Serum Hepcidin as a Biomarker of Subclinical Atherosclerosis in Peritoneal Dialysis: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Correlation Analysis
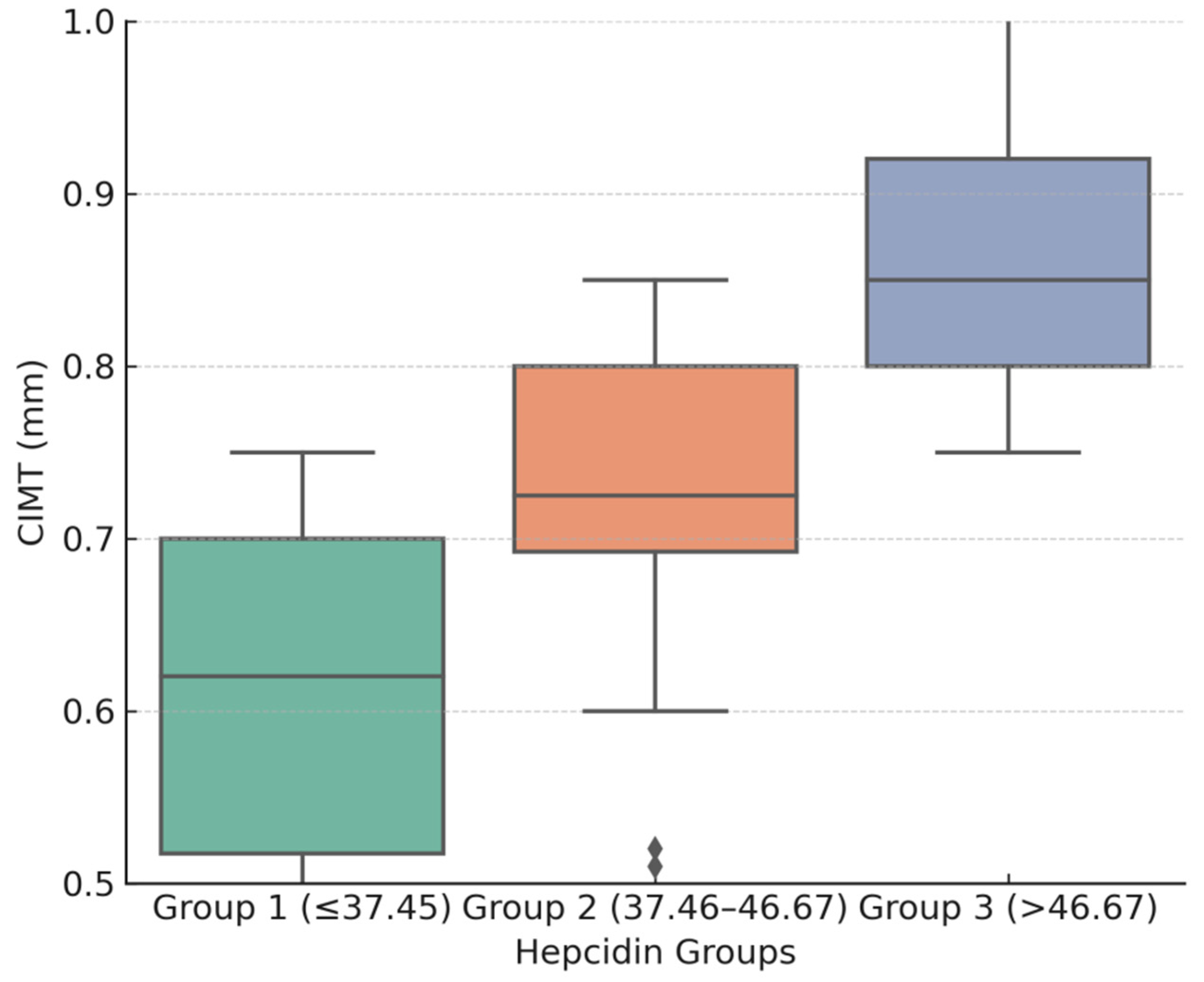
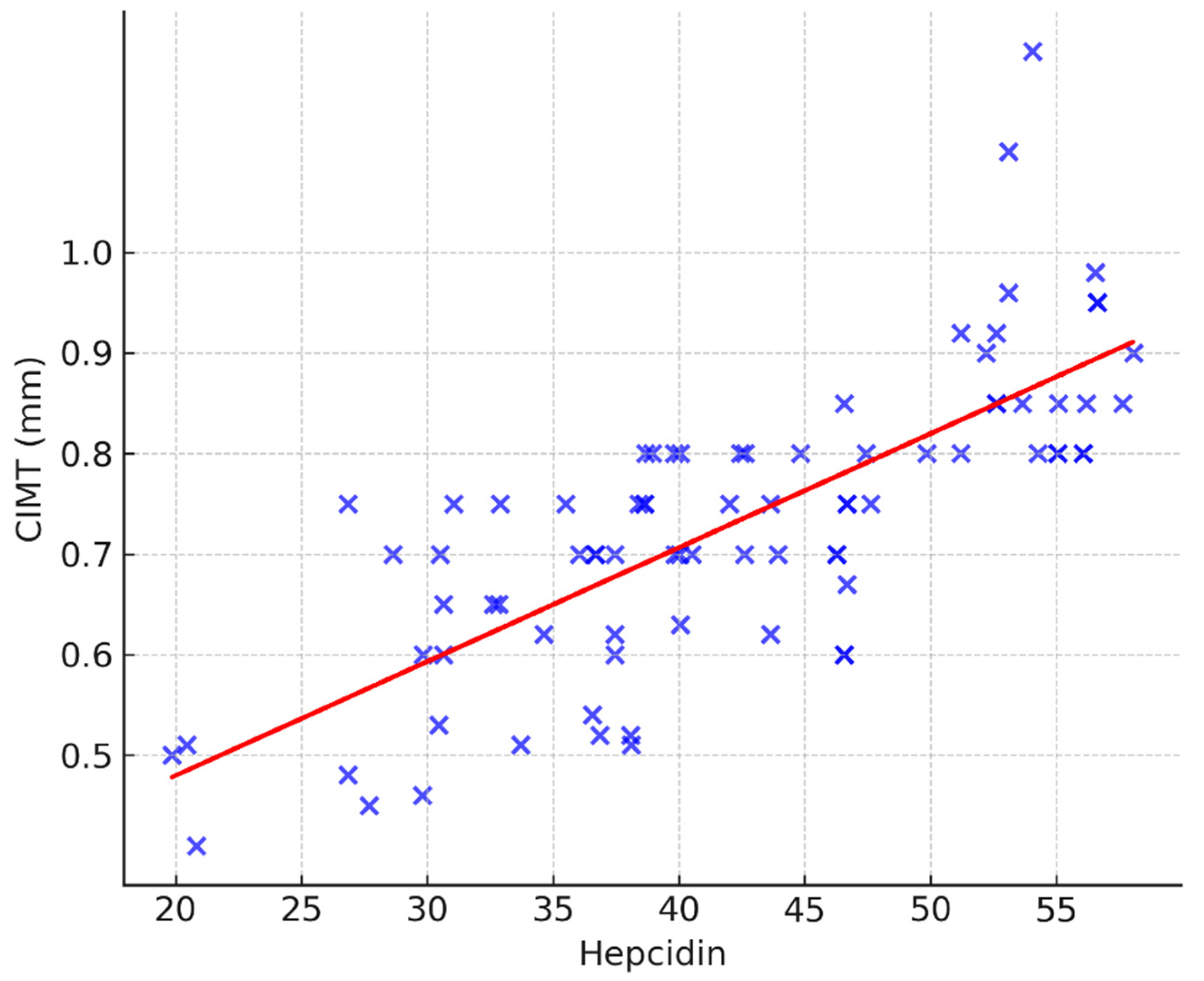
3.2. Hepcidin and CIMT Relationship
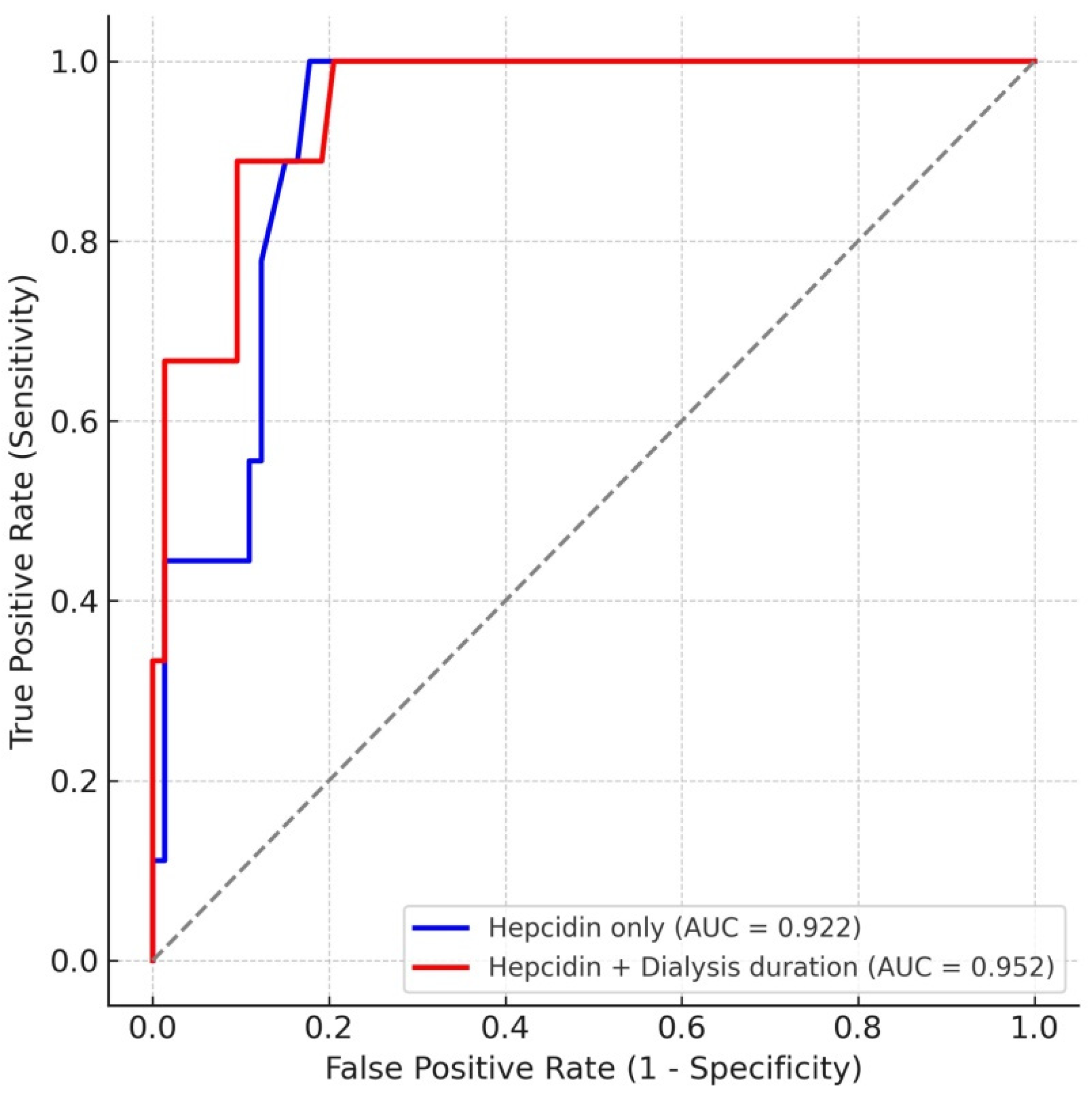
3.3. ROC Curve Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johansen, K.L.; Chertow, G.M.; Foley, R.N.; Gilbertson, D.T.; Herzog, C.A.; Ishani, A.; Israni, A.K.; Ku, E.; Tamura, M.K.; Li, S.; et al. US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2021, 77 (Suppl. 1), A7–A8. [Google Scholar] [CrossRef]
- Verbeke, F.; Van Biesen, W.; Honkanen, E.; Wikström, B.; Jensen, P.B.; Krzesinski, J.-M.; Rasmussen, M.; Vanholder, R.; Rensma, P.L. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: Outcome of the calcification outcome in renal disease (CORD) study. Clin. J. Am. Soc. Nephrol. 2011, 6, 153–159. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.L.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Chiu, Y.W.; Mehrotra, R. Can we reduce the cardiovascular risk in peritoneal dialysis patients? Indian J. Nephrol. 2010, 20, 59–67. [Google Scholar] [CrossRef]
- van der Weerd, N.C.; Grooteman, M.P.; Nubé, M.J.; Ter Wee, P.M.; Swinkels, D.W.; Gaillard, C.A. Hepcidin in chronic kidney disease: Not an anaemia management tool, but promising as a cardiovascular biomarker. Neth. J. Med. 2015, 73, 108–118. [Google Scholar]
- Fleming, R.E.; Sly, W.S. Hepcidin: A putative iron-regulatory hormone relevant to hereditary hemochromatosis and the anemia of chronic disease. Proc. Natl. Acad. Sci. USA 2001, 98, 8160–8162. [Google Scholar] [CrossRef]
- Muckenthaler, M.U. Fine tuning of hepcidin expression by positive and negative regulators. Cell Metab. 2008, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Papastergiou, E.; Rallis, D.; Papagianni, A.; Cholevas, V.; Katzilakis, N.; Siomou, E.; Stiakaki, E.; Makis, A. Intact FGF23 and Markers of Iron Homeostasis, Inflammation, and Bone Mineral Metabolism in Acute Pediatric Infections. Biology 2024, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Michihata, T.; Shishido, K.; Takahashi, K.; Takahashi, G.; Hosaka, N.; Ikeda, M.; Sanada, D.; Shibata, T. High fibroblast growth factor 23 levels are associated with decreased ferritin levels and increased intravenous iron doses in hemodialysis patients. PLoS ONE 2017, 12, e0176984. [Google Scholar] [CrossRef] [PubMed]
- David, V.; Martin, A.; Isakova, T.; Spaulding, C.; Qi, L.; Ramirez, V.; Zumbrennen-Bullough, K.B.; Sun, C.C.; Lin, H.Y.; Babitt, J.L.; et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016, 89, 135–146. [Google Scholar] [CrossRef]
- Nam, K.H.; Kim, H.; An, S.Y.; Lee, M.; Cha, M.-U.; Park, J.T.; Yoo, T.-H.; Lee, K.-B.; Kim, Y.-H.; Sung, S.-A.; et al. Circulating Fibroblast Growth Factor-23 Levels are Associated with an Increased Risk of Anemia Development in Patients with Nondialysis Chronic Kidney Disease. Sci. Rep. 2018, 8, 7294. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, Y.; Tanaka, K.; Matsui, T.; Sakaguchi, T.; Yamagishi, S.I.; Motomiya, Y. Fibroblast Growth Factor 23 Contributes to Regulation of Hepcidin/Ferroportin Axis. Austin J. Pharmacol. Ther. 2020, 8, 1118. [Google Scholar] [CrossRef]
- Sullivan, J.L. Macrophage iron, hepcidin, and atherosclerotic plaque stability. Exp. Biol. Med. 2007, 232, 1014–1020. [Google Scholar] [CrossRef]
- de Oliveira Junior, W.V.; Silva, A.P.F.; de Figueiredo, R.C.; Gomes, K.B.; Silva, A.C.S.E.; Dusse, L.M.S.; Rios, D.R.A. Association between dyslipidemia and CCL2 in patients undergoing hemodialysis. Cytokine 2020, 125, 154858. [Google Scholar] [CrossRef]
- Gluba-Brzózka, A.; Franczyk, B.; Olszewski, R.; Rysz, J. The Influence of Inflammation on Anemia in CKD Patients. Int. J. Mol. Sci. 2020, 21, 725. [Google Scholar] [CrossRef] [PubMed]
- van Venrooij, N.A.; Pereira, R.C.; Tintut, Y.; Fishbein, M.C.; Tumber, N.; Demer, L.L.; Salusky, I.B.; Wesseling-Perry, K. Fgf23 protein expression in coronary arteries is associated with impaired kidney function. Nephrol. Dial. Transplant. 2014, 29, 1525–1532. [Google Scholar] [CrossRef]
- Scialla, J.J.; Lau, W.L.; Reilly, M.P.; Isakova, T.; Yang, H.-Y.; Crouthamel, M.H.; Chavkin, N.W.; Rahman, M.; Wahl, P.; Amaral, A.P.; et al. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013, 83, 1159–1168. [Google Scholar] [CrossRef]
- Marathe, P.H.; Gao, H.X.; Close, K.L. American Diabetes Association Standards of Medical Care in Diabetes 2017. J. Diabetes 2017, 9, 320–324. [Google Scholar] [CrossRef]
- McMurray, J.J.; Parfrey, P.S.; Adamson, J.W.; Aljama, P.; Berns, J.S.; Bohlius, J.; Drüeke, T.B.; Finkelstein, F.O.; Fishbane, S.; Ganz, T.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012, 2, 279–335. [Google Scholar]
- Touboul, P.-J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez, R.H.; et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.L. Iron and the sex difference in heart disease risk. Lancet 1981, 1, 1293–1294. [Google Scholar] [CrossRef]
- Silva, G.; Jeney, V.; Chora, A.; Larsen, R.; Balla, J.; Soares, M.P. Oxidized hemoglobin is an endogenous proinflammatory agonist that targets vascular endothelial cells. J. Biol. Chem. 2009, 284, 29582–29595. [Google Scholar] [CrossRef]
- Tuomainen, T.P.; Punnonen, K.; Nyyssonen, K.; Salonen, J.T. Association between body iron stores and the risk of acute myocardial infarction in men. Circulation 1998, 97, 1461–1466. [Google Scholar] [CrossRef]
- Syrovatka, P.; Kraml, P.; Hulikova, K.; Fialova, L.; Vejrazka, M.; Crkovska, J.; Potockova, J.; Andel, M. Iron stores are associated with asymptomatic atherosclerosis in healthy men of primary prevention. Eur. J. Clin. Investig. 2011, 41, 846–853. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin and iron regulation, 10 years later. Blood 2011, 117, 4425–4433. [Google Scholar] [CrossRef]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- van Swelm, R.P.L.; Wetzels, J.F.M.; Swinkels, D.W. The multifaceted role of iron in renal health and disease. Nat. Rev. Nephrol. 2020, 16, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Dongiovanni, P.; Motta, B.M.; Swinkels, D.W.; Bonara, P.; Rametta, R.; Burdick, L.; Frugoni, C.; Fracanzani, A.L.; Fargion, S. Serum hepcidin and macrophage iron correlate with MCP-1 release and vascular damage in patients with metabolic syndrome alterations. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 683–690. [Google Scholar] [CrossRef]
- Galesloot, T.E.; Holewijn, S.; Kiemeney, L.A.; de Graaf, J.; Vermeulen, S.H.; Swinkels, D.W. Serum hepcidin is associated with presence of plaque in postmenopausal women of a general population. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 446–456. [Google Scholar] [CrossRef]
- Manolov, V.; Petrova, J.; Bogov, B.; Hadjidekova, S.; Vasilev, V.; Yonova, D.; Petrova, M.; Kunchev, T.; Jelev, Y.; Jeliazkov, P.; et al. Evaluation of Hepcidin and Atherosclerosis in Dialysis Patients. Clin. Lab. 2017, 63, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Anupama, K.V.; Yadla, M. Serum hepcidin levels and cardiovascular outcomes in patients on maintenance hemodialysis: A study from South India. J. Egypt. Soc. Nephrol. Transplant. 2022, 22, 209. [Google Scholar] [CrossRef]
- Erdoğan, B.; Eser, B.; Yayar, Ö.; Aylı, M.D. The association between serum hepcidin-25 level and subclinical atherosclerosis in peritoneal dialysis patients. Turk. Kardiyol. Dern. Ars. 2018, 46, 121–128. [Google Scholar] [CrossRef]
- Yayar, Ö.; Eser, B.; Kılıç, H. Relation between high serum hepcidin-25 level and subclinical atherosclerosis and cardiovascular mortality in hemodialysis patients. Anatol. J. Cardiol. 2018, 19, 117–122. [Google Scholar] [CrossRef]
- Zhong, Z.; Luo, D.; Luo, N.; Li, B.; Fu, D.; Fan, L.; Li, Z.; Chen, W.; Mao, H. Serum Hepcidin-25 and Risk of Mortality in Patients on Peritoneal Dialysis. Front. Med. 2021, 8, 684548. [Google Scholar] [CrossRef]
- Ruchala, P.; Nemeth, E. The pathophysiology and pharmacology of hepcidin. Trends Pharmacol. Sci. 2014, 35, 155–161. [Google Scholar] [CrossRef]
- Arroyo, D.; Betriu, A.; Martinez-Alonso, M.; Vidal, T.; Valdivielso, J.M.; Fernández, E. Observational multicenter study to evaluate the prevalence and prognosis of subclinical atheromatosis in a Spanish chronic kidney disease cohort: Baseline data from the NEFRONA study. BMC Nephrol. 2014, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Kali, A.; Yayar, O.; Erdogan, B.; Eser, B.; Buyukbakkal, M.; Ercan, Z.; Merhametsiz, O.; Haspulat, A.; Oğuz, E.G.; Canbakan, B.; et al. Is hepcidin-25 a predictor of atherosclerosis in hemodialysis patients? Hemodial. Int. 2016, 20, 191–197. [Google Scholar] [CrossRef]
- Fu, S.; Chen, J.; Liu, B.; Liang, P.; Zeng, Y.; Feng, M.; Xu, Z.; Zheng, G.; Yang, S.; Xu, A.; et al. Systemic inflammation modulates the ability of serum ferritin to predict all-cause and cardiovascular mortality in peritoneal dialysis patients. BMC Nephrol. 2020, 21, 237. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kang, S.H.; Do, J.Y. Effects of low-glucose degradation product solution on peritoneal membrane characteristics in peritoneal dialysis patients: A 3-year follow-up study. Iran. J. Kidney Dis. 2014, 8, 58–64. [Google Scholar] [PubMed]
- Bartosova, M.; Schmitt, C.P. Biocompatible Peritoneal Dialysis: The Target Is Still Way Off. Front. Physiol. 2019, 9, 1853. [Google Scholar] [CrossRef]
- Cho, Y.; Hawley, C.M.; Johnson, D.W. Clinical causes of inflammation in peritoneal dialysis patients. Int. J. Nephrol. 2014, 2014, 909373. [Google Scholar] [CrossRef]
- Wu, C.-L.; Wu, H.-M.; Chiu, P.-F.; Liou, H.-H.; Chang, C.-B.; Tarng, D.-C.; Chang, C.-C. Associations between the duration of dialysis, endotoxemia, monocyte chemoattractant protein-1, and the effects of a short-dwell exchange in patients requiring continuous ambulatory peritoneal dialysis. PLoS ONE 2014, 9, e109558. [Google Scholar] [CrossRef]
- Głogowski, T.; Wojtaszek, E.; Malyszko, J. Iron status and anemia control are related to peritoneal membrane properties in peritoneally dialyzed patients. Front. Med. 2023, 10, 1148094. [Google Scholar] [CrossRef]
- Kane, J.; Vos, W.G.; Bosmans, L.A.; van Os, B.W.; Toom, M.D.; Hoeksema-Hackmann, S.; Wit, D.M.; Gijbels, M.J.; Beckers, L.; Grefhorst, A.; et al. Peritoneal Dialysis Aggravates and Accelerates Atherosclerosis in Uremic ApoE-/- Mice. J. Am. Heart Assoc. 2024, 13, e034066. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Messa, P.; Pelusi, S.; Campostrini, N.; Girelli, D. Hepcidin levels in chronic hemodialysis patients: A critical evaluation. Clin. Chem. Lab. Med. 2014, 52, 613–619. [Google Scholar] [CrossRef]
- Asicioglu, E.; Velioglu, A.; Arikan, H.; Koc, M.; Tuglular, S.; Ozener, C. Baseline carotid intima-media thickness is associated with cardiovascular morbidity and mortality in peritoneal dialysis patients. Ther. Apher. Dial. 2021, 25, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Hu, H.; Li, J.; Tian, T. Relationship between serum hepcidin levels and cardiovascular disease in patients with maintenance hemodialysis. Physiol. Int. 2020, 107, 491–500. [Google Scholar] [CrossRef] [PubMed]
- El Sewefy, D.A.; Farweez, B.A.; Behairy, M.A.; Yassin, N.R. Impact of serum hepcidin and inflammatory markers on resistance to erythropoiesis-stimulating therapy in haemodialysis patients. Int. Urol. Nephrol. 2019, 51, 325–334. [Google Scholar] [CrossRef]
- van der Weerd, N.C.; Grooteman, M.P.; Bots, M.L.; van den Dorpel, M.A.; Hoedt, C.H.D.; Mazairac, A.H.; Nubé, M.J.; Penne, E.L.; Wetzels, J.F.; Wiegerinck, E.T.; et al. Hepcidin-25 is related to cardiovascular events in chronic haemodialysis patients. Nephrol. Dial. Transplant. 2013, 28, 3062–3071. [Google Scholar] [CrossRef]
| Variable | |
|---|---|
| Age (years) | 55.85 ± 11.79 |
| Gender (male) % | 59.76% |
| PD duration (months) | 41.23 ± 15.38 |
| Hypertension (%) | 48.78% |
| DM (%) | 57.32% |
| Smoking history% | 37.80% |
| BMI (kg/m2) | 23.85 ± 3.41 |
| Medications | |
| ESA | 48.78% |
| IV iron treatment | 28.05% |
| Variable | Mean ± SD |
|---|---|
| Total cholesterol (mmol/L) | 5.91 ± 1.36 |
| LDL-C (mmol/L) | 3.73 ± 1.13 |
| HDL-C (mmol/L) | 1.12 ± 0.3 |
| Triglycerides (mmol/L) | 2.01 ± 0.84 |
| Ca (mmol/L) | 2.19 ± 0.22 |
| P (mmol/L) | 1.44 ± 0.42 |
| Ca X P | 3.16 ± 0.94 |
| Hct (L/L) | 0.35 ± 0.06 |
| Hgb (g/L) | 111.61 ± 13.17 |
| Fe (µmol/L) | 11.87 ± 1.9 |
| TIBC | 43.69 ± 7.68 |
| TSAT (%) | 28.5 ± 11.24 |
| Ferritin (µg/L) | 281.06 ± 109.62 |
| CRP (mg/L) | 5.14 ± 2.35 |
| Hepcidin (ng/mL) | 42.12 ± 9.79 |
| CIMTav (mm) | 0.73± 0.15 |
| Variable | Group 1 (≤37.45) | Group 2 (37.46–46.67) | Group 3 (>46.67) | p-Value |
|---|---|---|---|---|
| Age (years) | 56.07 ± 15.45 | 55.29 ± 10.65 | 56.23 ± 8.35 | 0.99 |
| Gender (male) | 78.6% | 68.1% | 31.2% | 0.009 |
| PD duration (months) | 32.50 ± 15.40 | 39.68 ± 15.13 | 47.77 ± 15.36 | 0.0025 |
| Hypertension (%) | 29.4% | 65.7% | 81.2% | <0.001 |
| DM (%) | 18.1% | 43% | 88.2% | <0.001 |
| Smoking history (%) | 7.1% | 29.2% | 80.8% | <0.001 |
| BMI (kg/m2) | 23.80 ± 2.86 | 23.59 ± 3.18 | 24.17 ± 4.21 | 0.84 |
| ESA | 21.4% | 71.4% | 61.5% | 0.063 |
| IV iron treatment | 39.3% | 92.9% | 84.6% | 0.0088 |
| Total cholesterol (mmol/L) | 5.85 ± 1.05 | 6.38 ± 1.40 | 5.48 ± 1.49 | 0.17 |
| LDL-C (mmol/L) | 3.88 ± 1.00 | 3.47 ± 1.09 | 3.84 ± 1.30 | 0.24 |
| HDL-C (mmol/L) | 1.16 ± 0.26 | 1.13 ± 0.31 | 1.05 ± 0.33 | 0.15 |
| Triglycerides (mmol/L) | 2.00 ± 0.92 | 1.86 ± 0.74 | 2.18 ± 0.86 | 0.43 |
| Ca (mmol/L) | 2.19 ± 0.2 | 2.27 ± 0.24 | 2.11 ± 0.18 | 0.03 |
| P (mmol/L) | 1.4 ± 0.37 | 1.43 ± 0.32 | 1.49 ± 0.55 | 0.79 |
| Ca X P | 3.07 ± 0.86 | 3.25 ± 0.79 | 3.15 ± 1.16 | 0.64 |
| Hgb (g/L) | 105.24 ± 9.89 | 113.42 ± 11.36 | 118.0 ± 11.41 | 0.002 |
| Hct (L/L) | 0.32 ± 0.05 | 0.37 ± 0.06 | 0.36 ± 0.07 | 0.0013 |
| Fe (µmol/L) | 11.06 ± 1.4 | 12.3 ± 1.16 | 12.29 ± 2.64 | 0.016 |
| Ferritin (µg/L) | 196.50 ± 82.06 | 262.25 ± 72.48 | 392.37 ± 68.53 | <0.001 |
| TIBC (µmol/L) | 43.0 ± 8.5 | 44.1 ± 7.3 | 43.8 ± 7.3 | 0.9184 |
| TSAT (%) | 25.7 ± 10.6 | 29.0 ± 11.2 | 31.0 ± 11.1 | 0.0433 |
| CRP (mg/L) | 3.46 ± 1.69 | 5.09 ± 2.29 | 7.02 ± 1.64 | <0.001 |
| Hepcidin (ng/mL) | 31.48 ± 5.15 | 42.18 ± 3.16 | 53.5 ± 3.1 | <0.001 |
| CIMTav (mm) | 0.60 ± 0.1 | 0.71 ± 0.05 | 0.88 ± 0.1 | <0.001 |
| Variable | Correlation Coefficient (ρ) | p-Value |
|---|---|---|
| Age | 0.181 | 0.103 |
| Gender | −0.288 | 0.008 ** |
| PD duration | 0.25 | 0.023 * |
| Hypertension | 0.51 | <0.001 *** |
| DM | 0.059 | <0.001 *** |
| Smoking history | 0.0639 | <0.001 *** |
| BMI | 0.127 | 0.2579 |
| ESA | −0.221 | 0.043 * |
| IV iron treatment | 0.288 | 0.009 ** |
| Total cholesterol | −0.207 | 0.062 |
| LDL-C | −0.191 | 0.085 |
| HDL-C | −0.14 | 0.211 |
| Triglycerides | 0.075 | 0.502 |
| Ca | −0.136 | 0.224 |
| P | 0.076 | 0.499 |
| Ca x P | 0.092 | 0.4109 |
| Hct | 0.216 | 0.05 * |
| Hgb | 0.224 | 0.043 * |
| Fe | 0.253 | 0.002 ** |
| TSAT | 0.42 | <0.001 *** |
| Ferritin | 0.675 | <0.001 *** |
| CRP | 0.563 | <0.001 *** |
| Hepcidin | 0.788 | <0.001 |
| Variable | Univariate Linear Regression | Multivariable Linear Regression | |||
|---|---|---|---|---|---|
| Beta (95% CI) | p-Value | R2 | Beta (95% CI) | p-Value | |
| Gender | −0.0873 (−0.1502–−0.0245) | 0.0071 | 0.087 | −0.0023 (−0.0496–0.045) | 0.9228 |
| PD duration | 0.0028 (0.0008–0.0048) | 0.0075 | 0.086 | 0.0018 (0.0004–0.0032) | 0.0153 |
| Hypertension | 0.1345 (0.0766–0.1924) | <0.001 | 0.211 | 0.0553 (0.0076–0.103) | 0.0237 |
| DM | 0.1284 (0.0705–0.1862) | <0.001 | 0.196 | 0.0322 (−0.0231–0.0875) | 0.2499 |
| Smoking history | 0.1464 (0.0884–0.2044) | <0.001 | 0.24 | −0.0189 (−0.0786–0.0407) | 0.5285 |
| ESA | −0.0464 (−0.1101–0.0173) | 0.1514 | 0.026 | 0.02 (−0.0274–0.0674) | 0.4034 |
| IV iron therapy | −0.094 (−0.1627–−0.0253) | 0.0079 | 0.085 | −0.0127 (−0.0653–0.04) | 0.6326 |
| Hct | 0.1275 (0.022–0.233) | 0.041 | 0.051 | 0.1042 (0.021–0.187) | 0.062 |
| Hgb | 0.0021 (0.0005–0.0037) | 0.043 | 0.049 | 0.0012 (−0.0001–0.0025) | 0.071 |
| TSAT | 0.0023 (0.0007–0.0039) | 0.005 | 0.061 | 0.0020 (0.0001 to 0.0039) | 0.052 |
| Ferritin | 0.0009 (0.0006–0.0011) | <0.001 | 0.424 | 0.0002 (−0.0001–0.0006) | 0.1222 |
| CRP | 0.0362 (0.025–0.0474) | <0.001 | 0.341 | 0.0095 (−0.0021–0.0211) | 0.1062 |
| Hepcidin | 0.0113 (0.0092–0.0135) | <0.001 | 0.579 | 0.0057 (0.0013–0.0101) | 0.0122 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostić, E.; Dimitrijević, Z.; Apostolović, B.; Paunović, K.; Mitić, B. Serum Hepcidin as a Biomarker of Subclinical Atherosclerosis in Peritoneal Dialysis: A Cross-Sectional Study. J. Clin. Med. 2025, 14, 7905. https://doi.org/10.3390/jcm14227905
Kostić E, Dimitrijević Z, Apostolović B, Paunović K, Mitić B. Serum Hepcidin as a Biomarker of Subclinical Atherosclerosis in Peritoneal Dialysis: A Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(22):7905. https://doi.org/10.3390/jcm14227905
Chicago/Turabian StyleKostić, Emina, Zorica Dimitrijević, Branislav Apostolović, Karolina Paunović, and Branka Mitić. 2025. "Serum Hepcidin as a Biomarker of Subclinical Atherosclerosis in Peritoneal Dialysis: A Cross-Sectional Study" Journal of Clinical Medicine 14, no. 22: 7905. https://doi.org/10.3390/jcm14227905
APA StyleKostić, E., Dimitrijević, Z., Apostolović, B., Paunović, K., & Mitić, B. (2025). Serum Hepcidin as a Biomarker of Subclinical Atherosclerosis in Peritoneal Dialysis: A Cross-Sectional Study. Journal of Clinical Medicine, 14(22), 7905. https://doi.org/10.3390/jcm14227905






