Personalized Prediction of Clozapine Treatment Response Using Therapeutic Drug Monitoring Data in Japanese Patients with Treatment-Resistant Schizophrenia
Abstract
1. Introduction
- (1)
- Examine clozapine dosage and blood concentrations in patients with treatment-resistant schizophrenia (TRS);
- (2)
- Investigate the effects of sex and age on dosage and blood concentrations;
- (3)
- Evaluate blood profiles and neutrophil counts;
- (4)
- Assess clinical response to clozapine treatment;
- (5)
- Develop a random forest model to predict therapeutic response using clinical and TDM data.
2. Materials and Methods
2.1. Design and Participant
2.2. Measurement of Blood Levels
2.3. Clinical Assessment
2.4. Statistical Analysis
2.5. Machine Learning
3. Results
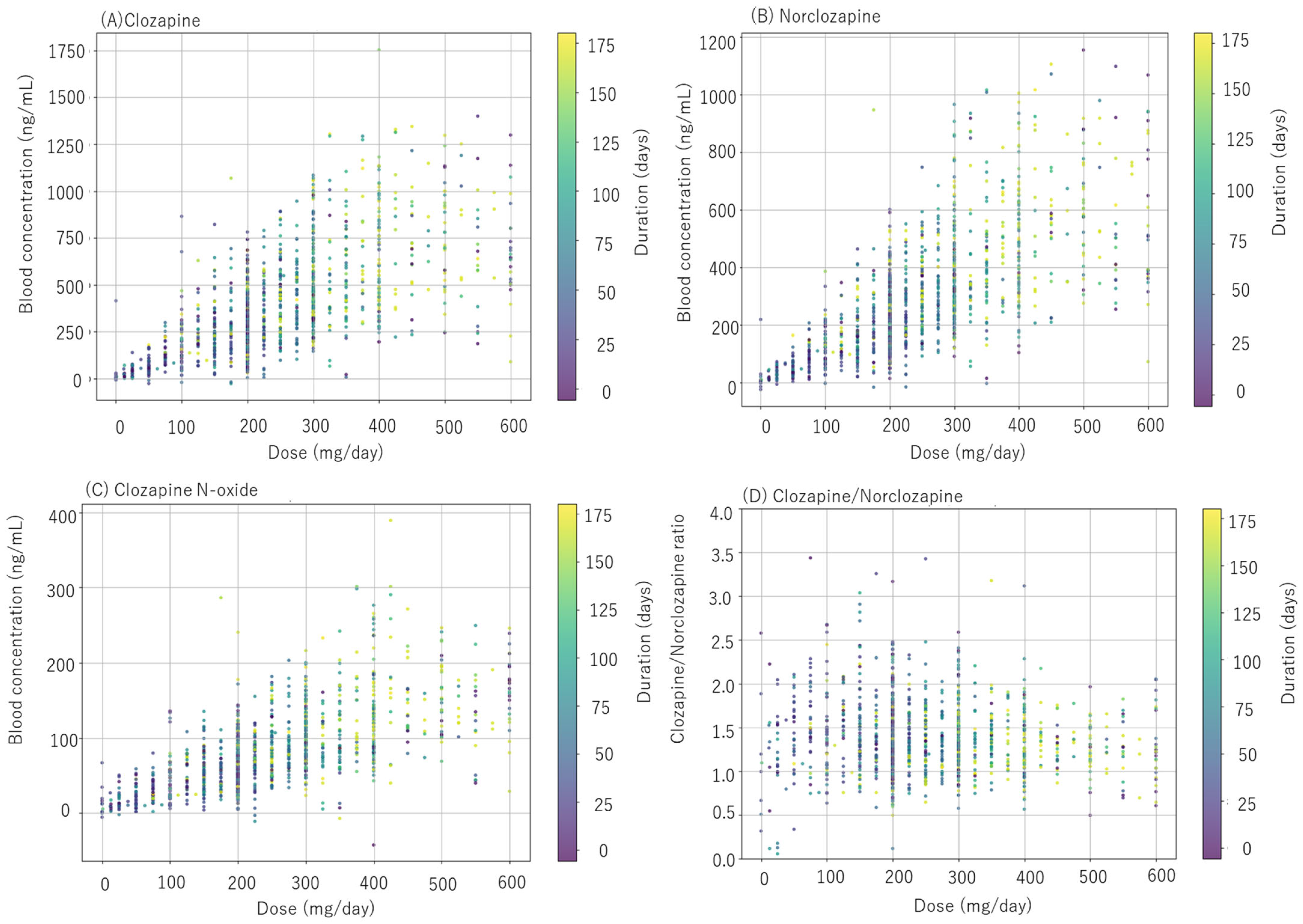
3.1. Clozapine Dosage and Concentration in Blood
3.2. Sex and Age Effects on Dosage and Blood Concentrations
3.3. Blood Profiles and Cell Counts
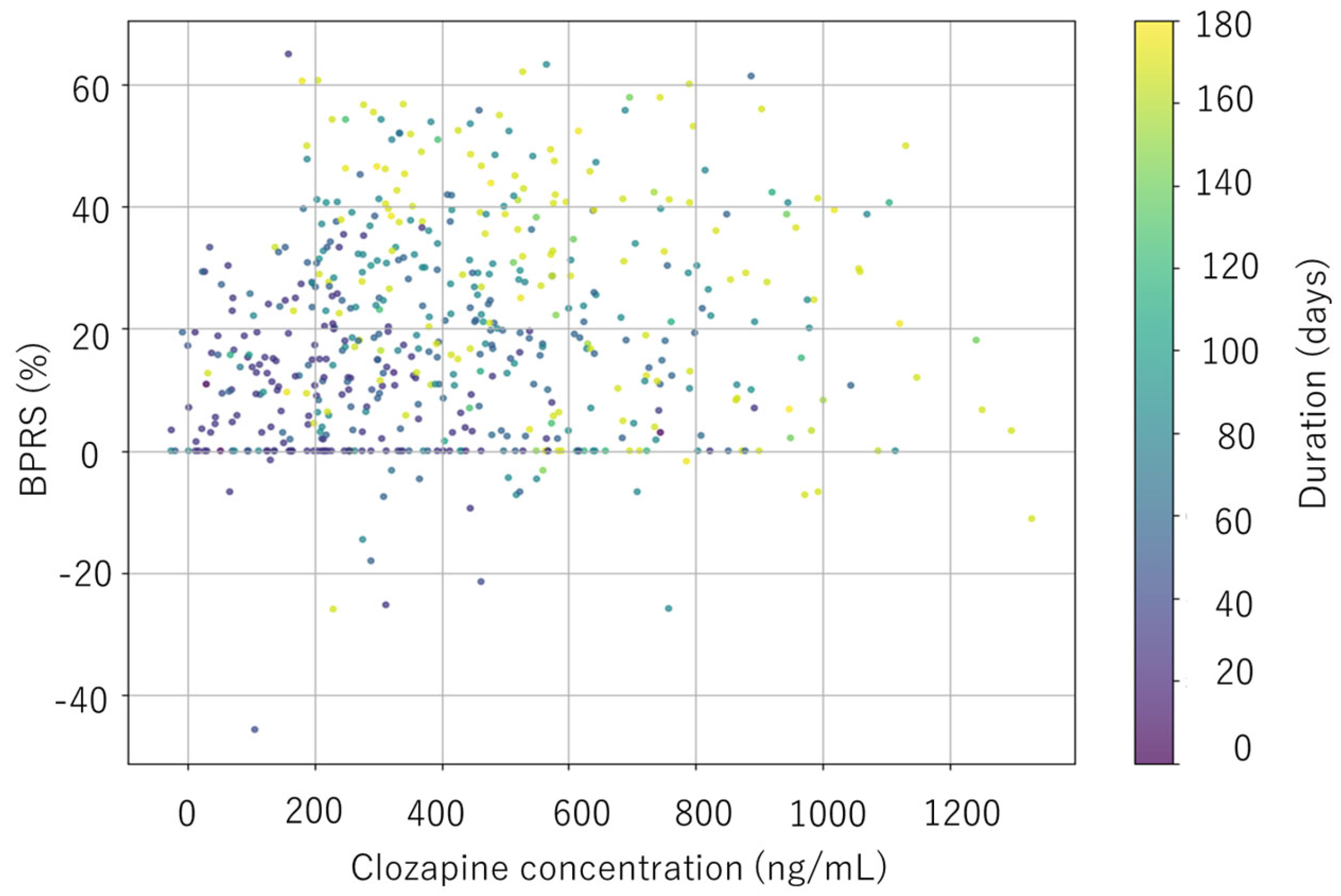
3.4. Clinical Response
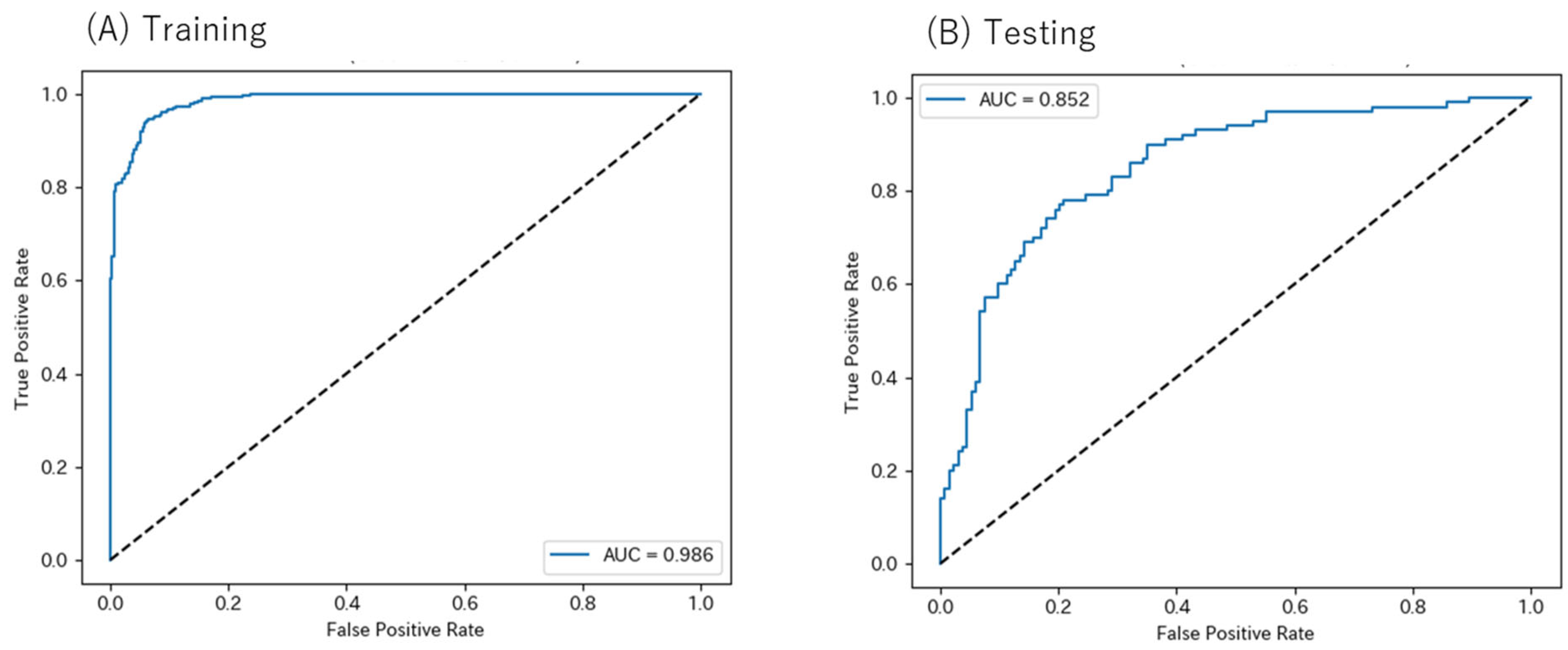
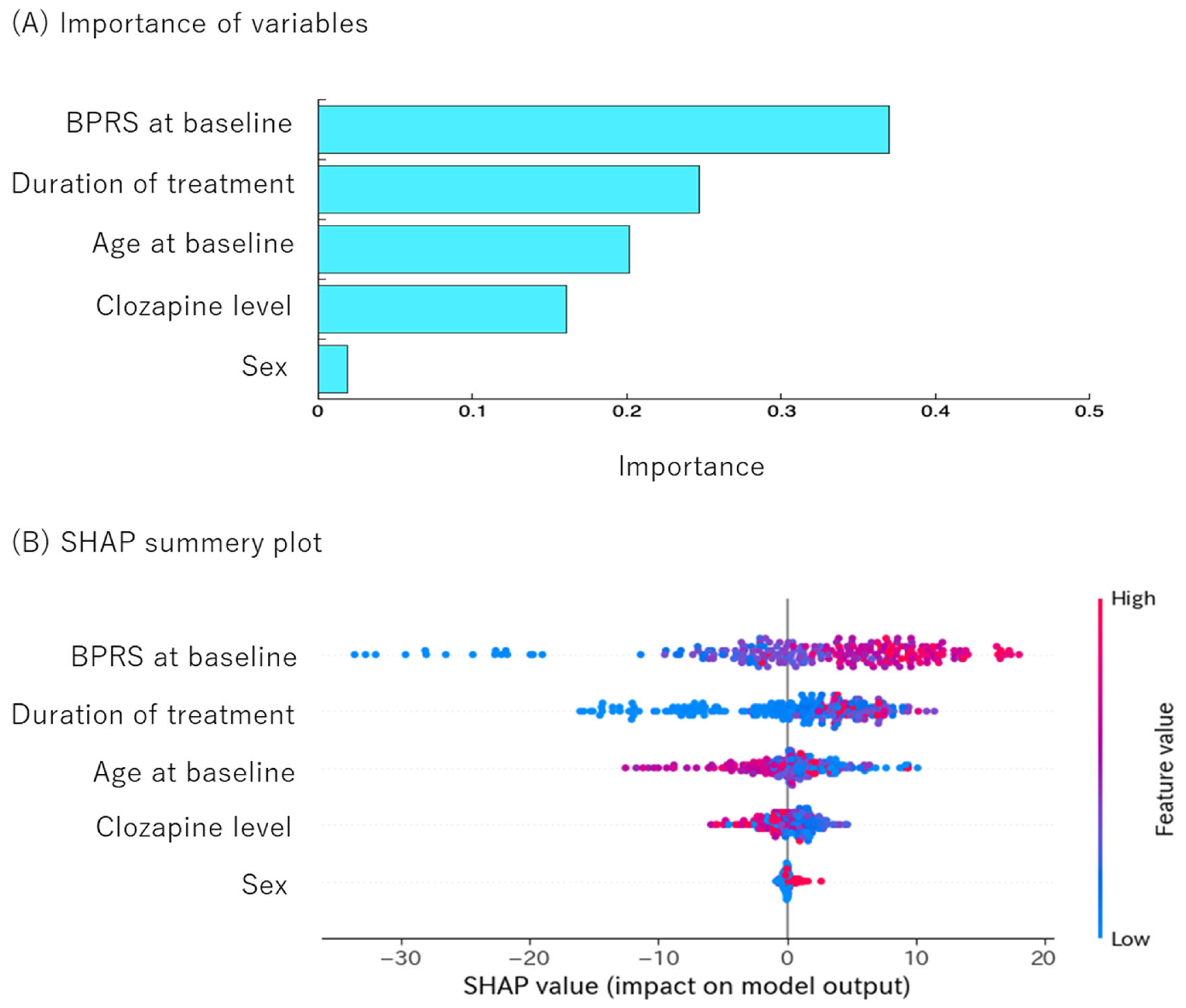
3.5. Random Forest Model Performance for Predicting Clozapine Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kane, J.M.; Schoretsanitis, G.; Rubio, J.M.; Correll, C.U. Clozapine in treatment-resistant schizophrenia: Reflections from the Hallmark US clinical trial and beyond. Schizophr. Res. 2024, 268, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [CrossRef] [PubMed]
- Northwood, K.; Pearson, E.; Arnautovska, U.; Kisely, S.; Pawar, M.; Sharma, M.; Vitangcol, K.; Wagner, E.; Warren, N.; Siskind, D. Optimising plasma clozapine levels to improvetreatment response: An individual patient data meta-analysis and receiver operating characteristic curve analysis. Br. J. Psychiatry 2023, 222, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Couchman, L.; Bowskill, S.V.; Handley, S.; Patel, M.X.; Flanagan, R.J. Plasma clozapine and norclozapine in relation to prescribed dose and other factors in patients aged < 18 years: Data from a therapeutic drug monitoring service, 1994–2010. Early Interv. Psychiatry 2013, 7, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Nomura, N.; Kitagawa, K.; So, R.; Misawa, F.; Kodama, M.; Takeuchi, H.; Bies, R.; Straubinger, T.; Banker, C.; Mizuno, Y.; et al. Comprehensive assessment of exposure to clozapine in association with side effects among patients with treatment-resistant schizophrenia: A population pharmacokinetic study. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211016189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krivoy, A.; Whiskey, E.; Webb-Wilson, H.; Joyce, D.; Derek, K.; Tracy, D.K.; Gaughran, F.; MacCabe, J.H.; Shergill, S.S. Outcomes in treatment-resistant schizophrenia: Symptoms, function and clozapine plasma concentrations. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211037179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aissa, A.; Jouini, R.; Ouali, U.; Zgueb, Y.; Nacef, F.; Hechmi, Z.E. Clinical predictors of response to clozapine in Tunisian patients with treatment resistant schizophrenia. Compr. Psychiatry 2022, 112, 152280. [Google Scholar] [CrossRef] [PubMed]
- Karampas, A.; Florou, D.; Markozannes, G.; Asimakopoulos, A.; Georgiou, G.; Plakoutsis, M.; Hyphantis, T.; Boumba, V.; Petrikis, P. Clozapine/norclozapine plasma concentrationsand their ratio in treatment resistant, early psychosis patients. Psychiatriki 2025, 36, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Llorca, P.M.; Lancon, C.; Disdier, B.; Farisse, J.; Sapin, C.; Auquier, P. Effectiveness of clozapine in neuroleptic-resistant schizophrenia: Clinical response and plasma concentrations. J. Psychiatry Neurosci. 2002, 27, 30–37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yada, Y.; Kitagawa, K.; Sakamoto, S.; Ozawa, A.; Nakada, A.; Kashiwagi, H.; Okahisa, Y.; Takao, S.; Takaki, M.; Kishi, Y.; et al. The relationship between plasma clozapine concentration and clinical outcome: A cross-sectional study. Acta Psychiatr. Scand. 2021, 143, 227–237. [Google Scholar] [CrossRef] [PubMed]
- de Haas, H.J.; Cohen, D.; de Koning, M.B.; van Weringh, G.; Petrovic, V.; de Haan, L.; Touw, D.J.; Ristic, D.I. Clozapine levels and outcomes in Serbian patients with treatment-resistant psychotic disorders previously treated without measuring clozapine levels (CLOSER). Psychiatry Res. 2024, 339, 116070. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Kim, S.; Park, K.; Yu, U.; Jang, Y.; Kim, B.H.; Lee, J.H.; Kim, E. Predictors of clozapine concentration and psychiatric symptoms in patients with Schizophrenia. PLoS ONE 2025, 20, e0319037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Freitas, D.F.; Kadra-Scalzo, G.; Agbedjro, D.; Francis, E.; Ridler, I.; Pritchard, M.; Shetty, H.; Segev, A.; Casetta, C.; Smart, S.E.; et al. Using a statistical learning approach to identify sociodemographic and clinical predictors of response to clozapine. J. Psychopharmacol. 2022, 36, 498–506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Podichetty, J.T.; Silvola, R.M.; Rodriguez-Romero, V.; Bergstrom, R.F.; Vakilynejad, M.; Bies, R.R.; Stratford, R.E., Jr. Application of machine learning to predict reduction in total PANSS score and enrich enrollment in schizophrenia clinical trials. Clin. Transl. Sci. 2021, 14, 1864–1874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soldatos, R.F.; Cearns, M.; Nielsen, M.Ø.; Kollias, C.; Xenaki, L.A.; Stefanatou, P.; Ralli, I.; Dimitrakopoulos, S.; Hatzimanolis, A.; Kosteletos, I.; et al. Prediction of early symptom remission in two independent samples of first-episode psychosis patients using machine learning. Schizophr. Bull. 2022, 48, 122–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, C.S.; Luedtke, A.R.; Sadikova, E.; Tsai, H.J.; Liao, S.C.; Liu, C.C.; Gau, S.F.; VanderWeele, T.J.; Kessler, R.C. Development and validation of a machine learning individualized treatment rule in first-episode schizophrenia. JAMA Netw. Open 2020, 3, 1921660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Zhang, L.; Zhang, Y.; Wen, H.; Huang, J.; Shen, Y.; Li, H. A random forest model for predicting social functional improvement in Chinese patients with schizophrenia after 3 months of atypical antipsychotic monopharmacy: A cohort study. Neuropsychiatr. Dis. Treat. 2021, 17, 847–857. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Del Fabro, L.; Bondi, E.; Serio, F.; Maggioni, E.; D’Agostino, A.; Brambilla, P. Machine learning methods to predict outcomes of pharmacological treatment in psychosis. Transl. Psychiatry 2023, 13, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schneider-Thoma, J.; Hamza, T.; Chalkou, K.; Siafis, S.; Dong, S.; Bighelli, I.; Hansen, W.P.; Scheuring, E.; Davis, J.M.; Priller, J.; et al. Efficacy of clozapine versus second-generation antipsychotics in people with treatment-resistant schizophrenia: A systematic review and individual patient data meta-analysis. Lancet Psychiatry 2025, 12, 254–265. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Kane, J.M.; Agid, O.; Baldwin, M.L.; Howes, O.; Lindenmayer, J.P.; Marder, S.; Olfson, M.; Potkin, S.G.; Correll, C.U. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J. Clin. Psychiatry 2019, 80, 18com12123. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Otani, N.; Ueno, T.; Hashimoto, K. Development of a hematocrit insensitive device to collect accurate volumes of dried blood spots without specialized skills for measuring clozapine and its metabolites as model analytes. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1087–1088, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Oh, H.S.; Lee, B.J.; Lee, Y.S.; Jang, O.J.; Nakagami, Y.; Inada, T.; Kato, T.A.; Kanba, S.; Chong, M.Y.; Lin, S.K.; et al. Machine learning algorithm-based prediction model for the augmented use of clozapine with electroconvulsive therapy in patients with schizophrenia. J. Pers. Med. 2022, 12, 969. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Bobadilla, A.V.; Schmitt, V.; Maier, C.S.; Mensing, S.; Stodtmann, S. Practical guide to SHAP analysis: Explaining super vised machine learning model predictions in drug development. Clin. Transl. Sci. 2024, 17, e70056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patteet, L.; Maudens, K.E.; Vermeulen, Z.; Dockx, G.; De Doncker, M.; Morrens, M.; Sabbe, B.; Neels, H. Retrospective evaluation of therapeutic drug monitoring of clozapine and norclozapine in Belgium using a multidrug UHPLC-MS/MS method. Clin. Biochem. 2014, 47, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Bowskill, S.; Couchman, L.; MacCabe, J.H.; Flanagan, R.J. Plasma clozapine and norclozapine in relation to prescribed dose and other factors in patients aged 65 years and over: Data from a therapeutic drug monitoring service, 1996–2010. Hum. Psychopharmacol. 2012, 27, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Piwowarska, J.; Radziwoń-Zaleska, M.; Dmochowska, M.; Szepietowska, E.; Matsumoto, H.; Sygitowicz, G.; Pilc, A.; Łukaszkiewicz, J. The usefulness of monitored therapy using clozapine concentration in the blood serum for determining drug dose in polish schizophrenic patients. Pharmacol. Rep. 2016, 68, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Mayerova, M.; Ustohal, L.; Jarkovsky, J.; Pivnicka, J.; Kasparek, T.; Ceskova, E. Influence of dose, gender, and cigarette smoking on clozapine plasma concentrations. Neuropsychiatr. Dis. Treat. 2018, 14, 1535–1543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olsson, E.; Edman, G.; Bertilsson, L.; Hukic, D.S.; Lavebratt, C.; Eriksson, S.V.; Ösby, U. Genetic and clinical factors affecting plasma clozapine concentration. Prim. Care Companion CNS Disord. 2015, 17, 25016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flanagan, R.J.; Yusufi, B.; Barnes, T.R. Comparability of whole-blood and plasma clozapine and norclozapine concentrations. Br. J. Clin. Pharmacol. 2003, 56, 135–138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piatkov, I.; Caetano, D.; Assur, Y.; Lau, S.L.; Jones, T.; Boyages, S.C.; McLean, M. ABCB1 and ABCC1 single-nucleotide polymorphisms in patients treated with clozapine. Pharmgenomics Pers. Med. 2017, 10, 235–242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bailey, L.; Varma, S.; Ahmad, N.; Gee, S.; Taylor, D.M. Factors predicting use of laxatives in outpatients stabilized on clozapine. Ther. Adv. Psychopharmacol. 2015, 5, 256–262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- García, C.I.; Alonso, A.I.; Bobes, J. Concentrations in plasma clozapine levels in schizophrenic and schizoaffective patients. Rev. Psiquiatr. Salud Ment. 2017, 10, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, N.H.; Leinonen, E.V.; Lehtonen, M.L.; Kivistö, K.T. Clozapine serum concentrations are lower in smoking than in non-smoking schizophrenic patients. Pharmacol. Toxicol. 1999, 85, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, R.J.; Obee, S.J.; Kim, A.H.M.; Every-Palmer, S. Plasma clozapine and N –desmethylclozapine (norclozapine) concentrations and the clozapine/norclozapine ratio: Effect of dose, sex, and cigarette smoking. J. Clin. Psychopharmacol. 2024, 44, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Lane, H.Y.; Chang, Y.C.; Chang, W.H.; Lin, S.K.; Tseng, Y.T.; Jann, M.W. Effects of gender and age on plasma levels of clozapine and its metabolites: Analyzed by critical statistics. J. Clin. Psychiatry 1999, 60, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Baumann, B.; Wolf, R.; Lehmann, D.; Peters, B.; Bogerts, B.; Meyer, F.P. Therapeutic drug monitoring of clozapine and relapse—A retrospective study of routine clinical data. Int. J. Clin. Pharmacol. Ther. 2003, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Mao, P.; Li, F.M.; Li, W.; Chen, Q.; Jiang, F.; Cai, Z.; Mitchell, P.B. Gender, age, smoking behaviour and plasma clozapine concentrations in 193 Chinese inpatients with Schizophrenia. Br. J. Clin. Pharmacol. 2007, 64, 49–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alharbi, N.S.; Hamdy, N.A.; Aboubakr, E.M.; Alharb, M.; Ali, M.A.; Alharbi, G.; ElMallah, A.I. Comparing the metabolic, sytemic, and neuropsychiatric impacts of olanzapine and clozapine in patients with schizophrenia. Pharmaceuticals 2025, 18, 314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vasudev, K.; Choi, Y.H.; Norman, R.; Kim, R.B.; Schwarz, U.I. Genetic determinants of clozapine-induced metabolic side effects. Can. J. Psychiatry 2017, 62, 138–149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Procyshyn, R.M.; Wasan, K.M.; Thornton, A.E.; Barr, A.M.; Chen, E.Y.; Pomarol-Clotet, E.; Stip, E.; Williams, R.; Macewan, G.W.; Birmingham, C.L. Changes in serum lipids, independent of weight, are associated with changes in symptoms during long-term clozapine treatment. J. Psychiatry Neurosci. 2007, 32, 331–338.PMID. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oyewumi, L.K.; Cernovsky, Z.Z.; Freeman, D.J.; Streiner, D.L. Relation of blood counts during clozapine treatment to serum concentrations of clozapine and norclozapine. Can. J. Psychiatry 2002, 47, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, T.; McCutcheon, R.A.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A.; et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poweleit, E.A.; Vinks, A.A.; Mizuno, T. Artificial intelligence and machine learning approaches to facilitate therapeutic drug management and model-informed precision dosing. Ther. Drug Monit. 2023, 45, 143–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siskind, D.; McCartney, L.; Goldschlager, R.; Kisely, S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: Systematic review and meta-analysis. Br. J. Psychiatry 2016, 209, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Samara, M.; Lappas, A.S.; Pinioti, E.; Glarou, E.; Fober, I.; Christogiannis, C.; Siafis, S.; Christodoulou, N.; Helfer, B.; Mavridis, D.; et al. Efficacy and tolerability of pharmacological interventions for schizophrenia non-responsive to prior treatment: A systematic review and network meta-analysis. EClinicalMedicine 2025, 84, 103291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smucny, J.; Lesh, T.A.; Albuquerque, M.D.; Rhilinger, J.P.; Carter, C.S. Predicting Clinical Improvement in Early Psychosis Using Circuit-Based Resting-State Functional Magnetic Resonance Imaging. Schizophr. Bull. 2024, 50, 1349–1356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Griffiths, K.; Millgate, E.; Egerton, A.; MacCabe, J.H. Demographic and clinical variables associated with response to clozapine in schizophrenia: A systematic review and meta-analysis. Psychol. Med. 2021, 51, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Okhuijsen-Pfeifer, C.; Sterk, A.Y.; Horn, I.M.; Terstappen, J.; Kahn, R.S.; Luykx, J.J. Demographic and clinical features as predictors of clozapine response in patients with schizophrenia spectrum disorders: A systematic review and metaanalysis. Neurosci. Biobehav. Rev. 2020, 111, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Siskind, D.; Sharma, M.; MPawar, M.; Pearson, E.; Wagner, E.; Warren, N.; Kisely, S. Clozapine levels as a predictor for therapeutic response: A systematic review and meta-analysis. Acta Psychiatr. Scand. 2021, 144, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Moscou, T.T.; Veerman, S.R.T. Clozapine/norclozapine plasma level ratio and cognitive functioning in patients with schizophrenia spectrum disorders: A systematic review. Ther. Adv. Psychopharmacol. 2024, 14, 20451253241302603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jacobson, T.A.; Kler, J.S.; Bae, Y.; Chen, J.; Ladror, D.T.; Iyer, R.; Nunes, D.A.; Montgomery, N.D.; Pleil, J.D.; Funk, W.E. A state-of-the-science review and guide for measuring environmental exposure biomarkers in dried blood spots. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 505–523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toyoda, K.; Hata, T.; Yamauchi, S.; Kinoshita, S.; Nishihara, M.; Uchiyama, K.; Inada, K.; Kanazawa, T. A descriptive study of 10-year clozapine use from the nationwide database in Japan. Psychiatry Res. 2021, 297, 113764. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.W.; Dong, M.; Zhang, Q.; Yang, S.Y.; Chen, L.Y.; Sim, K.; He, Y.L.; Chiu, H.F.; Sartorius, N.; Tan, C.H.; et al. Clozapine prescription pattern in patients with schizophrenia in Asia: The REAP survey (2016). Psychiatry Res. 2020, 287, 112271. [Google Scholar] [CrossRef] [PubMed]

| Mean | SD | Range | |
|---|---|---|---|
| Patients, sample number, and treatment duration (n = 167) | |||
| Age (y) | 40.6 | 11.4 | 18–72 |
| Number of samples/patients | 4.53 | 1.53 | 1–11 |
| Treatment duration (w) | 38.4 | 17.0 | 4–72 |
| Dose and concentrations before endpoint (n = 587) | |||
| Dose (mg/day) | 275 | 126 | 13–600 |
| Clozapine (ng/mL) | 457 | 282 | 38–1372 |
| Norclozapine (ng/mL) | 340 | 221 | 36–1319 |
| Clozapine N-oxide (ng/mL) | 90 | 50 | 10–390 |
| Clozapine/norclozapine ratio | 1.39 | 0.51 | 0.78–7.57 |
| Clozapine/dose ratio | 1.68 | 0.79 | 0.37–5.48 |
| Dose and concentrations at endpoint (n = 167) | |||
| Dose (mg/day) | 324 | 122 | 75–600 |
| Clozapine (ng/mL) | 526 | 261 | 106–1515 |
| Norclozapine (ng/mL) | 399 | 200 | 101–1131 |
| Clozapine N-oxide | 108 | 52 | 33–295 |
| Clozapine/norclozapine ratio | 1.35 | 0.33 | 0.79–2.48 |
| Clozapine/dose ratio | 1.70 | 0.80 | 0.10–5.05 |
| Sex | Men (n = 100) | Women (n = 67) | p | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | ||
| Dose and concentrations before endpoint (n = 373/214) | |||||||
| Dose (mg/day) | 288 | 130 | 13–600 | 252 | 115 | 50–600 | <0.001 |
| Clozapine (ng/mL) | 463 | 271 | 45–1372 | 446 | 300 | 38–1331 | 0.205 |
| Norclozapine (ng/mL) | 349 | 216 | 38–1100 | 326 | 229 | 33–1319 | 0.094 |
| Clozapine N-oxide (ng/mL) | 90 | 45 | 10–242 | 89 | 58 | 25–390 | 0.197 |
| Clozapine/norclozapine ratio | 1.37 | 0.48 | 0.80–3.43 | 1.42 | 0.56 | 0.78–7.57 | 0.215 |
| Clozapine/dose ratio | 1.63 | 0.75 | 0.37–5.04 | 1.76 | 0.85 | 0.37–5.48 | 0.063 |
| Dose and concentrations at end points (n = 100/67) | |||||||
| Dose (mg/day) | 344 | 125 | 100–600 | 295 | 114 | 75–525 | 0.011 |
| Clozapine (ng/mL) | 525 | 228 | 161–1119 | 528 | 307 | 106–1515 | 0.752 |
| Norclozapine (ng/mL) | 398 | 177 | 129–913 | 401 | 231 | 101–1131 | 0.753 |
| Clozapine N-oxide (ng/mL) | 110 | 46 | 39–263 | 106 | 60 | 33–295 | 0.255 |
| Clozapine/norclozapine ratio | 1.36 | 0.33 | 0.83–2.48 | 1.33 | 0.32 | 0.79–2.41 | 0.795 |
| Clozapine/dose ratio | 1.61 | 0.65 | 0.52–3.46 | 1.82 | 0.97 | 0.10–5.05 | 0.199 |
| Dose, Blood Levels, and Ratio | Age < 40 | Age ≧ 40 | p | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | ||
| Dose and concentrations before endpoint (n = 290/297) | |||||||
| Dose (mg/day) | 279 | 124 | 13–600 | 271 | 127 | 13–600 | 0.380 |
| Clozapine (ng/mL) | 461 | 297 | 43–1372 | 453 | 267 | 38–1330 | 0.976 |
| Norclozapine (ng/mL) | 344 | 234 | 38–1319 | 337 | 207 | 36–1016 | 0.901 |
| Clozapine N-oxide (ng/mL) | 92 | 51 | 12–253 | 88 | 50 | 10–390 | 0.254 |
| Clozapine/norclozapine ratio | 1.39 | 0.61 | 0.80–3.43 | 1.39 | 0.40 | 0.78–3.43 | 0.585 |
| Clozapine/dose ratio | 1.65 | 0.80 | 0.43–5.48 | 1.70 | 0.78 | 0.37–5.48 | 0.512 |
| Dose and concentrations at end points (n = 83/84) | |||||||
| Dose (mg/day) | 326 | 129 | 75–600 | 322 | 117 | 125–600 | 0.829 |
| Clozapine (ng/mL) | 527 | 242 | 88–1119 | 524 | 280 | 106–1515 | 0.720 |
| Norclozapine (ng/mL) | 401 | 189 | 101–844 | 397 | 212 | 89–1131 | 0.665 |
| Clozapine N-oxide (ng/mL) | 110 | 50 | 35–234 | 107 | 54 | 33–295 | 0.659 |
| Clozapine/norclozapine ratio | 1.36 | 0.34 | 0.79–2.48 | 1.34 | 0.31 | 0.50–2.41 | 0.736 |
| Clozapine/dose ratio | 1.70 | 0.72 | 0.10–5.48 | 1.68 | 0.87 | 0.16–5.05 | 0.358 |
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Endpoint | Baseline | Endpoint | |||||||
| Mean | SD (n) | Mean | SD (n) | p | Mean | SD (n) | Mean | SD (n) | p | |
| Body weight (kg) | 69.9 | 17.1 (89) | 66.9 | 14.9 (89) | 0.109 | 62.3 | 14.0 (62) | 59.9 | 13.9 (62) | 0.288 |
| BMI (kg/m2) | 24.2 | 5.1 (86) | 23.2 | 4.4 (86) | 0.161 | 25.6 | 6.4 (58) | 24.2 | 5.3 (57) | 0.252 |
| Glucose (mg/dL) | 95.5 | 17.9 (72) | 103.3 | 22.5 (70) | 0.018 * | 94.9 | 20.8 (37) | 104.1 | 28.1 (45) | 0.023 * |
| HbA1c (%) | 5.39 | 1.41 (74) | 5.47 | 0.39 (72) | 0.145 | 5.52 | 0.40 (37) | 5.62 | 0.73 (44) | 0.519 |
| TC (mg/dL) | 176 | 35 (63) | 179 | 42 (62) | 0.540 | 186 | 31 (36) | 189 | 39 (39) | 0.663 |
| HDL (mg/dL) | 51.5 | 12.5 (57) | 56.5 | 11.2 (48) | 0.025 * | 57.3 | 16.4 (28) | 59.3 | 15.8 (24) | 0.440 |
| TG (mg/dL) | 123 | 78 (63) | 136 | 79 (66) | 0.224 | 96.0 | 38.0 (35) | 107.1 | 41.8 (35) | 0.254 |
| WBC (/µL) | 6540 | 2033 (75) | 6800 | 2239 (97) | 0.091 | 6080 | 2112 (39) | 6615 | 1881 (65) | 0.184 |
| NEUT (%) | 59.8 | 10.1 (75) | 61.7 | 9.5 (96) | 0.128 | 59.5 | 10.8 (38) | 63.1 | 10.8 (64) | 0.116 |
| NEUT (/µL) | 3938 | 1802 (75) | 4302 | 2032 (95) | 0.090 | 3794 | 1629 (37) | 4271 | 1769 (64) | 0.091 |
| ALT (U/L) | 29.3 | 17.8 (57) | 33.6 | 20.3 (67) | 0.227 | 23.6 | 14.4 (33) | 29.3 | 17.4 (39) | 0.075 |
| AST (U/L) | 23.7 | 10.5 (57) | 26.9 | 16.7 (70) | 0.980 | 21.4 | 11.3 (33) | 23.6 | 10.9 (39) | 0.116 |
| γ-GTP (U/L) | 34.4 | 26.1 (38) | 28.5 | 20.0 (50) | 0.330 | 21.1 | 10.7 (23) | 24.0 | 20.0 (25) | 0.917 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakahara, T.; Harada, Y.; Nakayama, N.; Hashimoto, K.; Kida, N.; Onitsuka, T.; Noda, H.; Murasugi, K.; Takimoto, Y.; Omori, W.; et al. Personalized Prediction of Clozapine Treatment Response Using Therapeutic Drug Monitoring Data in Japanese Patients with Treatment-Resistant Schizophrenia. J. Clin. Med. 2025, 14, 7892. https://doi.org/10.3390/jcm14217892
Nakahara T, Harada Y, Nakayama N, Hashimoto K, Kida N, Onitsuka T, Noda H, Murasugi K, Takimoto Y, Omori W, et al. Personalized Prediction of Clozapine Treatment Response Using Therapeutic Drug Monitoring Data in Japanese Patients with Treatment-Resistant Schizophrenia. Journal of Clinical Medicine. 2025; 14(21):7892. https://doi.org/10.3390/jcm14217892
Chicago/Turabian StyleNakahara, Tatsuo, Yukiko Harada, Naho Nakayama, Kijiro Hashimoto, Naoya Kida, Toshiaki Onitsuka, Hiroo Noda, Kenji Murasugi, Yoshihiro Takimoto, Wataru Omori, and et al. 2025. "Personalized Prediction of Clozapine Treatment Response Using Therapeutic Drug Monitoring Data in Japanese Patients with Treatment-Resistant Schizophrenia" Journal of Clinical Medicine 14, no. 21: 7892. https://doi.org/10.3390/jcm14217892
APA StyleNakahara, T., Harada, Y., Nakayama, N., Hashimoto, K., Kida, N., Onitsuka, T., Noda, H., Murasugi, K., Takimoto, Y., Omori, W., Sukegawa, T., Shiraishi, J., Tanaka, K., Maesato, H., & Ueno, T. (2025). Personalized Prediction of Clozapine Treatment Response Using Therapeutic Drug Monitoring Data in Japanese Patients with Treatment-Resistant Schizophrenia. Journal of Clinical Medicine, 14(21), 7892. https://doi.org/10.3390/jcm14217892






