A Simplified Three-Item Clinical Score to Identify Exertional Hypoxemia in Fibrotic Interstitial Lung Disease: A Real-World Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Population and Inclusion Criteria
2.3. Sampling
2.4. Data Sources
- Clinical characteristics: age, sex, presence of cough, and smoking history;
- Physiological parameters: forced vital capacity (FVC% predicted) and diffusing capacity for carbon monoxide (DLCO% predicted);
- Oxygenation: peripheral oxygen saturation (SpO2) at rest and during exertion;
2.5. Outcome
2.6. Statistical Analysis
- Univariable ROC analyses (reference markers): ROC curves for FVC% predicted and DLCO% predicted were generated, reporting AUC and Youden-optimal cut-offs. Shapiro–Wilk results guided descriptive summaries (mean ± SD vs. median [IQR]);
- Multivariable logistic regression model: A conventional logistic model was constructed including FVC% predicted, DLCO% predicted, and cough as independent predictors. Regression coefficients (β) were estimated from the cohort data, and the linear predictor was used for discrimination and performance metrics.Equation—Logit(p) = β0 + β1·FVC + β2·DLCO + β3·Cough
- Stepwise logistic regression model: A stepwise selection procedure (bidirectional, based on Akaike information criterion) was applied to identify the most informative subset of predictors among the same candidate variables. Final model coefficients were derived from this data-driven selection.Equation—Logit(p) = β0 + β1·FVC + β2·DLCO
- Simplified clinical score (0–3): A point-based score was created by assigning 1 point each for FVC% predicted ≤ 61, DLCO% predicted ≤ 53, and presence of cough (total range 0–3 points). Cut-offs for FVC ≤ 61% and DLCO ≤ 53% were derived from the present cohort using Youden’s index from univariate ROC curves. This pragmatic scoring system was designed for bedside triage, with ≥2 points prespecified as the threshold for high risk.Equation—Score = (FVC ≤ 61) + (DLCO ≤ 53) + (Cough present)
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full term |
| 6MWT | 6 min walk test |
| ATS | American Thoracic Society |
| AUC | Area under the curve |
| BMI | Body mass index |
| CI | Confidence interval |
| COPD | Chronic obstructive pulmonary disease |
| CPFE | Combined pulmonary fibrosis and emphysema |
| CTD-ILD | Connective tissue disease–associated interstitial lung disease |
| DLCO | Diffusing capacity for carbon monoxide |
| EMR | Electronic medical record |
| ERS | European Respiratory Society |
| FVC | Forced vital capacity |
| HR | Heart rate |
| ILD | Interstitial lung disease |
| IMC | Body mass index (Índice de massa corporal) |
| IPF | Idiopathic pulmonary fibrosis |
| IQR | Interquartile range |
| LTOT | Long-term oxygen therapy |
| MCTD | Mixed connective tissue disease |
| NPV | Negative predictive value |
| O2 | Oxygen |
| PaO2 | Partial pressure of arterial oxygen |
| PPF | Progressive pulmonary fibrosis |
| PPV | Positive predictive value |
| PFT | Pulmonary function test |
| RA | Rheumatoid arthritis |
| ROC | Receiver operating characteristic |
| SBP | Systolic blood pressure |
| SD | Standard deviation |
| SOP | Standard operating procedure |
| SpO2 | Peripheral oxygen saturation |
| SSc | Systemic sclerosis |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| V | Cramér’s V (effect size) |
References
- Jacobs, S.S.; Krishnan, J.A.; Lederer, D.J.; Ghazipura, M.; Hossain, T.; Tan, A.M.; Carlin, B.; Drummond, M.B.; Ekström, M.; Garvey, C.; et al. Home Oxygen Therapy for Adults with Chronic Lung Disease. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e121–e141, Erratum in Am. J. Respir. Crit. Care Med. 2021, 203, 1045–1046. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Sundh, J.; Bornefalk-Hermansson, A.; Ekström, M. Long-Term Oxygen Therapy 24 vs 15 h/day and Mortality in Chronic Obstructive Pulmonary Disease. PLoS ONE 2016, 11, e0163293. [Google Scholar] [CrossRef]
- Cordeiro, R.; Nunes, A.; Smith, O.; Renzoni, E.A. Oxygen in interstitial lung diseases. Breathe 2023, 19, 220271. [Google Scholar] [CrossRef]
- Koudstaal, T.; Wijsenbeek, M.S. Idiopathic pulmonary fibrosis. La Presse Médicale 2023, 52, 104166. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.C.; Cox, N.S.; Goh, N.; Glaspole, I.; Westall, G.P.; Watson, A.; Holland, A.E. Oxygen therapy for interstitial lung disease: A systematic review. Eur. Respir. Rev. 2017, 26, 160080. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.P.; Degenholtz, H.B.; Lindell, K.O.; Kass, D.J. Supplemental Oxygen Therapy in Interstitial Lung Disease: A Narrative Review. Ann. Am. Thorac. Soc. 2023, 20, 1541–1549. [Google Scholar] [CrossRef]
- Otake, K.; Misu, S.; Fujikawa, T.; Sakai, H.; Tomioka, H. Exertional Desaturation Is More Severe in Idiopathic Pulmonary Fibrosis Than in Other Interstitial Lung Diseases. Phys. Ther. Res. 2023, 26, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Sandot, A.; Bunel, V.; Mal, H. Indications de l’oxygénothérapie à long terme chez les patients avec BPCO ou PID [Indications of home oxygen therapy for patients with COPD or ILD]. Rev. Mal. Respir. 2024, 41, 751–761. [Google Scholar] [CrossRef]
- Ekström, M.; Andersson, A.; Papadopoulos, S.; Kipper, T.; Pedersen, B.; Kricka, O.; Sobrino, P.; Runold, M.; Palm, A.; Blomberg, A.; et al. REDOX Collaborative Research Group. Long-Term Oxygen Therapy for 24 or 15 Hours per Day in Severe Hypoxemia. N. Engl. J. Med. 2024, 391, 977–988. [Google Scholar] [CrossRef]
- Khor, Y.H.; Smith, D.J.; Johannson, K.A.; Renzoni, E. Oxygen for interstitial lung diseases. Curr. Opin. Pulm. Med. 2020, 26, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Nathan, S.D.; Kim, H.H.; Kim, H.C. Clinical implications of six-minute walk test in idiopathic pulmonary fibrosis: A retrospective cohort study. Ther. Adv. Respir. Dis. 2024, 18, 17534666241275329. [Google Scholar] [CrossRef]
- Mullholand, J.B.; Grossman, C.E.; Perelas, A. Non-Pharmacological Management of Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2025, 14, 1317. [Google Scholar] [CrossRef]
- Ryerson, C.J.; Adegunsoye, A.; Piciucchi, S.; Hariri, L.P.; Khor, Y.H.; Wijsenbeek, M.S.; Wells, A.U.; Sharma, A.; Cooper, W.A.; Antoniou, K.; et al. Update of the International Multidisciplinary Classification of the Interstitial Pneumonias: An ERS/ATS Statement. Eur. Respir. J. 2025, 2500158. [Google Scholar] [CrossRef]
- Knudson, R.; Lebowitz, M.; Holberg, C.; Burrows, B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am. Rev. Respir. Dis. 1983, 127, 725–734. [Google Scholar]
- Neder, J.; Andreoni, S.; Peres, C.; Nery, L. Reference values for lung function tests. III. Carbon monoxide diffusing capacity (transfer factor). Braz. J. Med. Biol. Res. 1999, 32, 729–737. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117, Erratum in Am. J. Respir. Crit. Care Med. 2016, 193, 1185. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Lim, R.K.; Humphreys, C.; Morisset, J.; Holland, A.E.; Johannson, K.A.; O2 Delphi Collaborators. Oxygen in patients with fibrotic interstitial lung disease: An international Delphi survey. Eur. Respir. J. 2019, 54, 1900421. [Google Scholar] [CrossRef]
- Morice, A.H.; Millqvist, E.; Bieksiene, K.; Birring, S.S.; Dicpinigaitis, P.; Ribas, C.D.; Boon, M.H.; Kantar, A.; Lai, K.; McGarvey, L.; et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur. Respir. J. 2020, 55, 1901136, Erratum in Eur. Respir. J. 2020, 56, 1951136. [Google Scholar] [CrossRef]
- Zweig, M.H.; Campbell, G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin. Chem. 1993, 39, 561–577, Erratum in Clin. Chem. 1993, 39, 1589. [Google Scholar] [CrossRef]
- Kataoka, K.; Oda, K.; Takizawa, H.; Ogura, T.; Miyamoto, A.; Inoue, Y.; Akagawa, S.; Hashimoto, S.; Kishaba, T.; Sakamoto, K.; et al. Cohort study to evaluate prognostic factors in idiopathic pulmonary fibrosis patients introduced to oxygen therapy. Sci. Rep. 2023, 13, 13664. [Google Scholar] [CrossRef] [PubMed]
- Oldham, J.M.; Neely, M.L.; Wojdyla, D.M.; Gulati, M.; Li, P.; Patel, D.C.; Palmer, S.M.; Todd, J.L.; IPF-PRO Registry Investigators. Changes in Lung Function and Mortality Risk in Patients With Idiopathic Pulmonary Fibrosis. Chest 2025, 168, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Selman, M.; Inoue, Y.; Wong, A.W.; Corte, T.J.; Flaherty, K.R.; Han, M.K.; Jacob, J.; Johannson, K.A.; Kitaichi, M.; et al. Syndrome of Combined Pulmonary Fibrosis and Emphysema: An Official ATS/ERS/JRS/ALAT Research Statement. Am. J. Respir. Crit. Care Med. 2022, 206, e7–e41. [Google Scholar] [CrossRef]
- Hirons, B.; Rhatigan, K.; Wright, L.; Kesavan, H.; Mackay, E.; Cho, P.S.P.; Birring, S.S.; Myall, K.J. Patient Perception of Cough in Interstitial Lung Disease; Impact of Cough Hypersensitivity. Lung 2024, 202, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Wakwaya, Y.; Ramdurai, D.; Swigris, J.J. Managing Cough in Idiopathic Pulmonary Fibrosis. Chest 2021, 160, 1774–1782. [Google Scholar] [CrossRef]
- Robertson, L.; Sylvester, K.P.; Newman, J. Shorter walk test durations to detect ambulatory oxygen desaturation in interstitial lung disease: An observational cohort study. ERJ Open Res. 2025, 11, 00891-2024. [Google Scholar] [CrossRef]
- Bloem, A.E.; Dolk, H.M.; Wind, A.E.; van der Vis, J.J.; Kampen, M.J.; Custers, J.W.; Spruit, M.A.; Veltkamp, M. Prognostic value of the 6-min walk test derived attributes in patients with idiopathic pulmonary fibrosis. Respir. Med. 2025, 236, 107862. [Google Scholar] [CrossRef]
- Ley, B.; Ryerson, C.J.; Vittinghoff, E.; Ryu, J.; Tomassetti, S.; Lee, J.S.; Poletti, V.; Buccioli, M.; Elicker, B.M.; Jones, K.D.; et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 2012, 156, 684–691. [Google Scholar] [CrossRef]
- Salisbury, M.L.; Xia, M.; Zhou, Y.; Zhou, Y.; Murray, S.; Tayob, N.; Brown, K.K.; Wells, A.U.; Schmidt, S.L.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis: Gender-Age-Physiology Index Stage for Predicting Future Lung Function Decline. Chest 2016, 149, 491–498. [Google Scholar] [CrossRef]
- Saleem, F.; Ryerson, C.J.; Sarma, N.; Johannson, K.; Marcoux, V.; Fisher, J.; Assayag, D.; Manganas, H.; Khalil, N.; Morisset, J.; et al. Predicting New-onset Exertional and Resting Hypoxemia in Fibrotic Interstitial Lung Disease. Ann. Am. Thorac. Soc. 2023, 20, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Zanini, U.; Ding, J.; Luppi, F.; Kaur, K.; Anzani, N.; Franco, G.; Ferrara, G.; Kalluri, M.; Mura, M. Percent Predicted vs. Absolute Six-Minute Walk Distance as Predictors of Lung Transplant-Free Survival in Fibrosing Interstitial Lung Diseases. Lung 2024, 202, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.L.; Maher, A.; Gale, N.; Crawshaw, A.; Thickett, D.; Turner, A.M. Ambulatory Oxygen for Pulmonary Fibrosis (OxyPuF): A randomised controlled trial and acceptability study. Health Technol. Assess. 2025; 1–33, ahead of print. [Google Scholar] [CrossRef]
| Home Oxygen Supply (n.101) | No Home Oxygen Supply (n.49) | p-Value | Test | |
|---|---|---|---|---|
| Sex: F/M | 71 (70.3%):30 (29.7%) | 29 (59.2%)/20 (40.8%) | 0.176 | χ2 |
| Race: White/Non-white | 37 (36.6%):64 (63.4%) | 19 (38.8%)/30 (61.2%) | 0.799 | χ2 |
| Smoking: Ever | 59 (58.4%) | 15 (30.6%) | 0.001 | χ2 |
| Smoking: Never | 42 (41.6%) | 34 (69.4%) | 0.001 | χ2 |
| Age (years) | 65.0 (56.0–72.0) | 65.0 (60.0–75.0) | 0.439 | Mann–Whitney |
| Years on oxygen | 1.0 (1.0–2.0) | — | — | — |
| SpO2 at rest (%) | 95.0 (92.8–97.0) | 97.0 (96.0–98.0) | <0.001 | Mann–Whitney |
| SpO2 end of 6MWT (%) | 84.0 (79.0–87.0) | 92.0 (89.8–94.2) | <0.001 | Mann–Whitney |
| FVC (%) | 50.8 ± 14.9 | 66.1 ± 16.9 | <0.001 | Welch t-test |
| DLCO (%) | 37.0 (25.0–52.0) | 54.0 (39.0–66.0) | <0.001 | Mann–Whitney |
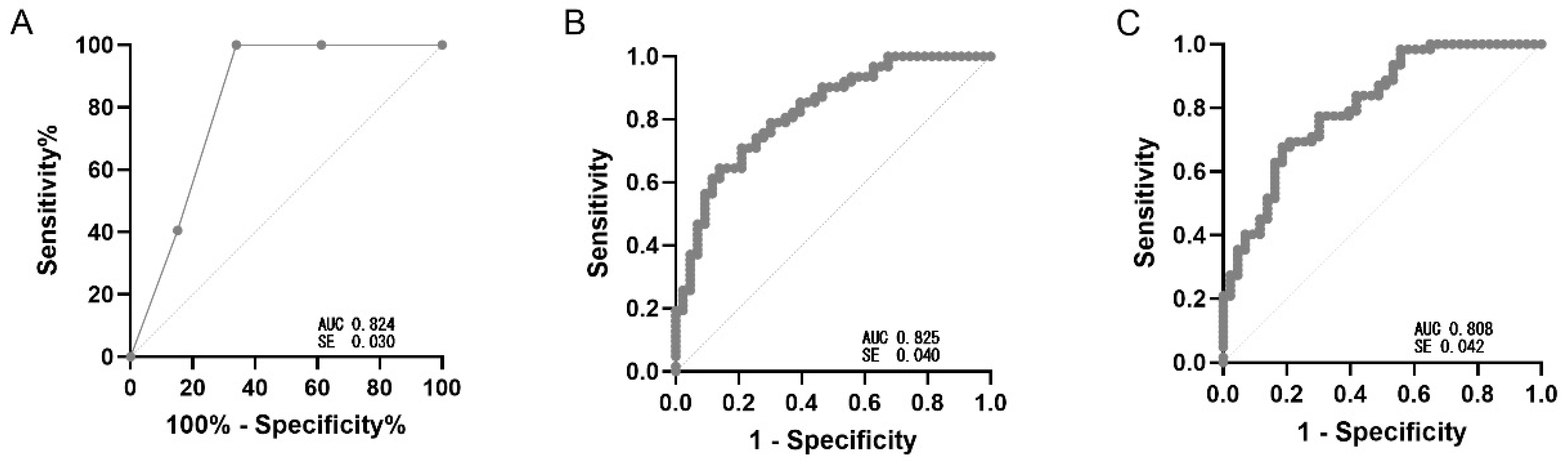
| Model | AUC | Cut-Off | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Logistic | 0.825 (SE 0.040) | ≥0.38 | 0.90 | 0.46 | 0.73 | 0.75 | 0.70 |
| Stepwise | 0.805 (SE 0.042) | ≥0.38 | 0.84 | 0.55 | 0.72 | 0.73 | 0.70 |
| Simplified clinical score (0–3) | 0.824 (SE 0.030) | ≥2.0 | 1.00 | 0.66 | 0.72 | 0.76 | 0.64 |
| Score (0–3) | n | Events (SpO2 ≤ 88%) | Observed Risk (%) | 95% CI | Probability Band |
|---|---|---|---|---|---|
| 0–1 | 35 | 6 | 17.1% | 6.6–33.7 | Low (0–40%) |
| 2 | 29 | 17 | 58.6% | 38.9–76.5 | Intermediate (40–70%) |
| 3 | 41 | 39 | 95.1% | 83.5–99.4 | High (>70%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rufino, R.; Serpa, I.T.; Palermo, L.; Bessa, E.; Rangel, B.; Lopes, M.; Lopes, A.J.; Rufino, M.C.; Costa, C.H.d.; Faria, A.C. A Simplified Three-Item Clinical Score to Identify Exertional Hypoxemia in Fibrotic Interstitial Lung Disease: A Real-World Cohort Study. J. Clin. Med. 2025, 14, 7858. https://doi.org/10.3390/jcm14217858
Rufino R, Serpa IT, Palermo L, Bessa E, Rangel B, Lopes M, Lopes AJ, Rufino MC, Costa CHd, Faria AC. A Simplified Three-Item Clinical Score to Identify Exertional Hypoxemia in Fibrotic Interstitial Lung Disease: A Real-World Cohort Study. Journal of Clinical Medicine. 2025; 14(21):7858. https://doi.org/10.3390/jcm14217858
Chicago/Turabian StyleRufino, Rogerio, Isabela Tamiozzo Serpa, Leonardo Palermo, Elizabeth Bessa, Bruno Rangel, Mariana Lopes, Agnaldo José Lopes, Mariana Costa Rufino, Cláudia Henrique da Costa, and Anamelia Costa Faria. 2025. "A Simplified Three-Item Clinical Score to Identify Exertional Hypoxemia in Fibrotic Interstitial Lung Disease: A Real-World Cohort Study" Journal of Clinical Medicine 14, no. 21: 7858. https://doi.org/10.3390/jcm14217858
APA StyleRufino, R., Serpa, I. T., Palermo, L., Bessa, E., Rangel, B., Lopes, M., Lopes, A. J., Rufino, M. C., Costa, C. H. d., & Faria, A. C. (2025). A Simplified Three-Item Clinical Score to Identify Exertional Hypoxemia in Fibrotic Interstitial Lung Disease: A Real-World Cohort Study. Journal of Clinical Medicine, 14(21), 7858. https://doi.org/10.3390/jcm14217858







