Understanding the Transvalvular Gradient in Aortic Stenosis: A Multifaceted Perspective
Abstract
1. Introduction
2. Transvalvular Aortic Gradient: A Multifaceted Parameter of Aortic Stenosis
2.1. Transvalvular Aortic Gradient and Pressure Recovery
2.2. Flow and Transvalvular Aortic Gradient Relationship
2.2.1. Phenotyping Flow Conditions in Aortic Stenosis
Challenges in SV Estimation
2.2.2. Influence of Hemodynamic Factors
2.2.3. Comorbid Cardiac Conditions
2.2.4. Refining Flow State: Stroke Volume vs. Flow Rate
3. Flow-Related Transvalvular Aortic Gradient/Aortic Valve Area Matching
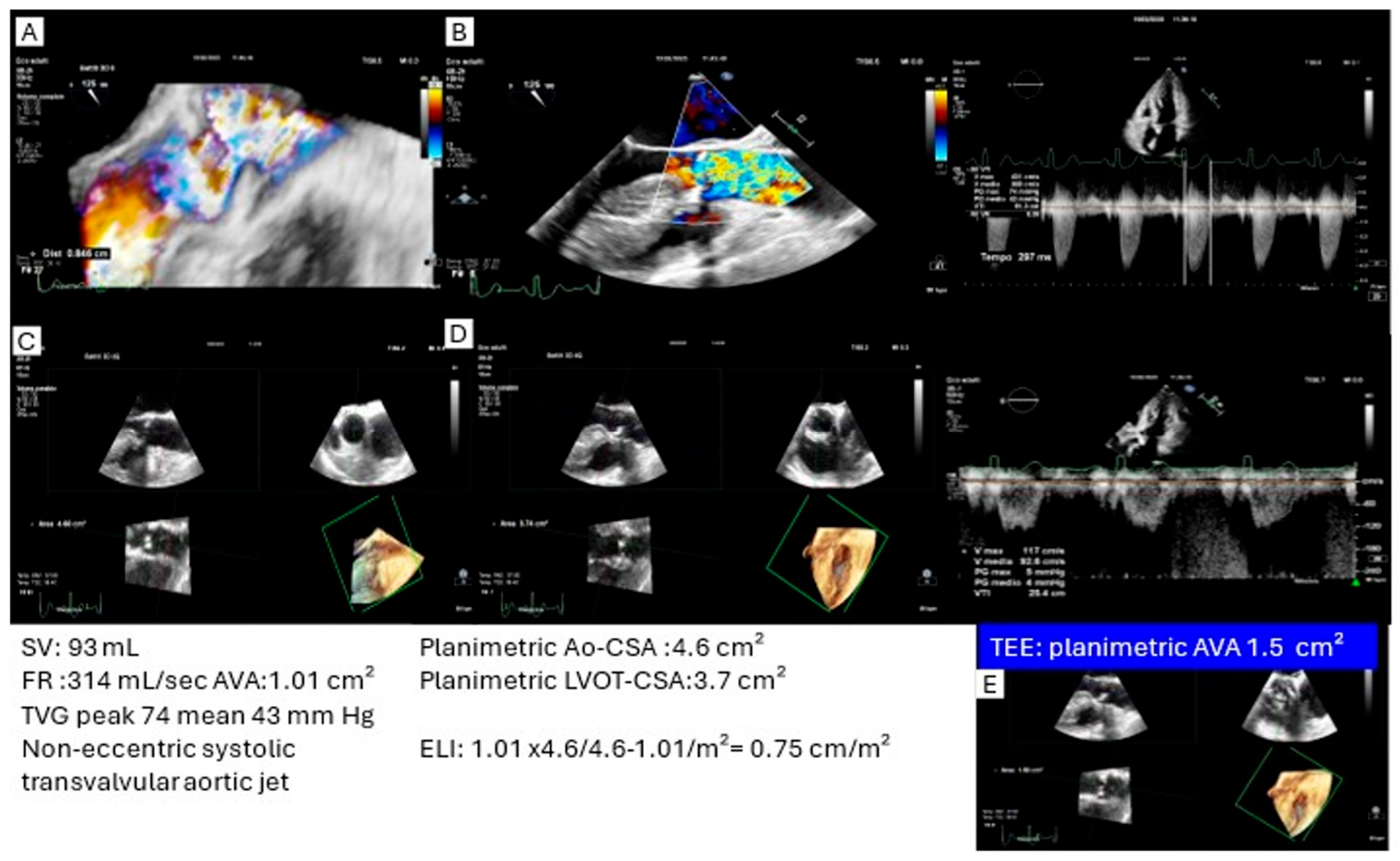
3.1. High Transvalvular Gradient with Discordant Aortic Valve Area (>1 cm2)
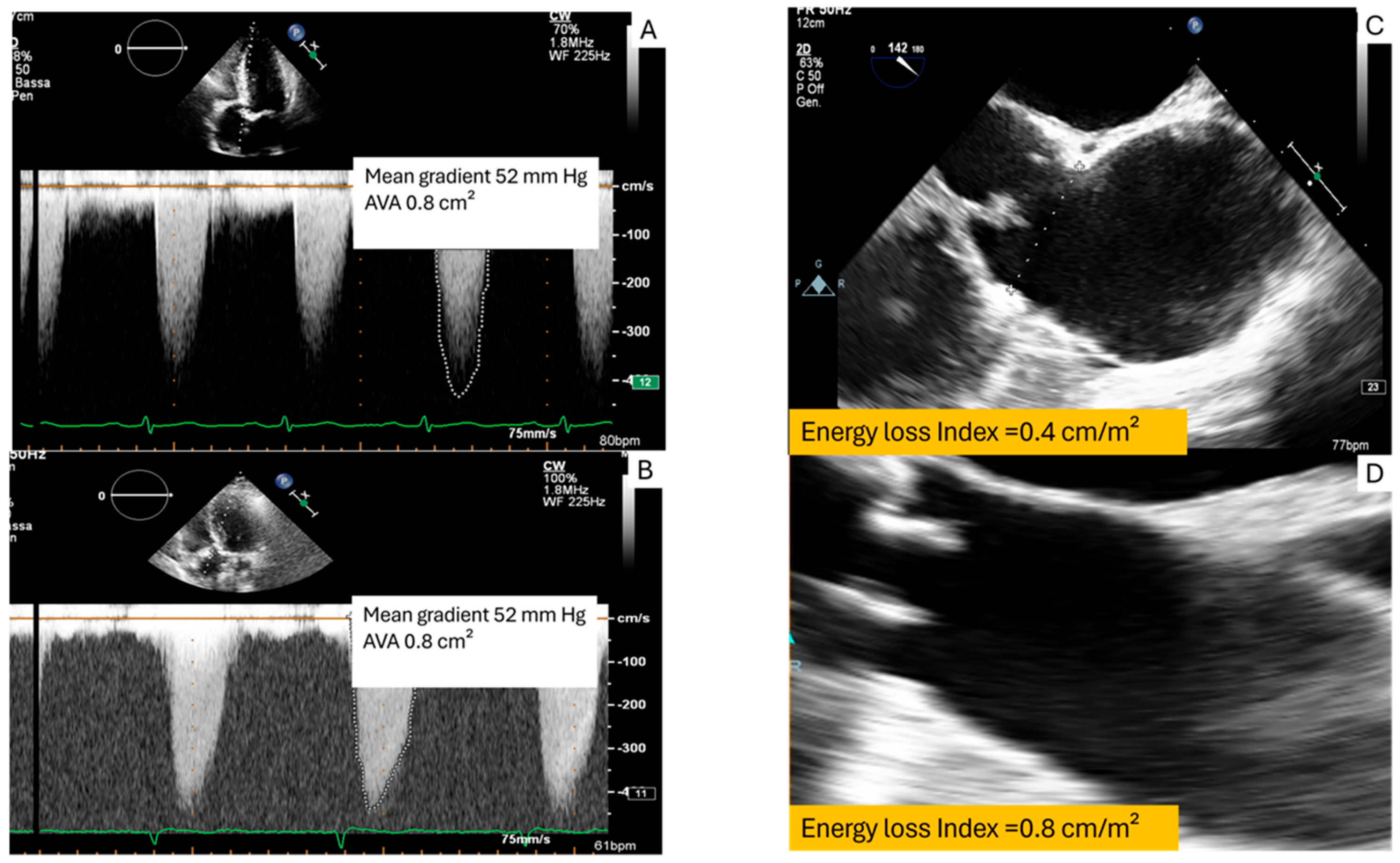
3.2. High Transvalvular Gradient with Concordant Aortic Valve (<1 cm2): What Is the Ultimate Degree of Aortic Stenosis?
3.3. Low-Gradient (Mean Gradient < 40 mm Hg) with Discordant Aortic Valve Area (<1 cm2)
3.4. Low-Low-Ejection-Fraction with Discordant Aortic Valve Area (<1 cm2)
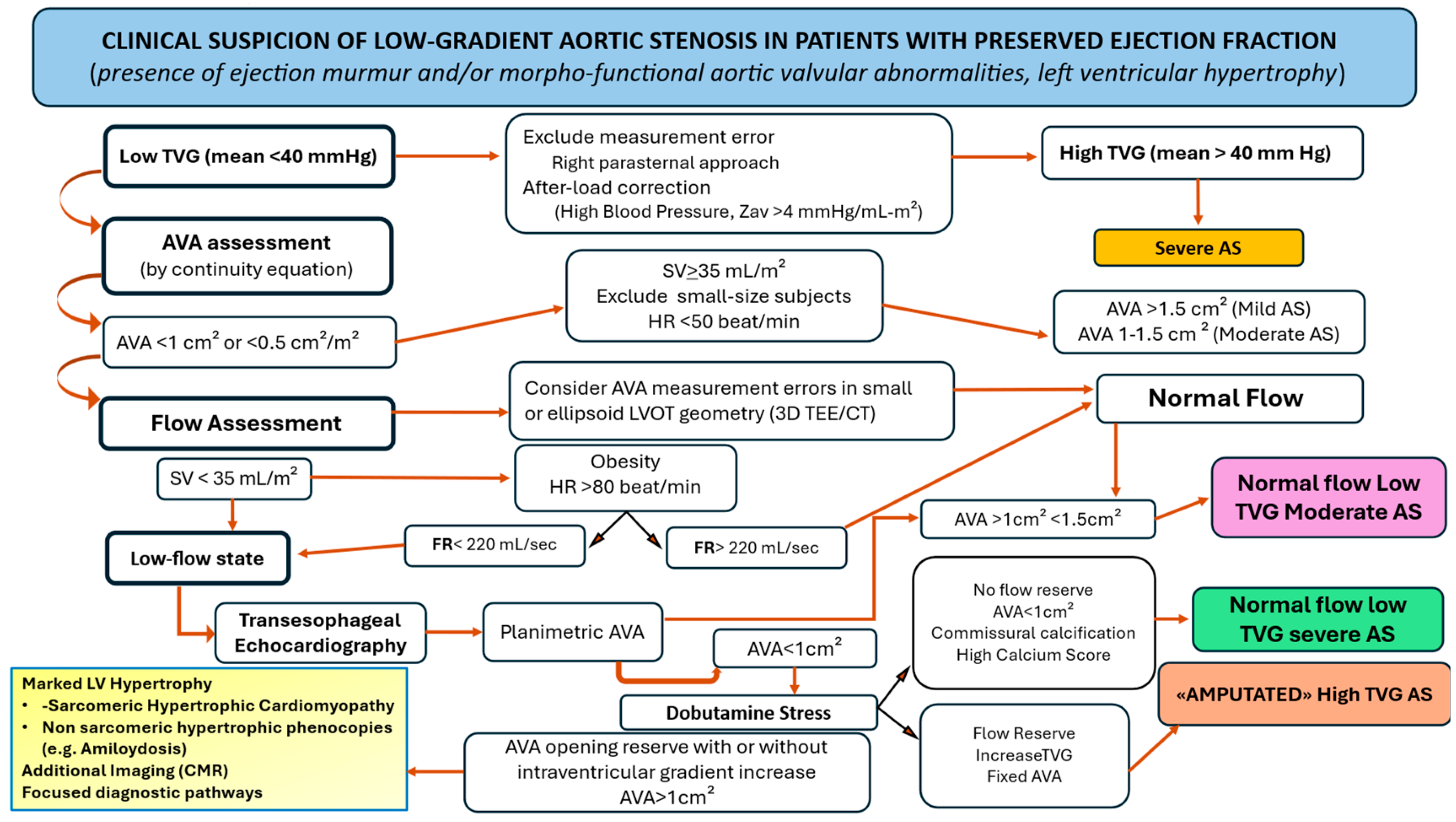
3.5. Normal Flow Low Gradient with Discordant Aortic Valve Area (<1 cm2)
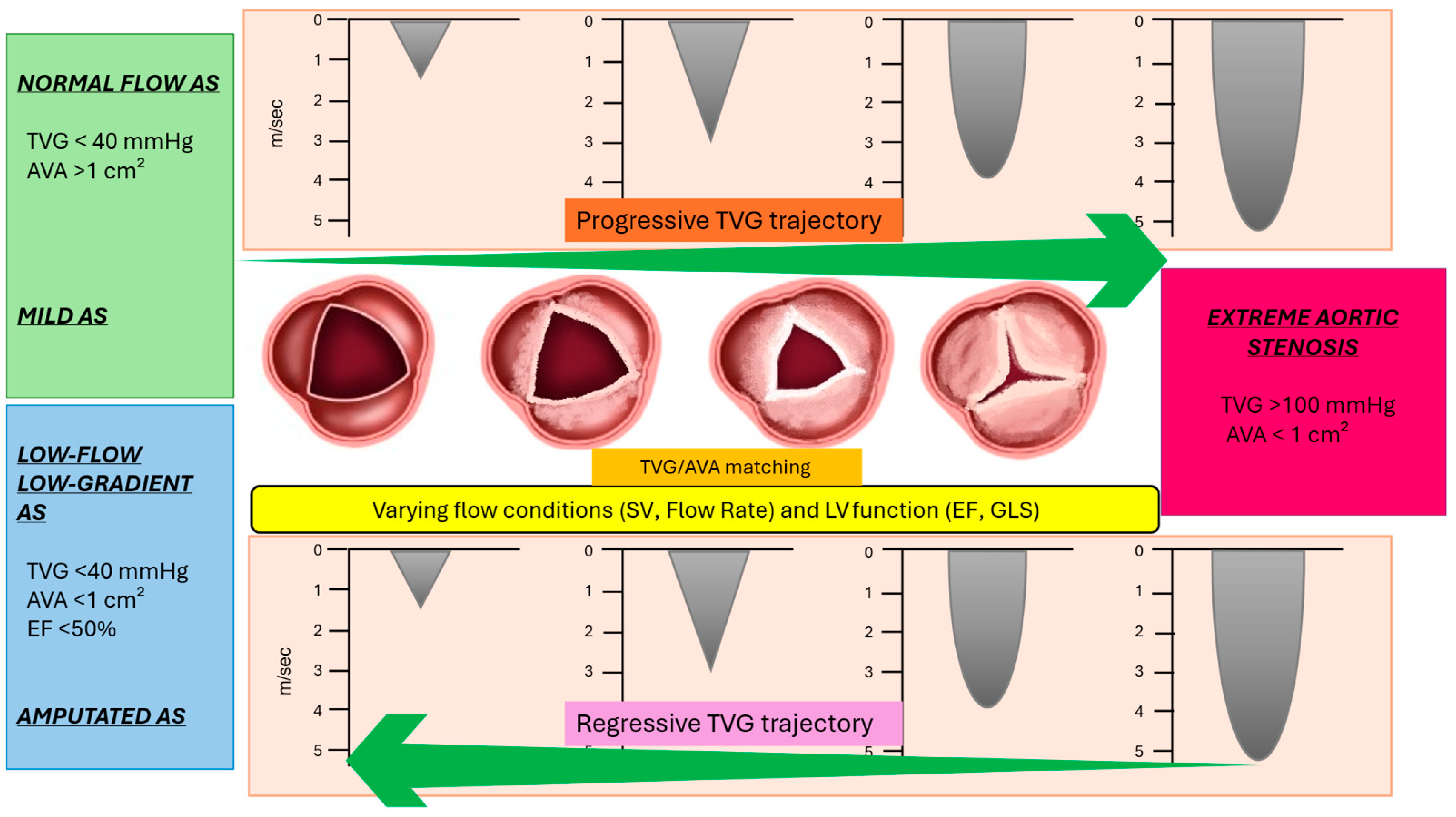
4. Trajectory of Transvalvular Aortic Gradient
5. A Comprehensive Approach to Multifaceted Transvalvular Gradient in Aortic Stenosis
6. Challenges in Diagnosing Aortic Stenosis in Complex Clinical Contexts
6.1. Hypertrophic Cardiomyopathy (Personal Observation)
6.2. Aortic Stenosis and Atrial Fibrillation
7. Perspectives
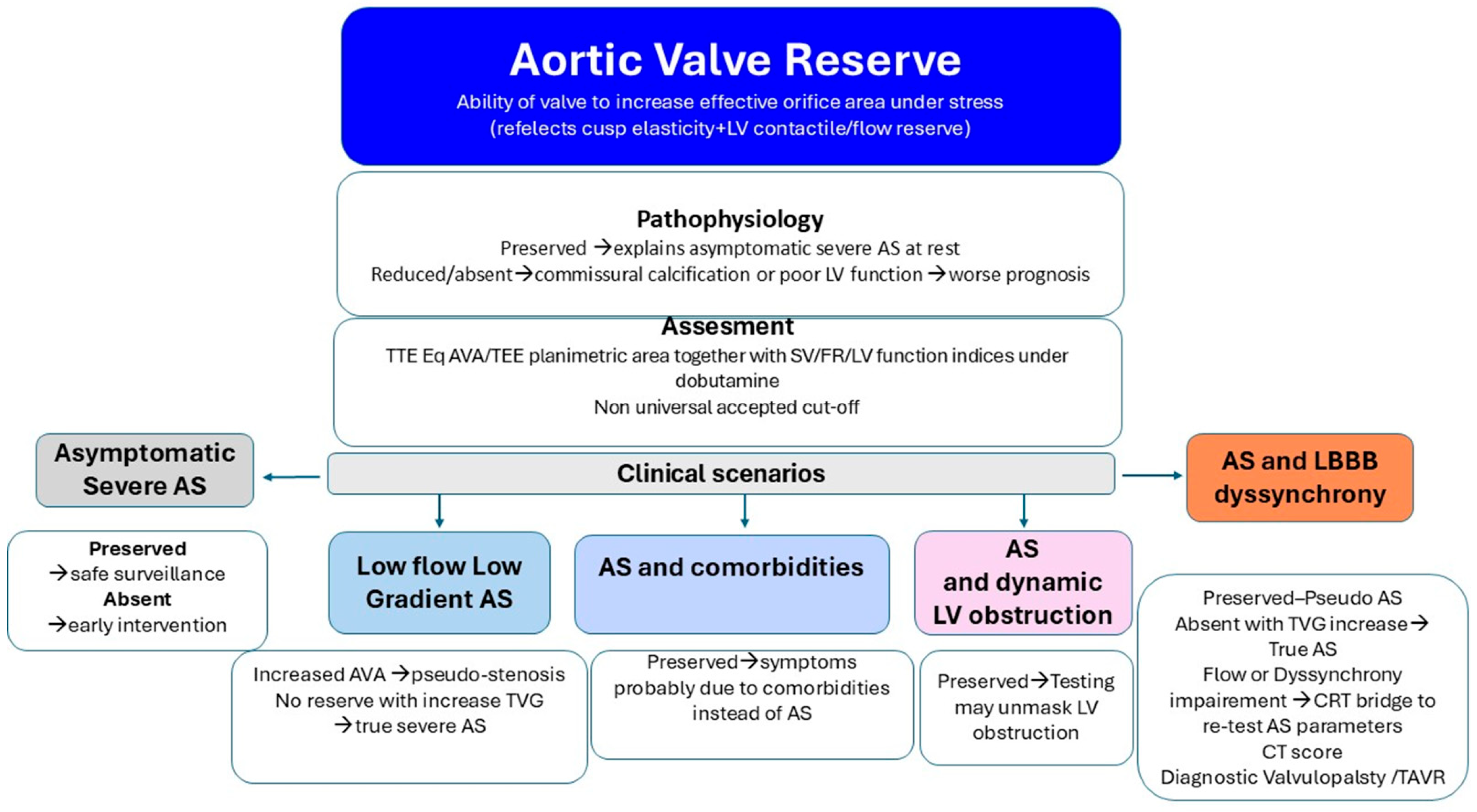
7.1. The Concept of Aortic Valvular Reserve
7.2. Potential Clinical Implications of Aortic Valve Reserve
- ‑
- Asymptomatic Severe AS. Emerging data suggest that early valve replacement in patients with asymptomatic severe aortic stenosis may improve outcomes compared with a surveillance strategy, which cannot reliably predict the onset of acute valve syndromes [99]. In patients with severe AS parameters at rest, preserved valve reserve may justify watchful waiting with close follow-up. Conversely, absent reserve can support earlier intervention despite a lack of overt symptoms.
- ‑
- Low-Flow, Low-Gradient AS vs. Pseudo-Stenosis. Recruitment of LV contractile reserve during inotropic stimulation may enhance valve opening in patients with reduced baseline excursion, supporting a diagnosis of pseudo-stenosis. In contrast, absent valve reserve with increased TVG indicates true (“masked”) severe AS requiring valve replacement.
- ‑
- Symptoms in Severe AS with Comorbidity. Several studies have shown a high rate of readmission for heart failure or a lack of symptomatic improvement following TAVI, suggesting a potentially underrecognized burden of comorbidities [100,101,102]. Valve reserve assessment acknowledges that static measurements may underestimate the hemodynamic impact of obstruction or fail to capture the valve’s latent adaptability. Demonstration of preserved valve reserve suggests that comorbid conditions may be the primary drivers of symptoms, highlighting the importance of comprehensive evaluation in complex and often challenging clinical settings.
- ‑
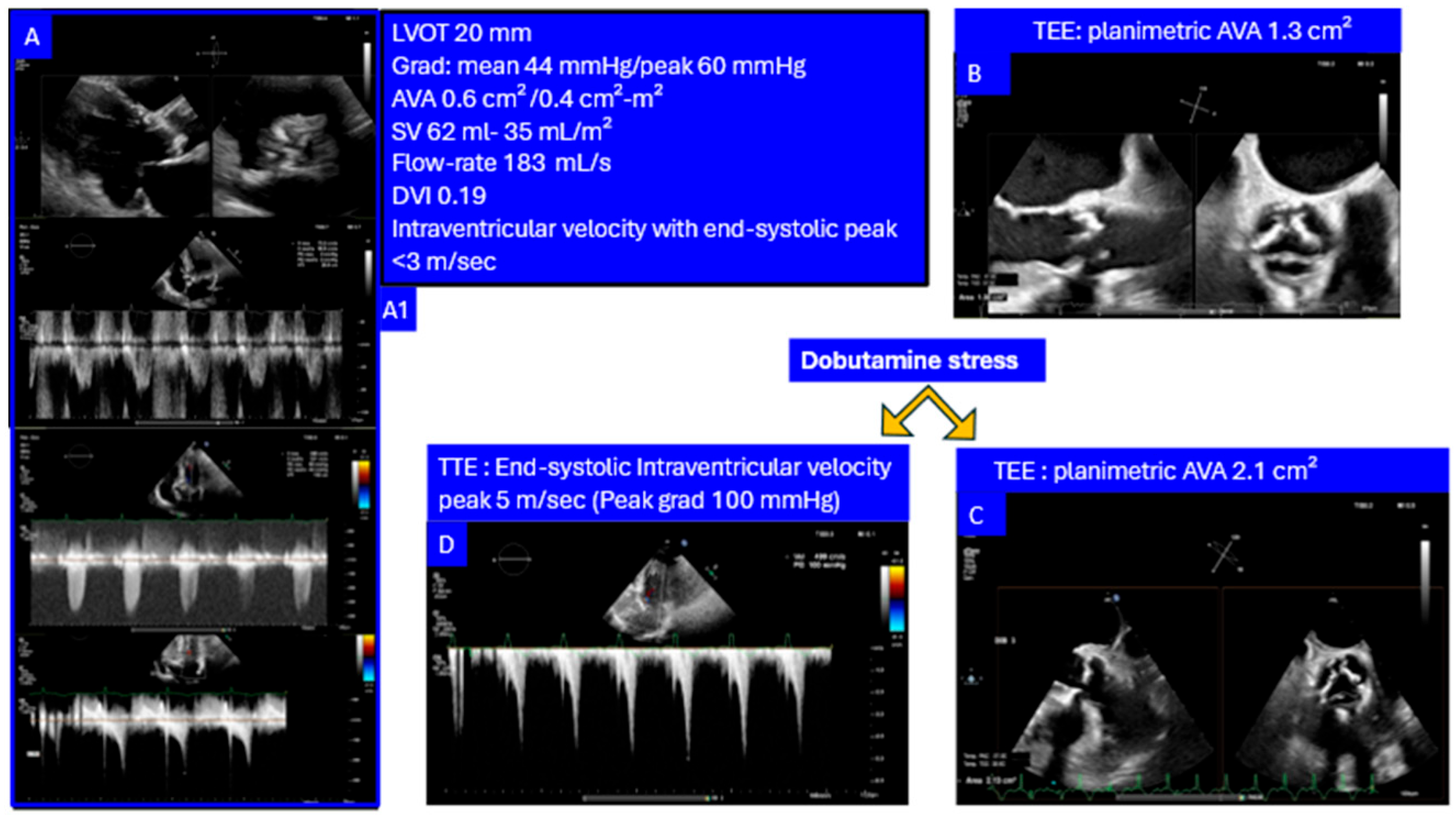
- AS with Intraventricular Dynamic Obstruction. Increased afterload from AS can obscure dynamic intraventricular gradients, which may become apparent after valve replacement. Conversely, preserved valve reserve during dobutamine stress may unmask the dynamic component before intervention supporting a diagnosis of non-significant AS with inducible dominant dynamic LV obstruction as a main cause of symptoms (Figure 6).
7.3. Refinement of Flow Assessment
7.4. Fluid Dynamics: A New Exploratory Frontier in Aortic Stenosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Coffey, S.; Roberts-Thomson, R.; Brown, A.; Carapetis, J.; Chen, M.; Enriquez-Sarano, M.; Zühlke, L.; Prendergast, B.D. Global epidemiology of valvular heart disease. Nat. Rev. Cardiol. 2021, 18, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Benfari, G.; Essayagh, B.; Michelena, H.I.; Ye, Z.; Inojosa, J.M.; Ribichini, F.L.; Crestanello, J.; Messika-Zeitoun, D.; Prendergast, B.; Wong, B.F.; et al. Severe aortic stenosis: Secular trends of incidence and outcomes. Eur. Heart J. 2024, 45, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2021, 143, e72–e227.28. [Google Scholar]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 372–392. [Google Scholar] [CrossRef]
- Lancellotti, P.; Donal, E.; Magne, J.; Moonen, M.; O’Connor, K.; Daubert, J.C.; Pierard, L.A. Risk stratification in asymptomatic moderate to severe aortic stenosis: The importance of the valvular, arterial and ventricular interplay. Heart 2010, 96, 1364–1371. [Google Scholar] [CrossRef]
- Bermejo, J.; Odreman, R.; Feijoo, J.; Moreno, M.M.; Gomez-Moreno, P.; Garcia-Fernandez, M.A. Clinical efficacy of Doppler-echocardiographic indices of aortic valve stenosis: A comparative test-based analysis of outcome. J. Am. Coll. Cardiol. 2003, 41, 142–151. [Google Scholar] [CrossRef]
- Sandeep, B.; Liu, X.; Wu, Q.; Gao, K.; Xiao, Z. Recent updates on asymptomatic and symptomatic aortic valve stenosis its diagnosis, pathogenesis, management and future perspectives. Curr. Probl. Cardiol. 2024, 49, 102631. [Google Scholar] [CrossRef]
- Lindman, B.R.; Dweck, M.R.; Lancellotti, P.; Généreux, P.; Piérard, L.A.; O’Gara, P.T.; O Bonow, R. Management of Asymptomatic severe aortic stenosis: Evolving concepts in timing of valve replacement. JACC Cardiovasc. Imaging 2020, 13, 481–493. [Google Scholar] [CrossRef]
- Abbas, A.E.; Pibarot, P. Hemodynamic characterization of aortic stenosis states. Catheter Cardiovasc Interv. 2019, 93, 1002–1023. [Google Scholar] [CrossRef]
- Hatle, L.; Angelsen, B.A.; Tromsdal, A. Non-invasive assessment of aortic stenosis by Doppler ultrasound. Br. Heart J. 1980, 43, 284–292. [Google Scholar] [CrossRef]
- Firstenberg, M.S.; Abel, E.E.; Papadimos, T.J.; Tripathi, R.S. Nonconvective forces: A critical and often ignored component in the echocardiographic assessment of transvalvular pressure gradients. Cardiol. Res. Pract. 2012, 2012, 383217. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Improving assessment of aortic stenosis. J. Am. Coll. Cardiol. 2012, 60, 169–180. [Google Scholar] [CrossRef]
- Rosenhek, R.; Zilberszac, R.; Schemper, M.; Czerny, M.; Mundigler, G.; Graf, S.; Bergler-Klein, J.; Grimm, M.; Gabriel, H.; Maurer, G. Natural history of very severe aortic stenosis. Circulation 2010, 121, 151–156. [Google Scholar] [CrossRef]
- Rong, L.Q.; Hameed, I.; Di Franco, A.; Rahouma, M.M.; Khan, F.M.; Demetres, M.; Weinsaft, J.W.; Devereux, R.B.; Gaudino, M.A. Pairwise meta-analytic comparison of aortic valve area determined by planimetric versus hemodynamic methods in aortic stenosis. Int. J. Cardiol. 2021, 322, 77–85. [Google Scholar] [CrossRef]
- Pappalardo, O.; Benfari, G.; Jenkins, W.; Foley, T.; Araoz, P.; Redaelli, A.; Onorati, F.; Faggian, G.; Michelena, H.I.; Votta, E.; et al. Quantification of anatomical aortic valve area by multi-detector computed tomography: A pilot 3D-morphological modeling of the stenotic aortic valve. Int. J. Cardiol. 2024, 413, 132–322. [Google Scholar] [CrossRef] [PubMed]
- Minners, J.; Allgeier, M.; Gohlke-Baerwolf, C.; Kienzle, R.P.; Neumann, F.J.; Jander, N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur. Heart J. 2008, 29, 1043. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Pibarot, P.; Dumesnil, J.G.; Sakr, F.; Durand, L.G. Assessment of aortic valve severity: A new index based on the energy loss concept. Circulation 2000, 101, 765–771. [Google Scholar] [CrossRef]
- Garcia, D.; Dumesnil, J.G.; Durand, L.-G.; Kadem, L.; Pibarot, P. Discrepancies Between Catheter and Doppler Estimates of Valve Effective Orifice Area Can Be Predicted from the Pressure Recovery Phenomenon. Practical Implications with Regard to Quantification of Aortic Stenosis Severity. J. Am. Coll. Cardiol. 2003, 41, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Garcia, D.; Dumesnil, J.G. Energy loss index in aortic stenosis: From fluid mechanics concept to clinical application. Circulation 2013, 127, 1101–1104. [Google Scholar] [CrossRef]
- VanAuker, M.D.; Chandra, M.; Shirani, J.; Strom, J.A. Jet eccentricity: A misleading source of agreement between Doppler/catheter pressure gradients in aortic stenosis. J. Am. Soc. Echocardiogr. 2001, 14, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.E.; Franey, L.M.; Lester, S.; Raff, G.; Gallagher, M.J.; Hanzel, G.; Safian, R.D.; Pibarot, P. The role of jet eccentricity in generating disproportionately elevated transaortic pressure gradients in patients with aortic stenosis. Echocardiography 2015, 32, 372–382. [Google Scholar] [CrossRef]
- Bahlmann, E.; Gerdts, E.; Cramariuc, D.; Gohlke-Baerwolf, C.; Nienaber, C.A.; Wachtell, K.; Seifert, R.; Chambers, J.B.; Kuck, K.H.; Ray, S. Prognostic value of energy loss index in asymptomatic aortic stenosis. Circulation 2013, 127, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Magne, J.; Donal, E.; Davin, L.; Kim O’Connor, K.; Rosca, M.; Szymanski, C.; Cosyns, B.; Piérard, L.A. Clinical outcome in asymptomatic severe aortic stenosis: Insights from the new proposed aortic stenosis grading classification. J. Am. Coll. Cardiol. 2012, 59, 235–243. [Google Scholar] [CrossRef]
- Rusinaru, D.; Bohbot, Y.; Ringle, A.; Maréchaux, S.; Diouf, M.; Tribouilloy, C. Impact of low stroke volume on mortality in patients with severe aortic stenosis and preserved left ventricular ejection fraction. Eur. Heart J. 2018, 39, 1992–1999. [Google Scholar] [CrossRef]
- Lønnebakken, M.T.; De Simone, G.; Saeed, S.; Boman, K.; Rossebø, A.B.; Bahlmann, E.; Gohlke-Bärwolf, C.; Gerdts, E. Impact of stroke volume on cardiovascular risk during progression of aortic valve stenosis. Heart 2017, 103, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Guzzetti, E.; Poulin, A.; Annabi, M.-S.; Zhang, B.; Kalavrouziotis, D.; Couture, C.; Dagenais, F.; Pibarot, P.; Clavel, M.-A. Trans-valvular flow, sex, and survival after valve replacement surgery in patients with severe aortic stenosis. J. Am. Coll. Cardiol. 2020, 75, 1897–1909. [Google Scholar] [CrossRef]
- Bahlmann, E.; Gerdts, E.; Einarsen, E.; Midtbø, H.; Pedersen, E.R.; Rossebø, A.; Willems, S.; Cramariuc, D. Impact of sex-specific thresholds for low flow in assessment of prognosis in concordantly and discordantly graded aortic valve stenosis. Eur. Heart J. Cardiovasc. Imaging 2025, 26, 280–286. [Google Scholar] [CrossRef]
- Kardos, A.; Rusinaru, D.; Maréchaux, S.; Alskaf, E.; Prendergast, B.; Tribouilloy, C. Implementation of a CT-derived correction factor to refine the measurement of aortic valve area and stroke volume using Doppler echocardiography improves grading of severity and prediction of prognosis in patients with severe aortic stenosis. Int. J. Cardiol. 2022, 363, 129–137. [Google Scholar] [CrossRef]
- Briand, M.; Dumesnil, J.G.; Kadem, L.; Tongue, A.G.; Rieu, R.; Garcia, D.; Pibarot, P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: Implications for diagnosis and treatment. J. Am. Coll. Cardiol. 2005, 46, 291–298. [Google Scholar] [CrossRef]
- Vamvakidou, A.; Jin, W.; Danylenko, O.; Chahal, N.; Khattar, R.; Senior, R. Low transvalvular flow rate predicts mortality in patients with low-gradient aortic stenosis following aortic valve intervention. JACC Cardiovasc. Imaging 2019, 12, 1715–1724. [Google Scholar] [CrossRef]
- Saeed, S.; Senior, R.; Chahal, N.S.; Lønnebakken, M.T.; Chambers, J.B.; Bahlmann, E.; Gerdts, E. Lower transaortic flow rate is associated with increased mortality in aortic valve stenosis. JACC Cardiovasc Imaging 2017, 10, 912–920. [Google Scholar] [CrossRef]
- Vamvakidou, A.; Annabi, M.-S.; Pibarot, P.; Plonska-Gosciniak, E.; Almeida, A.G.; Ezequiel Guzzetti, E.; Dahou, A.; Burwash, I.G.; Koschutnik, M.; Bartko, P.E. Clinical value of stress transaortic flow rate during dobutamine echocardiography in reduced left ventricular ejection fraction, low-gradient aortic stenosis: A multicenter study. Circ. Cardiovasc. Imaging 2021, 14, e012809. [Google Scholar] [CrossRef]
- Bache, R.J.; Wang, Y.; Greenfield, J.C., Jr. Left ventricular ejection time in valvular aortic stenosis. Circulation 1973, 47, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Sen, J.; Huynh, Q.; Stub, D.; Neil, C.; Marwick, T.H. Prognosis of severe low-flow, low-gradient aortic stenosis by stroke volume index and transvalvular flow rate. JACC Cardiovasc. Imaging 2021, 14, 915–927. [Google Scholar] [CrossRef]
- Bansal, P.; Maini, A.; Abbas, A.; Pibarot, P.; Maini, B.; Khalili, H. Transaortic Flow in Aortic Stenosis: Stroke Volume Index versus Transaortic Flow Rate. J. Am. Soc. Echocardiogr. 2021, 34, 1317–1320. [Google Scholar] [CrossRef]
- Saeed, S.; Vamvakidou, A.; Zidros, S.; Papasozomenos, G.; Lysne, V.; Khattar, R.S.; Senior, R. Sex differences in transaortic flow rate and association with all-cause mortality in patients with severe aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Gallone, G.; Islas, F.; Gorla, R.; Melillo, F.; Leone, P.P.; Cimaglia, P.; Pastore, M.C.; Franzone, A.; Landra, F.; Bruno, F. Stroke volume index and transvalvular flow rate trajectories in severe aortic stenosis treated with TAVR. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, M.; He, W.; Churchill, T.W.; Capoulade, R.; Liu, S.; Lee, H.; Danik, J.S.; Picard, M.H.; Pibarot, P.; Levine, R.A.; et al. Transvalvular flow rate determines prognostic value of aortic valve area in aortic stenosis. J. Am. Coll. Cardiol. 2020, 75, 1758–1769. [Google Scholar] [CrossRef]
- Unger, P.; Powers, A.; Le Nezet, E.; Lacasse-Rioux, E.; Galloo, X.; Clavel, M.A. Prevalence and outcomes of patients with discordant high-gradient aortic stenosis. J. Am. Coll. Cardiol. 2024, 83, 1109–1119. [Google Scholar] [CrossRef]
- Ito, S.; Oh, J.K.; Michelena, H.I.; Egbe, A.C.; Connolly, H.M.; Pellikka, A.P.; Nkomo, V.T.; Lewis, B.R.; Miranda, W.R. High-Gradient Aortic Stenosis with Valve Area > 1.0 cm2. The “Forgotten” Discordant Hemodynamic Phenotype. JACC Cardiovasc. Imaging 2025, 18, 166–176. [Google Scholar] [CrossRef]
- Zoghbi, W.A. High-Gradient “Moderate” Aortic Stenosis. Is it Moderate or Severe? JACC Cardiovac. Imaging 2025, 18, 176–177. [Google Scholar]
- Machida, T.; Izumo, M.; Suzuki, K.; Yoneyama, K.; Kamijima, R.; Mizukoshi, K.; Takai, M.; Kobayashi, Y.; Harada, T.; Miyake, F. Value of anatomical aortic valve area using real-time three-dimensional transoesophageal echocardiography in patients with aortic stenosis: A comparison between tricuspid and bicuspid aortic valves. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1120–1128. [Google Scholar] [CrossRef]
- Burwash, I.G.; Thomas, D.D.; Sadahiro, M.; Pearlman, A.S.; Verrier, E.D.; Thomas, R.; Kraft, C.D.; Otto, C.M. Dependence of Gorlin formula and continuity equation valve areas on transvalvular volume flow rate in valvular aortic stenosis. Circulation 1994, 89, 827–835. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J. Am. Coll. Cardiol. 2012, 60, 1845–1853. [Google Scholar] [CrossRef]
- Hachicha, Z.; Dumesnil, J.G.; Bogaty, P.; Pibarot, P. Paradoxical low flow, low gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007, 115, 2856–2864. [Google Scholar] [CrossRef] [PubMed]
- Mohty, D.; Magne, J.; Deltreuil, M.; Aboyans, V.; Echahidi, N.; Cassat, C.; Pibarot, P.; Laskar, M.; Virot, P. Outcome and impact of surgery in paradoxical low-flow, low-gradient severe aortic stenosis and preserved left ventricular ejection fraction: A cardiac catheterization study. Circulation 2013, 128, 235–242. [Google Scholar] [CrossRef]
- Dumesnil, J.G.; Pibarot, P.; Carabello, B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: Implications for diagnosis and treatment. Eur. Heart J. 2010, 31, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Jander, N.; Minners, J.; Holme, I.; Gerdts, E.; Boman, K.; Brudi, P.; Chambers, J.B.; Egstrup, K.; Kesaniemi, Y.A.; Malbecq, W. Outcome of patients with low-gradient ‘severe’ aortic stenosis and preserved ejection fraction. Circulation 2011, 123, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Eleid, M.F.; Nishimura, R.A.; Sorajja, P.; Borlaug, B.A. Systemic hypertension in low gradient severe aortic stenosis with preserved ejection fraction. Circulation 2013, 128, 1349–1353. [Google Scholar] [CrossRef]
- Roberts, W.C.; Ko, J.M. Relation of weights of operatively excised stenotic aortic valves to preoperative transvalvular peak systolic pressure gradients and to calculated aortic valve areas. J. Am. Coll. Cardiol. 2004, 44, 1847–1855. [Google Scholar] [CrossRef]
- Clavel, M.A.; Côté, N.; Mathieu, P.; Dumesnil, J.G.; Audet, A.; Pépin, A.; Couture, C.; Fournier, D.; Trahan, S.; Pagé, S. Paradoxical low-flow, low-gradient aortic stenosis despite preserved left ventricular ejection fraction: New insights from weights of operatively excised aortic valves. Eur. Heart J. 2014, 35, 2655–2662. [Google Scholar] [CrossRef]
- Clavel, M.A.; Messika-Zeitoun, D.; Pibarot, P.; Aggarwal, S.; Malouf, J.; Araoz, P.; Michelena, H.I.; Cueff, C.; Larose, E.; Capoulade, R.; et al. The complex nature of discordant severe calcified aortic valve disease grading: New insights from combined Doppler-echocardiographic and computed tomographic study. J. Am. Coll. Cardiol. 2013, 62, 2329–2338. [Google Scholar] [CrossRef]
- Clavel, M.A.; Burwash, I.G.; Pibarot, P. Cardiac Imaging for Assessing Low-Gradient Severe Aortic Stenosis. JACC Cardiovasc Imaging 2017, 10, 185–202. [Google Scholar] [CrossRef]
- Alluri, K.; Carabello, B.A.; Nekkanti, R. Imaging Strategies for Evaluating Low-Flow, Low-Gradient Aortic Stenosis with Reduced and Preserved Left Ventricular Ejection Fraction. Curr. Cardiol. Rep. 2019, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.A.; Ennezat, P.V.; Maréchaux, S.; Dumesnil, J.G.; Capoulade, R.; Hachicha, Z.; Mathieu, P.; Bellouin, A.; Bergeron, S.; Meimoun, P. Stress echocardiography to assess stenosis severity and predict outcome in patients with paradoxical low-flow, low-gradient aortic stenosis and preserved LVEF. JACC Cardiovasc. Imaging 2013, 6, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Thaden, J.J.; Nkomo, V.T.; Lee, K.J.; Oh, J.K. Doppler Imaging in Aortic Stenosis: The Importance of the Nonapical Imaging Windows to Determine Severity in a Contemporary Cohort. J. Am. Soc. Echocardiogr. 2015, 28, 780–785. [Google Scholar] [CrossRef]
- Hachicha, Z.; Dumensil, J.D.; Pibarot, P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J. Am. Coll. Cardiol. 2009, 54, 2003–2011. [Google Scholar] [CrossRef]
- Chin, C.W.; Khaw, H.J.; Luo, E.; Tan, S.; White, A.C.; Newby, D.E.; Dweck, M.R. Echocardiography underestimates stroke volume and aortic valve area: Implications for patients with small area low-gradient aortic stenosis. Can. J. Cardiol. 2014, 30, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Michelena, H.I.; Margaryan, E.; Miller, F.A.; Eleid, M.; Maalouf, J.; Suri, R.; Messika-Zeitoun, D.; Pellikka, P.A.; Enriquez-Sarano, M. Inconsistent echocardiographic grading of aortic stenosis: Is the left ventricular outflow tract important? Heart 2013, 99, 921–931. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahsan, M.J.; Newlun, M.; Sand, M.; Rmilah, A.A.; Yousaf, A.; Shabbir, M.A.; Malik, S.A.; Goldsweig, A.M. Outcomes of aortic stenosis in patients with cardiac amyloidosis: A systematic review and meta-analysis. Cardiovasc. Revasc. Med. 2025, 73, 98–106. [Google Scholar] [CrossRef]
- Shively, B.K.; Charlton, G.A.; Crawford, M.H.; Chaney, R.K. Flow Dependence of Valve Area in Aortic Stenosis: Relation to Valve Morphology. J. Am. Coll. Cardiol. 1998, 31, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.-A.; Magne, J.; Pibarot, P. Low-gradient aortic stenosis. Eur. Heart J. 2016, 37, 2645–2657. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Grantham, J.A.; Connolly, H.M.; Schaff, H.V.; Higano, S.T.; Holmes, D.R., Jr. Low-output, low-gradient aortic stenosis in patients with depressed left ventricular systolic function: The clinical utility of the dobutamine challenge in the catheterization laboratory. Circulation 2002, 106, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.-A.; Burwash, I.G.; Mundigler, G.; Dumesnil, J.G.; Baumgartner, H.; Bergler-Klein, J.; Sénéchal, M.; Mathieu, P.; Couture, C.; Beanlands, R.; et al. Validation of Conventional and Simplified Methods to Calculate Projected Valve Area at Normal Flow Rate in Patients with Low Flow, Low Gradient Aortic Stenosis: The Multicenter TOPAS (True or Pseudo Severe Aortic Stenosis) Study. J. Am. Soc. Echocardiogr. 2010, 23, 380–386. [Google Scholar] [CrossRef]
- Blais, C.; Burwash, I.G.; Mundigler, G.; Dumesnil, J.G.; Loho, N.; Rader, F.; Baumgartner, H.; Beanlands, R.S.; Chayer, B.; Kadem, L. Projected valve area at normal flow rate improves the assessment of stenosis severity in patients with low-flow, low-gradient aortic stenosis: The multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) study. Circulation 2006, 113, 711–721. [Google Scholar] [CrossRef]
- Chahal, N.S.; Drakopoulou, M.; Gonzalez-Gonzalez, A.M.; Manivarmane, R.; Khattar, R.; Senior, R. Resting aortic valve area at normal transaortic flow rate reflects true valve area in suspected low-gradient severe aortic stenosis. JACC Cardiovasc. Imaging 2015, 8, 1133–1139. [Google Scholar] [CrossRef]
- Vamvakidou, A.; Chahal, N.; Senior, R. Lack of stroke volume determined flow reserve does not always preclude assessment of severity of aortic stenosis in low-flow low-gradient state during Dobutamine Echocardiography. JACC Cardiovasc. Imaging 2017, 10, 491–493. [Google Scholar] [CrossRef]
- Elkaryoni, A.; Huded, C.P.; Saad, M.; Altibi, A.M.; Chhatriwalla, A.K.; Abbott, J.D.; Arnold, S.V. Normal-Flow Low-Gradient Aortic Stenosis. Comparing the U.S. and European Guidelines. ACC Cardiovasc Imaging 2024, 17, 926–936. [Google Scholar] [CrossRef]
- Eleid, M.F.; Sorajja, P.; Michelena, H.I.; Malouf, J.F.; Scott, C.G.; Pellikka, P.A. Flow-gradient patterns in severe aortic stenosis with preserved ejection fraction: Clinical characteristics and predictors of survival. Circulation 2013, 128, 1781–1789. [Google Scholar] [CrossRef]
- Guzzetti, E.; Pibarot, P.; Clavel, M.A. Normal-flow low-gradient severe aortic stenosis is a frequent and real entity. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1102–1104. [Google Scholar] [CrossRef]
- Maes, F.; Pierard, S.; de Meester, C.; Boulif, J.; Amzulescu, M.; Vancraeynest, D.; Pouleur, A.-C.; Pasquet, A.; Gerber, B.; Vanoverschelde, J.-L. Impact of left ventricular outflow tract ellipticity on the grading of aortic stenosis in patients with normal ejection fraction. J. Cardiovasc. Magn. Reson. 2017, 19, 37. [Google Scholar] [CrossRef]
- Côté, N.; Simard, L.; Zenses, A.-S.; Tastet, L.; Shen, M.; Clisson, M.; Clavel, M.-A. Impact of vascular hemodynamics on aortic stenosis evaluation: New insights into the pathophysiology of normal flow-small aortic valve area-low gradient pattern. J. Am. Heart Assoc. 2017, 6, e006276. [Google Scholar] [CrossRef]
- Saeed, S.; Vamvakidou, A.; Seifert, R.; Khattar, R.; Li, W.; Senior, R. The impact of aortic valve replacement on survival in patients with normal flow low gradient severe aortic stenosis: A propensity-matched comparison. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Zusman, O.; Pressman, G.S.; Banai, S.; Finkelstein, A.; Topilsky, Y. Intervention versus observation in symptomatic patients with normal flow low gradient severe aortic stenosis. JACC Cardiovasc. Imaging 2018, 11, 1225–1232. [Google Scholar] [CrossRef]
- Chadha, G.; Bohbot, Y.; Rusinaru, D.; Maréchaux, S.; Tribouilloy, C. Outcome of normal-flow low gradient severe aortic stenosis with preserved left ventricular ejection fraction: A propensitymatched study. J. Am. Heart Assoc. 2019, 8, e012301. [Google Scholar] [CrossRef]
- Kang, D.H.; Jang, J.Y.; Park, S.J. Watchful observation versus early aortic valve replacement for symptomatic patients with normal flow, low-gradient severe aortic stenosis. Heart 2015, 101, 1375–1381. [Google Scholar] [CrossRef]
- Kim, K.; Cho, I.; Ko, K.-Y.; Lee, S.-E.; Lee, S.; Hong, G.-R.; Ha, J.-W.; Shim, C.Y. Early aortic valve replacement in symptomatic normal-flow, low-gradient severe aortic stenosis: A Propensity score Matched retrospective cohort study. Korean Circ. J. 2023, 53, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.; Suppah, M.; Awad, K.; Farina, J.; Heon, B.J.; Wraith, R.; Abraham, B.; Kaldas, S.; Nkomo, V.; Arsanjani, R.; et al. Reevaluating Normal-Flow Low-Gradient Severe Aortic Stenosis: Clinical Phenotypes and Outcomes in Severe Aortic Stenosis Among Transcatheter Aortic Valve Replacement Patients. J. Am. Soc. Echocardiogr. 2025, 38, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, I.; Harris, A.W.; Seth, M.; Sukul, D.; Deeb, G.M.; Joseph, M.S.; Grossman, P.M.; Fukuhara, S.; Chetcuti, S. Quality of Life After Transcatheter Aortic Valve Replacement in Normal-Flow, Low-Gradient Aortic Stenosis. JACC Adv. 2023, 2, 100641. [Google Scholar] [CrossRef]
- Généreux, P.; Lindmanm, B.R.; Pibarot, P. New Classification to Describe Clinical Presentation in Aortic Stenosis: Stable, Progressive, and Acute Valve Syndrome. Circulation 2025, 151, 1627–1629. [Google Scholar] [CrossRef]
- Mehrotra, P.; Jansen, K.; Flynn, A.W.; Tan, T.C.; Elmariah, S.; Picard, M.H.; Hung, J. Differential left ventricular remodelling and longitudinal function distinguishes low flow from normal-flow preserved ejection fraction low-gradient severe aortic stenosis. Eur. Heart J. 2013, 34, 1906–1914. [Google Scholar] [CrossRef]
- Kwak, S.; Everett, R.J.; Treibel, T.A.; Yang, S.; Hwang, D.; Ko, T.; Williams, M.C.; Bing, R.; Singh, T.; Joshi, S. Markers of Myocardial Damage Predict Mortality in Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2021, 78, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Springhetti, P.; Tomaselli, M.; Benfari, G.; Milazzo, S.; Ciceri, L.; Penso, M.; Pilan, M.; Clement, A.; Rota, A.; Del Sole, P.A. Peak atrial longitudinal strain and risk stratification in moderate and severe aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 947–957. [Google Scholar] [CrossRef]
- Fortuni, F.; Bax, J.J.; Delgado, V. Changing the paradigm in the management of valvular heart disease: In addition to left ventricular ejection fraction, focus on the myocardium. Circulation 2021, 143, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.S.; Julakanti, R.; Ali, M.; Scott, C.G.; Padang, R.; Pellikka, P.A. Cardiac damage in early aortic stenosis: Is the valve to blame? JACC Cardiovasc. Imaging 2024, 17, 1031–1040. [Google Scholar] [CrossRef]
- Tastet, L.; Tribouilloy, C.; Maréchaux, S.; Vollema, E.M.; Delgado, V.; Salaun, E.; Shen, M.; Capoulade, R.; Clavel, M.A.; Arsenault, M. Staging cardiac damage in patients with asymptomatic aortic valve stenosis. J. Am. Coll. Cardiol. 2019, 74, 550–563. [Google Scholar] [CrossRef]
- Généreux, P.; Pibarot, P.; Redfors, B.; Bax, J.J.; Zhao, Y.; Makkar, R.R.; Kapadia, S.; Thourani, V.H.; Mack, M.J.; Nazif, T.M. Evolution and prognostic impact of cardiac damage after aortic valve replacement. J. Am. Coll. Cardiol. 2022, 80, 783–800. [Google Scholar] [CrossRef]
- Ajmone Marsan, N.; Delgado, V.; Shah, D.J.; Pellikka, P.; Bax, J.J.; Treibel, T.; Cavalcante, J.L. Valvular heart disease: Shifting the focus to the myocardium. Eur. Heart J. 2023, 44, 28–40. [Google Scholar] [CrossRef]
- Lancellotti, P.; Lebois, F.; Simon, M.; Tombeux, C.; Chauvel, C.; Luc, A.; Pierard, L.A. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005, 112 (Suppl. I), I-377–I-382. [Google Scholar] [CrossRef]
- Alkurashi, A.K.; Thaden, J.J.; Naser, J.A.; El-Am, E.A.; Pislaru, S.V.; Greason, K.L.; Negrotto, S.M.; Clavel, M.-A.; Pellikka, P.A.; Maleszewski, J.J.; et al. Underestimation of Aortic Stenosis Severity by Doppler Mean Gradient during Atrial Fibrillation: Insights from Aortic Valve Weight. J. Am. Soc. Echocardiogr. 2023, 36, 53–59. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M. Echocardiographic Assessment of Valve Stenosis: EAE/ASE Recommendations for clinical practice. J. Am. Soc. Echocardiogr. 2009, 22, 1–23. [Google Scholar] [CrossRef]
- Niemi, H.J.; Suihko, S.; Kylmala, M.; Rajala, H.; Syvaranta, S.; Kivisto, S.; Lommi, J. Impact of Atrial Fibrillation on the Symptoms and Echocardiographic Evaluation of Patients with Aortic Stenosis. Am. J. Cardiol. 2024, 211, 122–129. [Google Scholar] [CrossRef]
- Alsidawi, S.; Khan, S.; Pislaru, S.V.; Thaden, J.T.; El-Am, E.A.; Scott, G.C.; Morant, K.; Oguz, D.; Luis, S.A.; Padang, R. High Prevalence of Severe Aortic Stenosis in Low-Flow State Associated with Atrial Fibrillation. Circ. Cardiovasc. Imaging 2021, 14, e012453. [Google Scholar] [CrossRef]
- Das, P.; Rimington, H.; Smeeton, N.; Chambers, J. Determinants of symptoms and exercise capacity in aortic stenosis: A comparison of resting haemodynamics and valve compliance during dobutamine stress. Eur. Heart J. 2003, 24, 1254–1263. [Google Scholar] [CrossRef]
- Paulus, W.J.; Sys, S.U.; Heyndrickx, G.R.; Andries, E. Orifice variability of the stenotic aortic valve: Evaluation before and after balloon aortic valvuloplasty. J. Am. Coll. Cardiol. 1991, 17, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Banovic, M.; Kang, D.H.; Giustino, G.; Prendergast, B.D.; Lindman, B.R.; Newby, D.E.; Pibarot, P.; Redfors, B.; Craig, N.J. Aortic Valve Replacement vs Clinical Surveillance in Asymptomatic Severe Aortic Stenosis: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2025, 85, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Doutriaux, M.; Bettinger, N.; Tron, C.; Fauvel, C.; Bauer, F.; Dacher, J.N.; Bouhzam, N.; Litzler, P.Y.; Cribier, A.; et al. Incidence, Prognostic Impact, and Predictive Factors of Readmission for Heart Failure After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Bakhti, A.; Leurent, G.; Bedossa, M.; Tomasi, J.; Belhaj Soulami, R.; Verhoye, J.P.; Donal, E.; Galli, E.; Loirat, A. Determinants and Impact of Heart Failure Readmission Following Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2020, 13, e008959. [Google Scholar] [CrossRef]
- Van Bergeijk, K.H.; Venema, C.S.; Ophuis, B.; Plekkenpol, L.H.; Tomei, M.; Al-Barwary, H.; Tromp, J.; Hummel, Y.M.; Ouwerkerk, W.; van den Heuvel, A.F.M. Less Symptom Improvement in Patients Undergoing TAVI with Concomitant COPD, Atrial Fibrillation and Heart Failure. Catheter. Cardiovasc. Interv. 2025, 106, 1828–1836. [Google Scholar] [CrossRef]
- Garnier, F.; Eicher, J.C.; Jazayeri, S.; Bertaux, G.; Bouchot, O.; Aho, L.S.; Wolf, J.E.; Laurent, G. Usefulness and limitations of contractile reserve evaluation in patients with low-flow, low-gradient aortic stenosis eligible for cardiac resynchronization therapy. Eur. J. Heart Fail. 2014, 16, 648–654. [Google Scholar] [CrossRef]
- Lancellotti, P.; Szymanski, C.; Moonen, M.; Garweg, C.; O’Connor, K.; Tribouilloy, C.; Piérard, L.A. Dynamic left ventricular dyssynchrony: A potential cause of no contractile reserve in patients with low-gradient aortic stenosis. Eur. J. Echocardiogr. 2009, 10, 880–883. [Google Scholar] [CrossRef]
- Hu, H.; Huang, H.; Li, M.; Gao, X.; Yin, L.; Qi, R.; Wu, R.S.; Chen, X.; Ma, Y.; Shi, K. A wearable cardiac ultrasound imager. Nature 2023, 613, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, G.; Piazzese, C.; Lang, R.M.; Muratori, M.; Chiorino, E.; Mapelli, M.; Fusini, L.; Ali, S.G.; Gripari, P.; Pontone, G. Feasibility and Accuracy of Automated Software for Transthoracic Three-Dimensional Left Ventricular Volume and Function Analysis: Comparisons with Two-Dimensional Echocardiography, Three-Dimensional Transthoracic Manual Method, and Cardiac Magnetic Resonance Imaging. J. Am. Soc. Echocardiogr. 2017, 30, 1049–1058. [Google Scholar]
- Pedrizzetti, G.; La Canna, G.; Alfieri, O.; Tonti, G. The vortex—An early predictor of cardiovascular outcome? Nat. Rev. Cardiol. 2014, 11, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Demirkiran, A.; van Ooij, P.; Westenberg, J.J.M.; Hofman, M.B.M.; van Assen, H.C.; Schoonmade, L.J.; Asim, U.; Blanken, C.P.S.; Nederveen, A.J.; van Rossum, A.C. Clinical intra-cardiac 4D flow CMR: Acquisition, analysis, and applications. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 154–165. [Google Scholar] [CrossRef]
- Kilner, P.J.; Yang, G.Z.; Wilkes, A.J.; Mohiaddin, R.H.; Firmin, D.N.; Yacoub, M.H. Asymmetric redirection of flow through the heart. Nature 2000, 404, 759–761. [Google Scholar] [CrossRef]
- Garcia, J.; Barker, A.J.; Markl, M. The role of imaging of flow patterns by 4D flow MRI in aortic stenosis. JACC Cardiovasc. Imaging 2019, 12, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, H.; Andiapen, M.; Baumbach, A.; Mathur, A.; Kerswell, R.R. Wall shear stress and pressure patterns in aortic stenosis patients with and without aortic dilation captured by high-performance image-based computational fluid dynamics. PLoS Comput. Biol. 2023, 19, e1011479. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, V.; Arun Kolanjiyil, A.; Wanna, C.; Kim, H.; Prakash, S.; Krishnan, B.; Chandran, P.; McPherson, D.D.; Johnson, N.P. Biomechanical Evaluation of Aortic Valve Stenosis by Means of a Virtual Stress Test: A Fluid–Structure Interaction Study. Ann. Biomed. Eng. 2024, 52, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Markl, M.; Sathananthan, J.; Sellers, S.L.; Medur, C.; Cavalcante, J. Restoration of flow in the aorta: A novel therapeutic target in aortic valve intervention. Nat. Rev. Cardiol. 2024, 21, 264–273. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Canna, G.; Habjan, S.; Scarfò, I. Understanding the Transvalvular Gradient in Aortic Stenosis: A Multifaceted Perspective. J. Clin. Med. 2025, 14, 7916. https://doi.org/10.3390/jcm14227916
La Canna G, Habjan S, Scarfò I. Understanding the Transvalvular Gradient in Aortic Stenosis: A Multifaceted Perspective. Journal of Clinical Medicine. 2025; 14(22):7916. https://doi.org/10.3390/jcm14227916
Chicago/Turabian StyleLa Canna, Giovanni, Sara Habjan, and Iside Scarfò. 2025. "Understanding the Transvalvular Gradient in Aortic Stenosis: A Multifaceted Perspective" Journal of Clinical Medicine 14, no. 22: 7916. https://doi.org/10.3390/jcm14227916
APA StyleLa Canna, G., Habjan, S., & Scarfò, I. (2025). Understanding the Transvalvular Gradient in Aortic Stenosis: A Multifaceted Perspective. Journal of Clinical Medicine, 14(22), 7916. https://doi.org/10.3390/jcm14227916




