Outcomes of Lobar and Sublobar Resection for Clinical Stage I Lung Neuroendocrine Tumors: An ENETS Center of Excellence Experience †
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Surgical Treatment
3.3. Lymph Node Assessment
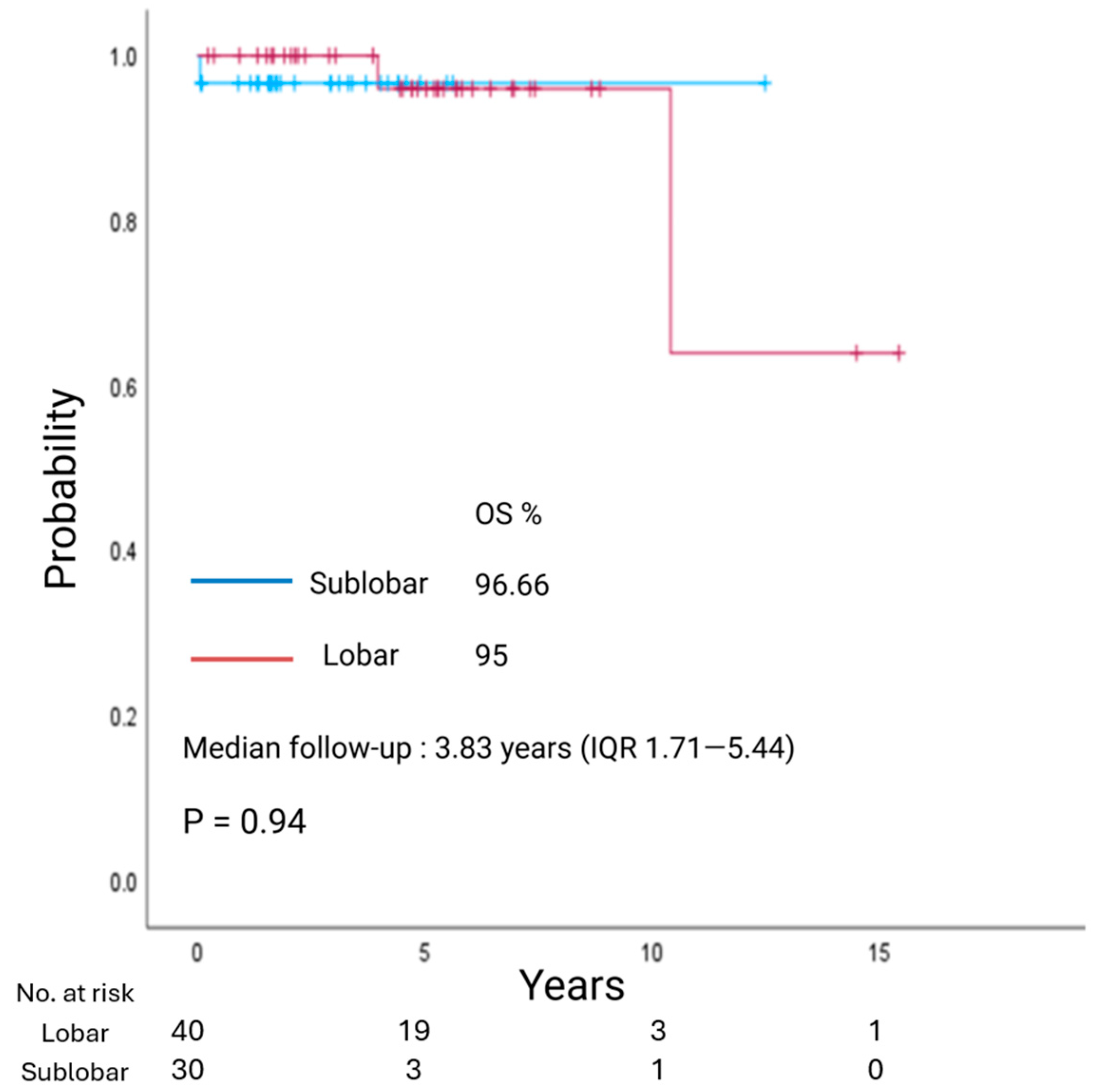
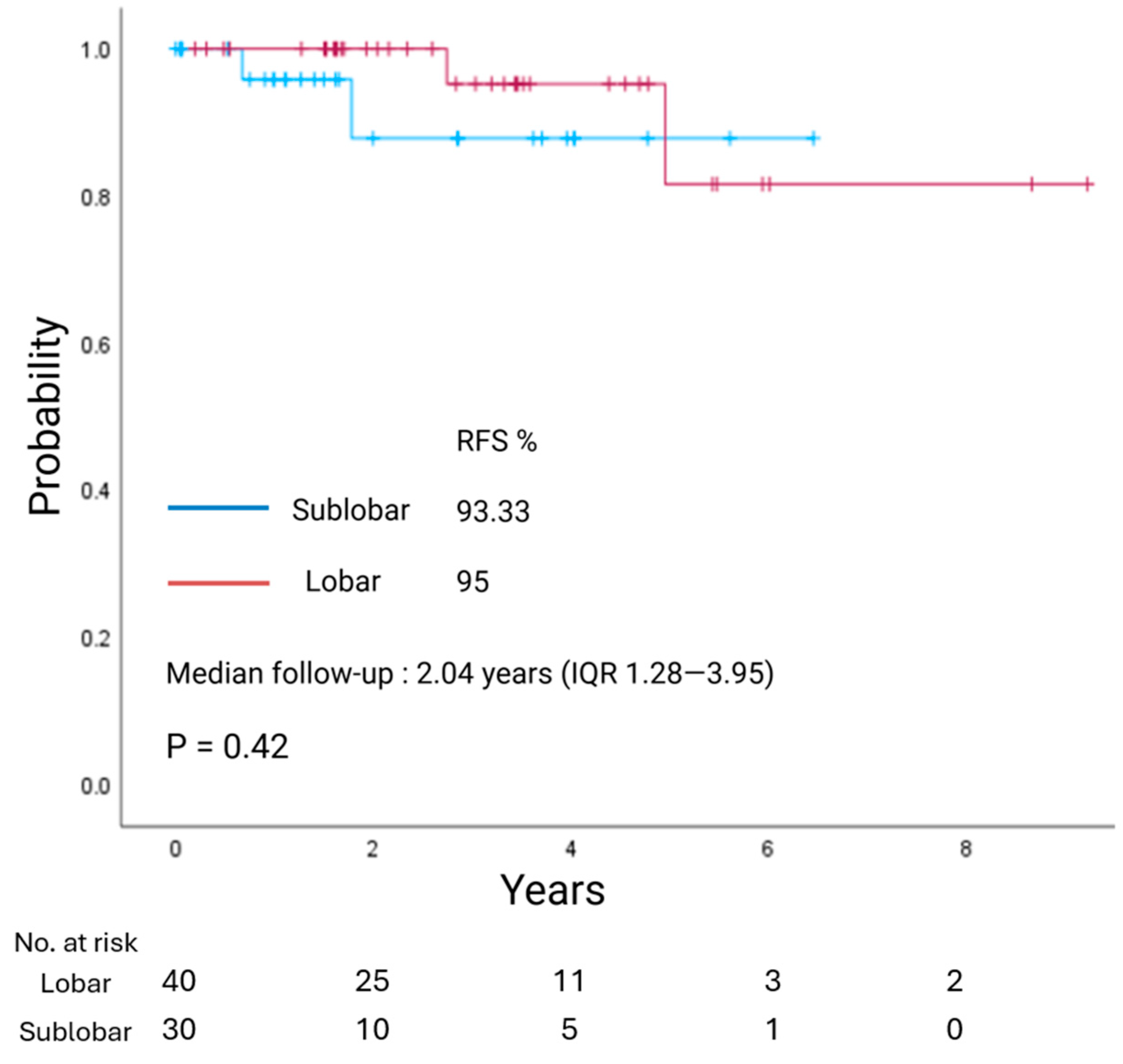
3.4. Survival Analysis
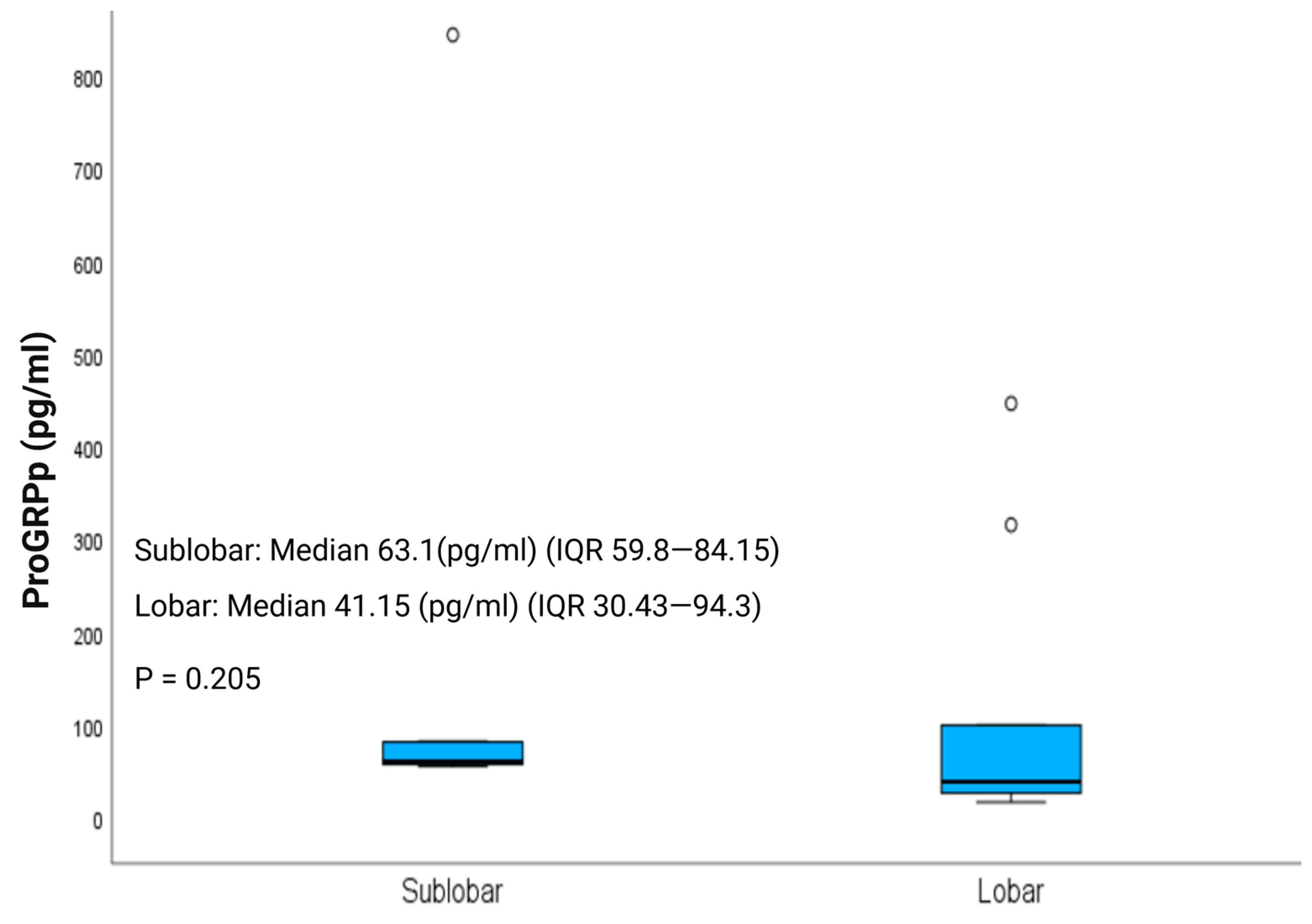
3.5. Post-Operative Plasma Progastrin-Releasing Peptide (ProGRPp)
3.6. Prognostic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | atypical carcinoid |
| ACTH | adrenocorticotropic hormone |
| DIPNECH | diffuse idiopathic pulmonary neuroendocrine cell hyperplasia |
| LCNEC | large cell neuroendocrine carcinoma |
| LNEC(s) | lung neuroendocrine carcinoma(s) |
| LNET(s) | lung neuroendocrine tumor(s) |
| LR | lobar resection |
| NSCLC | non–small cell lung cancer |
| OS | overall survival |
| PC(s) | pulmonary carcinoid(s) |
| PET | positron emission tomography |
| ProGRPp | plasma progastrin-releasing peptide |
| RFS | recurrence-free survival |
| SCLC | small cell lung carcinoma |
| SLR | sublobar resection |
| TC | typical carcinoid |
References
- Broder, M.S.; Cai, B.; Chang, E.; Neary, M.P.; Papoyan, E. Incidence and prevalence of neuroendocrine tumors of the lung: Analysis of a US commercial insurance claims database. BMC Pulm. Med. 2018, 18, 135. [Google Scholar] [CrossRef]
- Modlin, I.M.; Lye, K.D.; Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003, 97, 934–959. [Google Scholar] [CrossRef]
- World Health Organization. Classification of Tumours Editorial Board. In Thoracic Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021. [Google Scholar]
- Sun, T.Y.; Hwang, G.; Pancirer, D.; Hornbacker, K.; Codima, A.; Lui, N.S.; Raj, R.; Kunz, P.; Padda, S.K. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: Clinical characteristics and progression to carcinoid tumour. Eur. Respir. J. 2022, 59, 2101058. [Google Scholar] [CrossRef]
- Swarts, D.R.A.; Ramaekers, F.C.S.; Speel, E.J.M. Molecular and cellular biology of neuroendocrine lung tumors: Evidence for separate biological entities. Biochim. Biophys. Acta 2012, 1826, 255–271. [Google Scholar] [CrossRef]
- Baudin, E.; Caplin, M.; Garcia-Carbonero, R.; Fazio, N.; Ferolla, P.; Filosso, P.; Frilling, A.; de Herder, W.; Hörsch, D.; Knigge, U.; et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Russ, D.H.; Barta, J.A.; Evans, N.R.; Stapp, R.T.; Kane, G.C. Volume Doubling Time of Pulmonary Carcinoid Tumors Measured by Computed Tomography. Clin Lung Cancer. 2022, 23, e453–e459. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Brascia, D.; Marulli, G. Surgical principles in the management of lung neuroendocrine tumors: Open questions and controversial technical issues. Curr. Treat. Options Oncol. 2022, 23, 1645–1663. [Google Scholar] [CrossRef]
- Caplin, M.E.; Baudin, E.; Ferolla, P.; Filosso, P.; Garcia-Yuste, M.; Lim, E.; Oberg, K.; Pelosi, G.; Perren, A.; Rossi, R.E.; et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann. Oncol. 2015, 26, 1604–1620. [Google Scholar] [CrossRef]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O’DOrisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 863–881. [Google Scholar] [CrossRef]
- Singh, S.; Bergsland, E.K.; Card, C.M.; Hope, T.A.; Kunz, P.L.; Laidley, D.T.; Lawrence, B.; Leyden, S.; Metz, D.C.; Michael, M.; et al. Commonwealth Neuroendocrine Tumour Research Collaboration and the North American Neuroendocrine Tumor Society Guidelines for the Diagnosis and Management of Patients With Lung Neuroendocrine Tumors: An International Collaborative Endorsement and Update of the 2015 European Neuroendocrine Tumor Society Expert Consensus Guidelines. J Thorac Oncol. 2020, 15, 1577–1598. [Google Scholar] [CrossRef]
- Kneuertz, P.J.; Kamel, M.K.; Stiles, B.M.; Lee, B.E.; Rahouma, M.; Harrison, S.W.; Altorki, N.K.; Port, J.L. Incidence and Prognostic Significance of Carcinoid Lymph Node Metastases. Ann. Thorac. Surg. 2018, 106, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother. Psychosom. 2022, 91, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Nisman, B.; Oleinikov, K.; Nechushtan, H.; Maimon, O.; Atlan, K.; Peled, N.; Gross, D.; Peretz, T.; Meirovitz, A.; Grozinsky-Glasberg, S. Plasma Progastrin-Releasing Peptide and Chromogranin A Assays for Diagnosing and Monitoring Lung Well-Differentiated Neuroendocrine Tumors: A Brief Report. J. Thorac. Oncol. 2023, 18, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Wang, Z.; Zhang, M. Segmentectomy and wedge resection are equivalent for the treatment of early-stage pulmonary carcinoid tumors: A retrospective cohort study. Sci. Rep. 2024, 14, 17742. [Google Scholar] [CrossRef]
- Ernani, V.; Appiah, A.K.; Rodriguez, D.; Kusne, Y.; Beamer, S.E.; Ravanbakhsh, S.; Jaroszewski, D.; dos Santos, P.R.; Sio, T.T.; Yu, N.; et al. Lobar versus sublobar resection for atypical lung carcinoid: An analysis from the National Cancer Database. Cancer 2023, 129, 860–866. [Google Scholar] [CrossRef]
- Xu, S.; Li, X.; Ren, F.; He, J.; Zhao, S.; Wang, Y.; Ren, D.; Zhu, S.; Lei, X.; Chen, G.; et al. Sublobar Resection Versus Lobectomy for Early-Stage Pulmonary Carcinoid Tumors ≤ 3 cm in Size: A SEER Population-Based Study. Ann. Surg. 2022, 276, e991–e999. [Google Scholar] [CrossRef]
- Yang, H.; Xiao, X.; Mei, T.; Zhou, P. Long-term survival analysis of sublobar resection versus lobectomy for older patients with early-stage pulmonary carcinoid tumour: A database-based propensity score-matched study. Aging Clin. Exp. Res. 2022, 34, 1925–1934. [Google Scholar] [CrossRef]
- Yang, H.; Mei, T. Sublobar resection versus lobectomy for patients with stage T1-2N0M0 pulmonary typical carcinoid tumours: A population-based propensity score matching analysis. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac125. [Google Scholar] [CrossRef] [PubMed]
- Cattoni, M.; Vallières, E.; Brown, L.M.; Sarkeshik, A.A.; Margaritora, S.; Siciliani, A.; Filosso, P.L.; Guerrera, F.; Imperatori, A.; Rotolo, N.; et al. Sublobar Resection in the Treatment of Peripheral Typical Carcinoid Tumors of the Lung. Ann. Thorac. Surg. 2019, 108, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wang, K.; Liu, J.; Zeng, Y.; Bie, F.; Wang, G.; Du, J. Wedge resection is equal to segmental resection for pulmonary typical carcinoid patients at localized stage: A population-based analysis. PeerJ 2019, 7, e7519. [Google Scholar] [CrossRef]
- Brown, L.M.; Cooke, D.T.; Jett, J.R.; David, E.A. Extent of Resection and Lymph Node Assessment for Clinical Stage T1aN0M0 Typical Carcinoid Tumors. Ann. Thorac. Surg. 2018, 105, 207–213. [Google Scholar] [CrossRef]
- Furqan, M.; Tien, Y.-Y.; Schroeder, M.C.; Parekh, K.R.; Keech, J.; Allen, B.G.; Thomas, A.; Zhang, J.; Clamon, G.; Abu Hejleh, T. Lobar versus sub-lobar surgery for pulmonary typical carcinoid, a population-based analysis. J. Thorac. Dis. 2018, 10, 5850–5859. [Google Scholar] [CrossRef]
- Fox, M.; Van Berkel, V.; Bousamra, M.; Sloan, S.; Martin, R.C., 2nd. Surgical management of pulmonary carcinoid tumors: Sublobar resection versus lobectomy. Am. J. Surg. 2013, 205, 200–208. [Google Scholar] [CrossRef]
- Yendamuri, S.; Gold, D.; Jayaprakash, V.; Dexter, E.; Nwogu, C.; Demmy, T. Is sublobar resection sufficient for carcinoid tumors? Ann. Thorac. Surg. 2011, 92, 1774–1779. [Google Scholar] [CrossRef] [PubMed]
- Steuer, C.E.; Behera, M.; Kim, S.; Chen, Z.; Saba, N.F.; Pillai, R.N.; Owonikoko, T.K.; Khuri, F.R.; Ramalingam, S.S. Atypical carcinoid tumor of the lung: A surveillance, epidemiology, and end results database analysis. J. Thorac. Oncol. 2015, 10, 479–485. [Google Scholar] [CrossRef]
- Chen, X.; Pang, Z.; Wang, Y.; Bie, F.; Zeng, Y.; Wang, G.; Du, J. The role of surgery for atypical bronchopulmonary carcinoid tumor: Development and validation of a model based on Surveillance, Epidemiology, and End Results (SEER) database. Lung Cancer 2020, 139, 94–102. [Google Scholar] [CrossRef]
- Cañizares, M.A.; Matilla, J.M.; Cueto, A.; Algar, J.; Muguruza, I.; Moreno-Mata, N.; Moreno-Balsalobre, R.; Guijarro, R.; Arrabal, R.; Garcia-Fontan, E.; et al. Atypical carcinoid tumours of the lung: Prognostic factors and patterns of recurrence. Thorax 2014, 69, 648–653. [Google Scholar] [CrossRef]
- Fink, G.; Krelbaum, T.; Yellin, A.; Bendayan, D.; Saute, M.; Glazer, M.; Kramer, M.R. Pulmonary carcinoid: Presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001, 119, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, G.; Sera, F.; Di Martino, M.; Graziano, P.; Giunti, R.; Carbone, L.; Facciolo, F.; Martelli, M. Bronchial carcinoid tumors: Nodal status and long-term survival after resection. Ann. Thorac. Surg. 2004, 77, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- García-Yuste, M.; Matilla, J.M.; Cueto, A.; Paniagua, J.M.R.; Ramos, G.; Cañizares, M.A.; Muguruza, I. Typical and atypical carcinoid tumours: Analysis of the experience of the Spanish Multi-centric Study of Neuroendocrine Tumours of the Lung. Eur. J. Cardiothorac. Surg. 2007, 31, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Metovic, J.; Barella, M.; Pelosi, G. Neuroendocrine neoplasms of the lung: A pathology update. Memo 2021, 14, 381–385. [Google Scholar] [CrossRef]
| Parameter | Total (n = 70) | Typical (n = 50) | Atypical (n = 20) | p-Value |
|---|---|---|---|---|
| Demographics and Clinical Features | ||||
| Sex (F/M, % F) | 44/26 (63%) | 30/20 (60%) | 14/6 (70%) | 0.43 |
| Age (years), mean ± SD | 56.76 ± 16 | 57.50 ± 15.80 | 55 ± 17.10 | 0.51 |
| CCI, median (IQR) | 2 (1–4) | 2 (0.75–4.00) | 2.50 (1.00–3.75) | 0.80 |
| Preoperative FEV1 (% predicted), median (IQR) | 92 (80–99) | 92 (80–99) | 88 (81.50–101) | 0.86 |
| Smoking * | 22 (35.48%) | 14 (28%) | 8 (40%) | 0.33 |
| Previous malignancy | 19 (32.25%) | 14 (32%) | 5 (28%) | 0.80 |
| Tumor location (C/P, % C) | 25/45 (35.71%) | 20/30 (40%) | 5/15 (25%) | 0.94 |
| Functional tumor ** | 3 (4.29%) | 2 (4%) | 1 (5%) | 1.00 |
| Minimally invasive approach *** | 33 (47.14%) | 22 (44%) | 11 (55%) | 0.40 |
| LOS (days), median (IQR) | 4 (3–5) | 4 (3–5) | 5 (3–6) | 0.78 |
| 30-day mortality | 1 (1.43%) | 1 (2%) | 0 | 1.00 |
| Adjuvant therapy (SSA) | 16 (22.86%) | 5 (10%) | 11 (55%) | <0.001 |
| Nuclear Imaging | ||||
| FDG SUVmax, median (IQR) | 2.96 (2–4.40) | 2.78 (2–3.49) | 4.35 (2.62–5.42) | 0.08 |
| 68Ga-DOTATATE SUVmax, median (IQR) | 9.10 (1.80–34.06) | 12.20 (2.38–30.10) | 2.00 (0–44.81) | 0.59 |
| Type of Surgery | 0.07 | |||
| Lobectomy | 40 (57.14%) | 25 (50%) | 15 (75%) | |
| Sublobar resection | 30 (42.86%) | 25 (50%) | 5 (25%) | |
| Segmentectomy | 15 (21.43%) | 13 (26%) | 2 (10%) | |
| Wedge resection | 15 (21.43%) | 12 (24%) | 3 (15%) | |
| Pathological Parameters | ||||
| Mitotic index (per 2 mm2), mean ± SD | 1.47 ± 1.95 | 0.67 ± 0.60 | 3.47 ± 2.66 | <0.001 |
| Necrosis | 15 (22.73%) | 0 | 15 (75%) | <0.001 |
| Ki-67 index (%), mean ± SD | 4.62 ± 4.80 | 2.36 ± 1.72 | 9.94 ± 5.54 | <0.001 |
| DIPNECH | 17 (24.29%) | 12 (24%) | 5 (26%) | 1.00 |
| Lymphovascular invasion | 14 (21.21%) | 6 (13%) | 8 (40%) | 0.02 |
| Perineural invasion | 8 (12.70%) | 3 (7%) | 5 (28%) | 0.04 |
| Visceral pleural invasion | 3 (4.55%) | 1 (2%) | 2 (10%) | 0.19 |
| Direct invasion of adjacent structures | 7 (10.61%) | 2 (4%) | 5 (25%) | 0.02 |
| Margins (R0/R1, %R0) | 63/5 (92.65%) | 45/3 (93.75%) | 18/2 (90%) | 0.62 |
| Clinical Staging | ||||
| T category | 0.04 | |||
| T1a (≤1 cm) | 15 (21.43%) | 12 (24%) | 3 (15%) | |
| T1b (>1 but ≤2 cm) | 41 (58.57%) | 32 (64%) | 9 (45%) | |
| T1c (>2 but ≤3 cm) | 11 (15.71%) | 4 (8%) | 7 (35%) | |
| T2a (>3 but ≤4 cm) | 3 (4.29%) | 2 (4%) | 1 (5%) | |
| T2b–T4 (>4 cm) | 0 | 0 | 0 | |
| N category | ||||
| N0 | 69 (98.57%) | 49 (98%) | 20 (100%) | |
| N1–N3 | 0 | 0 | 0 | |
| Nx | 1 (1.43%) | 1 (2%) | 0 | |
| M category | ||||
| M0 | 70 (100%) | 50 (100%) | 20 (100%) | |
| M1 | 0 | 0 | 0 | |
| Clinical Stage **** | ||||
| Stage I | 70 (100%) | 50 (100%) | 20 (100%) | |
| Stage II–IV | 0 | 0 | 0 | |
| Pathological Staging | ||||
| T category | <0.01 | |||
| T1a (≤1 cm) | 15 (21.43%) | 13 (26%) | 2 (10%) | |
| T1b (>1 but ≤2 cm) | 34 (48.57%) | 27 (54%) | 7 (35%) | |
| T1c (>2 but ≤3 cm) | 13 (18.57%) | 4 (8%) | 9 (45%) | |
| T2a (>3 but ≤4 cm) | 8 (11.43%) | 6 (12%) | 2 (10%) | |
| T2b–T4 (>4 cm) | 0 | 0 | 0 | |
| N category | <0.01 | |||
| N0 | 50 (71.43%) | 40 (80%) | 10 (50%) | |
| N1 | 11 (15.71%) | 3 (6%) | 8 (40%) | |
| N2 | 2 (2.86%) | 1 (2%) | 1 (5%) | |
| N3 | 0 | 0 | 0 | |
| Nx | 7 (10%) | 6 (12%) | 1 (5%) | |
| Pathological Stage **** | 0.01 | |||
| Stage I | 57 (81.43%) | 45 (90%) | 12 (60%) | |
| Stage II | 9 (12.86%) | 4 (8%) | 5 (25%) | |
| Stage III | 4 (5.71%) | 1 (2%) | 3 (15%) | |
| Stage IV | 0 | 0 | 0 | |
| LN Assessment and Upstaging | ||||
| LN assessment ***** | 63 (90%) | 45 (90%) | 18 (90%) | 1.00 |
| Pathologic upstaging | 13 (18.57%) | 5 (10%) | 8 (40%) | <0.01 |
| LN upstaging | 13 (18.57%) | 5 (10%) | 8 (40%) | <0.01 |
| Parameter | Total (n = 70) | Lobar (n = 40) | Sublobar (n = 30) | p-Value |
|---|---|---|---|---|
| Demographics and Clinical Features | ||||
| Sex (F/M, % F) | 44/26 (63%) | 21/19 (52.50%) | 23/7 (76.67%) | 0.07 |
| Age (years), mean ± SD | 56.76 ± 16 | 50.58 ± 17.20 | 64.50 ± 9.72 | <0.001 |
| CCI, median (IQR) | 2 (1–4) | 1.50 (0–4) | 3 (2–4.75) | <0.01 |
| Preoperative FEV1 (% predicted), median (IQR) | 92 (80–99) | 92 (79.50–97) | 92 (85–99) | 0.81 |
| Smoking * | 22 (35.48%) | 14 (38.89%) | 8 (30.77%) | 0.70 |
| Previous malignancy | 19 (32.25%) | 8 (22.22%) | 11 (42.30%) | 0.16 |
| Tumor location (C/P, % C) | 25/45 (35.71%) | 21/19 (52.50%) | 4/26 (13.33%) | <0.001 |
| Functional tumor ** | 3 (4.29%) | 1 (2.50%) | 2 (6.67%) | 0.57 |
| Minimally invasive approach *** | 33 (47.14%) | 13 (32.50%) | 20 (66.67%) | 0.01 |
| LOS (days), median (IQR) | 4 (3–5) | 5 (4–6) | 3 (2–3) | <0.01 |
| 30-day mortality | 1 (1.43%) | 0 | 1 (3.33%) | 0.43 |
| Adjuvant therapy (SSA) | 16 (22.86%) | 10 (25%) | 6 (20%) | 0.78 |
| Nuclear Imaging | ||||
| FDG SUVmax, median (IQR) | 2.96 (2–4.40) | 3.10 (2.50–5) | 2.80 (2–4.25) | 0.46 |
| 68Ga-DOTATATE SUVmax, median (IQR) | 9.10 (1.80–34.06) | 24.75 ± 30.10 | 13.45 ± 15.47 | 0.31 |
| Pathological Parameters | ||||
| Typical/atypical (% typical) | 50/20 (71.43%) | 25/15 (62.50%) | 25/5 (83.33%) | 0.10 |
| Mitotic index (per 2 mm2), mean ± SD | 1.47 ± 1.95 | 1.58 ± 1.92 | 1.33 ± 2.01 | 0.94 |
| Necrosis | 15 (22.73%) | 11 (28.94%) | 4 (14.29%) | 0.24 |
| Ki-67 index (%), mean ± SD | 4.62 ± 4.80 | 5.17 ± 5.19 | 3.86 ± 4.17 | 0.17 |
| DIPNECH | 17 (24.29%) | 6 (15%) | 11 (36.67%) | 0.20 |
| Lymphovascular invasion | 14 (21.21%) | 9 (23.68%) | 5 (17.86%) | 0.76 |
| Perineural invasion | 8 (12.70%) | 7 (20%) | 1 (3.57%) | 0.13 |
| Visceral pleural invasion | 3 (4.55%) | 2 (5.41%) | 1 (3.45%) | 1.00 |
| Direct invasion of adjacent structures | 7 (10.61%) | 6 (16.22%) | 1 (3.45%) | 0.23 |
| Margins (R0/R1, %R0) | 63/5 (92.65%) | 35/4 (89.74%) | 28/1 (96.55%) | 0.62 |
| Clinical Staging | ||||
| T category | 0.16 | |||
| T1a (≤1 cm) | 15 (21.43%) | 6 (15%) | 9 (30%) | |
| T1b (>1 but ≤2 cm) | 41 (58.57%) | 23 (57.50%) | 18 (60%) | |
| T1c (>2 but ≤3 cm) | 11 (15.71%) | 8 (20%) | 3 (10%) | |
| T2a (>3 but ≤4 cm) | 3 (4.29%) | 3 (7.50%) | 0 | |
| T2b–T4 (>4 cm) | 0 | 0 | 0 | |
| N category | ||||
| N0 | 69 (98.57%) | 40 (100%) | 29 (96.67%) | |
| N1–N3 | 0 | 0 | 0 | |
| Nx | 1 (1.43%) | 0 | 1 (3.33%) | |
| M category | ||||
| M0 | 70 (100%) | 40 (100%) | 30 (100%) | |
| M1 | 0 | 0 | 0 | |
| Clinical Stage **** | ||||
| Stage I | 70 (100%) | 40 (100%) | 30 (100%) | |
| Stage II–IV | 0 | 0 | 0 | |
| Pathological Staging | ||||
| T category | 0.16 | |||
| T1a (≤1 cm) | 15 (21.43%) | 6 (15%) | 9 (30%) | |
| T1b (>1 but ≤2 cm) | 34 (48.57%) | 18 (45%) | 16 (53.33%) | |
| T1c (>2 but ≤3 cm) | 13 (18.57%) | 10 (25%) | 3 (10%) | |
| T2a (>3 but ≤4 cm) | 8 (11.43%) | 6 (15%) | 2 (6.67%) | |
| T2b–T4 (>4 cm) | 0 | 0 | 0 | |
| N category | <0.01 | |||
| N0 | 50 (71.43%) | 29 (72.50%) | 21 (70%) | |
| N1 | 11 (15.71%) | 10 (25%) | 1 (3.33%) | |
| N2 | 2 (2.86%) | 1 (2.50%) | 1 (3.33%) | |
| N3 | 0 | 0 | 0 | |
| Nx | 7 (10%) | 0 | 7 (23.33%) | |
| Pathological Stage **** | 0.08 | |||
| Stage I | 57 (81.43%) | 29 (72.50%) | 28 (93.33%) | |
| Stage II | 9 (12.86%) | 8 (20%) | 1 (3.33%) | |
| Stage III | 4 (5.71%) | 3 (7.50%) | 1 (3.33%) | |
| Stage IV | 0 | 0 | 0 | |
| LN Assessment and Upstaging | ||||
| LN assessment ***** | 63 (90%) | 40 (100%) | 23 (76.67%) | <0.01 |
| Pathologic upstaging | 13 (18.57%) | 11 (27.50%) | 2 (6.67%) | 0.03 |
| LN upstaging | 13 (18.57%) | 11 (27.50%) | 2 (6.67%) | 0.03 |
| LN Assessed * | Total (n = 70) | Lobar (n = 40) | Sublobar (n = 30) | p-Value |
|---|---|---|---|---|
| Hilar LN assessed (N1) Median (IQR) | 2.5 (1–5) | 4 (2–6.5) | 2 (0–3) | <0.001 |
| Mediastinal LN assessed (N2) Median (IQR) | 1 (0–3) | 2 (0–4) | 0 (0–1) | <0.01 |
| Total LN assessed | <0.001 | |||
| 0 | 7 (10%) | 0 (0%) | 7 (23.3%) | |
| 1–9 LN | 42 (60%) | 23 (57.5%) | 19 (63.3%) | |
| ≥10 LN | 13 (18.6%) | 12 (30%) | 1 (3.3%) | |
| Not quantified | 8 (11.4%) | 5 (12.5%) | 3 (10%) | |
| Median (IQR) | 4 (2–8.8) | 6 (3.5–11.5) | 2 (0.5–4) | <0.001 |
| Predictor | OS (p-Value) | RFS (p-Value) |
|---|---|---|
| Clinical variables | ||
| Sex | 0.714 | 0.678 |
| Age | 0.266 | 0.324 |
| CCI | 0.907 | 0.147 |
| Preoperative FEV1 (%) | 0.775 | 0.075 |
| Smoking (ever 2 vs. never) | 0.366 | 0.420 |
| Previous malignancy | 0.458 | 0.122 |
| Tumor Location (central/peripheral) | 0.960 | 0.575 |
| Clinical tumor size (>2 cm vs. ≤2 cm) | 0.351 | 0.307 |
| Surgical approach (thoracotomy vs. VATS/RATS) | 0.816 | 0.969 |
| Extent of resection (lobar/sub-lobar) | 0.940 | 0.420 |
| PET 18F-FDG SUVmax | 0.183 | 0.963 |
| PET 68Ga-DOTATATE SUVmax | 0.591 | 0.382 |
| Adjuvant therapy (SSA) | 0.459 | 0.003 |
| Functional tumor 3 | <0.001 | 0.781 |
| Pathologic features | ||
| Histologic grade (typical/atypical) | 0.548 | 0.002 |
| Necrosis | 0.396 | 0.001 |
| Ki-67 index (%) | 0.032 | 0.002 |
| DIPNECH | 0.434 | 0.861 |
| Lymphovascular invasion | 0.336 | 0.305 |
| Perineural invasion | 0.703 | 0.518 |
| Visceral pleural invasion | 0.006 | <0.001 |
| Resection margin status (R0 vs. R1) | 0.739 | 0.712 |
| LN assessment | 0.464 | 0.438 |
| Pathologic upstaging | 0.467 | 0.049 |
| Nodal upstaging | 0.467 | 0.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojerat, R.; Idais, I.; Aviel, G.; Bel-Ange, A.; Grozinsky-Glasberg, S.; Ben-Haim, S.; Nisman, B.; Maimon, O.; Atlan, K.; Shapira, O.M.; et al. Outcomes of Lobar and Sublobar Resection for Clinical Stage I Lung Neuroendocrine Tumors: An ENETS Center of Excellence Experience. J. Clin. Med. 2025, 14, 7927. https://doi.org/10.3390/jcm14227927
Hojerat R, Idais I, Aviel G, Bel-Ange A, Grozinsky-Glasberg S, Ben-Haim S, Nisman B, Maimon O, Atlan K, Shapira OM, et al. Outcomes of Lobar and Sublobar Resection for Clinical Stage I Lung Neuroendocrine Tumors: An ENETS Center of Excellence Experience. Journal of Clinical Medicine. 2025; 14(22):7927. https://doi.org/10.3390/jcm14227927
Chicago/Turabian StyleHojerat, Ranin, Islam Idais, Gal Aviel, Anat Bel-Ange, Simona Grozinsky-Glasberg, Simona Ben-Haim, Benjamin Nisman, Ofra Maimon, Karine Atlan, Oz M. Shapira, and et al. 2025. "Outcomes of Lobar and Sublobar Resection for Clinical Stage I Lung Neuroendocrine Tumors: An ENETS Center of Excellence Experience" Journal of Clinical Medicine 14, no. 22: 7927. https://doi.org/10.3390/jcm14227927
APA StyleHojerat, R., Idais, I., Aviel, G., Bel-Ange, A., Grozinsky-Glasberg, S., Ben-Haim, S., Nisman, B., Maimon, O., Atlan, K., Shapira, O. M., Korach, A., Izhar, U., Pines, G., & Wald, O. (2025). Outcomes of Lobar and Sublobar Resection for Clinical Stage I Lung Neuroendocrine Tumors: An ENETS Center of Excellence Experience. Journal of Clinical Medicine, 14(22), 7927. https://doi.org/10.3390/jcm14227927







