Infective Endocarditis After TAVR—Surgical Challenges and Outcomes
Abstract
1. Introduction
2. Methods
2.1. Study Design und Study Population, Definitions
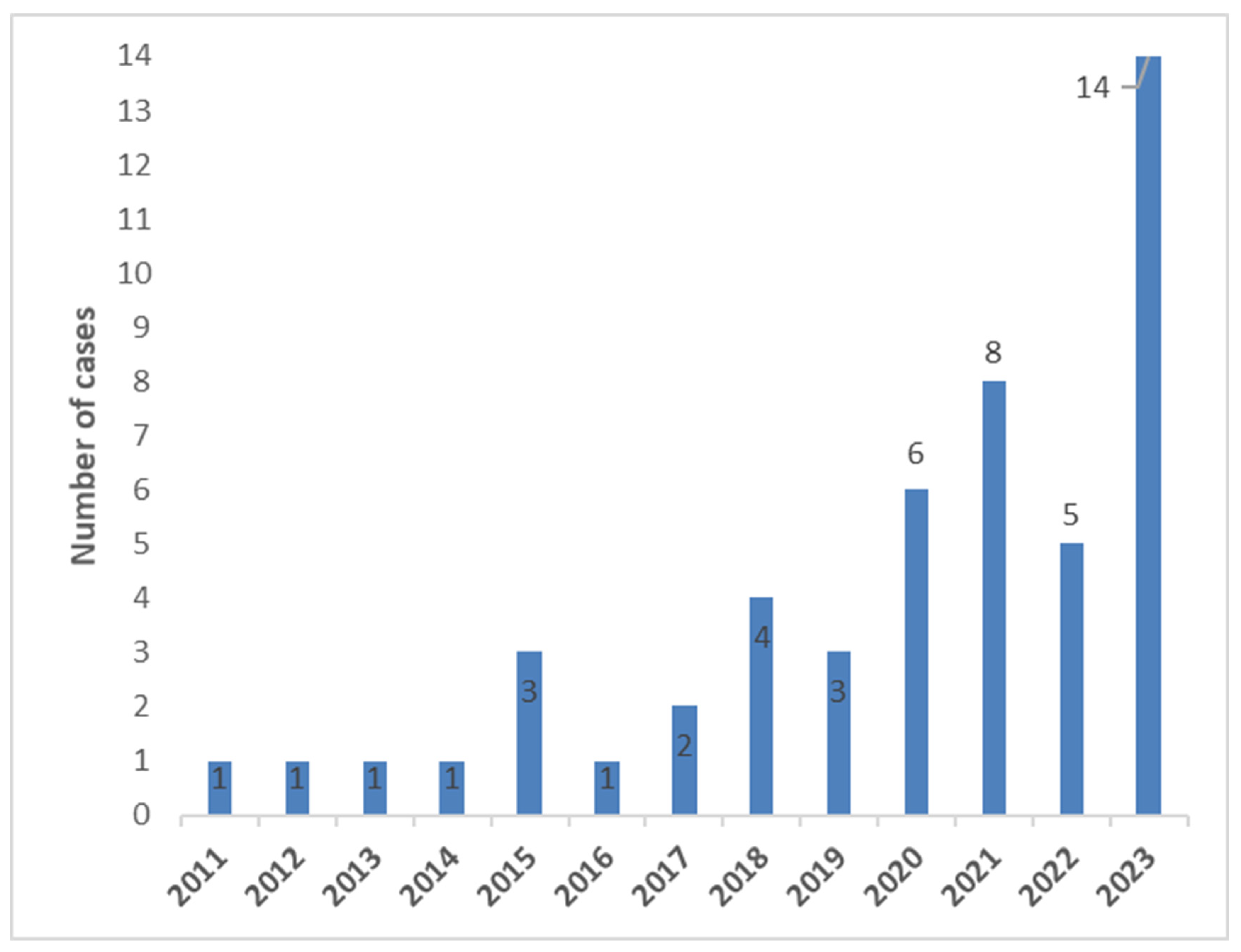
2.2. Cases Group
2.3. Control Group
2.4. Study Aims
2.5. Statistical Analysis
2.6. Ethical Statement
3. Results
3.1. Patient Characteristics/Population
3.2. Causative Microorganism
3.3. Periprocedural Characteristics
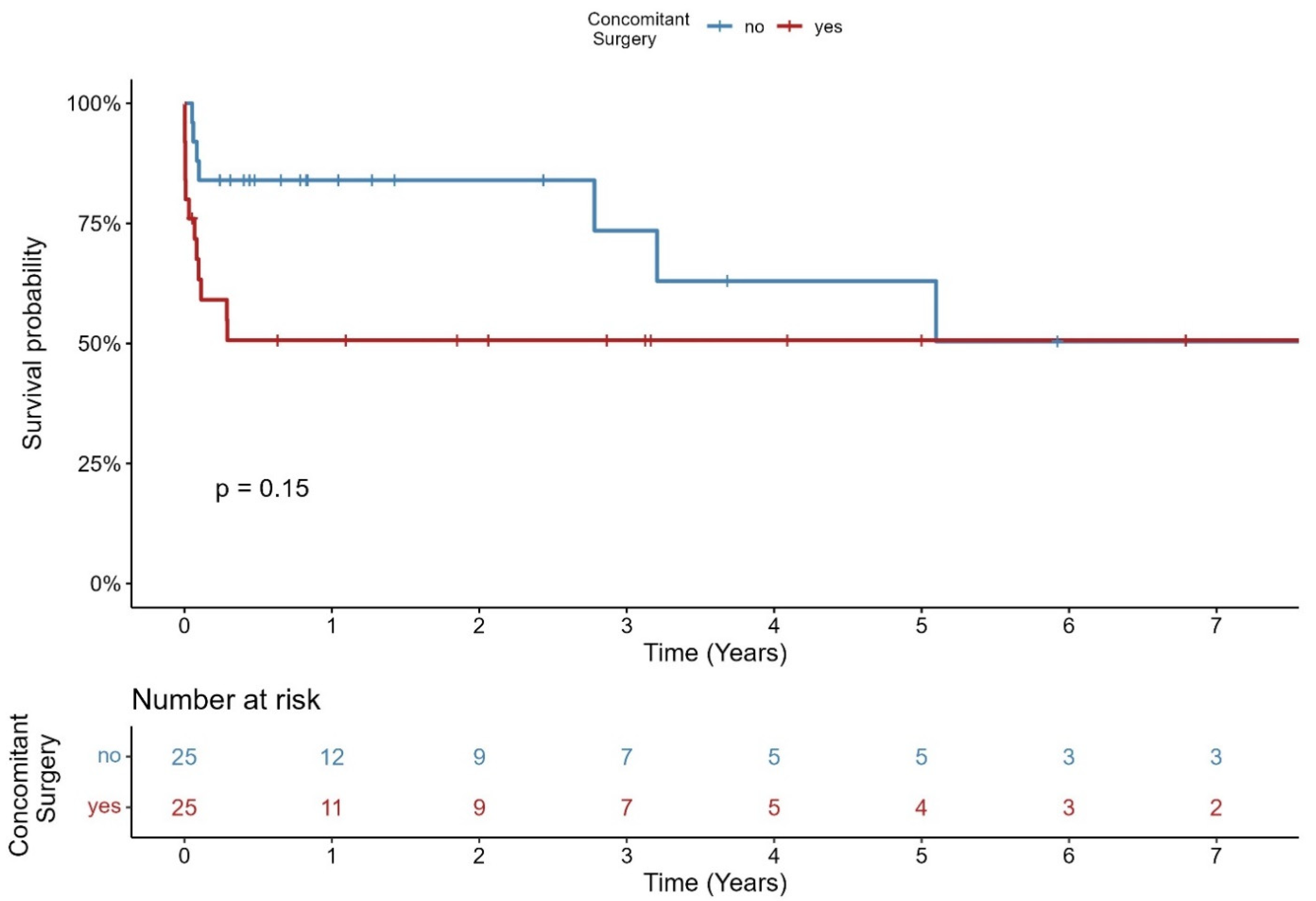
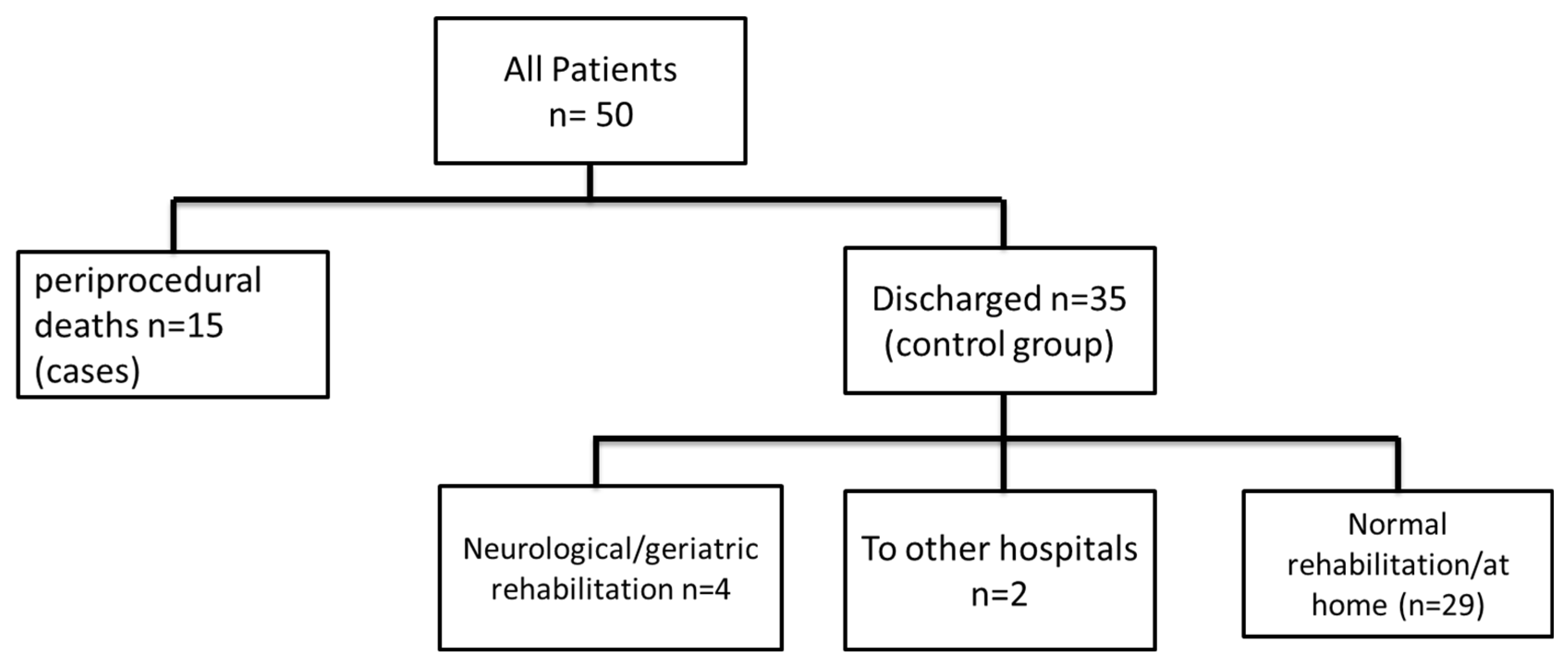
3.4. Inhospital Clinical Outcome
3.5. Follow Up Beyond Discharge
3.6. Early and Late IE
4. Discussion
- (1)
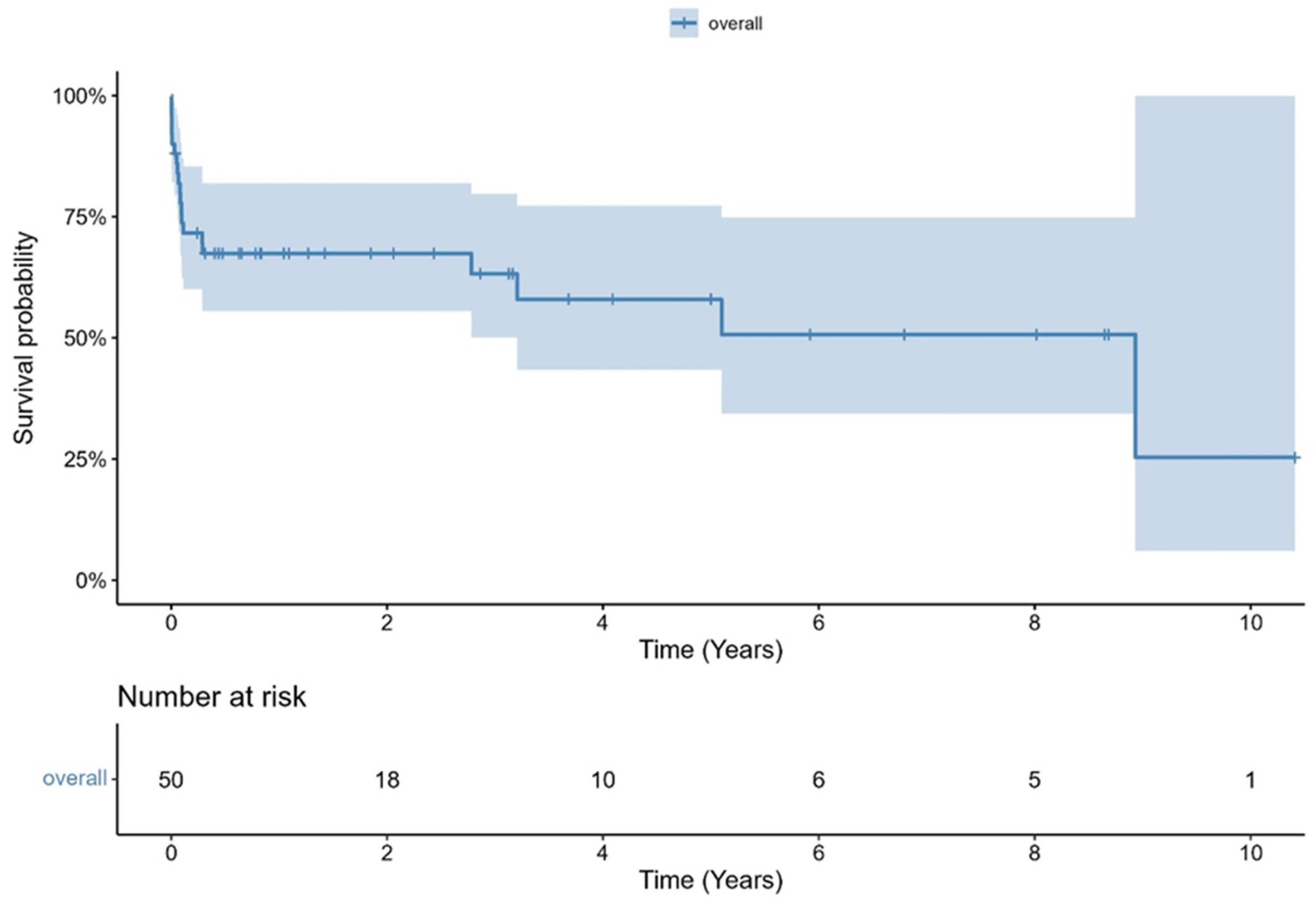
- Overall survival was 81.9% at 30 days and 67.4% at one year.
- (2)
- Patients with low STS-PROM at the time of TAVR explantation had better survival compared to those with intermediate or high operative risk.
- (3)
- Early mortality was associated with significantly higher logistic EuroScore and STS-PROM, and higher rates of postoperative renal failure, but not with causative microorganisms, cross-clamp time, or concomitant surgeries.
- (4)
- Most surviving patients recovered well and returned to independent living.
4.1. Mortality
4.2. Causative Microorganism
4.3. Early and Late IE
4.4. Periprocedural Aspects
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Writing Committee, M.; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Pibarot, P.; Hahn, R.T.; Genereux, P.; Kodali, S.K.; Kapadia, S.R.; Cohen, D.J.; Pocock, S.J.; et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N. Engl. J. Med. 2023, 389, 1949–1960. [Google Scholar] [CrossRef]
- Vekstein, A.M.; Wegermann, Z.K.; Manandhar, P.; Mack, M.J.; Cohen, D.J.; Hughes, G.C.; Harrison, J.K.; Kaneko, T.; Kapadia, S.R.; Stathogiannis, K.; et al. Outcomes of Transcatheter Aortic Valve Replacement in Low-Risk Patients in the United States: A Report From the STS/ACC TVT Registry. Circulation 2025, 151, 1134–1146. [Google Scholar] [CrossRef]
- Forrest, J.K.; Yakubov, S.J.; Deeb, G.M.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Crouch, J.; Merhi, W.; Wai Sang, S.L.; et al. 5-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients With Aortic Stenosis. J. Am. Coll. Cardiol. 2025, 85, 1523–1532. [Google Scholar] [CrossRef]
- Cahill, T.J.; Prendergast, B.D. Infective endocarditis. Lancet 2016, 387, 882–893. [Google Scholar] [CrossRef]
- Kolte, D.; Goldsweig, A.; Kennedy, K.F.; Abbott, J.D.; Gordon, P.C.; Sellke, F.W.; Ehsan, A.; Sodha, N.; Sharaf, B.L.; Aronow, H.D. Comparison of Incidence, Predictors, and Outcomes of Early Infective Endocarditis after Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement in the United States. Am. J. Cardiol. 2018, 122, 2112–2119. [Google Scholar] [CrossRef]
- Alexis, S.L.; Malik, A.H.; George, I.; Hahn, R.T.; Khalique, O.K.; Seetharam, K.; Bhatt, D.L.; Tang, G.H.L. Infective Endocarditis After Surgical and Transcatheter Aortic Valve Replacement: A State of the Art Review. J. Am. Heart Assoc. 2020, 9, e017347. [Google Scholar] [CrossRef]
- Del Val, D.; Panagides, V.; Mestres, C.A.; Miro, J.M.; Rodes-Cabau, J. Infective Endocarditis After Transcatheter Aortic Valve Replacement: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 81, 394–412. [Google Scholar] [CrossRef]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Ajmone Marsan, N.; Barili, F.; Bonaros, N.; Cosyns, B.; et al. 2025 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2025. [Google Scholar] [CrossRef]
- Varc-3 Writing Committee; Genereux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Ali, A.; Schnackenburg, P.; Horke, K.M.; Oberbach, A.; Schlichting, N.; Sadoni, S.; Rizas, K.; Braun, D.; Luehr, M.; et al. Surgery for Aortic Prosthetic Valve Endocarditis in the Transcatheter Era. J. Clin. Med. 2022, 11, 3418. [Google Scholar] [CrossRef]
- Marin-Cuartas, M.; Tang, G.H.L.; Kiefer, P.; Fukuhara, S.; Lange, R.; Harrington, K.B.; Saha, S.; Hagl, C.; Kleiman, N.S.; Goel, S.S.; et al. Transcatheter heart valve explant with infective endocarditis-associated prosthesis failure and outcomes: The EXPLANT-TAVR international registry. Eur. Heart J. 2024, 45, 2519–2532. [Google Scholar] [CrossRef]
- Caceres Polo, M.; Thibault, D.; Jawitz, O.K.; Zwischenberger, B.A.; O’Brien, S.M.; Thourani, V.H.; Jacobs, J.P.; Hooker, R.L. Aortic Prosthetic Valve Endocarditis: Analysis of The Society of Thoracic Surgeons Database. Ann. Thorac. Surg. 2022, 114, 2140–2147. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar]
- Kang, D.H.; Kim, Y.J.; Kim, S.H.; Sun, B.J.; Kim, D.H.; Yun, S.C.; Song, J.M.; Choo, S.J.; Chung, C.H.; Song, J.K.; et al. Early surgery versus conventional treatment for infective endocarditis. N. Engl. J. Med. 2012, 366, 2466–2473. [Google Scholar] [CrossRef]
- Mangner, N.; del Val, D.; Abdel-Wahab, M.; Crusius, L.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; Gasior, T.; et al. Surgical Treatment of Patients with Infective Endocarditis After Transcatheter Aortic Valve Implantation. J. Am. Coll. Cardiol. 2022, 79, 772–785. [Google Scholar] [CrossRef]
- Lalani, T.; Chu, V.H.; Park, L.P.; Cecchi, E.; Corey, G.R.; Durante-Mangoni, E.; Fowler, V.G.; Jr Gordon, D.; Grossi, P.; Hannan, M.; et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern. Med. 2013, 173, 1495–1504. [Google Scholar] [CrossRef]
- Ghanta, R.K.; Pettersson, G.B. Surgical Treatment of Infective Endocarditis in Elderly Patients: The Importance of Shared Decision Making. J. Am. Heart Assoc. 2021, 10, e022186. [Google Scholar] [CrossRef]
- Ragnarsson, S.; Salto-Alejandre, S.; Strom, A.; Olaison, L.; Rasmussen, M. Surgery Is Underused in Elderly Patients with Left-Sided Infective Endocarditis: A Nationwide Registry Study. J. Am. Heart Assoc. 2021, 10, e020221. [Google Scholar] [CrossRef]
- Regueiro, A.; Linke, A.; Latib, A.; Ihlemann, N.; Urena, M.; Walther, T.; Husser, O.; Herrmann, H.C.; Nombela-Franco, L.; Cheema, A.N.; et al. Association Between Transcatheter Aortic Valve Replacement and Subsequent Infective Endocarditis and In-Hospital Death. JAMA 2016, 316, 1083–1092. [Google Scholar] [CrossRef]
- Del Val, D.; Abdel-Wahab, M.; Linke, A.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; Landt, M.; Auffret, V.; et al. Temporal Trends, Characteristics, and Outcomes of Infective Endocarditis After Transcatheter Aortic Valve Replacement. Clin. Infect. Dis. 2021, 73, e3750–e3758. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Jaber, W.A.; Reed, G.W.; Puri, R.; Krishnaswamy, A.; Yun, J.; Unai, S.; Kapadia, S.R. Surgical versus medical management of infective endocarditis after TAVR. Catheter. Cardiovasc. Interv. 2022, 99, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Panagides, V.; Del Val, D.; Abdel-Wahab, M.; Mangner, N.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; Gasior, T.; et al. Perivalvular Extension of Infective Endocarditis After Transcatheter Aortic Valve Replacement. Clin. Infect. Dis. 2022, 75, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Panagides, V.; Cuervo, G.; Llopis, J.; Abdel-Wahab, M.; Mangner, N.; Habib, G.; Regueiro, A.; Mestres, C.A.; Tornos, P.; Durand, E.; et al. Infective Endocarditis After Transcatheter Versus Surgical Aortic Valve Replacement. Clin. Infect. Dis. 2024, 78, 179–187. [Google Scholar] [CrossRef]
- Rosch, R.M.; Brendel, L.; Buschmann, K.; Vahl, C.F.; Treede, H.; Dohle, D.S. Surgery for Infective Endocarditis after Primary Transcatheter Aortic-Valve Replacement-A Retrospective Single-Center Analysis. J. Clin. Med. 2023, 12, 5177. [Google Scholar] [CrossRef]
- Heiro, M.; Helenius, H.; Hurme, S.; Savunen, T.; Metsarinne, K.; Engblom, E.; Nikoskelainen, J.; Kotilainen, P. Long-term outcome of infective endocarditis: A study on patients surviving over one year after the initial episode treated in a Finnish teaching hospital during 25 years. BMC Infect. Dis. 2008, 8, 49. [Google Scholar] [CrossRef]
- Saha, S.; Joskowiak, D.; Marin-Cuartas, M.; Diab, M.; Schwaiger, B.M.; Sandoval-Boburg, R.; Popov, A.F.; Weber, C.; Varghese, S.; Martens, A.; et al. Surgery for infective endocarditis following low-intermediate risk transcatheter aortic valve replacement-a multicentre experience. Eur. J. Cardiothorac. Surg. 2022, 62, ezac075. [Google Scholar] [CrossRef]
- Luehr, M.; Bauernschmitt, N.; Peterss, S.; Li, Y.; Heyn, O.; Dashkevich, A.; Oberbach, A.; Bagaev, E.; Pichlmaier, M.A.; Juchem, G.; et al. Incidence and Surgical Outcomes of Patients with Native and Prosthetic Aortic Valve Endocarditis. Ann. Thorac. Surg. 2020, 110, 93–101. [Google Scholar] [CrossRef]
- Nappi, F. Advancements and Challenges in the Management of Prosthetic Valve Endocarditis: A Review. Pathogens 2024, 13, 1039. [Google Scholar] [CrossRef] [PubMed]
- Hota, S.; Patil, S.R.; Mane, P.M. Enterococcus: Understanding Their Resistance Mechanisms, Therapeutic Challenges, and Emerging Threats. Cureus 2025, 17, e79628. [Google Scholar] [CrossRef] [PubMed]
- Del Val, D.; Abdel-Wahab, M.; Mangner, N.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; Gasior, T.; Wojakowski, W.; et al. Infective Endocarditis Caused by Staphylococcus aureus After Transcatheter Aortic Valve Replacement. Can. J. Cardiol. 2022, 38, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Ried, I.D.; Omran, H.; Potratz, M.; Rudolph, T.K.; Scholtz, S.; Bleiziffer, S.; Piper, C. Infective endocarditis after isolated aortic valve replacement: Comparison between catheter-interventional and surgical valve replacement. Clin. Res. Cardiol. 2024, 113, 336–352. [Google Scholar] [CrossRef]
- Stortecky, S.; Heg, D.; Tueller, D.; Pilgrim, T.; Muller, O.; Noble, S.; Jeger, R.; Toggweiler, S.; Ferrari, E.; Taramasso, M.; et al. Infective Endocarditis After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 75, 3020–3030. [Google Scholar] [CrossRef]
- Ando, T.; Ashraf, S.; Villablanca, P.A.; Telila, T.A.; Takagi, H.; Grines, C.L.; Afonso, L.; Briasoulis, A. Meta-Analysis Comparing the Incidence of Infective Endocarditis Following Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement. Am. J. Cardiol. 2019, 123, 827–832. [Google Scholar] [CrossRef]
- Pizzino, F.; Paradossi, U.; Trimarchi, G.; Benedetti, G.; Marchi, F.; Chiappino, S.; Conti, M.; Di Bella, G.; Murzi, M.; Di Sibio, S.; et al. Clinical Features and Patient Outcomes in Infective Endocarditis with Surgical Indication: A Single-Centre Experience. J. Cardiovasc. Dev. Dis. 2024, 11, 138. [Google Scholar] [CrossRef]
- Summers, M.R.; Leon, M.B.; Smith, C.R.; Kodali, S.K.; Thourani, V.H.; Herrmann, H.C.; Makkar, R.R.; Pibarot, P.; Webb, J.G.; Leipsic, J.; et al. Prosthetic Valve Endocarditis After TAVR and SAVR: Insights From the PARTNER Trials. Circulation 2019, 140, 1984–1994. [Google Scholar] [CrossRef]
- Prasitlumkum, N.; Thangjui, S.; Leesutipornchai, T.; Kewcharoen, J.; Limpruttidham, N.; Pai, R.G. Comparison of infective endocarditis risk between balloon and self-expandable valves following transcatheter aortic valve replacement: Systematic review and meta-analysis. Cardiovasc. Interv. Ther. 2021, 36, 363–374. [Google Scholar] [CrossRef]
- Zaid, S.; Kleiman, N.S.; Goel, S.S.; Szerlip, M.I.; Mack, M.J.; Marin-Cuartas, M.; Mohammadi, S.; Nazif, T.M.; Unbehaun, A.; Andreas, M.; et al. Impact of transcatheter heart valve type on outcomes of surgical explantation after failed transcatheter aortic valve replacement: The EXPLANT-TAVR international registry. EuroIntervention 2024, 20, e146–e157. [Google Scholar] [CrossRef]
- Fukuhara, S.; Brescia, A.A.; Shiomi, S.; Rosati, C.M.; Yang, B.; Kim, K.M.; Deeb, G.M. Surgical explantation of transcatheter aortic bioprostheses: Results and clinical implications. J. Thorac. Cardiovasc. Surg. 2021, 162, 539–547.e1. [Google Scholar] [CrossRef]


| Variable | All Patients (n = 50) | Cases Group (n = 15) | Control Group (n = 35) | p Value |
|---|---|---|---|---|
| age, years, median [range] | 78 [37–88] | 80 [37–86] | 77 [57–88] | 0.8 |
| sex (male), n (%) | 40 (80) | 12 (80) | 28 (80) | 1.0 |
| height (m) | 1.8 [1.5–1.9] | 1.8 [1.5–1.8] | 1.8 [1.6–1.9] | 0.4 |
| weight (kg) | 80 [43–150] | 80 [60–150] | 80 [43–125] | 0.7 |
| body-Mass-Index (kg/m2) | 26.7 [16.8–54.7] | 27.5 [18.5–54.7] | 26.5 [16.8–38.9] | 0.5 |
| white Blood Count (109/L) | 9.42 [3.82–45.58] | 9.92 [5.15–45.58] | 8.87 [3.82–19.33] | 0.12 |
| c reactive protein (mg/dL) | 69.85 [1.34–365] | 75.95 [20.8–365] | 64.7 [1.34–265] | 0.22 |
| creatinine (mg/dL) | 1.27 [0.49–10.40] | 1.35 [0.49–10.40] | 1.23 [0.56–6.16] | 0.13 |
| glomerular filtration rate (mL/min) | 53 [7–144] | 47 [7–100] | 54 [11–144] | 0.053 |
| cerebrovascular disease, n (%) | 13 (26) | 4 (26.7) | 9 (25.7) | 0.9 |
| TIA, n (%) | 8 (16) | 3 (20) | 5 (14.3) | |
| stroke, n (%) | 5 (10) | 1 (6.7) | 4 (11.4) | |
| COPD, n (%) | 10 (20) | 3 (20) | 7 (20) | 1.0 |
| diabetes mellitus | 14 (28) | 5 (33.3) | 9 (25.7) | 0.9 |
| NIDDM | 6 (12.0%) | 2 (13.3%) | 4 (11.4%) | |
| IDDM | 8 (16.0%) | 3 (20.0%) | 5 (14.3%) | |
| LV-ejection fraction (%) | 47.4 ± 10.4 | 42.7 ± 8.4 | 49.4 ± 10.6 | 0.024 |
| peripheral arterial disease, n (%) | 5 (10) | 2 (13.3) | 3 (8.6) | 0.6 |
| previous heart surgery, n (%) | 9 (18) | 1 (6.7) | 8 (22.9) | 0.2 |
| log Euroscore pre-TAVR, median, % [range] | 9.5 [1.3–48.3] | 8.1 [1.4–37.6] | 10.7 [1.3–48.3] | 0.6 |
| STS-Score pre TAVR, median, % [range] | 2.4 [0.8–5.9] | 2.7 [1.5–5.4] | 2.1 [0.8–5.9] | 0.1 |
| log Euroscore pre SAVR, median, % [range] | 28.5 [6.8–82.1] | 53.3 [20.1–82.1] | 26.6 [6.8–64.5] | <0.001 |
| STS PROM pre SAVR, median, % [range] | 6.5 [1.3–67.6] | 14.4 [3.9–67.6] | 4.8 [1.3–51.2] | 0.004 |
| time between TAVR and SAVR, median, days [range] | 342 [35–3248] | 490 [35–1634] | 328 [74–3248] | 0.6 |
| time between TAVR and SAVR (days) | 0.07 | |||
| <90 days, n (%) | 9 (18%) | 5 (33.3) | 4 (11.4) | |
| >90–<364 days, n (%) | 17 (34%) | 2 (13.3) | 15 (42.9) | |
| >365 days, n (%) | 24 (48%) | 8 (53.3) | 16 (45.7) |
| Causative Microorganism, n (%) | All Patients (n = 50) | Cases Group (n = 15) | Control Group (n = 35) |
|---|---|---|---|
| Staphylococcus aureus | 8 (16) | 4 (26.7) | 4 (11.4) |
| Coagulase-negative staphylococci | 13 (26) | 3 (20) | 10 (28.6) |
| Streptococcus viridans | 8 (16) | 0 | 8 (22.9) |
| Enterococci | 11 (22) | 4 (26.7) | 7 (20) |
| others | 6 (12) | 3 (20) | 3 (8.6) |
| no causative microorganism found | 3 (6) | 1 (6.7) | 2 (5.7) |
| Periprocedural Characteristics | All Patients (n = 50) | Cases Group (n = 15) | Control Group (n = 35) | p Value |
|---|---|---|---|---|
| CPB Time, min, median [range] | 137 [54–348] | 155 [54–348] | 136 [83–316] | 0.5 |
| cross-clamp Time, min, median [range] | 95 [36–233] | 89 [36–233] | 97 [52–220] | 0.9 |
| total operative time, min, median [range] | 258 [124–538] | 295 [133–538] | 255 [124–431] | 0.3 |
| explanted BEV-Device, n (%) | 39 (78) | 12 (80) | 27 (77.1) | |
| explanted SEV-Devices, n (%) | 11 (22) | 3 (20) | 8 (22.9) | 0.12 |
| concomitant surgery, n (%) | 25 (50.0%) | 11 (73.3%) | 14 (40.0%) | 0.062 |
| In-hospital Clinical Outcomes | All Patients (n = 50) | Cases Group (n = 15) | Control Group (n = 35) | p Value |
|---|---|---|---|---|
| acute renal failure/Dialysis n (%) | 22 (44.0%) | 13 (86.7%) | 9 (25.7%) | <0.001 |
| Stroke, n (%) | 5 (10) | 2 (13.3) | 3 (8.6) | 0.6 |
| new pacemaker implantation, n (%) | 10 (20) | 9 (25.7) | 1 (7.1) | 0.2 |
| Rethoracotomy, n (%) | 7 (14) | 1 (6.7) | 6 (17.1) | 0.7 |
| length of hospital stay, days, median [range] | 20 [2–95] | 25 [2–42] | 19 [3–95] | 0.9 |
| length of ICU stay, days, median [range] | 6 [1–95] | 23 [1–42] | 5 [1–95] | 0.07 |
| VARC3-Mortality, n, (%) | ||||
| alive n, (%) | 30 (60) | 0 | 30 (85.7) | |
| periprocedural n, (%) | 15 (30) | 15 (100) | 0 | |
| early n, (%) | 1 (2) | 0 | 1 (2.9) | |
| late n, (%) | 4 (8.0) | 0 | 4 (11.4) | |
| estimated 30 day mortality (95% CI), % | 81.9 ± 5.5 | |||
| estimated 1-year-mortality (95% CI), % | 67.4 ± 6.7 | |||
| follow up (days) | 294 [1–3802] | 22 [1–106] | 752 [19–3802] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiter, A.; Schreyer, J.; Burri, M.; Ruge, H.; Krane, M.; Puluca, N. Infective Endocarditis After TAVR—Surgical Challenges and Outcomes. J. Clin. Med. 2025, 14, 7859. https://doi.org/10.3390/jcm14217859
Reiter A, Schreyer J, Burri M, Ruge H, Krane M, Puluca N. Infective Endocarditis After TAVR—Surgical Challenges and Outcomes. Journal of Clinical Medicine. 2025; 14(21):7859. https://doi.org/10.3390/jcm14217859
Chicago/Turabian StyleReiter, Andrea, Julia Schreyer, Melchior Burri, Hendrik Ruge, Markus Krane, and Nazan Puluca. 2025. "Infective Endocarditis After TAVR—Surgical Challenges and Outcomes" Journal of Clinical Medicine 14, no. 21: 7859. https://doi.org/10.3390/jcm14217859
APA StyleReiter, A., Schreyer, J., Burri, M., Ruge, H., Krane, M., & Puluca, N. (2025). Infective Endocarditis After TAVR—Surgical Challenges and Outcomes. Journal of Clinical Medicine, 14(21), 7859. https://doi.org/10.3390/jcm14217859








