Development and Validation of a Clinical Decision Support Tool to Predict Disease Progression in Crohn’s Disease Treated with Ustekinumab
Abstract
1. Introduction
2. Materials and Methods
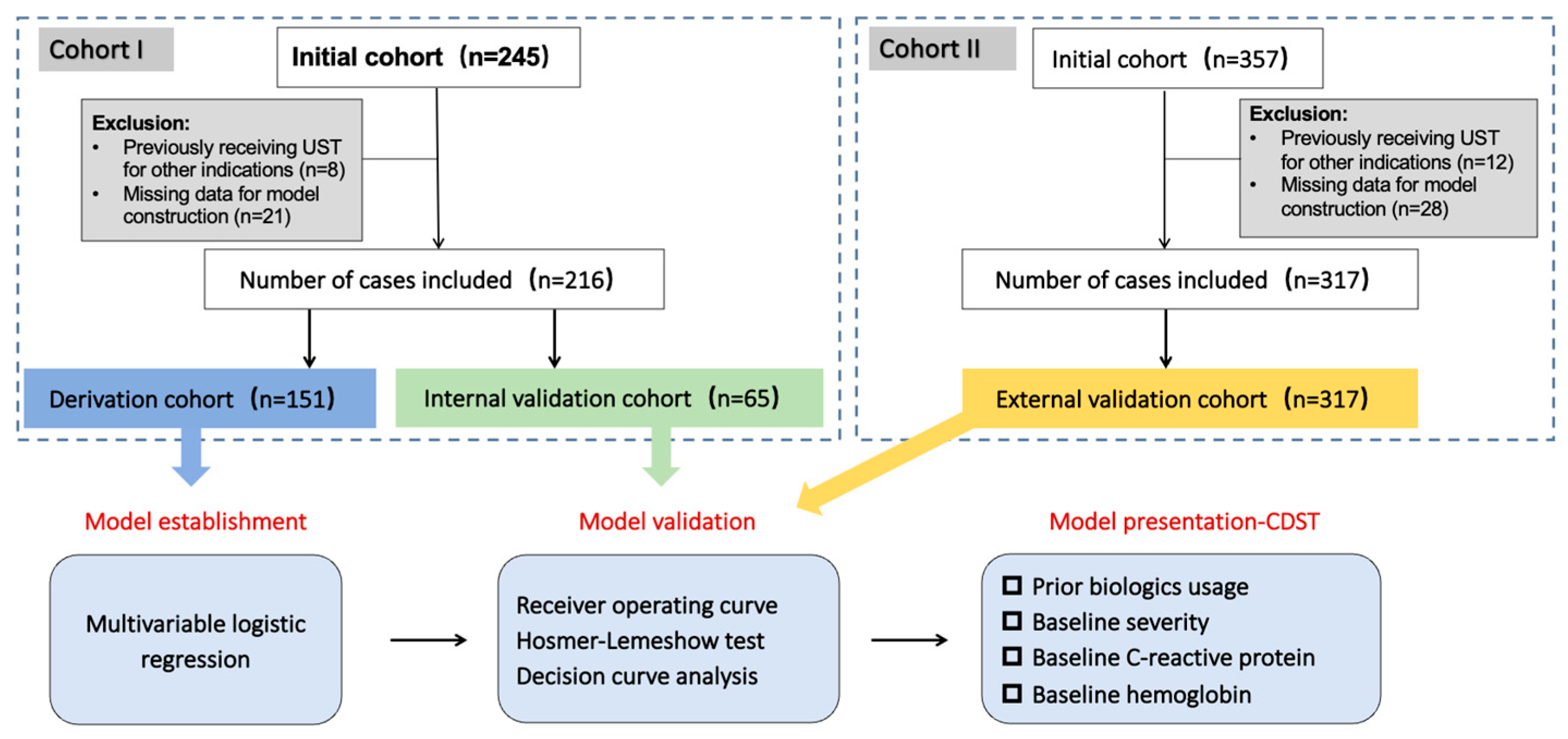
2.1. Study Design
2.2. Study Population
2.3. Predicting Variables
2.4. Clinical Outcomes and Definitions
2.5. Statistical Analysis
2.5.1. Handling of Missing Data
2.5.2. Random Split Verification
2.5.3. Model Establishment
2.5.4. Model Validation
2.5.5. Construction and Validation of the UST-CDST
3. Results
3.1. Patient Characteristics
3.2. Variable Selection
| Logit (Pr (Progression = Y) = −2.2395 (intercept) + [0.3877 when prior biologics exposure is true] + [0.0031 × disease severity as CDAI score] + [0.0069 × baseline CRP in mg/L] − [0.0145 × baseline hemoglobin in g/L] |
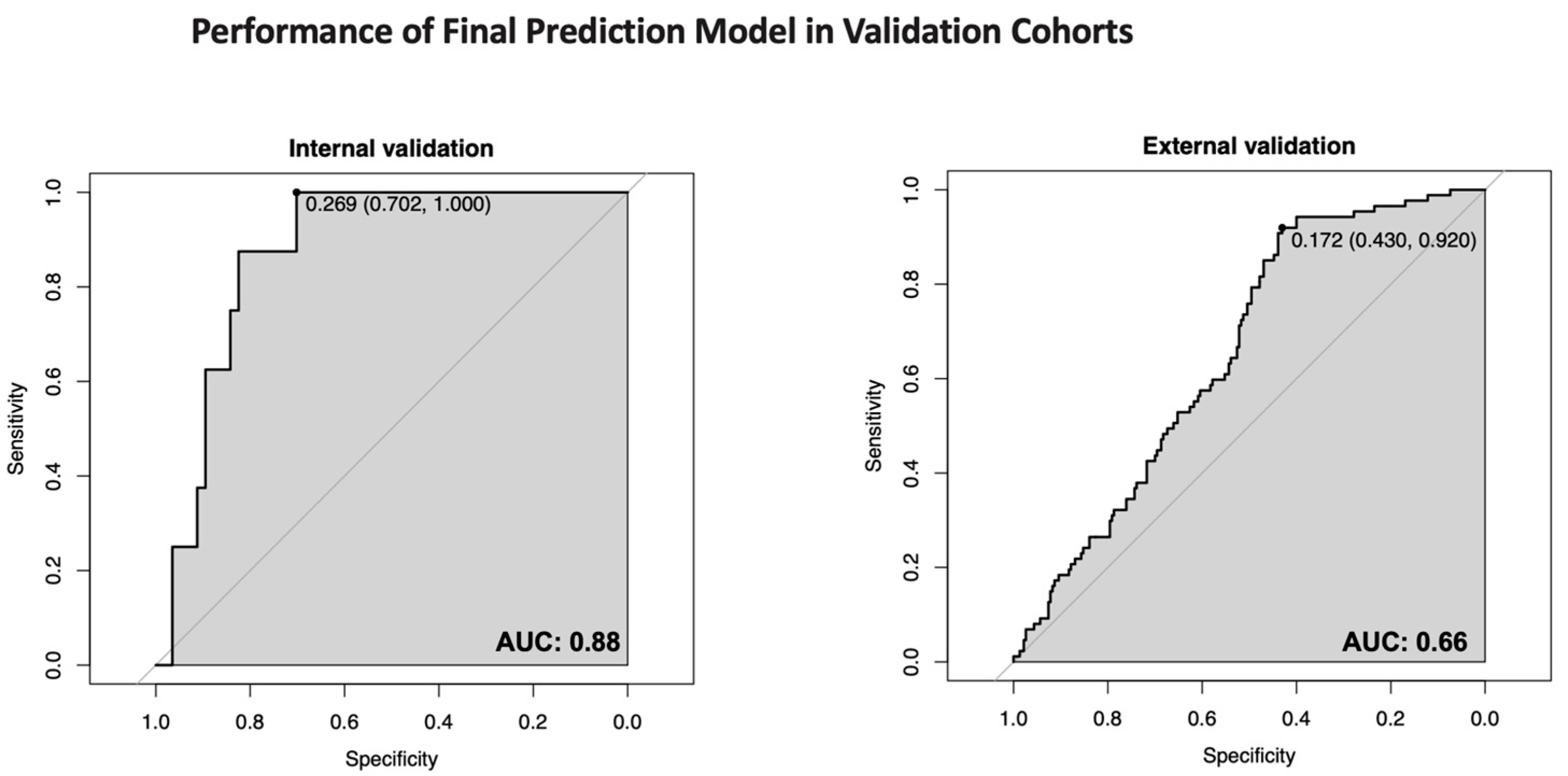
3.3. Model Performance and Validation
3.4. Clinical Decision Support Tool
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBD | Inflammatory bowel disease |
| CD | Crohn’s disease |
| UST | Ustekinumab |
| CDST | Clinical decision support tool |
| AUC | Area under the curve |
References
- Chaparro, M.; Baston-Rey, I.; Fernández Salgado, E.; González García, J.; Ramos, L.; Diz-Lois Palomares, M.T.; Argüelles-Arias, F.; Iglesias Flores, E.; Cabello, M.; Rubio Iturria, S.; et al. Using Interpretable Machine Learning to Identify Baseline Predictive Factors of Remission and Drug Durability in Crohn’s Disease Patients on Ustekinumab. J. Clin. Med. 2022, 11, 4518. [Google Scholar] [CrossRef]
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s Disease. Nat. Rev. Dis. Primers 2020, 6, 22. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Rebuck, R.; Wang, Y.; Zou, B.; Adedokun, O.J.; Gasink, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Ghosh, S.; et al. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: The IM-UNITI Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 578–590.e4. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, B.; Sun, Y.; Li, J.; Shen, P.; Hu, L.; Liu, G.; Wang, J.; Duan, L.; Zhan, S.; et al. Incidence of Inflammatory Bowel Disease in Urban China: A Nationwide Population-Based Study. Clin. Gastroenterol. Hepatol. 2023, 21, 3379–3386.e29. [Google Scholar] [CrossRef]
- Dulai, P.S.; Boland, B.S.; Singh, S.; Chaudrey, K.; Koliani-Pace, J.L.; Kochhar, G.; Parikh, M.P.; Shmidt, E.; Hartke, J.; Chilukuri, P.; et al. Development and Validation of a Scoring System to Predict Outcomes of Vedolizumab Treatment in Patients with Crohn’s Disease. Gastroenterology 2018, 155, 687–695.e10. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.-L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef]
- Yao, L.; Lin, X.; Tang, J.; Gao, H.; Gao, X.; Cao, Q.; Chen, M. Effectiveness, Treatment Pattern, and Safety of Ustekinumab in Treating Bio-Naïve Patients with Crohn’s Disease in the Real-World Clinical Setting in China. Chin. Med. J. 2025. [Google Scholar] [CrossRef]
- Wan, J.; Shen, J.; Wu, X.; Zhong, J.; Chen, Y.; Zhu, L.; Miao, Y.; Hu, N.; Chen, J.; Liang, J.; et al. Geographical Heterogeneity in the Disease Characteristics and Management of Patients with Inflammatory Bowel Disease, the Preliminary Results of a Chinese Database for IBD (CHASE-IBD). Ther. Adv. Gastroenterol. 2023, 16, 17562848231210367. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Sandborn, W.J.; Feagan, B.G.; Gasink, C.; Jacobstein, D.; Zou, B.; Johanns, J.; Adedokun, O.J.; Sands, B.E.; Rutgeerts, P.; et al. IM-UNITI: Three-Year Efficacy, Safety, and Immunogenicity of Ustekinumab Treatment of Crohn’s Disease. J. Crohn’s Colitis 2020, 14, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Barkai, L.J.; Gonczi, L.; Balogh, F.; Angyal, D.; Farkas, K.; Farkas, B.; Molnar, T.; Szamosi, T.; Schafer, E.; Golovics, P.A.; et al. Efficacy, Drug Sustainability, and Safety of Ustekinumab Treatment in Crohn’s Disease Patients over Three Years. Sci. Rep. 2024, 14, 14909. [Google Scholar] [CrossRef] [PubMed]
- Meserve, J.; Ma, C.; Dulai, P.S.; Jairath, V.; Singh, S. Effectiveness of Reinduction and/or Dose Escalation of Ustekinumab in Crohn’s Disease: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2728–2740.e1. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Neurath, M.F. Biomarkers for Personalizing IBD Therapy: The Quest Continues. Clin. Gastroenterol. Hepatol. 2024, 22, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Waljee, A.K.; Liu, B.; Sauder, K.; Zhu, J.; Govani, S.M.; Stidham, R.W.; Higgins, P.D.R. Predicting Corticosteroid-Free Endoscopic Remission with Vedolizumab in Ulcerative Colitis. Aliment. Pharmacol. Ther. 2018, 47, 763–772. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Predictors of Primary Response to Biologic Treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in Patients with Inflammatory Bowel Disease: From Basic Science to Clinical Practice. J. Crohn’s Colitis 2020, 14, 694–709. [Google Scholar] [CrossRef]
- Guizzetti, L.; Zou, G.; Khanna, R.; Dulai, P.S.; Sandborn, W.J.; Jairath, V.; Feagan, B.G. Development of Clinical Prediction Models for Surgery and Complications in Crohn’s Disease. J. Crohn’s Colitis 2018, 12, 167–177. [Google Scholar] [CrossRef]
- Dulai, P.; Guizzetti, L.; Ma, T.; Jairath, V.; Singh, S.; Feagan, B.G.; Gasink, C.; Pires, A.; Sandborn, W.J. 637 Clinical Prediction Model and Decision Support Tool for Ustekinumab in Crohn’s Disease. Am. J. Gastroenterol. 2019, 114, S373. [Google Scholar] [CrossRef]
- Park, J.; Chun, J.; Yoon, H.; Cheon, J.H. Feasibility of a Clinical Decision Support Tool for Ustekinumab to Predict Clinical Remission and Relapse in Patients with Crohn’s Disease: A Multicenter Observational Study. Inflamm. Bowel Dis. 2023, 29, 548–554. [Google Scholar] [CrossRef]
- Shin, S.Y.; Park, S.J.; Kim, Y.; Im, J.P.; Kim, H.J.; Lee, K.-M.; Kim, J.W.; Jung, S.-A.; Lee, J.; Kang, S.-B.; et al. Clinical Outcomes and Predictors of Response for Adalimumab in Patients with Moderately to Severely Active Ulcerative Colitis: A KASID Prospective Multicenter Cohort Study. Intest. Res. 2022, 20, 350–360. [Google Scholar] [CrossRef]
- Kucharzik, T.; Ellul, P.; Greuter, T.; Rahier, J.F.; Verstockt, B.; Abreu, C.; Albuquerque, A.; Allocca, M.; Esteve, M.; Farraye, F.A.; et al. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 879–913. [Google Scholar] [CrossRef]
- Inflammatory Bowel Disease Group of the Chinese Society of Gastroenterology. Chinese Consensus on Diagnosis and Treatment in Inflammatory Bowel Disease. Chin. J. Dig. 2018, 38, 292–311. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Chinese Society of Gastroenterology; Chinese Medical Association. Consensus on Biological Agents in Treating Patients with Inflammatory Bowel Disease. Chin. J. Dig. 2021, 41, 366–378. [Google Scholar]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A Simulation Study of the Number of Events per Variable in Logistic Regression Analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the Performance of Prediction Models: A Framework for Traditional and Novel Measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef]
- McCurdy, J.D.; Israel, A.; Hasan, M.; Weng, R.; Mallick, R.; Ramsay, T.; Carrier, M. A Clinical Predictive Model for Post-Hospitalisation Venous Thromboembolism in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2019, 49, 1493–1501. [Google Scholar] [CrossRef]
- Stoltzfus, J.C. Logistic Regression: A Brief Primer. Acad. Emerg. Med. 2011, 18, 1099–1104. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Anti-TNF Agents and New Biological Agents (Vedolizumab and Ustekinumab) in the Prevention and Treatment of Postoperative Recurrence After Surgery in Crohn’s Disease. Drugs 2023, 83, 1179–1205. [Google Scholar] [CrossRef] [PubMed]
- Golovics, P.A.; Lakatos, L.; Mandel, M.D.; Lovasz, B.D.; Vegh, Z.; Kurti, Z.; Szita, I.; Kiss, L.S.; Pandur, T.; Lakatos, P.L. Prevalence and Predictors of Hospitalization in Crohn’s Disease in a Prospective Population-Based Inception Cohort from 2000–2012. World J. Gastroenterol. 2015, 21, 7272–7280. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.-F.; Sands, B.E.; Gasink, C.; Yeager, B.; Adedokun, O.J.; Izanec, J.; Ma, T.; Gao, L.-L.; Lee, S.D.; Targan, S.R.; et al. Evolution of Symptoms After Ustekinumab Induction Therapy in Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2024, 22, 144–153.e2. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-Reactive Protein: A Critical Update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Sakurai, T.; Saruta, M. Positioning and Usefulness of Biomarkers in Inflammatory Bowel Disease. Digestion 2023, 104, 30–41. [Google Scholar] [CrossRef]
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in Inflammatory Bowel Disease. Clin. Chim. Acta 2020, 510, 556–565. [Google Scholar] [CrossRef]
- Alric, H.; Amiot, A.; Kirchgesner, J.; Tréton, X.; Allez, M.; Bouhnik, Y.; Beaugerie, L.; Carbonnel, F.; Meyer, A. Vedolizumab Clinical Decision Support Tool Predicts Efficacy of Vedolizumab But Not Ustekinumab in Refractory Crohn’s Disease. Inflamm. Bowel Dis. 2022, 28, 218–225. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Adler, J.; Chachu, K.A.; Nguyen, N.H.; Siddique, S.M.; Weiss, J.M.; Sultan, S.; Velayos, F.S.; Cohen, B.L.; Singh, S.; et al. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Crohn’s Disease. Gastroenterology 2023, 165, 1367–1399. [Google Scholar] [CrossRef] [PubMed]
- Hirata, I. Usefulness of Fecal Lactoferrin and Hemoglobin in Diagnosis of Colorectal Diseases. World J. Gastroenterol. 2007, 13, 1569–1574. [Google Scholar] [CrossRef]
- Dulai, P.S.; Amiot, A.; Peyrin-Biroulet, L.; Jairath, V.; Serrero, M.; Filippi, J.; Singh, S.; Pariente, B.; Loftus, E.V.; Roblin, X.; et al. A Clinical Decision Support Tool May Help to Optimise Vedolizumab Therapy in Crohn’s Disease. Aliment. Pharmacol. Ther. 2020, 51, 553–564. [Google Scholar] [CrossRef]
- Forss, A.; Clements, M.; Myrelid, P.; Strid, H.; Söderman, C.; Wagner, A.; Andersson, D.; Hjelm, F.; The PROSE SWIBREG study group; Olén, O.; et al. Ustekinumab Is Associated with Real-World Long-Term Effectiveness and Improved Health-Related Quality of Life in Crohn’s Disease. Dig. Dis. Sci. 2023, 68, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Vande Casteele, N.; Meserve, J.; Winters, A.; Chablaney, S.; Aniwan, S.; Shashi, P.; Kochhar, G.; Weiss, A.; et al. Development and Validation of Clinical Scoring Tool to Predict Outcomes of Treatment with Vedolizumab in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2952–2961.e8. [Google Scholar] [CrossRef]
- Yang, H.; Huang, Z.; Li, M.; Zhang, H.; Fu, L.; Wang, X.; Yang, Q.; He, Y.; Wu, W.; Jiang, T.; et al. Comparative Effectiveness of Ustekinumab vs. Vedolizumab for Anti-TNF-Naïve or Anti-TNF-Exposed Crohn’s Disease: A Multicenter Cohort Study. eClinicalMedicine 2023, 66, 102337. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Shen, B.; Zhu, W. Selection of Treatment Modalities for Intestinal Stenosis in Crohn’s Disease. Chin. J. Inflamm. Bowel Dis. 2019, 3, 169–172. [Google Scholar] [CrossRef]

| Characteristics | Internal (n = 216) | External (n = 317) | p Value |
|---|---|---|---|
| Male, n (%) | 96 (44.44) | 76 (23.97) | 0.072 |
| Median age at diagnosis (IQR), years | 31.35 ± 12.06 | 27.80 ± 11.46 | 0.118 |
| Median age at first UST (IQR), years | 35.60 ± 12.70 | 30.00 ± 11.98 | 0.022 |
| BMI (IQR), kg/m2 | 19.28 ± 2.90 | 19.71 ± 3.11 | 0.112 |
| Smoking status, n (%) | <0.001 | ||
| Never | 196 (90.74) | 280 (88.33) | |
| Current smoker | 11 (5.10) | 35 (11.04) | |
| Smoked previously | 9 (4.16) | 2 (0.63) | |
| Median interval from onset to diagnosis, years | 2.41 ± 3.39 | 3.33 ± 3.77 | 0.004 |
| Severity (CDAI score) | 211.55 ± 62.96 | 151.89 ± 103.36 | <0.001 |
| Location, n (%) | <0.001 | ||
| Ileal | 67 (31.02) | 85 (26.82) | |
| Colonic | 3 (1.39) | 18 (5.68) | |
| Ileocolonic | 91 (42.13) | 184 (58.04) | |
| Upper GI | 55 (25.46) | 30 (9.46) | |
| Behavior, n (%) | <0.001 | ||
| Nonstricturing, nonpenetrating | 72 (33.33) | 164 (51.74) | |
| Stricturing | 97 (44.91) | 96 (30.28) | |
| Penetrating | 47 (21.76) | 57 (17.98) | |
| Perianal involvement, n (%) | 147 (71.36) | 164 (51.74) | <0.001 |
| EIMs, n (%) | 44 (20.37) | 42 (13.25) | 0.028 |
| Prior 5-ASA usage, n (%) | 95 (43.98) | 173 (54.57) | 0.016 |
| Prior steroid usage, n (%) | 52 (24.07) | 113 (35.65) | 0.004 |
| Prior immunosuppressant usage, n (%) | 91 (42.12) | 182 (57.41) | <0.001 |
| Prior biologics usage, n (%) | 126 (58.33) | 172 (54.26) | 0.35 |
| History of gastrointestinal surgery, n (%) | 96 (44.44) | 80 (25.24) | <0.001 |
| History of perianal surgery, n (%) | 80 (37.04) | 111 (35.02) | 0.633 |
| C-reactive protein, mg/L | 16.39 ± 26.72 | 18.61 ± 27.47 | 0.346 |
| Erythrocyte sedimentation rate, mm/h | 18.47 ± 17.22 | 23.35 ± 23.50 | 0.008 |
| White blood cell, 109/L | 5.97 ± 2.13 | 6.72 ± 2.47 | <0.001 |
| Neutrophil, 109/L | 3.93 ± 1.74 | 4.17 ± 1.82 | 0.113 |
| Blood platelet count, 109/L | 259.74 ± 22.35 | 306.57 ± 103.83 | <0.001 |
| Hemoglobin, g/L | 126.67 ± 22.35 | 123.22 ± 21.51 | 0.072 |
| Albumin, g/L | 39.02 ± 5.02 | 39.40 ± 6.17 | 0.460 |
| Concomitant drug usage, n (%) | 17 (7.87) | 37 (11.88) | 0.154 |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Sex | 0.66 (0.3–1.43) | 0.29 | ||
| Median age at diagnosis | 1.01 (0.98–1.04) | 0.36 | ||
| Median age at first UST | 1.02 (0.99–1.04) | 0.25 | ||
| BMI | 0.97 (0.86–1.10) | 0.68 | ||
| Smoking status | ||||
| Never | (reference) | |||
| Current smoker | 0.70 (0.14–3.46) | 0.66 | ||
| Smoked previously | 1.68 (0.38–7.40) | 0.49 | ||
| Severity (CDAI score) | 1.00 (1.00–1.01) | 0.10 | 1.00 (0.99–1.01) | 0.30 |
| Median interval from onset to diagnosis, years | 1.01 (0.92–1.12) | 0.79 | ||
| Location | ||||
| Ileal | (reference) | |||
| Colonic | 2.25 (0.18–27.96) | 0.53 | ||
| Ileocolonic | 1.97 (0.78–4.95) | 0.15 | ||
| Upper GI | 1.80 (0.62–5.20) | 0.28 | ||
| Behavior | ||||
| Nonstricturing, nonpenetrating | (reference) | |||
| Stricturing | 2.29 (0.92–5.72) | 0.28 | ||
| Penetrating | 2.45 (0.86–6.96) | 0.08 | ||
| Perianal involvement | 0.86 (0.39–1.91) | 0.72 | ||
| EIMs | 0.71 (0.27–1.91) | 0.50 | ||
| Prior 5-ASA usage | 0.76 (0.36–1.59) | 0.47 | ||
| Prior steroid usage | 1.15 (0.49–2.66) | 0.75 | ||
| Prior immunosuppressant usage | 1.23 (0.60–2.55) | 0.57 | ||
| Prior biologics usage | 1.58 (0.74–3.39) | 0.14 | 1.47 (0.67–3.34) | 0.30 |
| History of gastrointestinal surgery | 1.47 (0.71–3.03) | 0.30 | ||
| History of perianal surgery | 1.36 (0.65–2.85) | 0.41 | ||
| Concomitant drug usage | 1.43 (0.41–5.04) | 0.58 | ||
| C-reactive protein (mg/L) | 1.01 (1.00–1.02) | 0.13 | 1.01 (0.99–1.02) | 0.30 |
| Erythrocyte sedimentation rate | 1.00 (0.98–1.02) | 0.83 | ||
| White blood cell | 0.80 (0.66–0.97) | 0.03 | ||
| Hemoglobin (g/L) | 0.98 (0.97–1.00) | 0.03 | 0.99 (0.97–1.00) | 0.07 |
| Blood platelet count | 1.00 (0.99–1.00) | 0.63 | ||
| Neutrophil | 0.78 (0.62–0.99) | 0.05 | ||
| Albumin | 0.90 (0.83–0.96) | 0.01 | ||
| Characteristics | Final Model |
|---|---|
| Nagelkerke R2 | 0.08 |
| Brier score | 0.11 |
| ROC-AUC (95% CI) internal validation | 0.88 (0.78–0.97) |
| ROC-AUC (95% CI) external validation | 0.66 (0.60–0.72) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Cao, Y.; Bai, C.; Liu, R.; Yang, W.; Chao, K.; Huang, Z.; Qiu, Y.; Gao, X.; Chen, M.; et al. Development and Validation of a Clinical Decision Support Tool to Predict Disease Progression in Crohn’s Disease Treated with Ustekinumab. J. Clin. Med. 2025, 14, 7919. https://doi.org/10.3390/jcm14227919
Yao L, Cao Y, Bai C, Liu R, Yang W, Chao K, Huang Z, Qiu Y, Gao X, Chen M, et al. Development and Validation of a Clinical Decision Support Tool to Predict Disease Progression in Crohn’s Disease Treated with Ustekinumab. Journal of Clinical Medicine. 2025; 14(22):7919. https://doi.org/10.3390/jcm14227919
Chicago/Turabian StyleYao, Lingya, Yushu Cao, Chenhao Bai, Rongbei Liu, Wenjing Yang, Kang Chao, Zhaopeng Huang, Yun Qiu, Xiang Gao, Minhu Chen, and et al. 2025. "Development and Validation of a Clinical Decision Support Tool to Predict Disease Progression in Crohn’s Disease Treated with Ustekinumab" Journal of Clinical Medicine 14, no. 22: 7919. https://doi.org/10.3390/jcm14227919
APA StyleYao, L., Cao, Y., Bai, C., Liu, R., Yang, W., Chao, K., Huang, Z., Qiu, Y., Gao, X., Chen, M., & Cao, Q. (2025). Development and Validation of a Clinical Decision Support Tool to Predict Disease Progression in Crohn’s Disease Treated with Ustekinumab. Journal of Clinical Medicine, 14(22), 7919. https://doi.org/10.3390/jcm14227919








