel, K.; Meertens, R.; Obotiba, A.D.; Alharbi, M.; Knapp, K.M.; Strain, W.D. Bone Mineral Density, Bone Biomarkers, and Joints in Acute, Post, and Long COVID-19: A Systematic Review. Viruses 2024, 16, 1694. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.W.; Gong, C.L.; Jiao, X.; Zawadzki, N.K.; Zawadzki, R.S.; Pickard, A.S.; Xie, F.; Crawford, S.A.; Gu, N.Y. A US population health survey on the impact of COVID-19 using the EQ-5D-5L. J. Gen. Intern. Med. 2021, 36, 1292–1301. [Google Scholar] [CrossRef]
- Wright, J.; Astill, S.L.; Sivan, M. The Relationship between Physical Activity and Long COVID: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 5093. [Google Scholar] [CrossRef]
- Carlile, O.; Briggs, A.; Henderson, A.D.; Butler-Cole, B.F.; Tazare, J.; Tomlinson, L.A.; Marks, M.; Jit, M.; Lin, L.-Y.; Bates, C. Impact of long COVID on health-related quality-of-life: An OpenSAFELY population cohort study using patient-reported outcome measures (OpenPROMPT). Lancet Reg. Health–Eur. 2024, 40, 100908. [Google Scholar] [CrossRef]
- Moens, M.; Duarte, R.V.; De Smedt, A.; Putman, K.; Callens, J.; Billot, M.; Roulaud, M.; Rigoard, P.; Goudman, L. Health-related quality of life in persons post-COVID-19 infection in comparison to normative controls and chronic pain patients. Front. Public Health 2022, 10, 991572. [Google Scholar] [CrossRef]
- Shanbehzadeh, S.; Zanjari, N.; Yassin, M.; Yassin, Z.; Tavahomi, M. Association between long COVID, functional activity, and health-related quality of life in older adults. BMC Geriatr. 2023, 23, 40. [Google Scholar] [CrossRef]
- Oliver-Mas, S.; Matias-Guiu, J.A.; Delgado-Alonso, C.; Cuevas, C.; Alcalá Ramírez del Puerto, J.M.; López-Carbonero, J.I.; Matias-Guiu, J.; Diez-Cirarda, M. Differential Fatigue Profile in Patients with Post-COVID Condition, Fibromyalgia, and Multiple Sclerosis. J. Clin. Med. 2025, 14, 952. [Google Scholar] [CrossRef]
- Diez-Cirarda, M.; Yus-Fuertes, M.; Polidura, C.; Gil-Martinez, L.; Delgado-Alonso, C.; Delgado-Alvarez, A.; Gomez-Ruiz, N.; Gil-Moreno, M.J.; Jorquera, M.; Oliver-Mas, S. Neural basis of fatigue in post-COVID syndrome and relationships with cognitive complaints and cognition. Psychiatry Res. 2024, 340, 116113. [Google Scholar] [CrossRef]
- Haudenschild, A.K.; Christiansen, B.A.; Orr, S.; Ball, E.E.; Weiss, C.M.; Liu, H.; Fyhrie, D.P.; Yik, J.H.; Coffey, L.L.; Haudenschild, D.R. Acute bone loss following SARS-CoV-2 infection in mice. J. Orthop. Res. 2023, 41, 1945–1952. [Google Scholar] [CrossRef]
- Qiao, W.; Lau, H.E.; Xie, H.; Poon, V.K.-M.; Chan, C.C.-S.; Chu, H.; Yuan, S.; Yuen, T.T.-T.; Chik, K.K.-H.; Tsang, J.O.-L. SARS-CoV-2 infection induces inflammatory bone loss in golden Syrian hamsters. Nat. Commun. 2022, 13, 2539. [Google Scholar] [CrossRef] [PubMed]
- Awosanya, O.D.; Dalloul, C.E.; Blosser, R.J.; Dadwal, U.C.; Carozza, M.; Boschen, K.; Klemsz, M.J.; Johnston, N.A.; Bruzzaniti, A.; Robinson, C.M. Osteoclast-mediated bone loss observed in a COVID-19 mouse model. Bone 2022, 154, 116227. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Junior, C.M.; Santos, A.C.; Gonçalves, M.R.; Brito, C.B.; Barrioni, B.; Almeida, P.J.; Gonçalves-Pereira, M.H.; Silva, T.; Oliveira, S.R.; Pereira, M.M. Acute coronavirus infection triggers a TNF-dependent osteoporotic phenotype in mice. Life Sci. 2023, 324, 121750. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Morrone, M.C.; Patrono, C.; Santoro, M.G.; Schiaffino, S.; Remuzzi, G.; Bussolati, G.; Cappuccinelli, P.; Fitzgerald, G.; Bacci, M.L.; et al. Long Covid: Where we stand and challenges ahead. Cell Death Differ. 2022, 29, 1891–1900. [Google Scholar] [CrossRef]
- Arrieta, F.; Martinez-Vaello, V.; Bengoa, N.; Rosillo, M.; de Pablo, A.; Voguel, C.; Pintor, R.; Belanger-Quintana, A.; Mateo-Lobo, R.; Candela, A. Stress hyperglycemia and osteocalcin in COVID-19 critically ill patients on artificial nutrition. Nutrients 2021, 13, 3010. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Wang, H.; Gao, Y.; Hu, X.; Li, X.; Zhang, S.; Xu, Y.; Wei, W. Characteristics of laboratory indexes in COVID-19 patients with non-severe symptoms in Hefei City, China: Diagnostic value in organ injuries. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2447–2455. [Google Scholar] [CrossRef]
- Hu, M.; Song, T.; Gong, Z.; Che, Q.; Guo, J.; Chen, L.; Zhang, H.; Li, H.; Liang, N.; Zhao, G. Symptom Trajectories and Clinical Subtypes in Post–COVID-19 Condition: Systematic Review and Clustering Analysis. JMIR Public Health Surveill. 2025, 11, e72221. [Google Scholar] [CrossRef]
- Demko, Z.O.; Yu, T.; Mullapudi, S.K.; Varela Heslin, M.G.; Dorsey, C.A.; Payton, C.B.; Tornheim, J.A.; Blair, P.W.; Mehta, S.H.; Thomas, D.L. Two-year longitudinal study reveals that long COVID symptoms peak and quality of life nadirs at 6–12 months postinfection. Open Forum Infect. Dis. 2024, 11, ofae027. [Google Scholar] [CrossRef]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; Offringa, C.; Bloemers, F.W.; van Weeghel, M.; Schomakers, B.V.; et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat. Commun. 2024, 15, 17. [Google Scholar] [CrossRef]
- Charlton, B.T.; Goulding, R.P.; Jaspers, R.T.; Appelman, B.; van Vugt, M.; Wüst, R.C.I. Skeletal muscle adaptations and post-exertional malaise in long COVID. Trends Endocrinol. Metab. 2025, 36, 614–622. [Google Scholar] [CrossRef]
- Marques, K.C.; Quaresma, J.A.S.; Falcão, L.F.M. Cardiovascular autonomic dysfunction in “Long COVID”: Pathophysiology, heart rate variability, and inflammatory markers. Front. Cardiovasc. Med. 2023, 10, 1256512. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, T.; Kanerva, M.; Luukkonen, R.; Lantto, H.; Uusitalo, A.; Piirilä, P. Cardiopulmonary exercise testing in long covid shows the presence of dysautonomia or chronotropic incompetence independent of subjective exercise intolerance and fatigue. BMC Cardiovasc. Disord. 2024, 24, 413. [Google Scholar] [CrossRef] [PubMed]
- Astin, R.; Banerjee, A.; Baker, M.R.; Dani, M.; Ford, E.; Hull, J.H.; Lim, P.B.; McNarry, M.; Morten, K.; O’Sullivan, O. Long COVID: Mechanisms, risk factors and recovery. Exp. Physiol. 2023, 108, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Christodoulatos, G.S.; Papavasileiou, G.; Petropoulou, D.; Magkos, F.; Dalamaga, M. Laboratory Findings and Biomarkers in Long COVID: What Do We Know So Far? Insights into Epidemiology, Pathogenesis, Therapeutic Perspectives and Challenges. Int. J. Mol. Sci. 2023, 24, 10458. [Google Scholar] [CrossRef]
- Pasculli, P.; Zingaropoli, M.A.; Dominelli, F.; Solimini, A.G.; Masci, G.M.; Birtolo, L.I.; Pasquariello, L.; Paribeni, F.; Iafrate, F.; Panebianco, V.; et al. Insights into Long COVID: Unraveling Risk Factors, Clinical Features, Radiological Findings, Functional Sequelae and Correlations: A Retrospective Cohort Study. Am. J. Med. 2025, 138, 721–731. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A helpful immuno-modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef]
- Greiller, C.L.; Martineau, A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef]
- Laird, E.; Rhodes, J.; Kenny, R.A. Vitamin D and inflammation: Potential implications for severity of Covid-19. Ir. Med. J. 2020, 113, 81. [Google Scholar]
- Selmi, C.; Gershwin, M.E. Diagnosis and classification of reactive arthritis. Autoimmun. Rev. 2014, 13, 546–549. [Google Scholar] [CrossRef]
- Siva, C.; Velazquez, C.; Mody, A.; Brasington, R. Diagnosing acute monoarthritis in adults: A practical approach for the family physician. Am. Fam. Physician 2003, 68, 83–90. [Google Scholar]
- Parisi, S.; Borrelli, R.; Bianchi, S.; Fusaro, E. Viral arthritis and COVID-19. Lancet Rheumatol. 2020, 2, e655–e657. [Google Scholar] [CrossRef]
- Vassilopoulos, D.; Calabrese, L.H. Virally associated arthritis 2008: Clinical, epidemiologic, and pathophysiologic considerations. Arthritis Res. Ther. 2008, 10, 215. [Google Scholar] [CrossRef]
- Baimukhamedov, C.; Barskova, T.; Matucci-Cerinic, M. Arthritis after SARS-CoV-2 infection. Lancet Rheumatol. 2021, 3, e324–e325. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.F. Musculoskeletal complications of severe acute respiratory syndrome. Semin. Musculoskelet. Radiol. 2011, 15, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, N.; Minematsu, N.; Katano, H.; Suzuki, T. Case of acute arthritis following SARS-CoV-2 infection. Ann. Rheum. Dis. 2021, 80, e101. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Kishimoto, M.; Shimasaki, T.; Uchida, H.; Kurai, D.; Deshpande, G.A.; Komagata, Y.; Kaname, S. Reactive arthritis after COVID-19 infection. RMD Open 2020, 6, e001350. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19 (NG188): Management. Available online: https://www.nice.org.uk/guidance/ng188/chapter/5-Management (accessed on 30 October 2025).
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Adults aged ≥18 years of any gender and ethnicity. | Participants who had hospitalisation due to COVID-19 requiring intubation, ICU admission, or ventilatory support (to exclude post-intensive care syndrome). |
| WR participants with a history of SARS-CoV-2 infection confirmed via RT-PCR or antigen testing. | Individuals with pre-existing osteoporosis or metabolic bone diseases (e.g., primary hyperparathyroidism, osteogenesis imperfecta). |
| LC participants met the WHO and NICE definitions of long COVID or had a confirmed diagnosis of LC. | Those undergoing long-term corticosteroid therapy (≥5 mg prednisolone daily) or taking bisphosphonates, denosumab, or teriparatide. |
| Pregnant or breastfeeding women, due to the use of ionising radiation in DXA scans. | |
| Participants with recent fractures (<12 months) or conditions affecting joint health, such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE). |
| Participant Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Baseline | Follow-up | ||||||
| WR (n = 40) | LC (n = 45) | Test Value | p | WR (n = 30) | LC (n = 36) | Test Value | p | |
| Age (yr) (₽) | 51 ± 15.17 | 52.22 ± 9.94 | t (83) = −0.444 | 0.658 | 52.83 ± 14.85 | 53.28 ± 10.08 | t (64) = −0.144 | 0.885 |
| Gender (Female), n (%) (X) | 19 (47) | 38 (84.45) | X2 (1) = 13.084 | <0.001 * | 15 (50) | 29 (80.56) | X2 (1) = 6.875 | 0.009 * |
| BMI (kg/m2) (‡) | 26.6 (23.8; 30.65) | 27.9 (24.7; 33) | z = −1.242 | 0.214 | 25.55 (23.4; 29.3) | 28.8 (23.8; 34.45) | z = −1.707 | 0.087 |
| Ethnicity, n (%) (¥) | 0.459 | 1.00 | ||||||
| White or not stated | 39 (97.5) | 41 (91.1) | 30 (100) | 33 (91.7) | ||||
| Indian | 0 (0.0) | 2 (4.4) | 0 (0.0) | 1 (2.8) | ||||
| Pakistani | 0 (0.0) | 1 (2.2) | 0 (0.0) | 1 (2.8) | ||||
| Black African | 1 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Chinese | 0 (0.0) | 1 (2.22) | 0 (0.0) | 1 (2.8) | ||||
| Socio-economic, n (%) (X) | X2 (2) = 2.649 | 0.266 | X2 (2) = 1.602 | 0.449 | ||||
| Upper | 20 (50) | 23 (51.1) | 15 (50) | 18 (50) | ||||
| Upper Middle | 13 (32.5) | 19 (42.2) | 12 (40) | 17 (47.2) | ||||
| Lower Middle | 7 (17.5) | 3 (6.7) | 3 (10) | 1 (1.8) | ||||
| Smoking status, n (%) (¥) | 0.298 | 0.742 | ||||||
| Non-smoker | 26 (65) | 35 (77.8) | 21 (70) | 26 (72.2) | ||||
| Ex-smoker | 8 (20) | 8 (17.8) | 4 (13.3) | 6 (16.7) | ||||
| Light smoker (less than 10) | 2 (5) | 2 (4.44) | 2 (6.67) | 3 (8.3) | ||||
| Moderate smoker (10 to 19) | 3 (7.5) | 0 (0.0) | 1 (3.33) | 1 (2.8) | ||||
| Heavy smoker (20 or over) | 1 (2.5) | 0 (0.0) | 2 (6.67) | 0 (0.0) | ||||
| Alcohol status, n (%) (¥) | 0.526 | 0.396 | ||||||
| Non | 15 (37.5) | 22 (48.89) | 16 (53.33) | 21 (58.3) | ||||
| <1 unit per day | 12 (11.3) | 10 (22.2) | 7 (23.33) | 9 (25) | ||||
| 1–2 units per day | 9 (22.5) | 8 (17.8) | 4 (13.33) | 4 (11.1) | ||||
| 3–6 units per day | 1 (2.5) | 4 (8.9) | 3 (10) | 0 (0.0) | ||||
| 7–9 units per day | 1 (2.5) | 0 (0.0) | 0 (0.0) | 1 (2.8) | ||||
| >9 units per day | 2 (5) | 1 (2.22) | 0 (0.0) | 1 (2.8) | ||||
| Hormonal Replacement Therapy, n (%) (X) | 3 (7.5) | 8 (17.8) | X2 (1) = 1.985 | 0.159 | ||||
| Supplementation of Vitamin D, n (%) (X) | 6 (20) | 14 (38.9) | X2 (1) = 3.054 | 0.080 | ||||
| Bone Health Medication, n (%) (X) | - | - | - | 5 (16.7) | 1 (2.8) | X2 (1) = 1.985 | 0.084 | |
| Systemic Results Compared Between the LC and WR Groups at Baseline and Follow-Up | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | n | Baseline | n | Follow-up | |||||||||
| WR | LC | Test Value | p | 95% CI | WR | LC | Test Value | p | 95% CI | ||||
| (EQ-5D-5L) | Mobility (X) | 40/45 | 0 (0; 0) | 0.058 (0; 0.076) | X2 (3) = 38.995 | <0.001 * | 30/36 | 0 (0; 0) | 0.067 (0; 0.076) | X2 (3) = 28.308 | <0.001 * | ||
| Self-Care (X) | 0 (0; 0) | 0 (0; 0.05) | X2 (2) = 20.465 | <0.001 * | 0 (0; 0) | 0 (0; 0.05) | X2 (2) = 19.081 | <0.001 * | |||||
| Usual Activities (X) | 0 (0; 0) | 0.063 (0.05; 0.063) | X2 (4) = 59.792 | <0.001 * | 0 (0; 0) | 0.063 (0.05; 0.162) | X2 (4) = 48.796 | <0.001 * | |||||
| Pain/Discomfort (X) | 0 (0; 0.032) | 0.084 (0.063; 0.084) | X2 (4) = 42.561 | <0.001 * | 0 (0; 0.063) | 0.084 (0.063; 0.084) | X2 (3) = 26.886 | <0.001 * | |||||
| Anxiety/Depression (X) | 0 (0; 0.078) | 0.075 (0; 0.104) | X2 (3) = 17.111 | 0.001 * | 0 (0; 0) | 0.078 (0; 0.091) | X2 (3) = 18.172 | <0.001 * | |||||
| UI (‡) | 1 (0.922; 1) | 0.72 (0.55; 0.808) | z = 7.041 | <0.001 * | (−0.564; −0.415) | 1 (0.937; 1) | 0.697 (0.53; 0.809) | z = 6.632 | <0.001 * | (−0.584; −0.452) | |||
| VAS (‡) | 87.5 (80; 95) | 55 (40; 70) | z = 6.993 | <0.001 * | (−0.519; −0.423) | 90 (80; 97) | 60 (40; 70) | z = 6.362 | <0.001 * | (−0.532; −0.430) | |||
| Joint Pain | Hand Pain (¥) | 0 (0; 0) | 1 (0; 5) | 0.003 * | (0.208; 0.526) | 0(0; 0) | 0.5 (0; 4) | 0.039 | (0.244; 0.571) | ||||
| Knee Pain (¥) | 0 (0; 2) | 3 (0; 6) | 0.024 | (0.176; 0.434) | 0 (0; 2) | 3 (0; 6) | 0.093 | (0.087; 0.411) | |||||
| Biomarkers | TNF-α (‡) | 40/45 | 1.03 (0.68; 1.28) | 0.77 (0.61; 0.98) | z = 2.430 | 0.015 | (−0.0276; −0.032) | 29/35 | 0.96 (0.68; 1.39) | 0.86 (0.76; 0.98) | z = 1.396 | 0.162 | (−0.258; 0.053) |
| IL-6 (‡) | 1.03 (0.63; 1.41) | 1.08 (0.72; 1.66) | z = −0.977 | 0.328 | (−0.063; 0.0187) | 0.81 (0.48; 1.35) | 1.06 (0.62; 1.44) | z = −0.816 | 0.414 | (−0.085; 0.205) | |||
| IFN-γ (‡) | 1.03 (0.55; 1.53) | 0.73 (0.52; 1.44) | z = 1.083 | 0.278 | (−0.0195;0.057) | 0.82 (0.48; 1.23) | 0.95 (0.51; 1.40) | z = −0.573 | 0.566 | (−0.103; 0.188) | |||
| PINP/CTX ratio (‡) | 40/44 | 150.6 (124.5; 184.6) | 152.91 (125; 200.3) | z = −0.448 | 0.654 | (−0.098; 0.156) | 30/36 | 168.2 (132.6; 194.7) | 171.5 (144.6; 208.9) | z = −0.541 | 0.588 | (−0.106; 0.1844) | |
| 25 OH D ng/mL (‡) | 40/45 | 20.36 (15.995; 27.65) | 29.46 (23.75; 35.06) | z = −3.073 | 0.0021 * | (0.072; 0.318) | 30/36 | 29.58 (25.33; 41.74) | 35.89 (30.095; 41.16) | z = −1.648 | 0.099 | (−0.028; 0.267) | |
| Paired Analysis Within Groups from Baseline to Follow-Up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | WR (n = 30) | LC (n = 36) | |||||||||
| n | Baseline | Follow-Up | CI | p | n | Baseline | Follow-Up | CI | p | ||
| (EQ-5D-5L) | Mobility | 30 | 0 (0; 0) | 0 (0; 0) | 1.000 | 36 | 0.058 (0; 0.076) | 0.067 (0; 0.076) | 0.528 | ||
| Self-Care | 0 (0; 0) | 0 (0; 0) | 1.000 | 0 (0; 0.05) | 0 (0; 0.05) | 0.948 | |||||
| Usual Activities | 0 (0; 0) | 0 (0; 0) | 0.157 | 0.063 (0.05; 0.063) | 0.063 (0.05; 0.162) | 0.060 | |||||
| Pain/Discomfort | 0 (0; 0) | 0 (0; 0.063) | 0.058 | 0.084 (0.063; 0.084) | 0.084 (0.063; 0.084) | 0.867 | |||||
| Anxiety/Depression | 0 (0; 0.078) | 0 (0; 0) | 0.282 | 0.078 (0; 0.091) | 0.078 (0; 0.091) | 0.362 | |||||
| UI | 1 (0.922; 1) | 1 (0.937; 1) | −0.028; 0.019 | 0.946 | 0.725 (0.587; 0.809) | 0.697 (0.53; 0.809) | −0.057; 0.024 | 0.398 | |||
| VAS | 87.5 (80; 95) | 90 (80; 97) | −1.133; 3.666 | 0.264 | 57.5 (40; 70) | 60 (40; 70) | −5.653; 8.486 | 0.868 | |||
| Joint Pain | Hand Pain | 0 (0; 0) | 0 (0; 0) | −0.122; 0.755 | 0.917 | 0.5 (0; 5) | 0.5 (0; 4) | −0.782; 0.393 | 0.624 | ||
| Knee Pain | 0.5 (0; 2) | 0 (0; 2) | −0.805; 1.005 | 0.371 | 3 (0; 5) | 3 (0; 6) | −1.164; 0.553 | 0.573 | |||
| Biomarkers | TNF-α | 29 | 0.98 (0.67; 1.28) | 0.96 (0.68; 1.39) | −0.122; 0.262 | 0.991 | 35 | 0.77 (0.61; 0.98) | 0.86 (0.76; 0.98) | −0.107; 0.133 | 0.412 |
| IL-6 | 0.85 (0.58; 1.35) | 0.81 (0.48; 1.35) | −0.248; 0.190 | 0.566 | 1.25 (0.72; 1.88) | 1.06 (0.62; 1.44) | −0.489; 0.012 | 0.076 | |||
| IFN-γ | 0.91 (0.43; 1.52) | 0.82 (0.48; 1.23) | −0.862; 0.210 | 0.112 | 0.84 (0.55; 1.63) | 0.95 (0.51; 1.4) | −0.444; 0.210 | 0.831 | |||
| PINP/CTX ratio | 30 | 143.2 (124.2; 182.6) | 168.151 (132.6; 194.7) | −8.227; 38.819 | 0.192 | 149.2 (113.2; 190.1) | 169.4 (143.6; 206.2) | 3.583; 31.629 | 0.011 | ||
| 25 OH D ng/mL | 30 | 21.4 (16.34; 27.89) | 29.58 (25.33; 41.74) | 4.889; 11.603 | <0.001 * | 36 | 32.695 (23.665; 35.1) | 35.89 (30.1; 41.2) | 2.752; 9.259 | 0.0023 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, F.; Meertens, R.; Obotiba, A.D.; Harries, L.W.; Appleby, S.; Mokbel, K.; Knapp, K.M.; Strain, W.D. Assessment of Health-Related Quality of Life and Biomarkers in Long COVID: A 12-Month Longitudinal Feasibility Cohort. J. Clin. Med. 2025, 14, 7931. https://doi.org/10.3390/jcm14227931
Alghamdi F, Meertens R, Obotiba AD, Harries LW, Appleby S, Mokbel K, Knapp KM, Strain WD. Assessment of Health-Related Quality of Life and Biomarkers in Long COVID: A 12-Month Longitudinal Feasibility Cohort. Journal of Clinical Medicine. 2025; 14(22):7931. https://doi.org/10.3390/jcm14227931
Chicago/Turabian StyleAlghamdi, Fahad, Robert Meertens, Abasiama Dick Obotiba, Lorna W. Harries, Sarah Appleby, Kinan Mokbel, Karen M. Knapp, and William David Strain. 2025. "Assessment of Health-Related Quality of Life and Biomarkers in Long COVID: A 12-Month Longitudinal Feasibility Cohort" Journal of Clinical Medicine 14, no. 22: 7931. https://doi.org/10.3390/jcm14227931
APA StyleAlghamdi, F., Meertens, R., Obotiba, A. D., Harries, L. W., Appleby, S., Mokbel, K., Knapp, K. M., & Strain, W. D. (2025). Assessment of Health-Related Quality of Life and Biomarkers in Long COVID: A 12-Month Longitudinal Feasibility Cohort. Journal of Clinical Medicine, 14(22), 7931. https://doi.org/10.3390/jcm14227931







