A Cohort Study Characterizing the Outcomes Following an Acute SARS-CoV-2 Infection in Pregnancy
Abstract
1. Introduction
2. Methods
2.1. Human Subjects Protection
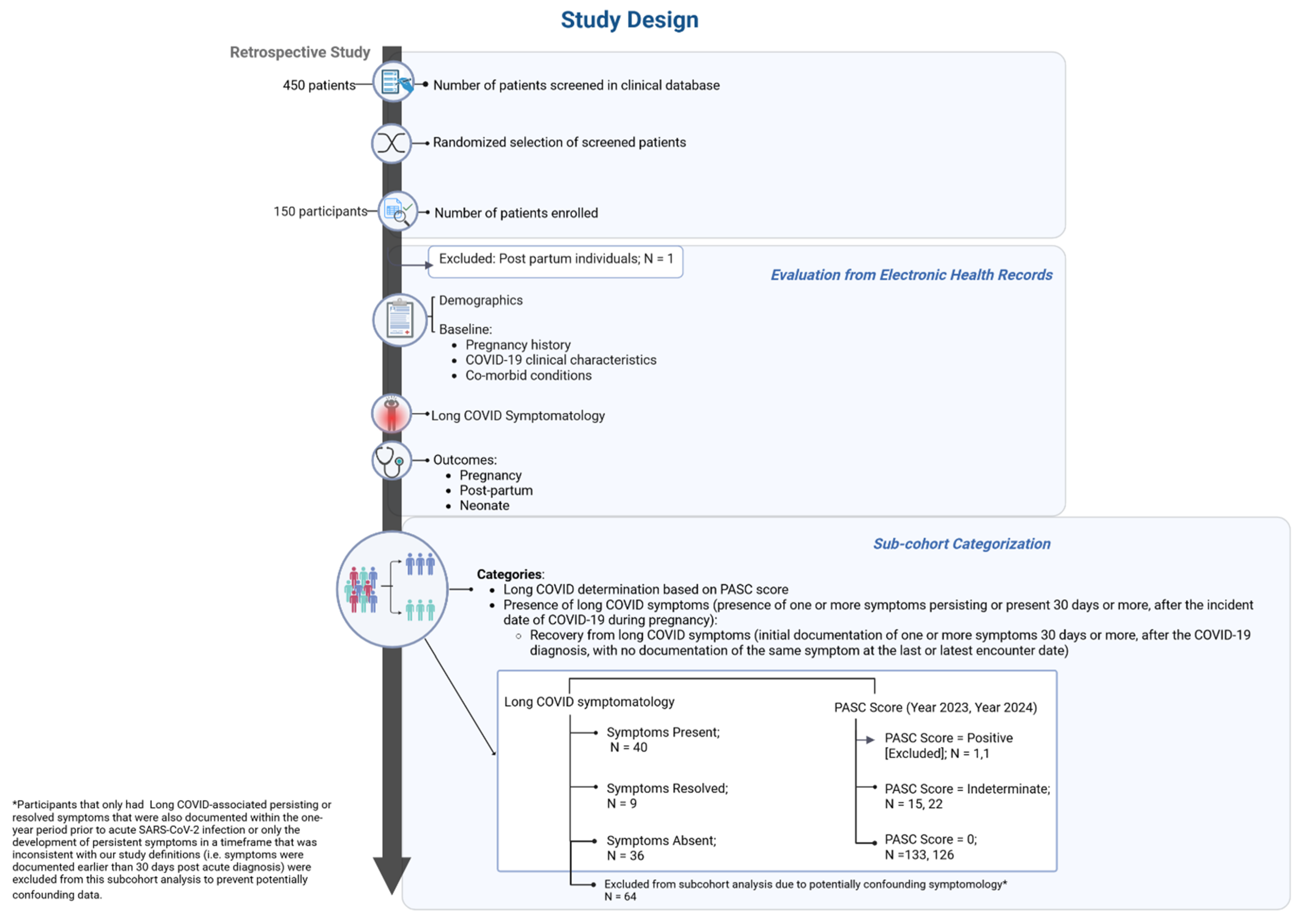
2.2. Cohort Selection and Definitions
2.3. Study Period
2.4. EHR Review
2.5. Data Collection Variables
2.6. Cohort Classification
2.6.1. Overall Symptom Presence
2.6.2. PASC Score
2.7. Statistical Analysis
3. Results
3.1. Subcohorts Based on Overall Symptom Presence and PASC Score Classification
3.1.1. Overall Symptom Presence
3.1.2. PASC Score
3.2. Documentation of Symptoms Prior to Acute COVID-19 Diagnosis
3.3. Symptoms Following Acute SARS-CoV-2 Infection During Pregnancy
3.4. Pregnancy and Neonatal Outcomes
3.4.1. Outcomes Based on Overall Symptom Presence Classification
3.4.2. Outcomes Based on PASC Score Classification
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yong, E. COVID-19 Can Last for Several Months; The Atlantic: Washington, DC, USA, 2020. [Google Scholar]
- Centers for Disease Control and Prevention. Long COVID Basics; CDC: Atlanta, GA, USA, 2025.
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Global Health; Board on Health Sciences Policy; Committee on Examining the Working Definition for Long COVID. A Long COVID Definition: A Chronic, Systemic Disease State with Profound Consequences; Goldowitz, I., Worku, T., Brown, L., Fineberg, H.V., Eds.; National Academies Press (US): Washington, DC, USA, 2024. [Google Scholar]
- Thaweethai, T.; Jolley, S.E.; Karlson, E.W.; Levitan, E.B.; Levy, B.; McComsey, G.A.; McCorkell, L.; Nadkarni, G.N.; Parthasarathy, S.; Singh, U.; et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA 2023, 329, 1934–1946. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Post COVID-19 Condition (Long COVID). 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/post-covid-19-condition-(long-covid) (accessed on 8 April 2025).
- World Health Organization (WHO). Number of COVID-19 Cases Reported to WHO (Cumulative Total). Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 8 April 2025).
- National Center for Health Statistics. Household Pulse Survey, 2022–2024. Long COVID. 2024. Available online: https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm#print (accessed on 8 April 2025).
- Burns, A. As Recommendations for Isolation End, How Common is Long COVID? Kaiser Family Foundation: San Francisco, CA, USA, 2024. [Google Scholar]
- O’Hare, A.M.; Vig, E.K.; Iwashyna, T.J.; Fox, A.; Taylor, J.S.; Viglianti, E.M.; Butler, C.R.; Vranas, K.C.; Helfand, M.; Tuepker, A.; et al. Complexity and Challenges of the Clinical Diagnosis and Management of Long COVID. JAMA Netw. Open 2022, 5, e2240332. [Google Scholar] [CrossRef]
- Quach, T.C.; Miglis, M.G.; Tian, L.; Bonilla, H.; Yang, P.C.; Grossman, L.; Paleru, A.; Xin, V.; Tiwari, A.; Shafer, R.W.; et al. Post-COVID-19 Vaccination and Long COVID: Insights from Patient-Reported Data. Vaccines 2024, 12, 1427. [Google Scholar] [CrossRef] [PubMed]
- Maripuri, M.; Dey, A.; Honerlaw, J.; Hong, C.; Ho, Y.-L.; Tanukonda, V.; Chen, A.W.; Panickan, V.A.; Wang, X.; Zhang, H.G.; et al. Characterization of Post-COVID-19 Definitions and Clinical Coding Practices: Longitudinal Study. Online J. Public Health Inform. 2024, 16, e53445. [Google Scholar] [CrossRef]
- Khullar, D.; Zhang, Y.; Zang, C.; Xu, Z.; Wang, F.; Weiner, M.G.; Carton, T.W.; Rothman, R.L.; Block, J.P.; Kaushal, R. Racial/Ethnic Disparities in Post-acute Sequelae of SARS-CoV-2 Infection in New York: An EHR-Based Cohort Study from the RECOVER Program. J. Gen. Intern. Med. 2023, 38, 1127–1136. [Google Scholar] [CrossRef]
- Jacobs, M.M.; Evans, E.; Ellis, C. Racial, ethnic, and sex disparities in the incidence and cognitive symptomology of long COVID-19. J. Natl. Med. Assoc. 2023, 115, 233–243. [Google Scholar] [CrossRef]
- Metz, T.D.; Reeder, H.T.; Clifton, R.G.; Flaherman, V.; Aragon, L.V.; Baucom, L.C.M.; Beamon, C.J.; Braverman, A.; Brown, J.; Cao, T.; et al. Post-Acute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) After Infection During Pregnancy. Obstet. Gynecol. 2024, 144, 411–420. [Google Scholar] [CrossRef]
- Zang, C.; Guth, D.; Bruno, A.M.; Xu, Z.; Li, H.; Ammar, N.; Chew, R.; Guthe, N.; Hadley, E.; Kaushal, R.; et al. Long COVID after SARS-CoV-2 during pregnancy in the United States. Nat. Commun. 2025, 16, 3005. [Google Scholar] [CrossRef]
- Woodworth, K.R.; Olsen, E.O.; Neelam, V.; Lewis, E.L.; Galang, R.R.; Oduyebo, T.; Aveni, K.; Yazdy, M.M.; Harvey, E.; Longcore, N.D.; et al. Birth and Infant Outcomes Following Laboratory-Confirmed SARS-CoV-2 Infection in Pregnancy—SET-NET, 16 Jurisdictions, March 29–October 14, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1635–1640. [Google Scholar] [CrossRef]
- Strid, P.; Zapata, L.B.; Tong, V.T.; Zambrano, L.D.; Woodworth, K.R.; Riser, A.P.; Galang, R.R.; Gilboa, S.M.; Ellington, S.R. Coronavirus Disease 2019 (COVID-19) Severity Among Women of Reproductive Age with Symptomatic Laboratory-Confirmed Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection by Pregnancy Status—United States, 1 January 2020–25 December 2021. Clin. Infect. Dis. 2022, 75 (Suppl. S2), S317–S325. [Google Scholar] [CrossRef]
- Boettcher, L.B.; Metz, T.D. Maternal and neonatal outcomes following SARS-CoV-2 infection. Semin. Fetal Neonatal Med. 2023, 28, 101428. [Google Scholar] [CrossRef] [PubMed]
- Casella, C.; Giudice, M.L.; Niola, M.; DI Lorenzo, P.; Adamo, M.; Bianco, C.; Gragnano, E.; Saccone, G.; Guida, M. Need for fair inclusion of pregnant women in clinical trials: Scientific and ethical considerations about the lesson from the COVID-19 vaccines development. Minerva Obstet. Gynecol. 2022, 74, 112–113. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER). Pregnant Women: Scientific and Ethical Considerations for Inclusion in Clinical Trials Guidance for Industry; U.S. Department of Health and Human Services: Washington, DC, USA, 2018. Available online: https://www.fda.gov/media/112195/download (accessed on 26 August 2025).
- Sewell, C.A.; Sheehan, S.M.; Gill, M.S.; Henry, L.M.; Bucci-Rechtweg, C.; Gyamfi-Bannerman, C.; Lyerly, A.D.; McKinney, L.C.; Hatfield, K.P.; Baer, G.R.; et al. Scientific, ethical, and legal considerations for the inclusion of pregnant people in clinical trials. Am. J. Obstet. Gynecol. 2022, 227, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System. 2025. Available online: https://www.cdc.gov/maternal-mortality/php/pregnancy-mortality-surveillance-data/index.html?cove-tab=2 (accessed on 9 April 2025).
- Mehta, N.; Chen, K.; Hardy, E.; Powrie, R. Respiratory disease in pregnancy. Best Pr. Res. Clin. Obstet. Gynaecol. 2015, 29, 598–611. [Google Scholar] [CrossRef]
- Oseghale, O.; Vlahos, R.; O’leary, J.J.; Brooks, R.D.; Brooks, D.A.; Liong, S.; Selemidis, S. Influenza Virus Infection During Pregnancy as a Trigger of Acute and Chronic Complications. Viruses 2022, 14, 2729. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef]
- Berumen-Lechuga, M.G.; Leaños-Miranda, A.; Molina-Pérez, C.J.; García-Cortes, L.R.; Palomo-Piñón, S. Risk Factors for Severe-Critical COVID-19 in Pregnant Women. J. Clin. Med. 2023, 12, 5812. [Google Scholar] [CrossRef]
- Afshar, Y.; Gaw, S.L.; Flaherman, V.J.; Chambers, B.D.; Krakow, D.; Berghella, V.; A Shamshirsaz, A.; A Boatin, A.; Aldrovandi, G.; Greiner, A.; et al. Clinical Presentation of Coronavirus Disease 2019 (COVID-19) in Pregnant and Recently Pregnant People. Obstet. Gynecol. 2020, 136, 1117–1125. [Google Scholar] [CrossRef]
- Mammaro, A.; Carrara, S.; Cavaliere, A.; Ermito, S.; Dinatale, A.; Pappalardo, E.M.; Militello, M.; Pedata, R. Hypertensive disorders of pregnancy. J. Prenat. Med. 2009, 3, 1–5. [Google Scholar]
- Zajdenverg, L.; Negrato, C.A. Gestational diabetes mellitus and type 2 diabetes: Same disease in a different moment of life? Maybe not. Arch. Endocrinol. Metab. 2017, 61, 208–210. [Google Scholar] [CrossRef]
- Muñoz-Chápuli Gutiérrez, M.; Prat, A.S.; Vila, A.D.; Claverol, M.B.; Martínez, P.P.; Recarte, P.P.; Benéitez, M.V.; García, C.A.; Muñoz, E.C.; Navarro, M.; et al. Post-COVID-19 condition in pregnant and postpartum women: A long-term follow-up, observational prospective study. EClinicalMedicine 2024, 67, 102398. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Carvalho, M.A.; Nacul, L.; Cabar, F.R.; Fabri, A.W.; Peres, S.V.; Zaccara, T.A.; O’boyle, S.; Alexander, N.; Takiuti, N.H.; et al. Post-Viral Fatigue Following SARS-CoV-2 Infection during Pregnancy: A Longitudinal Comparative Study. Int. J. Environ. Res. Public Health 2022, 19, 5735. [Google Scholar] [CrossRef] [PubMed]
- Vásconez-González, J.; Fernandez-Naranjo, R.; Izquierdo-Condoy, J.S.; Delgado-Moreira, K.; Cordovez, S.; Tello-De-La-Torre, A.; Paz, C.; Castillo, D.; Izquierdo-Condoy, N.; Carrington, S.J.; et al. Comparative analysis of long-term self-reported COVID-19 symptoms among pregnant women. J. Infect. Public Health 2023, 16, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.N.; Erlandson, K.M.; Hornig, M.; Letts, R.; Selvaggi, C.; Ashktorab, H.; Atieh, O.; Bartram, L.; Brim, H.; Brosnahan, S.B.; et al. 2024 Update of the RECOVER-Adult Long COVID Research Index. JAMA 2025, 333, 694–700. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Cisewski, J.A.; Hoyert, D.L. Provisional Maternal Mortality Rates; National Center for Health Statistics, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2025. [CrossRef]
- Foster, T.B.; Porter, S.R.; Pharris-Ciurej, N. “Excess Mortality” During COVID19 Varied by Race, Ethnicity, Geography; United States Census Burea: Suitland, MD, USA, 2024.
- MacEwan, S.R.; Rahurkar, S.; Tarver, W.L.; Eiterman, L.P.; Melnyk, H.; Olvera, R.G.; Eramo, J.L.; Teuschler, L.; Gaughan, A.A.; Rush, L.J.; et al. The Impact of Long COVID on Employment and Well-Being: A Qualitative Study of Patient Perspectives. J. Gen. Intern. Med. 2025, 40, 1070–1077. [Google Scholar] [CrossRef]
- Silver, S.R.; Li, J.; Ford, N.D.; Saydah, S.H. Functional disabilities and adverse well-being by COVID-19 and Long COVID history and employment status: 2022 Behavioral Risk Factor Surveillance System. Am. J. Ind. Med. 2024, 67, 1089–1107. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, W.; Zhang, D.; Tam, K.W.; Li, Y.; Chan, D.C.C.; Yang, Z.; Wong, S.Y.S. Excess risks of long COVID symptoms compared with identical symptoms in the general population: A systematic review and meta-analysis of studies with control groups. J. Glob. Health 2024, 14, 05022. [Google Scholar] [CrossRef]
- Muluh, E.A.E.; McCormack, J.C.; Mo, Y.; Garratt, M.; Peng, M. Gustatory and olfactory shifts during pregnancy and the postpartum period: A systematic review and meta-analysis. Physiol. Behav. 2024, 273, 114388. [Google Scholar] [CrossRef]
- Mumtaz, A.; Sheikh, A.A.E.; Khan, A.M.; Khalid, S.N.; Khan, J.; Nasrullah, A.; Sagheer, S.; Sheikh, A.B. COVID-19 Vaccine and Long COVID: A Scoping Review. Life 2022, 12, 1066. [Google Scholar] [CrossRef]
- Hamilton, B.E.; Martin, J.A.; Osterman, M.J.K. Births: Provisional Data for 2024; U.S. Centers for Disease Control and Prevention: Atlanta, GA, USA, 2025; Volume 38.
- Bello-Chavolla, O.Y.; Fermín-Martínez, C.A.; Ramírez-García, D.; Vargas-Vázquez, A.; Fernández-Chirino, L.; Basile-Alvarez, M.R.; Sánchez-Castro, P.; Núñez-Luna, A.; Antonio-Villa, N.E. Prevalence and determinants of post-acute sequelae after SARS-CoV-2 infection (Long COVID) among adults in Mexico during 2022: A retrospective analysis of nationally representative data. Lancet Reg. Health Am. 2024, 30, 100688. [Google Scholar] [CrossRef]
- Zhang, H.G.; Honerlaw, J.P.; Maripuri, M.; Samayamuthu, M.J.; Beaulieu-Jones, B.R.; Baig, H.S.; L’yi, S.; Ho, Y.-L.; Morris, M.; Panickan, V.A.; et al. Potential pitfalls in the use of real-world data for studying long COVID. Nat. Med. 2023, 29, 1040–1043. [Google Scholar] [CrossRef]
- Ignacio, M.; Oesterle, S.; Rodriguez-González, N.; Lopez, G.; Ayers, S.; Carver, A.; Wolfersteig, W.; Williams, J.H.; Sabo, S.; Parthasarathy, S. Limited Awareness of Long COVID Despite Common Experience of Symptoms Among African American/Black, Hispanic/Latino, and Indigenous Adults in Arizona. J. Racial Ethn. Health Disparities 2025, 12, 3013–3023. [Google Scholar] [CrossRef]
- Sprague Martinez, L.; Sharma, N.; John, J.; Battaglia, T.A.; Linas, B.P.; Clark, C.R.; Hudson, L.B.; Lobb, R.; Betz, G.; O’nEill, S.O.O.; et al. Long COVID impacts: The voices and views of diverse Black and Latinx residents in Massachusetts. BMC Public Health 2024, 24, 2265. [Google Scholar] [CrossRef]


| Symptom List/Description |
|---|
| Poor appetite or overeating |
| Fatigue (being very tired) |
| Post-exertional malaise (Symptoms worse after even minor physical or mental effort) |
| Swelling of legs, Weakness in arms or legs, muscle cramps in legs and/or feet |
| Fever, chills, sweats or flushing |
| Loss of or change in smell or taste |
| Pain in any part of your body |
| Shortness of breath |
| Cough |
| Palpitations, racing heart, arrhythmia, skipped beats |
| Gastrointestinal (belly) symptoms (feeling full or vomiting after eating, diarrhea, constipation, cramping or colicky abdominal pain) |
| Bladder problems (incontinence, trouble passing urine or emptying bladder) |
| Nerve problems (tremor, shaking, abnormal movements, numbness, tingling, burning, cannot move part of body, new seizures) |
| Problems with anxiety, depression, stress, or trauma-related symptoms like nightmares or grief |
| Problems with sleep |
| Problems thinking or concentrating (“brain fog”), Feeling faint, dizzy, “goofy”; difficulty thinking soon after standing up from a sitting or lying position |
| Color changes in your skin, such as red, white or purple |
| Skin rash, sores |
| Excessively dry mouth, eyes |
| Excessive thirst |
| Vision problems (blurry, light sensitivity, difficulty reading or focusing, floaters, flashing lights, “snow”) |
| Problems with hearing (hearing loss, ringing in ears) |
| Hair loss |
| Problems with teeth |
| Changes to menstrual cycle, reports of heavy periods |
| Changes in fertility or difficulty getting pregnant |
| Any other symptoms that were attributed to COVID-19 in EHR documentation |
| Symptoms Absent | Symptoms Present | Symptoms Resolved | PASC Zero 2023 | PASC Indeterminate 2023 | PASC Zero 2024 | PASC Indeterminate 2024 | ||
|---|---|---|---|---|---|---|---|---|
| Number of participants | 36 | 40 | 9 | 133 | 15 | 126 | 22 | |
| Proportion of participant pool | 24.20% | 26.90% | 6.04% | 89.30% | 10.10% | 84.60% | 14.80% | |
| Age (years) | Range | 16–41 | 20–44 | 17–36 | 15–41 | 20–39 | 16–41 | 15–39 |
| Mean | 26.6 | 32.4 | 26.7 | 27.7 | 28.9 | 28.1 | 27.1 | |
| Race/Ethnicity | Black | 31% | 47.50% | 22% | 43% | 47% | 44% | 36% |
| White | 32% | 30% | 22% | 30% | 33% | 29% | 41% | |
| Latina | 32% | 17.50% | 56% | 22% | 13% | 22% | 14% | |
| Asian | 0% | 5% | 0% | 2% | 7% | 2% | 5% | |
| Native American or Alaska Native | 0% | 0% | 0% | 1% | 0% | 0% | 5% | |
| Native Hawaiian or Pacific Islander | 0% | 0% | 0% | 0% | 0% | 0% | 5% | |
| Multiracial | 5% | 0% | 0% | 2% | 0% | 2% | 0% | |
| Vaccination Status | Before COVID-19 diagnosis | 17% | 15% | 11% | 21% | 7% | 21% | 9% |
| After COVID-19 diagnosis | 19% | 27.50% | 33% | 20% | 40% | 21% | 32% | |
| Not documented | 53% | 17.50% | 33% | 33% | 13% | 33% | 18% | |
| Patient declined | 11% | 40% | 22% | 26% | 40% | 25% | 41% | |
| Trimester on date of COVID-19 Diagnosis | 1st | 3% | 12.50% | 0% | 5% | 20% | 5% | 14% |
| 2nd | 28% | 32.50% | 22% | 32% | 47% | 35% | 50% | |
| 3rd | 69% | 55% | 78% | 62% | 33% | 60% | 36% | |
| Gestational Age (weeks) | Mean | 31.9 | 28 | 33.6 | 29 | 25.7 | 29.5 | 25.6 |
| Trimester | Mean | 3rd | 3rd | 3rd | 3rd | 2nd | 3rd | 2nd |
| Symptoms Absent | Symptoms Present | ||||
|---|---|---|---|---|---|
| (n) | (%) | (n) | (%) | ||
| 36 | 47% | 40 | 53% | ||
| General Characteristics of Concurrent COVID-19 and Pregnancy | |||||
| Number of times pregnancy is documented in the patient’s EHR (including current/recent pregnancy, previous pregnancies, live births, miscarriages, stillbirths or abortions) | 1 | 7 | 19.44% | 2 | 5.00% |
| 2 | 11 | 30.56% | 10 | 25.00% | |
| 3 | 5 | 13.89% | 8 | 20.00% | |
| 4 | 9 | 25.00% | 6 | 15.00% | |
| 5 | 3 | 8.33% | 6 | 15.00% | |
| >5 | 1 | 2.78% | 8 | 20.00% | |
| Number of multiple gestations BEFORE pregnancy during COVID-19 | 1 | 3.45% | 1 | 2.50% | |
| Prior Vaginal Birth | 16 | 55.17% | 16 | 40.00% | |
| Adverse Outcomes of Prior Pregnancies | |||||
| Miscarriages | 1 | 2 | 6.90% | 9 | 23.68% |
| 2 | 1 | 3.45% | 2 | 5.26% | |
| 3 | 0 | 0.00% | 3 | 7.89% | |
| Still Birth | 0 | 0.00% | 1 | 2.63% | |
| HELLP | 0 | 0.00% | 1 | 2.63% | |
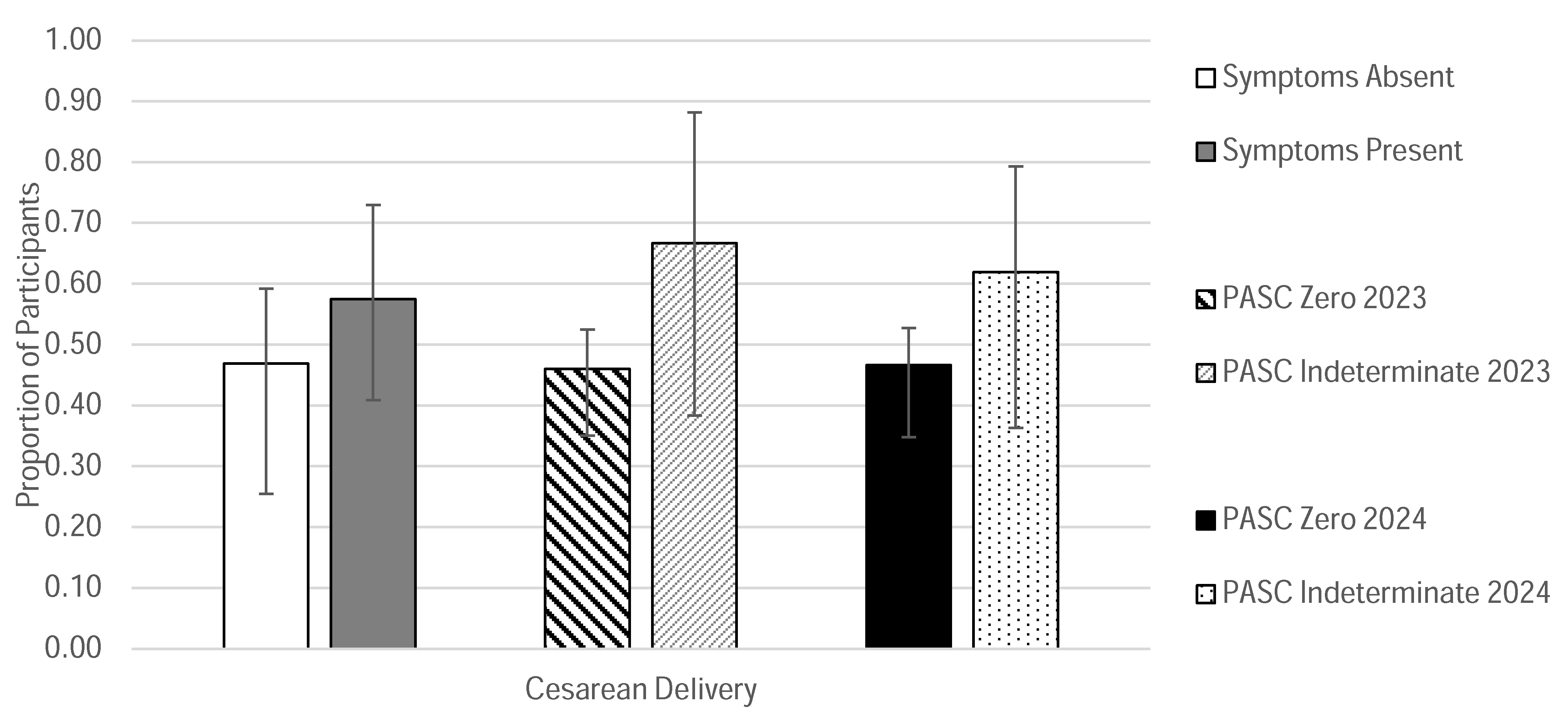
| Cesarean Section (CS) Delivery | 7 | 24.14% | 15 | 39.47% | |
| Gestational Diabetes | 0 | 0.00% | 4 | 10.53% | |
| Gestational Hypertension | 3 | 10.34% | 6 | 15.79% | |
| Preeclampsia | 5 | 17.24% | 3 | 7.89% | |
| Preterm birth | 2 | 6.90% | 5 | 13.16% | |
| COVID-19 Experience | |||||
| Stage at which acute COVID-19 was diagnosed | Pregnant | 20 | 55.56% | 31 | 77.50% |
| In labor/at delivery | 16 | 44.44% | 9 | 22.50% | |
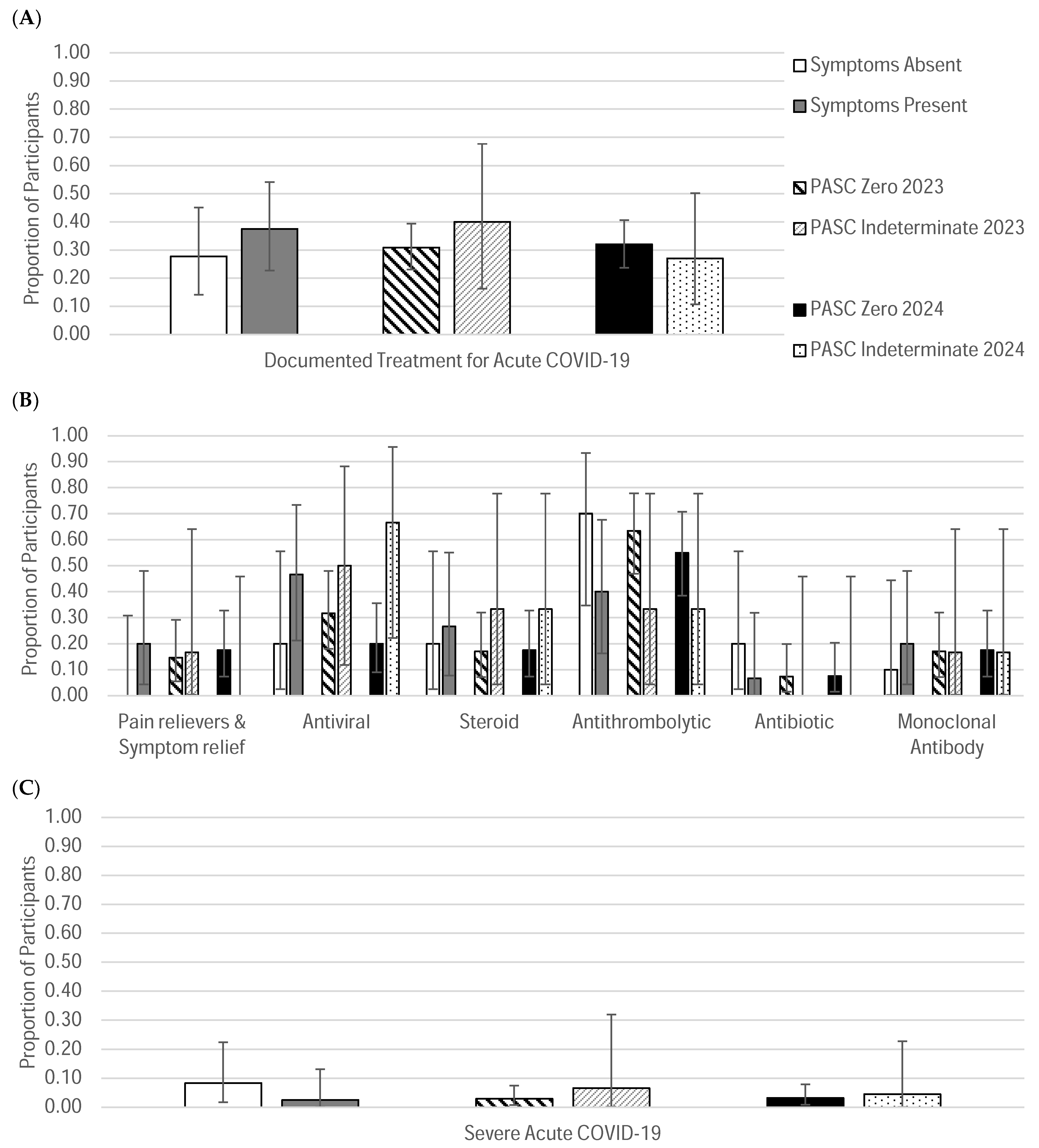
| Treated for acute COVID-19 | 10 | 27.78% | 15 | 37.50% | |
| Acute COVID-19 treatment given | Pain relievers and symptom relief | 0 | 0.00% | 3 | 20.00% |
| Antivirals | 2 | 20.00% | 7 | 46.67% | |
| Steroids | 2 | 20.00% | 4 | 26.67% | |
| Antithrombolytics | 7 | 70.00% | 6 | 40.00% | |
| Antibiotics | 2 | 20.00% | 1 | 6.67% | |
| Monoclonal Antibody | 1 | 10.00% | 3 | 20.00% | |
| No documented treatment for acute COVID-19 | 26 | 72.22% | 25 | 62.50% | |
| Documented reasons for lack of acute COVID-19 treatment | Asymptomatic | 8 | 30.77% | 5 | 20.00% |
| Patient declined | 1 | 3.85% | 4 | 16.00% | |
| Mild Symptoms | 4 | 15.38% | 2 | 8.00% | |
| Patient hospitalized and/or given oxygen due to acute COVID-19 | 3 | 8.33% | 1 | 2.25% | |
| Pregnancy Outcomes | |||||
| Number of multiple gestations DURING pregnancy during COVID-19 | 3 | 8.33% | 1 | 2.50% | |
| Live Birth | 32 | 89% | 40 | 100% | |
| Number of babies | 1 | 30 | 93.75% | 39 | 97.50% |
| 2 | 3 | 9.38% | 1 | 2.50% | |
| Sex distribution of neonate | M | 21 | 64.00% | 20 | 50.00% |
| F | 12 | 36.00% | 20 | 50.00% | |
| Miscarriages | 0 | 0.00% | 0 | 0.00% | |
| Still Birth | 2 | 5.56% | 0 | 0.00% | |
| Preterm birth | 11 | 30.56% | 13 | 32.50% | |
| CS Delivery | 15 | 46.88% | 23 | 57.50% | |
| Reason for CS Delivery | Planned cesarean delivery because of prior cesarean delivery | 6 | 40.00% | 9 | 39.13% |
| Abnormal progress in labor | 4 | 26.67% | 4 | 17.39% | |
| Concern about baby based on the heart monitor | 2 | 13.33% | 7 | 30.43% | |
| Baby was breech | 2 | 13.33% | 2 | 8.70% | |
| Emergency due to risk to baby or participant | 3 | 20.00% | 3 | 13.04% | |
| Patient too sick with COVID-19 to be in labor | 1 | 6.67% | 0 | 0.00% | |
| Other reasons for a Cesarean | 0 | 0.00% | 3 | 13.04% | |
| Forceps or Vacuum-assisted delivery | 0 | 0.00% | 6 | 15.00% | |
| Gestational diabetes | 6 | 16.67% | 3 | 7.50% | |
| Gestational hypertension | 2 | 5.56% | 7 | 17.50% | |
| Preeclampsia | 8 | 22.22% | 8 | 20.00% | |
| HELLP syndrome | 1 | 2.78% | 2 | 5.00% | |
| Seizures | 1 | 2.78% | 0 | 0.00% | |
| Placenta abruption | 3 | 8.33% | 1 | 2.50% | |
| Preterm premature rupture of membranes | 6 | 16.67% | 5 | 12.50% | |
| Oligohydramnios | 1 | 2.78% | 1 | 2.50% | |
| Hemorrhage or excessive bleeding | 4 | 11.76% | 3 | 7.50% | |
| Blood transfusion | 1 | 2.94% | 3 | 7.50% | |
| Uterine infection | 4 | 11.76% | 3 | 7.50% | |
| Blood clot in the legs of lungs requiring treatment with blood thinning medications | 0 | 0.00% | 1 | 2.50% | |
| Mechanical Ventilation | 1 | 2.94% | 0 | 0.00% | |
| Pneumonia | 1 | 2.94% | 2 | 5.00% | |
| Fetal Growth Restriction | 0 | 0.00% | 0 | 0.00% | |
| Congenital Anomalies | 1 | 3% | 1 | 3% | |
| Before COVID-19 Diagnosis | 1 | 100% | 0 | 0% | |
| After COVID-19 Diagnosis | 0 | 0% | 1 | 100% | |
| Category of Congenital Anomaly | Abdomen | 0 | 0% | 1 | 100% |
| Brain | 0 | 0% | 0 | 0% | |
| Face or lip | 0 | 0% | 0 | 0% | |
| Cardiac | 1 | 100% | 0 | 0% | |
| Trimester anomaly was observed | 2nd | 1 | 100% | 0 | 0% |
| 3rd | 0 | 0% | 0 | 0% | |
| At birth | 0 | 0% | 1 | 100% | |
| Number of multiple gestations AFTER pregnancy during COVID-19 | 0 | 0.00% | 0 | 0.00% | |
| Number of participants currently pregnant | 1 | 2.78% | 6 | 15.00% | |
| Neonatal Outcomes | |||||
| Neonatal Intensive Care Unit (NICU) admittance | 11 | 34.38% | 13 | 32.50% | |
| Non-Survival at discharge | 6 | 18.75% | 1 | 2.50% | |
| Non-Survival post-partum | 1 | 3.85% | 3 | 7.69% | |
| Average Birth Weight (for those born between 37 and 41 weeks of gestation) | 6.0 lbs 7.6 oz | 5.8 lbs 6.2 oz | |||
| Average Birth Weight (for all live births) | 6 lbs 7.6 oz | 5.8 lbs 6.0 oz | |||
| PASC 2023 = 0 | PASC Indeterminate 2023 | PASC 2024 = 0 | PASC Indeterminate 2024 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | ||
| 133 | 89% | 15 | 10% | 126 | 85% | 22 | 15% | ||
| General Characteristics of Concurrent COVID-19 and Pregnancy | |||||||||
| Number of times pregnancy is documented in the patient’s EHR (including current/recent pregnancy, previous pregnancies, live births, miscarriages, stillbirths or abortions) | 1 | 17 | 12.78% | 0 | 0.00% | 15 | 11.90% | 2 | 9.090% |
| 2 | 33 | 24.81% | 4 | 26.67% | 30 | 23.81% | 7 | 31.82% | |
| 3 | 22 | 16.54% | 3 | 20% | 19 | 15.08% | 6 | 27.27% | |
| 4 | 27 | 20.30% | 2 | 13.33% | 27 | 21.43% | 3 | 13.64% | |
| 5 | 13 | 9.77% | 3 | 20% | 13 | 10.32% | 3 | 13.64% | |
| >5 | 21 | 15.79% | 3 | 20% | 23 | 18.25% | 1 | 4.55% | |
| Number of multiple gestations BEFORE pregnancy during COVID-19 | 1 | 0.86% | 1 | 6.67% | 1 | 0.89% | 1 | 5.00% | |
| Prior Vaginal Birth | 57 | 49.14% | 5 | 33.33% | 57 | 50.89% | 5 | 25.00% | |
| Adverse Outcomes of Prior Pregnancies | |||||||||
| Miscarriages | 1 | 29 | 25.00% | 2 | 13.33% | 29 | 25.89% | 2 | 10.00% |
| 2 | 5 | 4.31% | 1 | 6.67% | 5 | 4.46% | 1 | 5.00% | |
| 3 | 6 | 5.17% | 1 | 6.67% | 7 | 6.25% | 0 | 0.00% | |
| Still Birth | 1 | 0.86% | 1 | 6.67% | 5 | 4.46% | 1 | 5.00% | |
| HELLP | 0 | 0.00% | 1 | 6.67% | 0 | 0.00% | 1 | 5.00% | |
| Cesarean Section (CS) Delivery | 41 | 35.34% | 5 | 33.33% | 38 | 33.93% | 8 | 40.00% | |
| Gestational Diabetes | 5 | 4.31% | 3 | 20.00% | 5 | 4.46% | 3 | 15.00% | |
| Gestational Hypertension | 12 | 10.34% | 3 | 20.00% | 11 | 9.82% | 4 | 20.00% | |
| Preeclampsia | 16 | 13.79% | 2 | 13.33% | 16 | 14.29% | 2 | 10.00% | |
| Fetal Growth Restriction | 3 | 2.59% | 0 | 0.00% | 3 | 2.68% | 0 | 0.00% | |
| Preterm birth | 13 | 11.21% | 3 | 20.00% | 37 | 33.04% | 2 | 10.00% | |
| COVID-19 Experience | |||||||||
| Stage at which acute COVID-19 was diagnosed | Pregnant | 89 | 66.92% | 12 | 80% | 82 | 65.08% | 19 | 86.36% |
| In labor/at delivery | 44 | 33.08% | 3 | 20% | 44 | 34.92% | 3 | 13.64% | |
| Treated for acute COVID-19 | 41 | 30.83% | 6 | 40% | 40 | 31.75 | 6 | 27.27 | |
| Acute COVID-19 treatment given | Pain relievers and symptom relief | 6 | 14.63% | 1 | 16.67% | 7 | 17.50% | 0 | 0.00% |
| Antivirals | 13 | 31.71% | 3 | 50.00% | 8 | 20.00% | 4 | 66.67% | |
| Steroids | 7 | 17.07% | 2 | 33.33% | 7 | 17.5% | 2 | 33.33% | |
| Antithrombolytics | 26 | 63.41% | 2 | 33.33% | 22 | 55.00% | 2 | 33.33% | |
| Antibiotics | 3 | 7.32% | 0 | 0.00% | 3 | 7.50% | 0 | 0.00% | |
| Monoclonal Antibody | 7 | 17.07% | 1 | 16.67% | 7 | 17.5% | 1 | 16.67% | |
| Not documented treatment for acute COVID-19 | 92 | 69.17% | 9 | 60.00% | 81 | 64.29% | 16 | 72.73% | |
| Documented reasons for lack of acute COVID-19 treatment | Asymptomatic | 30 | 32.61% | 1 | 11.11% | 25 | 30.86% | 4 | 25.00% |
| Patient declined | 7 | 7.61% | 3 | 33.33% | 6 | 7.41% | 3 | 18.75% | |
| Mild Symptoms | 10 | 10.87% | 0 | 0.00% | 9 | 11.11% | 1 | 6.25% | |
| Patient hospitalized and/or given oxygen due to acute COVID-19 | 4 | 3.00% | 1 | 6.67% | 4 | 3.17% | 1 | 4.55% | |
| Pregnancy Outcomes | |||||||||
| Number of multiple gestations DURING pregnancy during COVID-19 | 6 | 4.51% | 1 | 6.67% | 5 | 3.97% | 2 | 9.09% | |
| Live Birth | 126 | 95% | 15 | 100.00% | 118 | 94.00% | 21 | 95.45% | |
| Number of babies | 1 | 121 | 96.03% | 14 | 93.33% | 114 | 96.61% | 20 | 95.24% |
| 2 | 5 | 3.97% | 1 | 6.67% | 5 | 4.24% | 1 | 4.76% | |
| Sex distribution of neonate | M | 64 | 51.00% | 10 | 66.67% | 61 | 50.41% | 13 | 59.09% |
| F | 62 | 49.00% | 5 | 33.33% | 60 | 49.59% | 9 | 40.91% | |
| Miscarriage | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | |
| Still Birth | 3 | 2.26% | 0 | 0.00% | 3 | 2.38% | 0 | 0.00% | |
| Preterm birth | 39 | 29.32% | 2 | 13.33% | 37 | 29.37% | 4 | 8.18% | |
| CS Delivery | 58 | 46.03% | 10 | 66.67% | 55 | 46.61% | 13 | 61.90% | |
| Reason for CS Delivery | Planned cesarean delivery because of prior cesarean delivery | 25 | 43.10% | 4 | 40.00% | 22 | 40.00% | 7 | 53.85% |
| Abnormal progress in labor | 13 | 22.41% | 1 | 10.00% | 13 | 23.64% | 1 | 7.69% | |
| Concern about baby based on the heart monitor | 12 | 20.69% | 3 | 30.00% | 12 | 21.82% | 3 | 23.08% | |
| Baby was breech | 3 | 5.17% | 1 | 10.00% | 3 | 5.45% | 1 | 7.69% | |
| Uterine infection | 10 | 17.24% | 2 | 20.00% | 7 | 12.73% | 3 | 23.08% | |
| Emergency due to risk to baby or participant | 10 | 17.24% | 0 | 0.00% | 10 | 18.18% | 0 | 0.00% | |
| Patient too sick with COVID-19 to be in labor | 1 | 1.72% | 0 | 0.00% | 1 | 1.82% | 0 | 0.00% | |
| Other reason(s) | 6 | 10.34% | 0 | 0.00% | 6 | 10.91% | 0 | 0.00% | |
| Forceps or Vacuum-assisted delivery | 9 | 7.14% | 2 | 13.33% | 7 | 5.93% | 4 | 19.05% | |
| Gestational diabetes | 15 | 11.28% | 2 | 13.33% | 15 | 11.90% | 2 | 9.09% | |
| Gestational hypertension | 15 | 11.28% | 2 | 13.33% | 15 | 11.90% | 2 | 9.09% | |
| Preeclampsia | 29 | 21.80% | 1 | 6.67% | 27 | 21.43% | 3 | 13.64% | |
| HELLP syndrome | 2 | 1.50% | 1 | 6.67% | 2 | 1.59% | 1 | 4.55% | |
| Seizures | 1 | 0.75% | 0 | 0.00% | 1 | 0.79% | 0 | 0.00% | |
| Placenta abruption | 5 | 3.76% | 0 | 0.00% | 5 | 3.97% | 0 | 0.00% | |
| Preterm premature rupture of membranes | 14 | 10.53% | 1 | 6.67% | 13 | 10.32% | 2 | 9.09% | |
| Oligohydramnios | 2 | 1.50% | 1 | 6.67% | 3 | 2.38% | 0 | 0.00% | |
| Hemorrhage or excessive bleeding | 12 | 9.30% | 1 | 6.67% | 11 | 9.17% | 2 | 9.52% | |
| Blood transfusion | 4 | 3.10% | 1 | 6.67% | 2 | 1.67% | 3 | 14.29% | |
| Blood clot in the legs of lungs requiring treatment with blood thinning medications | 1 | 0.78% | 0 | 0.00% | 1 | 0.83% | 0 | 0.00% | |
| Mechanical Ventilation | 1 | 0.78% | 0 | 0.00% | 1 | 0.83% | 1 | 4.76% | |
| Pneumonia | 2 | 1.55% | 1 | 6.67% | 1 | 0.83% | 2 | 9.52% | |
| Fetal Growth Restriction | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | |
| Congenital Anomalies | 8 | 6.02% | 0 | 0.00% | 7 | 5.56% | 1 | 4.55% | |
| Before COVID-19 diagnosis | 7 | 87.50% | 0 | 0.00% | 6 | 85.71% | 1 | 100% | |
| After COVID-19 Diagnosis | 1 | 12.50% | 0 | 0.00% | 1 | 14.29% | 0 | 0% | |
| Category of Congenital Anomaly | Abdomen | 1 | 12.50% | 0 | 0.00% | 1 | 14.29% | 0 | 0% |
| Brain | 1 | 12.50% | 0 | 0.00% | 1 | 14.29% | 0 | 0% | |
| Face or lip | 1 | 12.50% | 0 | 0.00% | 1 | 14.29% | 0 | 0% | |
| Cardiac | 5 | 62.50% | 0 | 0.00% | 3 | 42.86% | 1 | 100% | |
| Lungs | 1 | 12.50% | 0 | 0.00% | 1 | 14.29% | 0 | 0% | |
| Limbs | 1 | 12.50% | 0 | 0.00% | 1 | 14.29% | 0 | 0% | |
| Trimester anomaly was observed | 2nd | 5 | 62.50% | 0 | 0.00% | 4 | 57.14% | 1 | 100% |
| 3rd | 0 | 0% | 0 | 0.00% | 0 | 0% | 0 | 0% | |
| At birth | 1 | 12.50% | 0 | 0.00% | 1 | 14.29% | 0 | 0% | |
| Number of multiple gestations AFTER pregnancy during COVID-19 | 1 | 0.75% | 0 | 0.00% | 1 | 0.79% | 0 | 0.00% | |
| Number of participants currently pregnant | 5 | 3.76% | 4 | 26.67% | 6 | 4.76% | 3 | 13.64% | |
| Neonatal Outcomes | |||||||||
| Neonatal Intensive Care Unit (NICU) admittance | 37 | 29.37% | 5 | 33.33% | 36 | 30.51% | 6 | 28.57% | |
| Non-Survival at discharge | 12 | 9.52% | 0 | 0.00% | 11 | 9.32% | 1 | 4.76% | |
| Non-Survival post-partum | 4 | 3.51% | 2 | 13.33% | 4 | 3.74% | 2 | 9.52% | |
| Average Birth Weight (for those born between 37 and 41 weeks of gestation) | 5.9 lb 8.2 oz | 6.2 lbs 5.4 oz | 5.8 lbs 8.2 oz | 5.8 lbs 8.2 oz | |||||
| Average Birth Weight (for all live births) | 5.8 lbs 8.9 oz | 5.9 lbs 6.1 oz | 5.9 lbs 7.6 oz | 5.7 lbs 7.3 oz | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeyemi, C.; Breuer, L.; Kodvawala, R.; Miller, D.; Powers-Fletcher, M.V. A Cohort Study Characterizing the Outcomes Following an Acute SARS-CoV-2 Infection in Pregnancy. J. Clin. Med. 2025, 14, 7869. https://doi.org/10.3390/jcm14217869
Adeyemi C, Breuer L, Kodvawala R, Miller D, Powers-Fletcher MV. A Cohort Study Characterizing the Outcomes Following an Acute SARS-CoV-2 Infection in Pregnancy. Journal of Clinical Medicine. 2025; 14(21):7869. https://doi.org/10.3390/jcm14217869
Chicago/Turabian StyleAdeyemi, Clementine, Leticia Breuer, Raghad Kodvawala, Delia Miller, and Margaret V. Powers-Fletcher. 2025. "A Cohort Study Characterizing the Outcomes Following an Acute SARS-CoV-2 Infection in Pregnancy" Journal of Clinical Medicine 14, no. 21: 7869. https://doi.org/10.3390/jcm14217869
APA StyleAdeyemi, C., Breuer, L., Kodvawala, R., Miller, D., & Powers-Fletcher, M. V. (2025). A Cohort Study Characterizing the Outcomes Following an Acute SARS-CoV-2 Infection in Pregnancy. Journal of Clinical Medicine, 14(21), 7869. https://doi.org/10.3390/jcm14217869






