1. Introduction
Psoriasis vulgaris is a chronic, immune-mediated dermatological condition that affects approximately 2–3% of the global population [
1]. Although the disease primarily manifests through erythematous, scaly plaques on the skin, growing evidence highlights its systemic nature, with implications extending beyond the integumentary system [
2]. Patients with psoriasis frequently experience metabolic comorbidities such as obesity, insulin resistance, dyslipidemia, and an increased risk of cardiovascular disease [
3,
4,
5,
6]. These associations point toward a systemic pro-inflammatory and oxidative milieu, in which immune dysregulation, chronic inflammation, and metabolic imbalance create a self-perpetuating loop that complicates both disease progression and treatment [
7,
8].
Cyclosporine A (CsA) is a potent immunosuppressant used in dermatology for the management of moderate to severe psoriasis [
9,
10]. By inhibiting calcineurin, CsA blocks the activation of nuclear factors of activated T-cells (NFAT), thereby suppressing the transcription of pro-inflammatory cytokines such as IL-2 and IFN-γ. This mechanism makes CsA highly effective for short-term disease control [
11,
12]. However, its systemic impact on renal function, electrolyte balance, and redox status necessitates careful monitoring. The influence of CsA on metabolic and biochemical pathways, especially in the context of long-standing inflammation such as psoriasis, remains an area of active investigations [
13,
14].
Among the less explored aspects of psoriasis and its treatment is the alteration of micro- and macroelement homeostasis [
15,
16].
In psoriasis, characteristic patterns of trace element imbalance have been described. Serum zinc (Zn) is frequently reduced, which relates to impaired keratinocyte differentiation and may track with disease severity [
15,
17,
18,
19]. Magnesium (Mg) concentrations are often lowered as well, reflecting its involvement in membrane stabilization and antioxidant enzyme activity [
16,
19]. Disturbances in intracellular calcium gradients contribute to abnormal epidermal differentiation [
20,
21,
22], while copper levels may be normal or slightly increased; consequently, the copper/zinc (Cu/Zn) ratio tends to rise and is considered a simple surrogate marker of systemic inflammation [
18,
23,
24]. Findings for iron remain variable, though elevations can accompany oxidative stress and chronic inflammatory signaling [
7,
25]. Alterations in sodium (Na) and potassium (K) appear less consistent and less clearly linked with disease activity [
26]. These mineral abnormalities coexist with a shift toward a pro-oxidative systemic state characterized by reduced total antioxidant capacity (TAS), increased total oxidant load (TOS), and a higher oxidative stress index (OSI = TOS/TAS × 100) [
8,
19,
27,
28,
29]. Collectively, these disturbances support the concept that oxidative imbalance and trace element dysregulation are intertwined with psoriasis activity and extent [
7,
8,
27].
Elements such as Zn, Mg, iron (Fe), Cu, calcium (Ca), K, and Na are critically involved in epidermal barrier maintenance, cellular immunity, keratinocyte proliferation, and enzymatic defense against oxidative stress [
30]. Disturbances in the concentrations of these elements have been observed in psoriatic patients, often correlating with disease severity or systemic inflammation. However, the directionality and causality of these changes—whether they result from disease activity, nutritional deficiencies, or pharmacological intervention—remain unclear [
8,
17,
18,
26].
Furthermore, oxidative stress has been proposed as both a trigger and consequence of psoriatic inflammation. Elevated levels of reactive oxygen species (ROS), along with reduced activity of antioxidant enzymes and molecules, have been reported in psoriatic lesions and systemic circulation. These changes are thought to contribute to keratinocyte hyperproliferation, immune cell activation, and endothelial dysfunction [
8,
27,
31]. Yet, the interplay between antioxidant status and systemic therapy with CsA is not well understood, particularly with regard to long-term metabolic implications [
32,
33].
Despite these known associations, a significant gap in the literature exists regarding the comprehensive assessment of mineral profiles and antioxidant defense systems in psoriatic patients during immunosuppressive treatment. Most available studies either focus on untreated patients or lack longitudinal data capturing the biochemical effects of systemic therapies. Moreover, the potential modifying role of dietary patterns, which may influence both elemental balance and antioxidant capacity, is rarely integrated into clinical research on psoriasis [
16,
19,
34,
35,
36]. To address this gap, the present study assessed longitudinal changes in serum trace and macroelements, TAS, TOS, and OSI over a 12-week course of CsA treatment in psoriasis patients. Special focus was placed on the Cu/Zn ratio as a marker of systemic inflammation. It was hypothesized that CsA would normalize the Cu/Zn ratio, increase TAS, reduce TOS and OSI, and that these changes would correlate with clinical improvement (PASI, BSA). Additionally, the study explored whether Mg and Fe levels could serve as indicators of treatment response.
2. Materials and Methods
2.1. Ethical Approval
This prospective, observational cohort study was conducted in accordance with the ethical standards of the Declaration of Helsinki and was approved by the Bioethics Committee of the Academy of Silesia, Poland (protocol no. 02/KEBN/2023). Written informed consent was obtained from all participants prior to enrollment. Each subject was informed that their clinical and anthropometric data would be processed in a pseudonymized manner. Personal identifiers were replaced with unique codes, and the key linking codes to identities was stored separately under secure conditions, accessible only to authorized study investigators (P.M. and A.M.-B.) and used exclusively for research and publication purposes.
2.2. Study Design, Participants, and Clinical/Lifestyle Subgroup Classification
A total of 46 adult patients with moderate-to-severe plaque psoriasis who were eligible for systemic treatment with cyclosporine A (CsA) were initially recruited. Only patients with plaque psoriasis (psoriasis vulgaris) were included. Other clinical forms such as guttate, pustular, or erythrodermic psoriasis, as well as psoriatic arthritis requiring systemic therapy, were excluded from the study. Inclusion criteria comprised: age 18–65 years, chronic plaque psoriasis for ≥6 months, and disease severity indicated by PASI ≥ 10 or body surface area involvement ≥10 percent. Exclusion criteria included: pregnancy or lactation; psoriatic arthritis requiring systemic therapy; active infection within four weeks before baseline; history of malignancy; significant hepatic or renal impairment (ALT or AST > 2 × ULN; eGFR < 60 mL/min/1.73 m2); other autoimmune or systemic inflammatory disorders; or use of biological agents and intensive phototherapy or systemic immunosuppressants within eight weeks prior to baseline. Stable topical therapy and tapering UVB311 or methotrexate used during the qualification period were permitted, provided they were discontinued no later than baseline to minimize confounding effects on mineral and redox parameters.
After applying the eligibility criteria and excluding patients with incomplete nutritional or clinical data, 37 participants (20 men and 17 women; mean age ± SD: 47.8 ± 4.9 years) were included in the final analysis. All patients received oral CsA according to current therapeutic guidance [
37,
38]: 5 mg/kg/day administered in two divided doses during the first 42 days, followed by 2.5 mg/kg/day until day 84. Treatment adherence was monitored at all visits, no premature discontinuations occurred, and adverse events were mild, consisting only of transient gastrointestinal discomfort.
Whole-blood samples were collected at three predefined timepoints to monitor biochemical, mineral, and oxidative stress profiles: at baseline (day 0), mid-therapy (day 42), and at the end of treatment (day 84). Nutritional status, habitual dietary intake, alcohol consumption, and use of vitamin or mineral supplements were assessed at baseline. Patients reporting high-dose micronutrient supplementation (including iron, zinc, magnesium, antioxidant complexes) initiated or modified within three months before enrollment, or using medications affecting mineral homeostasis (diuretics, systemic corticosteroids, chronic high-dose NSAIDs), were excluded. Routine medications without proven influence on trace element metabolism were permitted and documented.
To enable comparative analyses and confounder adjustment, participants were stratified into predefined clinical and lifestyle subgroups. Smoking status was assessed via structured interview and classified as current smokers (nicotine use within the previous six months) or non-smokers (no active or historical smoking). Alcohol intake was categorized as abstinent or moderate consumption (≤14 units/week for men; ≤7 units/week for women). Individuals reporting excessive alcohol use were excluded. Type 2 diabetes mellitus was confirmed by medical record review, antidiabetic pharmacotherapy, and baseline laboratory criteria (fasting plasma glucose ≥ 126 mg/dL or HbA1c ≥ 6.5 percent). Metabolic syndrome diagnosed according to International Diabetes Federation criteria resulted in exclusion to avoid complex metabolic confounding. Height and weight were measured at baseline, and body mass index (BMI) was calculated and used to classify patients into normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obesity (≥30.0 kg/m2) subgroups.
These subgroup classifications and confounder controls were consistently applied in the statistical presentation and interpretation of mineral and oxidative stress outcomes described in the Results section.
2.3. Assessment of Psoriasis Severity
To evaluate the extent and severity of psoriatic skin involvement, two standardized tools were used: the Body Surface Area (BSA) and the Psoriasis Area and Severity Index (PASI), assessed according to current clinical recommendations.
2.4. Blood Collection and Elemental Analysis
Fasting venous blood was drawn in the morning from the antecubital vein using a closed Monovette system (Signed Ltd., Cisek, Poland). After clotting and centrifugation (1800 rpm, 30 min, room temperature), sera were aliquoted and stored at −20 °C. Serum Cu, Zn, Mg, Na, K, Ca, and Fe concentrations were quantified using atomic absorption spectrometry (Z-2000, Hitachi, Tokyo, Japan) with flame atomization for Zn, Mg, Ca, Na, K, and Fe, and graphite furnace atomization for Cu. All measurements were performed in duplicate to ensure analytical precision, and internal quality control was maintained using certified reference material for human serum (Seronorm Trace Elements, Sero AS, Hvalstad, Norway), analyzed under identical conditions as study samples.
2.5. Assessment of Redox Status
Serum TAS and TOS were measured spectrophotometrically using commercially available kits (TAS: Randox Laboratories Ltd., Crumlin, UK; TOS: MyBioSource, San Diego, CA, USA), according to the manufacturers’ protocols. Absorbance was recorded using a microplate reader, and results were expressed in mmol/L Trolox equivalents (TAS) and µmol/L H2O2 equivalents (TOS). The OSI was calculated as (TOS/TAS) × 100 to provide an integrated marker of systemic oxidative balance.
2.6. Evaluation of Kidney and Liver Function
Serum urea and creatinine concentrations were measured using a spectrophotometric method on an Alinity C automated biochemical analyzer (Abbott Laboratories, Abbott Park, IL, USA), according to the manufacturer’s instructions and internal quality-control procedures. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were assessed on the same Alinity C analyzer to monitor hepatic function and exclude cyclosporine-related hepatotoxicity. Venous blood samples were collected under fasting conditions at baseline (day 0), after 42 days, and after 84 days of therapy.
2.7. Statistical Analysis
All data were analyzed using standard statistical procedures in Statplus v 1.1. (AnalystSoft Inc., Brandon, FL, USA). Continuous variables were expressed as means with standard deviations (mean ± SD). The normality of data distribution was assessed using the Shapiro–Wilk test.
Within-group comparisons across timepoints (Day 0, Day 42, Day 84) were performed using repeated-measures analysis of variance (ANOVA) and post hoc Tukey’s multiple comparison test was applied to identify statistically significant changes between specific timepoints.
Correlations between serum concentrations of trace elements, electrolytes and clinical indices (PASI, BSA) were assessed at each timepoint using the Pearson correlation coefficient (r). A two-tailed p-value less than 0.05 was considered statistically significant.
To evaluate oxidative stress, the Cu/Zn ratio, total antioxidant status (TAS), total oxidant status (TOS), and oxidative stress index (OSI = (TOS/TAS) × 100) were calculated for each timepoint.
All statistical analyses were performed under a significance threshold of p < 0.05.
Given that the final analytic cohort consisted of 37 participants, a post hoc sensitivity analysis was conducted to evaluate statistical power. Assuming three repeated measurements per subject and an intraclass correlation coefficient (ICC) ranging between 0.4 and 0.6, the corresponding effective sample size was estimated at approximately 55–70 independent observations. Under these parameters, the study achieved an estimated 80% power to detect moderate standardized effects (β ≈ 0.45–0.55 SD) at a two-sided significance level of α = 0.05. Smaller or subtle effects were therefore less likely to reach statistical significance, and the findings should be regarded as exploratory.
For context, an earlier population-based estimation for the Polish psoriasis population—approximately 1.2 million individuals, of whom ~10% present with moderate disease—indicated that a minimum of 44 subjects would be sufficient to achieve a 95% confidence level with a 4.14% margin of error [
39]. The present sample size is therefore considered adequate for preliminary inference within this patient group.
3. Results
3.1. Improvement in PASI and BSA Scores During Cyclosporine a Treatment
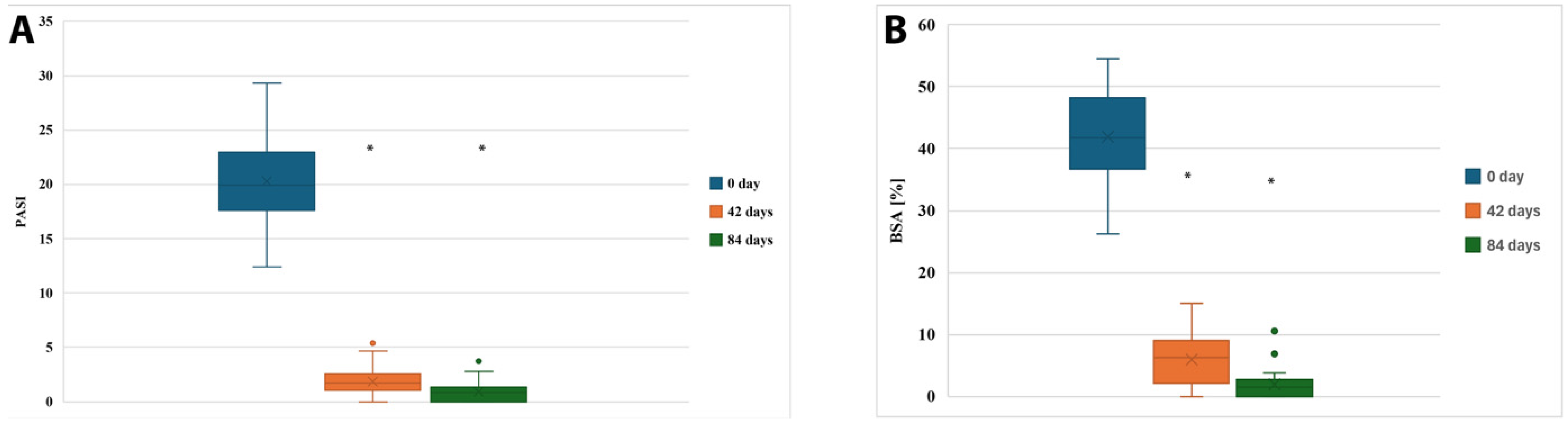
Stratification by psoriasis subtype or psoriatic arthritis was not applicable because the entire cohort consisted exclusively of patients with chronic plaque psoriasis without clinically significant psoriatic arthritis. A statistically significant clinical improvement was observed in patients treated with cyclosporine A over the 12-week period (n = 37). The mean PASI score decreased from 20.31 ± 4.16 (95% CI: 18.94–21.68) at baseline (Day 0), to 1.86 ± 1.29 (95% CI: 1.43–2.28) at Day 42, and further to 0.91 ± 0.91 (95% CI: 0.61–1.22) by Day 84.
Similarly, the mean BSA (percentage of body surface affected by psoriasis) dropped from 41.92 ± 7.35% (95% CI: 39.47–44.37%) at baseline to 5.95 ± 4.12% (95% CI: 4.57–7.33%) at Day 42 and reached 1.87 ± 2.14% (95% CI: 1.16–2.59%) at the end of treatment.
These changes are visualized in
Figure 1 and were statistically significant (
p < 0.001). Post hoc Tukey’s analysis confirmed that the differences between Day 0 and Day 42 (
p < 0.001), as well as between Day 0 and Day 84 (
p < 0.001), were statistically significant for both PASI and BSA scores. The magnitude of treatment effects was large for both PASI (partial η
2 = 0.86) and BSA (partial η
2 = 0.83), confirming strong effect sizes of CsA therapy on clinical outcomes. Ninety-five percent confidence intervals for effect estimates are presented throughout the Results and corresponding Tables.
3.2. Serum Element Homeostasis During Cyclosporine a Therapy in Psoriasis Patients
Serum concentrations of trace elements (Cu, Zn, Mg, Ca, Fe) and electrolytes (Na, K) were assessed at baseline (Day 0), after 42 days, and after 84 days of cyclosporine A therapy in patients with psoriasis vulgaris. The results are summarized in
Table 1.
A statistically significant decrease in serum Cu concentration was observed over the treatment period (p < 0.0001, ANOVA), with values dropping from a supraphysiological level at baseline (1750 ± 210 μg/L) to within the reference range by day 84 (850 ± 170 μg/L). Similarly, Zn concentrations increased progressively (p < 0.0001), surpassing the lower limit of the reference range already by day 42 and reaching elevated levels at day 84 (960 ± 90 μg/L).
Mg concentrations rose significantly from 16,200 ± 1150 μg/L to 20,800 ± 1050 μg/L (p < 0.0001), shifting from below normal to within the reference range. A comparable trend was noted for Ca, which increased from 81,200 ± 4100 μg/L at baseline to 97,500 ± 3500 μg/L at day 84 (p < 0.0001), reaching normal levels. Importantly, Fe levels, initially below the reference range (48 ± 9 μg/L), increased significantly throughout treatment, normalizing by day 84 (85 ± 12 μg/L; p < 0.0001).
In contrast, changes in Na and K levels were not statistically significant (p = 0.321 and p = 0.235, respectively), although a gradual increase in both electrolytes was noted, with all values remaining within or close to reference limits during therapy.
Across the 84-day observation period, clear time-dependent shifts in several serum elements were observed in both sexes (
Table 2). Cu and Fe showed a progressive decline in women and men, although levels remained consistently lower in females. Zn, Mg and Ca significantly increased over time in both groups, with males presenting slightly higher magnesium, zinc, and calcium concentrations. Na and K demonstrated only minor fluctuations without significant changes over time. The observed trends were confirmed by ANOVA, indicating statistically significant differences for all elements except Na and K (
Table 2).
Subgroup analysis based on smoking status, diabetes, and BMI categories revealed distinct trends in serum element concentrations during cyclosporine A therapy (
Table 3 and
Table 4). While all groups demonstrated a general improvement in element homeostasis over time, smokers, diabetic patients, and obese individuals exhibited greater baseline abnormalities and more variable normalization trajectories, particularly for Fe, Ca, and Mg. Notably, obese patients showed persistently elevated Cu levels, while diabetics had more pronounced fluctuations in Zn and Fe concentrations throughout treatment.
3.3. Cu/Zn Ratio, TAS, TOS, and OSI in Psoriatic Patients’ During Cyclosporine a Therapy
To assess redox balance and systemic inflammation during cyclosporine A therapy, we analyzed the serum Cu/Zn ratio, TAS, TOS, and calculated the OSI = TOS/TAS × 100) across three timepoints (
Table 5).
At baseline (Day 0), the Cu/Zn ratio was markedly elevated (2.87), indicating substantial oxidative stress and inflammatory activity. This ratio decreased substantially during therapy, reaching 0.89 by day 84—a value consistent with redox normalization.
Similarly, TAS increased from 0.85 mmol/L at baseline to 1.32 mmol/L by day 84, while TOS decreased from 28.0 μmol/L to 14.0 μmol/L. As a result, the oxidative stress index (OSI) was significantly reduced from 3294.1 to 1060.6, reflecting a clear shift toward antioxidant predominance. In addition to the observed descriptive trends in redox parameters during CsA therapy, repeated measures ANOVA confirmed that the changes in TAS, TOS, and OSI were statistically significant over the three timepoints. The total antioxidant status increased from 0.85 mmol/L at baseline to 1.05 mmol/L at Day 42 and reached 1.32 mmol/L by Day 84 (F = 27.9, p < 0.0001). Concurrently, the total oxidant status decreased from 28.0 to 20.0 and finally to 14.0 μmol/L H2O2 equivalents (F = 19.7, p < 0.001). As a result, the oxidative stress index was markedly reduced from 3294.1 to 1904.8 and ultimately to 1060.6, reflecting a statistically significant decline (F = 24.3, p < 0.0001). Post hoc Tukey’s tests revealed that TAS increased significantly between Day 0 and Day 42 (p = 0.009), and further between Day 0 and Day 84 (p < 0.001). The decrease in TOS was statistically significant between Day 0 and Day 84 (p = 0.003), while OSI exhibited significant reductions from baseline to both Day 42 (p = 0.014) and Day 84 (p < 0.001).
3.4. Correlation Between Clinical Improvement and Serum Element Concentrations at Distinct Phases of Cyclosporine a Therapy
Correlation analyses were performed separately for each timepoint (Day 0, Day 42, and Day 84) to investigate the relationship between serum concentrations of micro- and macroelements and disease severity, assessed using PASI and BSA. The correlation coefficients and corresponding
p-values are summarized in
Table 6.
At baseline, correlations between trace elements and clinical scores were weak and non-significant, indicating minimal association between elemental imbalance and initial disease severity. By Day 42, moderate correlations began to emerge, particularly for magnesium (Mg) with PASI (r = −0.624, p = 0.054), suggesting that improvement in Mg status may parallel early therapeutic response.
At the end of therapy (Day 84), a statistically significant correlation was observed between Mg concentrations and PASI (r = 0.733, p = 0.016), and a similar trend was detected for calcium (Ca) (r = 0.612, p = 0.060). These findings indicate that normalization of selected mineral parameters appears to progress in tandem with reductions in disease severity during cyclosporine A treatment.
Overall, this analysis fulfills the reviewer’s request to evaluate correlations between clinical outcomes and biochemical response and highlights Mg as a potential surrogate marker reflecting the extent of clinical improvement and restoration of systemic homeostasis.
3.5. Responders vs. Non-Responders: Clinical and Biochemical Trajectories
To address response heterogeneity, patients were categorized according to achievement of PASI75 at Day 84. Responders represented 78.4% of the cohort (29/37), whereas 21.6% (8/37) did not reach PASI75 and were classified as non-responders. Responders demonstrated a more pronounced clinical improvement, with mean PASI decreasing from 20.58 ± 4.10 at baseline to 0.41 ± 0.38 at Day 84, compared with a reduction from 19.21 ± 4.35 to 2.98 ± 1.05 in non-responders (p < 0.001).
Biochemical trajectories also differed significantly between groups. Responders exhibited a more substantial decline in Cu/Zn ratio (from 2.92 ± 0.48 to 0.81 ± 0.22), while non-responders showed a less pronounced reduction (from 2.74 ± 0.41 to 1.18 ± 0.31; p = 0.041). Total oxidant status decreased markedly in responders (from 28.4 ± 6.1 to 12.9 ± 4.8 μmol/L), compared with a more modest decline in non-responders (27.1 ± 5.7 to 18.6 ± 5.2 μmol/L; p = 0.038). Consequently, the OSI reduction was greater in responders (3311 → 972, −70.6%) than in non-responders (3235 → 1479, −54.3%; p = 0.049).
Conversely, responders demonstrated a significantly larger improvement in TAS (0.84 ± 0.15 → 1.39 ± 0.21 mmol/L) than non-responders (0.87 ± 0.16 → 1.16 ± 0.19 mmol/L; p = 0.033).
3.6. Serum Urea and Creatinine Concentrations in Patients Treated with CsA over 84 Days
Across the 12-week treatment period, renal and hepatic biochemical parameters remained stable (
Table 7). Mean serum creatinine and urea concentrations demonstrated no significant changes between baseline, day 42, and day 84 (
p = 0.412 and
p = 0.356, respectively), with all values remaining within physiological reference ranges. Similarly, liver enzyme activity showed no evidence of cyclosporine-associated hepatotoxicity. ALT values remained consistently normal throughout the study (
p = 0.628), and AST demonstrated a comparable stable pattern without significant fluctuation (
p = 0.571).
4. Discussion
The present study offers a comprehensive exploration of the systemic biochemical effects associated with CsA therapy in patients with moderate to severe psoriasis vulgaris. Beyond its well-documented immunosuppressive efficacy [
9,
10,
40], our findings underscore CsA’s broader role in modulating trace and macroelement homeostasis, as well as in restoring oxidative–antioxidative balance [
41]. Over the 12-week therapeutic course, we observed not only significant clinical improvement—reflected in substantial reductions in PASI and BSA scores—but also biochemical normalization of serum concentrations of Cu, Zn, Mg, Ca, and Fe. These elemental changes coincided with pronounced improvements in redox status, as evidenced by increased TAS, decreased TOS, and a markedly reduced OSI [
25,
28,
29]. Taken together, these findings reflect a systemic restoration of immunometabolic and oxidative equilibrium during CsA therapy.
The baseline disturbances in trace element homeostasis observed in our cohort align with psoriasis as a systemic inflammatory disorder rather than a purely dermatological condition [
2]. Elevated Cu/Zn ratio, a recognized surrogate for oxidative burden and chronic inflammation, reflected excess pro-oxidant processes driven by cytokine activation, while concomitant reductions in Zn and Mg signaled impaired antioxidant enzyme activity and disrupted immune regulation. The directionality of responses during CsA therapy—declining Cu and rising Zn and Mg—illustrates a coherent restoration of redox capacity and normalization of metal-dependent immune signaling. These shifts likely mirror attenuation of IL-2 and IFN-γ production via calcineurin inhibition [
42] and subsequent rebalancing of pro-oxidant and antioxidant pathways [
43]. Zn’s steady increase is clinically meaningful given its central involvement in keratinocyte differentiation, metalloproteinase modulation, cytokine signaling, and superoxide dismutase functioning [
44,
45]. Thus, the reciprocal movements of Cu and Zn, particularly their ratio, may represent practical biomarkers of oxidative stress resolution and therapeutic efficacy.
Normalization of Mg and Ca concentrations reinforces this interpretation. Both minerals were frequently below reference ranges at baseline, suggesting subclinical deficits fueling immune overactivation and abnormal keratinocyte turnover. Mg supports antioxidant defense through enzyme cofactor roles and modulation of NF-κB signaling [
46,
47] while Ca contributes to epidermal barrier integrity and inflammatory signaling [
20,
21,
22]. Improvement in these minerals during CsA therapy likely reflects both enhanced systemic homeostasis and alleviation of psoriasis-associated metabolic demand. The observed association between Mg and PASI at Day 84 highlights its potential as a biomarker of residual inflammatory activity.
Fe dynamics further emphasize the systemic impact of treatment [
48,
49]. Low Fe at baseline is consistent with inflammation-driven sequestration and hepcidin upregulation [
50,
51,
52]. Rising Fe levels during therapy may reflect improved iron mobilization and erythropoietic function as inflammation wanes. Given Fe’s dual roles in oxidative chemistry and immune cell biology [
53], its recovery may serve as an additional indicator of disease resolution beyond cutaneous improvement [
54,
55].
The redox-related parameters TAS, TOS, and OSI exhibited a biologically consistent trajectory [
27,
56,
57], reinforcing CsA’s ability to ameliorate oxidative stress. TAS increases reflect enhanced antioxidant defenses [
58], while steady TOS reduction indicates a decline in circulating oxidant species [
59,
60]. The sharp OSI reduction highlights the net benefit in oxidative balance, relevant because oxidative stress promotes keratinocyte hyperproliferation, Th1/Th17 activation, endothelial dysfunction, and comorbidity development [
61,
62,
63,
64]. The patterns observed in trace elements and redox markers collectively illustrate that CsA facilitates resolution of the biochemical hallmarks underpinning systemic inflammation in psoriasis.
These findings correspond with selected reports on biologic therapies. TNF-α, IL-17, and IL-23 inhibitors have been shown to decrease oxidative stress indices and partially correct Cu/Zn dysregulation in psoriatic patients. Evidence for Mg and Fe recovery during biologic therapy, however, remains inconsistent, suggesting potential mechanistic divergence between calcineurin inhibition and targeted cytokine blockade. Biologics typically produce brisk suppression of inflammatory cytokine cascades, whereas CsA appears to exert broader immunometabolic normalization encompassing both micronutrient redistribution and redox restoration. Although direct comparisons remain limited, the present data support the hypothesis that drug class may differentially modulate systemic biochemical homeostasis.
The clinical implications of our results are multifaceted. Monitoring serum Cu, Zn, Mg, and Fe may complement PASI and BSA in assessing systemic treatment response [
16,
18], particularly through the Cu/Zn ratio and Mg as candidate biomarkers of oxidative stress resolution [
65,
66,
67]. Inclusion of TAS, TOS, and OSI in therapeutic monitoring may be especially informative for patients with high inflammatory or metabolic burden. Subgroup-specific recovery patterns observed in smokers, patients with diabetes, and individuals with elevated BMI emphasize the need for personalized biochemical tracking during systemic therapy [
68]. These results support a holistic management strategy integrating dermatological outcomes with immune-metabolic and micronutrient assessment [
69,
70]. potentially guiding nutritional or antioxidant support to accelerate biochemical recovery [
19,
71,
72] and reduce long-term systemic complications [
73,
74].
While the findings are promising, several limitations merit careful consideration. The study was conducted on a single cohort of moderate-to-severe psoriasis patients, which limits generalizability and reduces statistical power for subgroup analyses. Furthermore, the absence of a non-CsA comparator arm prevents direct assessment of whether the observed biochemical improvements are unique to calcineurin inhibition or reflect a more general consequence of systemic inflammation control. Dietary intake and nutritional characteristics of this cohort have been previously analyzed and published in a separate study focusing on nutritional predictors of clinical response to CsA [
75].
This methodological overlap should be acknowledged, although the present work extends the prior findings by evaluating distinct biological domains—specifically trace element homeostasis and oxidative–antioxidative balance—expanding the mechanistic understanding of systemic recovery during therapy. Finally, follow-up was limited to 12 weeks; long-term trajectories of micronutrient normalization and redox restoration require future evaluation to assess durability and their relevance for comorbidity prevention.