Age-Related Comparative Study of In-Hospital Mortality, Functional Outcome, and Recurrence in a Large Cohort of Patients Surgically Treated for Chronic Subdural Hematoma
Abstract
1. Introduction
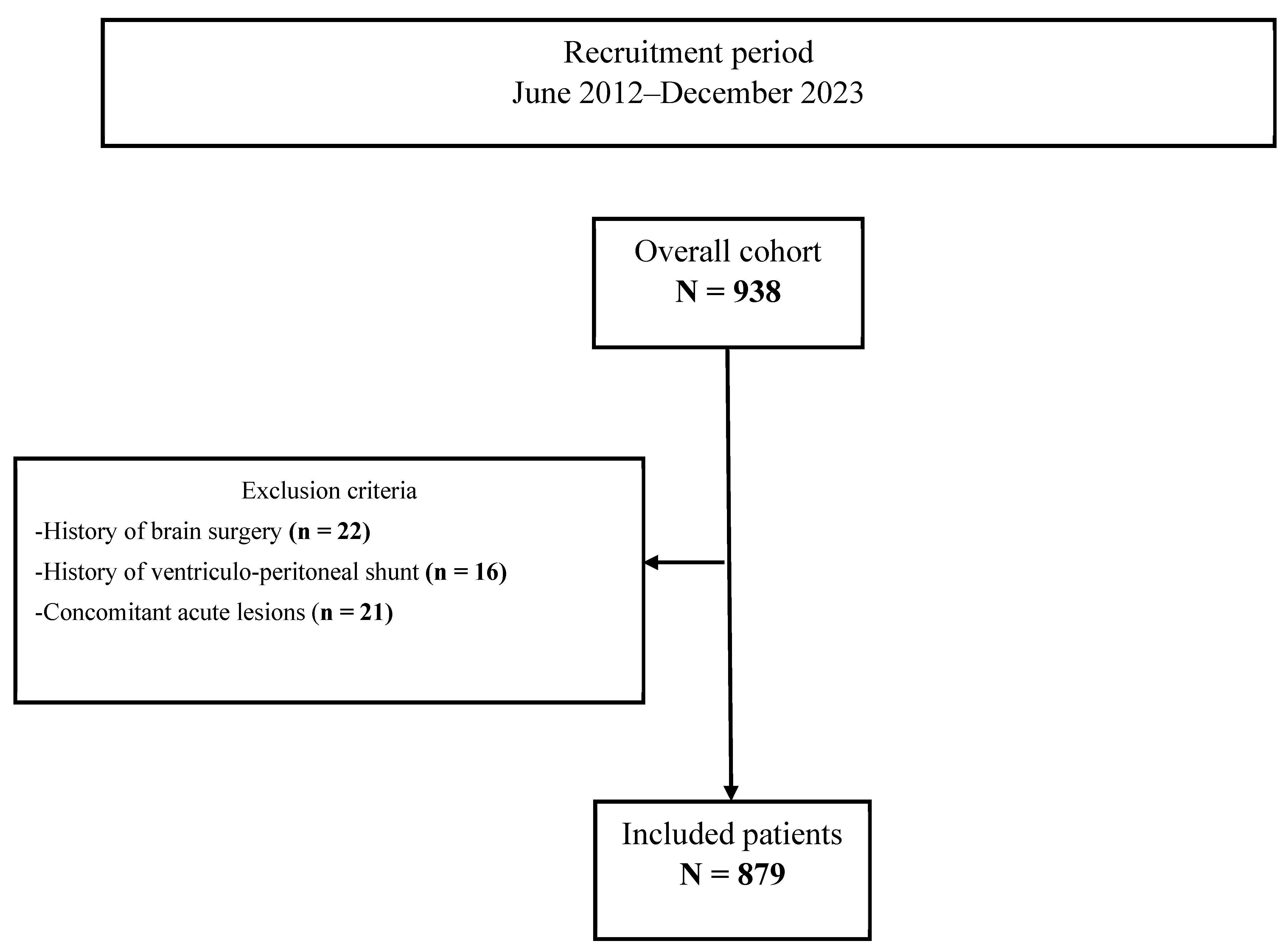
2. Methods and Materials
2.1. Study Population
2.2. Definition of Age-Related Groups in CSDH Patients
2.3. Patient Data Collection
2.4. Routine Clinical Care and Treatment Protocol of CSDH
2.5. Perioperative Management of AAT
2.6. Assessment of Radiographic Variables
2.7. Statistical Analyses
2.8. Use of GenAI in Writing and Editing
3. Results
3.1. Baseline Characteristics
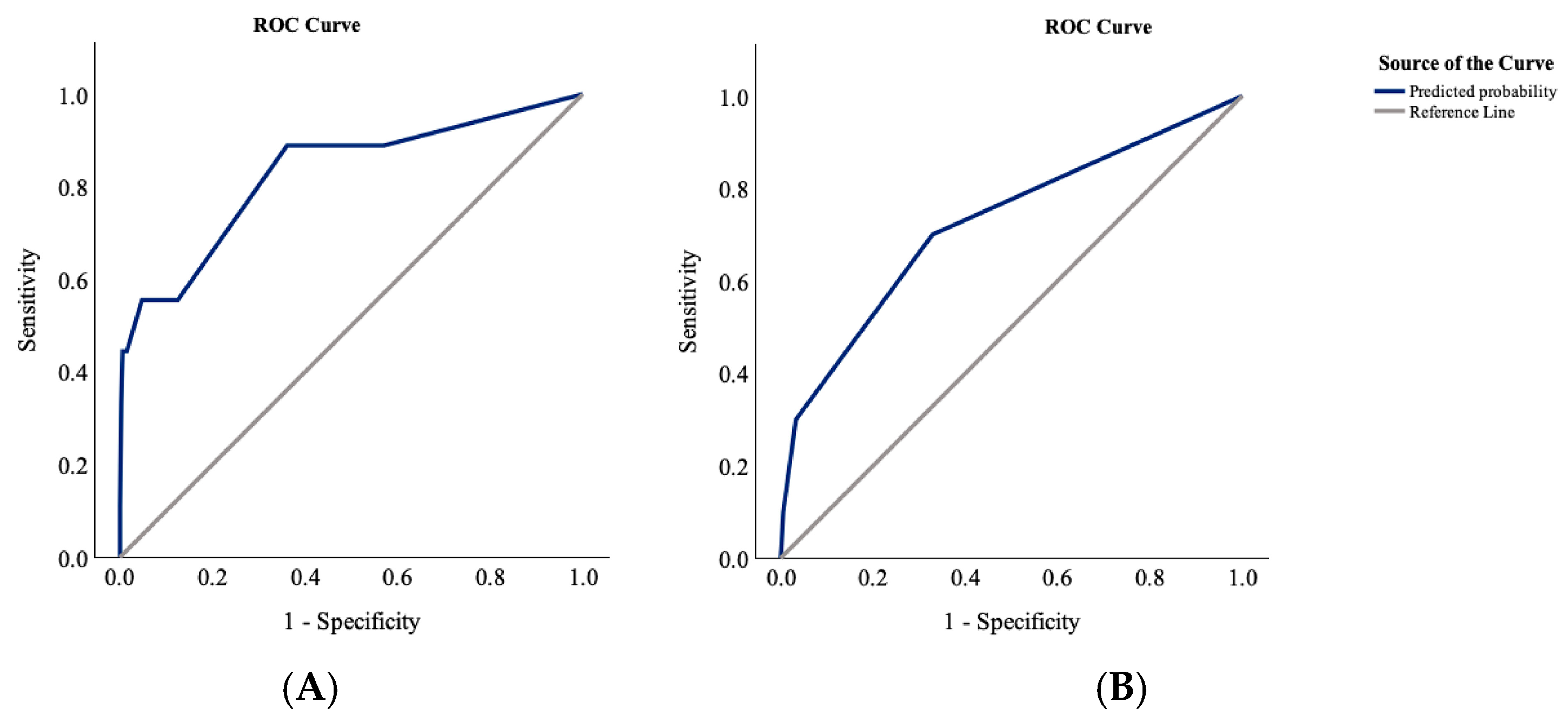
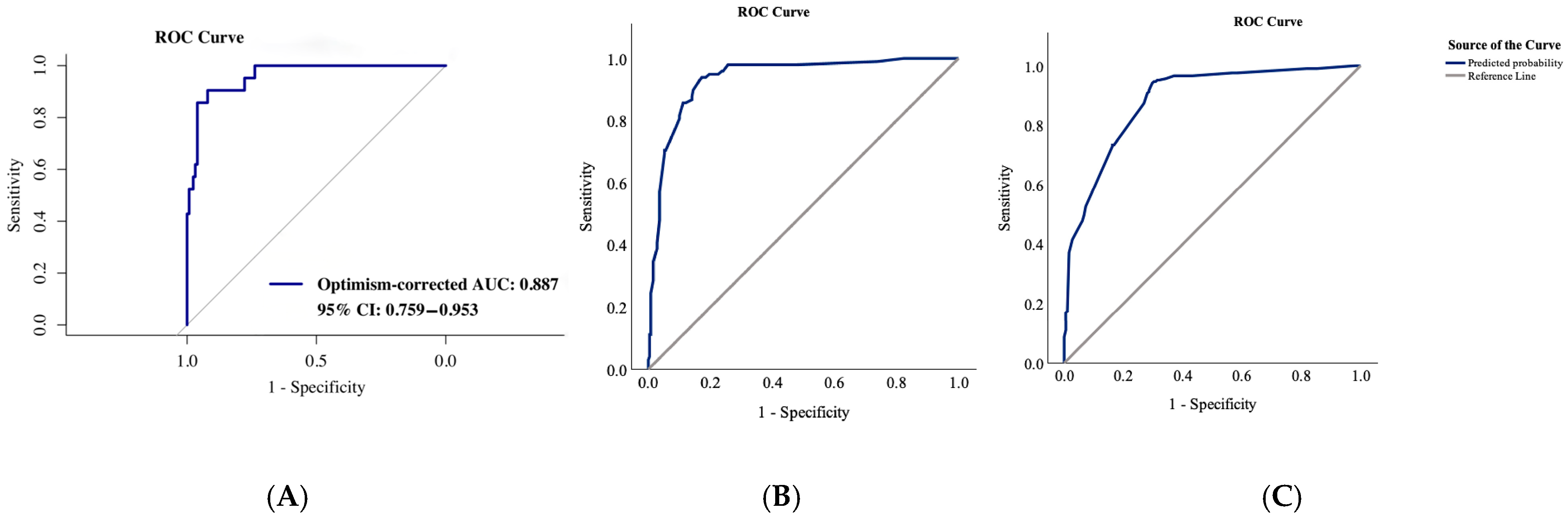
3.2. Characteristics and Predictors of In-Hospital Mortality
3.3. Characteristics and Predictors of Functional Outcome at Discharge
3.4. Characteristics of Recurrence
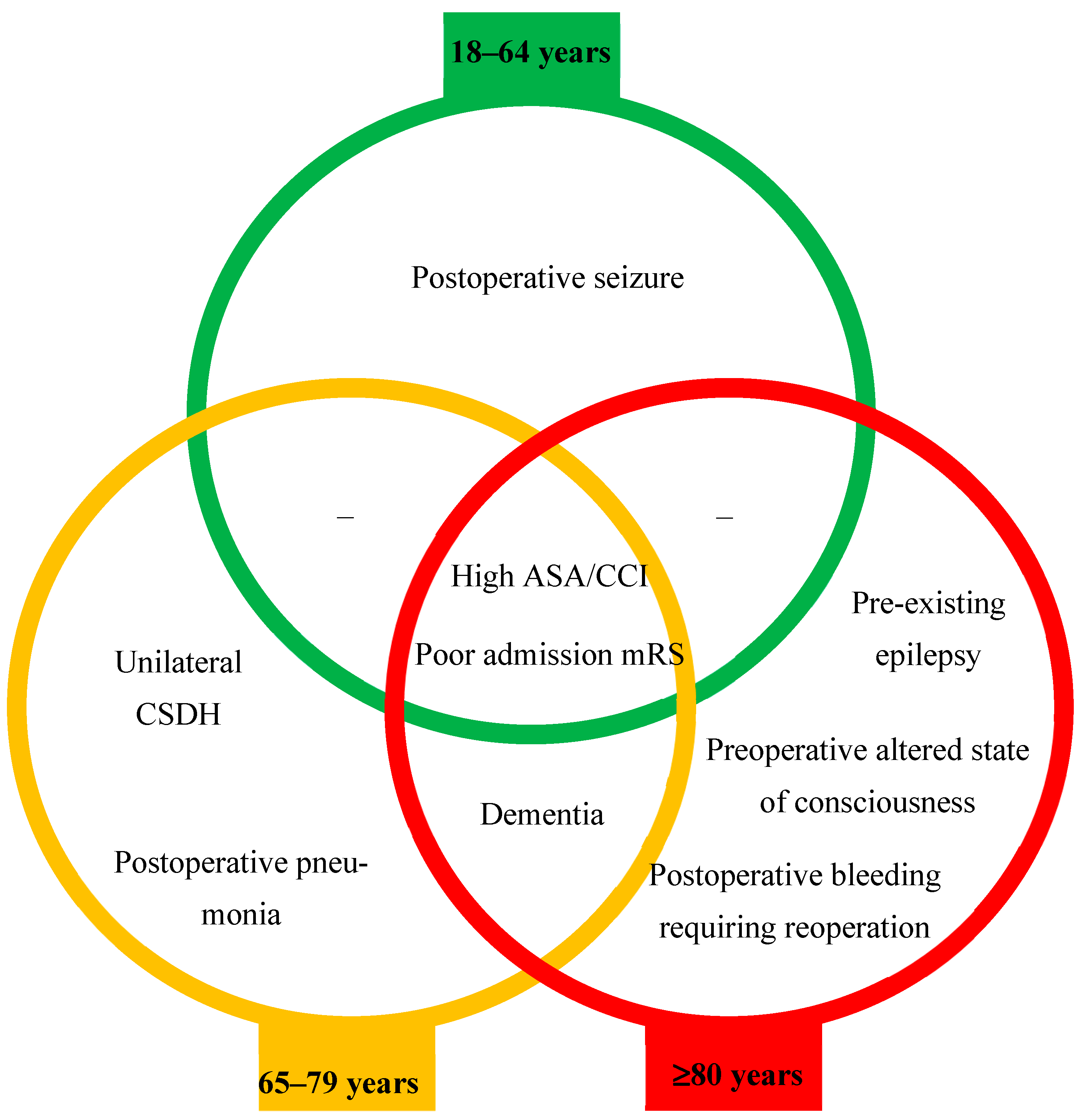
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rauhala, M.; Helén, P.; Huhtala, H.; Heikkilä, P.; Iverson, G.L.; Niskakangas, T.; Öhman, J.; Luoto, T.M. Chronic subdural hematoma—Incidence, complications, and financial impact. Acta Neurochir. 2020, 162, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Dong, J.; Wu, L.; Xu, L.; Wang, L.; Liu, B.; Li, J.; Liu, W. A comparative study of chronic subdural hematoma in three age ranges: Below 40 years, 41–79 years, and 80 years and older. Clin. Neurol. Neurosurg. 2019, 178, 63–69. [Google Scholar] [CrossRef]
- Kudo, H.; Kuwamura, K.; Izawa, I.; Sawa, H.; Tamaki, N. Chronic Subdural Hematoma in Elderly People: Present Status on Awaji Island and Epidemiological Prospect. Neurol. Med.-Chir. 1992, 32, 207–209. [Google Scholar] [CrossRef]
- Dobran, M. Spontaneous chronic subdural hematoma in young adult: The role of missing coagulation facto. G. Di Chir. J. Surg. 2017, 38, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Gondar, R.; Schaller, K.; Meling, T. Chronic Subdural Hematoma (cSDH): A review of the current state of the art. Brain Spine 2021, 1, 100300. [Google Scholar] [CrossRef]
- Bartek, J.; Sjåvik, K.; Dhawan, S.; Sagberg, L.M.; Kristiansson, H.; Ståhl, F.; Förander, P.; Chen, C.C.; Jakola, A.S. Clinical Course in Chronic Subdural Hematoma Patients Aged 18–49 Compared to Patients 50 Years and Above: A Multicenter Study and Meta-Analysis. Front. Neurol. 2019, 10, 311. [Google Scholar] [CrossRef]
- Ou, Y.; Dong, J.; Wu, L.; Xu, L.; Wang, L.; Liu, B.; Li, J.; Liu, W. The Clinical Characteristics, Treatment, and Outcomes of Chronic Subdural Hematoma in Young Patients. World Neurosurg. 2019, 125, e1241–e1246. [Google Scholar] [CrossRef]
- Won, Y.D.; Yi, H.-J.; Lee, Y.J.; Chun, H.-J.; Cho, H.; Bak, K.-H. Chronic Subdural Hematoma in Young Adult: An Age Comparison Study. Korean J. Neurotrauma 2013, 9, 6–11. [Google Scholar] [CrossRef]
- Liliang, P.-C.; Tsai, Y.-D.; Liang, C.-L.; Lee, T.-C.; Chen, H.-J. Chronic subdural haematoma in young and extremely aged adults: A comparative study of two age groups. Injury 2002, 33, 345–348. [Google Scholar] [CrossRef]
- Baechli, H.; Nordmann, A.; Bucher, H.C.; Gratzl, O. Demographics and prevalent risk factors of chronic subdural haematoma: Results of a large single-center cohort study. Neurosurg. Rev. 2004, 27, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Benek, H.B.; Akcay, E. Concomitant chronic subdural hematomas and arachnoid cysts in young adults. F1000Research 2022, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, K.; Sorimachi, T.; Honda, Y.; Ishizaka, H.; Baba, T.; Osada, T.; Nishiyama, J.; Inoue, G.; Matsumae, M. Chronic Subdural Hematomas Associated with Arachnoid Cysts: Significance in Young Patients with Chronic Subdural Hematomas. Neurol. Med.-Chir. 2015, 55, 727–734. [Google Scholar] [CrossRef]
- Younsi, A.; Riemann, L.; Habel, C.; Fischer, J.; Beynon, C.; Unterberg, A.W.; Zweckberger, K. Relevance of comorbidities and antithrombotic medication as risk factors for reoperation in patients with chronic subdural hematoma. Neurosurg. Rev. 2021, 45, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Leroy, H.-A.; Aboukaïs, R.; Reyns, N.; Bourgeois, P.; Labreuche, J.; Duhamel, A.; Lejeune, J.-P. Predictors of functional outcomes and recurrence of chronic subdural hematomas. J. Clin. Neurosci. 2015, 22, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Cheon, T.M.; Kim, K.M.; Bae, J.W.; Shim, Y.S.; Hyun, D.-K. Functional outcomes of surgically treated chronic subdural hematoma and the factors associated with outcomes: A retrospective study. J. Korean Soc. Geriatr. Neurosurg. 2023, 19, 39–44. [Google Scholar] [CrossRef]
- Chandran, R.S.; Nagar, M.; Sharmad, M.S.; Prabhakar, R.B.; Peethambaran, A.K.; Kumar, S.; Sharma, S.; Jain, S.K. Single Parietal Burr-hole Craniostomy with Irrigation and Drainage for Unilateral Chronic Subdural Hematoma in Young Adults <40 Years: A Rationale behind the Procedure. J. Neurosci. Rural. Pract. 2019, 8, 389–394. [Google Scholar] [CrossRef]
- Amipara, R.; Winders, H.R.; Justo, J.A.; Bookstaver, P.B.; Kohn, J.; Al-Hasan, M.N. Impact of follow up blood cultures on outcomes of patients with community-onset gram-negative bloodstream infection. EClinicalMedicine 2021, 34, 100811. [Google Scholar] [CrossRef]
- Fuchs, J.; Gaertner, B.; Perlitz, H.; Kuttig, T.; Klingner, A.; Baumert, J.; Hüther, A.; Kuhnert, R.; Wolff, J.; Scheidt-Nave, C. Study on Health of Older People in Germany (Gesundheit 65+): Objectives, design and implementation. J. Health Monit. 2023, 8, 61. [Google Scholar]
- Vrettos, I.; Anagnostopoulos, F.; Voukelatou, P.; Kyvetos, A.; Theotoka, D.; Niakas, D. Does old age comprise distinct subphases? Evidence from an analysis of the relationship between age and activities of daily living, comorbidities, and geriatric syndromes. Ann. Geriatr. Med. Res. 2024, 28, 65. [Google Scholar] [CrossRef]
- Wang, H.-y.; Lv, X.; Du, J.; Kong, G.; Zhang, L. Age-and gender-specific prevalence of frailty and its outcomes in the longevous population: The Chinese longitudinal healthy longevity study. Front. Med. 2021, 8, 719806. [Google Scholar] [CrossRef]
- Shenkin, S.D.; Harrison, J.K.; Wilkinson, T.; Dodds, R.M.; Ioannidis, J.P. Systematic reviews: Guidance relevant for studies of older people. Age Ageing 2017, 46, 722–728. [Google Scholar] [CrossRef]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Toi, H.; Kinoshita, K.; Hirai, S.; Takai, H.; Hara, K.; Matsushita, N.; Matsubara, S.; Otani, M.; Muramatsu, K.; Matsuda, S. Present epidemiology of chronic subdural hematoma in Japan: Analysis of 63,358 cases recorded in a national administrative database. J. Neurosurg. 2017, 128, 222–228. [Google Scholar] [CrossRef]
- Kanat, A.; Kayaci, S.; Kazdal, H.; Terzi, Y. Chronic subdural hematoma in adults: Why does it occur more often in males than females? Influence of patient’s sexual gender on occurrence. J. Neurosurg. Sci. 2010, 54, 99–103. [Google Scholar]
- Sioutas, G.S.; Sweid, A.; Chen, C.-J.; Becerril-Gaitan, A.; Al Saiegh, F.; El Naamani, K.; Abbas, R.; Amllay, A.; Birkenstock, L.; Cain, R.E. Surgical evacuation for chronic subdural hematoma: Predictors of reoperation and functional outcomes. World Neurosurg. X 2024, 21, 100246. [Google Scholar] [CrossRef]
- Ou, Y.; Fan, W.; Yu, X.; Wu, L.; Liu, W. A single-center analysis of sex differences in patients with chronic subdural hematoma in China. Front. Neurol. 2022, 13, 888526. [Google Scholar] [CrossRef]
- Posti, J.P.; Luoto, T.M.; Sipilä, J.O.; Rautava, P.; Kytö, V. Prognosis of patients with operated chronic subdural hematoma. Sci. Rep. 2022, 12, 7020. [Google Scholar] [CrossRef]
- Maeda, T.; Kikkawa, Y.; Ehara, T.; Tsuchiya, R.; Tabata, S.; Onodera, K.; Kimura, T.; Take, Y.; Suzuki, K.; Kurita, H. Clinical outcomes in elderly patients with chronic subdural hematoma: Validation of irrigation assignment based on hematoma characteristics. Life 2024, 14, 518. [Google Scholar] [CrossRef] [PubMed]
- De Bonis, P.; Olei, S.; Mongardi, L.; Cavallo, M.A.; Santantonio, M.; Trevisi, G.; Anile, C.; Mangiola, A. Chronic subdural hematoma in patients aged 80 years and older: A two-centre study. Clin. Neurol. Neurosurg. 2018, 170, 88–92. [Google Scholar] [CrossRef]
- Blaauw, J.; Jacobs, B.; Hertog, H.M.D.; van der Gaag, N.A.; Jellema, K.; Dammers, R.; Kho, K.H.; Groen, R.J.M.; van der Naalt, J.; Lingsma, H.F. Mortality after chronic subdural hematoma is associated with frailty. Acta Neurochir. 2022, 164, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Rohde, V.; Graf, G.; Hassler, W. Complications of burr-hole craniostomy and closed-system drainage for chronic subdural hematomas: A retrospective analysis of 376 patients. Neurosurg. Rev. 2002, 25, 89–94. [Google Scholar] [CrossRef]
- Nozawa, Y.; Harada, K.; Noma, K.; Katayama, Y.; Hamada, M.; Ozaki, T. Association Between Early Mobilization and Postoperative Pneumonia Following Robot-assisted Minimally Invasive Esophagectomy in Patients with Thoracic Esophageal Squamous Cell Carcinoma. Phys. Ther. Res. 2024, 27, 121–127. [Google Scholar] [CrossRef]
- Sigona, A.; Richman, D.C. Identifying and reducing risks of postoperative pulmonary complications. J. Oral Maxillofac. Anesth. 2023, 2, 30. [Google Scholar] [CrossRef]
- Chebib, N.; Cuvelier, C.; Malézieux-Picard, A.; Parent, T.; Roux, X.; Fassier, T.; Müller, F.; Prendki, V. Correction to: Pneumonia prevention in the elderly patients: The other sides. Aging Clin. Exp. Res. 2020, 33, 3149. [Google Scholar] [CrossRef]
- ten Cate, H.; De Bonis, P.; Trevisi, G.; de Waure, C.; Sferrazza, A.; Volpe, M.; Pompucci, A.; Anile, C.; Mangiola, A. Antiplatelet/Anticoagulant Agents and Chronic Subdural Hematoma in the Elderly. PLoS ONE 2013, 8, e68732. [Google Scholar] [CrossRef]
- Weimer, J.M.; Gordon, E.; Frontera, J.A. Predictors of Functional Outcome After Subdural Hematoma: A Prospective Study. Neurocritical Care 2016, 26, 70–79. [Google Scholar] [CrossRef]
- Wu, L.; Guo, X.; Ou, Y.; Yu, X.; Zhu, B.; Li, Y.; Liu, W. Seizure after chronic subdural hematoma evacuation: Associated factors and effect on clinical outcome. Front. Neurol. 2023, 14, 1190878. [Google Scholar] [CrossRef] [PubMed]
- Grobelny, B.T.; Ducruet, A.F.; Zacharia, B.E.; Hickman, Z.L.; Andersen, K.N.; Sussman, E.; Carpenter, A.; Connolly, E.S. Preoperative antiepileptic drug administration and the incidence of postoperative seizures following bur hole–treated chronic subdural hematoma. J. Neurosurg. 2009, 111, 1257–1262. [Google Scholar] [CrossRef]
- Lavergne, P.; Labidi, M.; Brunet, M.-C.; Lessard Bonaventure, P.; Zetchi, A.; Carrondo Cottin, S.; Simonyan, D.; Turmel, A. Efficacy of antiseizure prophylaxis in chronic subdural hematoma: A cohort study on routinely collected health data. J. Neurosurg. 2020, 132, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.M.; Kolias, A.G.; Joannides, A.J.; Shapey, J.; Marcus, H.J.; Gregson, B.A.; Grover, P.J.; Hutchinson, P.J.; Coulter, I.C. The management and outcome for patients with chronic subdural hematoma: A prospective, multicenter, observational cohort study in the United Kingdom. J. Neurosurg. 2017, 127, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, Y.; Zhao, X.; Yang, C.; Gu, J.; Weng, W.; Hui, J.; Mao, Q.; Gao, G.; Feng, J. Risk factors of hospital mortality in chronic subdural hematoma: A retrospective analysis of 1117 patients, a single institute experience. J. Clin. Neurosci. 2019, 67, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, J.H. Clinical Characteristics of Bilateral versus Unilateral Chronic Subdural Hematoma. Korean J. Neurotrauma 2014, 10, 49–54. [Google Scholar] [CrossRef]
- Park, H.S.; Park, E.S.; Park, J.B.; Kwon, S.C.; Lyo, I.U.; Kim, M.H.; Sim, H.B. Chronic Subdural Hematomas: Comparison between Unilateral and Bilateral Involvement. Korean J. Neurotrauma 2014, 10, 55–59. [Google Scholar] [CrossRef]
- Agawa, Y.; Mineharu, Y.; Tani, S.; Adachi, H.; Imamura, H.; Sakai, N. Bilateral Chronic Subdural Hematoma is Associated with Rapid Progression and Poor Clinical Outcome. Neurol. Med. Chir. 2016, 56, 198–203. [Google Scholar] [CrossRef]
- Raj, R.; Tommiska, P.; Luoto, T.; Leinonen, V.; Koivisto, T.; Tetri, S.; Posti, J.; Lönnrot, K. Failure to improve—Identifying risk factors for poor functional recovery following chronic subdural hematoma surgery. Age Ageing 2025, 54, afaf056. [Google Scholar] [CrossRef]
- Feinkohl, I.; Winterer, G.; Spies, C.D.; Pischon, T. Cognitive Reserve and the Risk of Postoperative Cognitive Dysfunction. Dtsch. Ärzteblatt Int. 2017, 114, 110. [Google Scholar] [CrossRef]
- Chen, L.; Au, E.; Saripella, A.; Kapoor, P.; Yan, E.; Wong, J.; Tang-Wai, D.F.; Gold, D.; Riazi, S.; Suen, C.; et al. Postoperative outcomes in older surgical patients with preoperative cognitive impairment: A systematic review and meta-analysis. J. Clin. Anesth. 2022, 80, 110883. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, H.; Zhao, J.; Wang, C.; Liu, H.; Wang, C.; Li, A.; Hu, J. Preoperative Fibrinogen Levels and Function as Predictive Factors for Acute Bleeding in the Hematoma Cavity After Burr Hole Drainage in Patients with CSDH. World Neurosurg. 2023, 180, e364–e375. [Google Scholar] [CrossRef]
- Pang, C.H.; Lee, S.E.; Kim, C.H.; Kim, J.E.; Kang, H.S.; Park, C.K.; Paek, S.H.; Kim, C.H.; Jahng, T.A.; Kim, J.W.; et al. Acute intracranial bleeding and recurrence after bur hole craniostomy for chronic subdural hematoma. J. Neurosurg. 2015, 123, 65–74. [Google Scholar] [CrossRef]
- Mishra, R.; Deora, H.; Florez-Perdomo, W.A.; Moscote-Salazar, L.R.; Garcia-Ballestas, E.; Rahman, M.M.; Shrivastava, A.; Raj, S.; Chavda, V.; Montemurro, N.; et al. Clinical and Radiological Characteristics for Recurrence of Chronic Subdural Hematoma: A Systematic Review and Meta-Analysis. Neurol. Int. 2022, 14, 683–695. [Google Scholar] [CrossRef] [PubMed]

| Variables | Age Groups (Years) | Total | p-Value | ||
|---|---|---|---|---|---|
|
18–64
(n = 147) |
65–79
(n = 347) |
≥80
(n = 385) | |||
| Age (mean ± SD) | 54.5 ± 9.6 | 73.4 ± 4.2 | 84.8 ± 3.6 | 75.2 ± 11.9 | -- |
| Sex (ratio female:male) | 1:3.2 | 1:2.4 | 1:1.7 | 1:2.1 | 0.001 |
| Charlson comorbidity index (median (IQR)) | 2 (1) | 4 (2) | 5 (2) | 4 (3) | <0.001 |
| ASA status (median (IQR)) | 3 (1) | 3 (1) | 3 (0) | 3 (0) | <0.001 |
| Recent head trauma | 91/61.9 | 254/73.2 | 322/83.6 | 667/75.9 | <0.001 |
| Epilepsy | 11/7.5 | 15/4.3 | 15/3.9 | 41/4.7 | 0.150 |
| Dementia | 2/1.4 | 41/11.8 | 94/24.4 | 137/15.6 | <0.001 |
| Arterial hypertension | 67/45.6 | 251/72.3 | 285/74 | 603/68.6 | <0.001 |
| Diabetes mellitus | 16/10.9 | 82/23.6 | 103/26.8 | 201/22.9 | <0.001 |
| Chronic kidney disease | 2/1.4 | 28/8.1 | 63/16.4 | 93/10.6 | <0.001 |
| Atrial fibrillation | 4/2.7 | 70/20.2 | 120/31.2 | 194/22.1 | <0.001 |
| Chronic heart disease | 12/8.2 | 66/19 | 96/24.9 | 174/19.8 | <0.001 |
| Cerebral vascular/transient ischemic accident | 11/7.5 | 47/13.5 | 69/17.9 | 127/14.4 | 0.002 |
| Operative technique (one burr hole) | 100/68 | 232/66.9 | 263/68.3 | 595/67.7 | 0.827 |
| CSDH location (bilateral) | 28/19 | 76/21.9 | 79/20.5 | 183/20.8 | 0.937 |
| Prior AAT use | 37/25.2 | 183/52.7 | 250/64.9 | 470/53.5 | <0.001 |
| -Aspirin | 22/15 | 81/23.3 | 121/31.4 | 224/25.5 | - |
| -Clopidogrel | 0/0 | 3/0.9 | 9/2.3 | 12/1.4 | - |
| -VKA | 4/2.7 | 61/17.6 | 58/15.1 | 123/14 | - |
| -DOAC | 6/4.1 | 18/5.2 | 34/8.9 | 58/6.6 | - |
| -Dual or triple therapy | 4/2.7 | 20/5.8 | 27/7 | 51/5.8 | - |
| -Heparin | 1/0.7 | 0/0 | 1/0.3 | 2/0.2 | - |
| Perioperative AAT short stop and heparin bridging | 0/0 | 5/1.4 | 4/1 | 9/1 | 0.451 |
| No perioperative stop of AAT | 3/2 | 8/2.3 | 7/1.8 | 18/2 | 0.949 |
| At admission: mRS > 3 | 36/24.5 | 131/37.8 | 247/64.2 | 414/47.1 | 0.0005 |
| Preoperative symptoms and signs | |||||
| -Nausea | 15/10.2 | 21/6.1 | 13/3.4 | 49/5.6 | 0.003 |
| -Headache | 91/61.9 | 123/35.4 | 97/25.2 | 311/35.4 | <0.001 |
| -Dizziness | 25/17 | 51/14.7 | 46/11.9 | 122/13.9 | 0.108 |
| -Change in behavior | 17/11.6 | 55/15.9 | 59/15.3 | 131/14.9 | 0.465 |
| -Cognitive deterioration | 39/26.5 | 114/32.9 | 148/38.4 | 301/34.2 | 0.008 |
| -Gait disorder | 55/37.4 | 161/46.4 | 221/57.4 | 437/49.7 | <0.001 |
| -Seizure < 24 h | 13/8.8 | 25/7.2 | 14/3.6 | 52/5.9 | 0.009 |
| -Neurological deficits | 74/50.3 | 213/61.4 | 259/67.3 | 546/62.1 | <0.001 |
| -Altered state of consciousness | 48/32.7 | 157/45.2 | 205/53.2 | 410/46.6 | <0.001 |
| -GCS score ≤ 7 | 2/1.4 | 6/1.7 | 5/1.3 | 13/1.5 | 0.784 |
| -Aphasia | 29/19.7 | 112/32.3 | 138/35.8 | 279/31.7 | 0.002 |
| -Dysarthria | 6/4.1 | 27/7.8 | 54/14 | 87/9.9 | <0.001 |
| -Hemiparesis | 63/42.9 | 187/53.9 | 237/61.6 | 487/55.4 | <0.001 |
| -Hemiplegia | 1/0.7 | 3/0.9 | 6/1.6 | 10/1.1 | 0.302 |
| -Sensitive deficit | 23/15.6 | 36/10.4 | 43/11.2 | 102/11.6 | 0.349 |
| Preoperative CT-based severity criteria | 136/92.5 | 307/88.5 | 833/87.8 | 781/88.9 | |
| -Hematoma thickness (mean ± SD in mm) | 22.9 ± 8.4 | 24.9 ± 9.2 | 26.8 ± 12.6 | 25.4 ± 10.8 | <0.001 |
| -Midline shift > 10 mm | 50/36.8 | 89/29 | 77/22.8 | 216/24.6 | 0.002 |
| -Presence of septations | 62/45.6 | 176/57.3 | 194/57.4 | 432/49.1 | 0.072 |
| -Density | |||||
| •hypodense | 29/21.3 | 50/16.3 | 58/17.2 | 137/15.6 | 0.005 |
| •isodense | 21/15.4 | 38/12.4 | 22/6.5 | 81/9.2 | |
| •hyperdense | 9/6.6 | 21/6.8 | 16/4.7 | 46/5.2 | |
| •mixed | 77/56.6 | 198/64.5 | 242/71.6 | 517/58.8 | |
| Postoperative complications | |||||
| -Seizure | 11/7.5 | 21/6.1 | 29/7.5 | 61/6.9 | 0.717 |
| -Bleeding requiring re-operation | 6/4.1 | 33/9.5 | 29/7.5 | 68/7.7 | 0.575 |
| -Pneumonia | 2/1.4 | 8/2.3 | 18/4.7 | 28/3.2 | 0.023 |
| -Pulmonary embolism | 0/0 | 1/0.3 | 4/1 | 5/0.6 | 0.096 |
| -Myocardial infarction | 0/0 | 0/0 | 3/0.8 | 3/0.3 | 0.067 |
| -Stroke | 1/0.7 | 0/0 | 2/0.5 | 3/0.3 | 0.752 |
| New neurological deficit at discharge | 6/4.1 | 15/4.3 | 15/3.9 | 36/4.1 | 0.841 |
| New motor deficit at discharge | 3/2 | 8/2.3 | 7/1.8 | 18/2 | 0.744 |
| New sensitive deficit at discharge | 2/1.4 | 0/0 | 1/0.3 | 3/0.3 | 0.231 |
| At discharge: mRS > 3 | 21/14.3 | 98/28.2 | 207/53.8 | 326/37.1 | 0.0005 |
| Total hospital stay in days | 10.4 ± 8.1 | 11.5 ± 7.3 | 11.5 ± 6.3 | 11.3 ± 7 | 0.008 |
| First Recurrence | 15/10.2 | 58/16.7 | 51/13.2 | 24/14.1 | 0.932 |
| Time to first recurrence in days (mean (95% CI)) | 11.2 (7.8–14.6) | 15.4 (12.8–18) | 21.4 (16.4–26.3) | 17.4 (14.9–19.9) | 0.011 |
| Second recurrence | 2/1.4 | 11/3.2 | 4/1 | 17/1.9 | 0.270 |
| Third recurrence | 0/0 | 1/0.3 | 1/0.3 | 2/0.2 | 0.699 |
| In-hospital mortality | 2/1.4 | 10/2.9 | 20/5.2 | 32/3.6 | 0.020 |
| (A) | |||||||||
| Variables | Age Groups (Years) | ||||||||
| 18–64 (n = 147) | 65–79 (n = 347) | ≥80 (n = 385) | |||||||
| Univariate Analysis | |||||||||
| Mean ± SD | p-Value | Mean ± SD | p-Value | Mean ± SD | p-Value | ||||
| Age (mean ± SD) | 63.5 ± 0.7 | 0.031 | 74.3 ± 4.4 | 0.457 | 85.9 ± 4.1 | 0.185 | |||
| Median (IQR) | p-value | Median (IQR) | p-value | Median (IQR) | p-value | ||||
| Charlson comorbidity index (median (IQR)) | 8 (0) | 0.002 | 4.5 (7) | 0.017 | 5.5 (3) | 0.129 | |||
| ASA status (median (IQR)) | 3 (0) | 0.403 | 3 (1) | 0.078 | 3 (0) | 0.043 | |||
| n/% | p-value | n/% | p-value | n/% | p-value | ||||
| Sex (female) | 0/0 | 1.000 | 3/3 | 1.000 | 6/4.2 | 0.637 | |||
| Recent head trauma | 1/1 | 1.000 | 5/2 | 0.140 | 17/5.3 | 1.000 | |||
| Epilepsy | 0/0 | 1.000 | 1/6.7 | 0.361 | 3/20 | 0.037 | |||
| Dementia | 0/0 | 1.000 | 3/7.3 | 0.102 | 8/8.5 | 0.110 | |||
| Arterial hypertension | 1/1.5 | 1.000 | 8/3.2 | 0.733 | 18/6.3 | 0.118 | |||
| Diabetes mellitus | 0/0 | 1.000 | 2/2.4 | 1.000 | 9/8.7 | 0.070 | |||
| Chronic kidney disease | 0/0 | 1.000 | 1/3.6 | 1.000 | 6/9.5 | 0.115 | |||
| Atrial fibrillation | 0/0 | 1.000 | 2/2.9 | 1.000 | 10/8.3 | 0.081 | |||
| Chronic heart disease | 0/0 | 1.000 | 2/3 | 1.000 | 6/6.3 | 0.599 | |||
| Cerebral vascular/transient ischemic accident | 0/0 | 1.000 | 0/0 | 0.369 | 3/4.3 | 0.782 | |||
| Operative technique (one burr hole) | 1/1 | 1.000 | 5/2.2 | 0.015 | 13/4.9 | 0.446 | |||
| Year of surgery | - | 0.595 | 0.203 | - | 0.356 | ||||
| CSDH location (bilateral) | 1/3.6 | 0.346 | 1/1.3 | 0.471 | 4/5.1 | 1.000 | |||
| Prior AAT use | 0/0 | 0.621 | 6/3.3 | 0.754 | 17/6.8 | 0.057 | |||
| Perioperative AAT short stop and heparin bridging | NA | NA | 0/0 | 1.000 | 1/25 | 0.193 | |||
| No perioperative stop of AAT | 0/0 | 1.000 | 0/0 | 1.000 | 0/0 | 1.000 | |||
| At admission: mRS > 3 | 1/2.8 | 0.431 | 8/6.1 | 0.007 | 16/6.5 | 0.155 | |||
| Preoperative symptoms and signs | |||||||||
| -Nausea | 0/0 | 1.000 | 0/0 | 0.648 | 1/7.7 | 1.000 | |||
| -Headache | 1/1.1 | 1.000 | 1/0.8 | 0.105 | 4/4.1 | 0.618 | |||
| -Dizziness | 0/0 | 1.000 | 1/2 | 1.000 | 1/2.2 | 0.490 | |||
| -Change in behavior | 0/0 | 1.000 | 2/3.6 | 1.000 | 3/5.1 | 1.000 | |||
| -Cognitive deterioration | 1/2.6 | 1.000 | 5/4.4 | 0.307 | 8/5.4 | 1.000 | |||
| -Gait disorder | 1/1.8 | 1.000 | 3/1.9 | 0.350 | 8/3.6 | 0.162 | |||
| -Seizure < 24 h | 0/0 | 1.000 | 2/8 | 0.157 | 0/0 | 0.628 | |||
| -Neurological deficits | 1/1.4 | 1.000 | 6/2.8 | 1.000 | 11/4.2 | 0.327 | |||
| -Altered state of consciousness | 1/2.1 | 1.000 | 8/5.1 | 0.048 | 14/6.8 | 0.167 | |||
| -GCS score ≤ 7 | 0/0 | 1.000 | 2/33.3 | 0.009 | 1/20 | 0.236 | |||
| -Aphasia | 0/0 | 1.000 | 4/3.6 | 0.733 | 11/8 | 0.092 | |||
| -Dysarthria | 0/0 | 1.000 | 1/3.7 | 1.000 | 3/5.6 | 1.000 | |||
| -Hemiparesis | 1/1.6 | 1.000 | 7/3.7 | 0.352 | 15/6.3 | 0.244 | |||
| -Hemiplegia | 0/0 | 1.000 | 1/33.3 | 0.084 | 0/0 | 1.000 | |||
| -Sensitive deficit | 0/0 | 1.000 | 0/0 | 0.403 | 1/2.3 | 0.494 | |||
| Preoperative CT-based severity criteria | |||||||||
| -Hematoma thickness (mean ± SD in mm) | 24.4 ± 2.3 | 0.556 | 19.9 ± 8.4 | 0.106 | 27.1 ± 9.5 | 0.768 | |||
| -Midline shift > 10 mm | 0/0 | 0.532 | 3/3.4 | 1.000 | 6/7.8 | 0.397 | |||
| -Presence of septations | 0/0 | 0.500 | 5/2.8 | 1.000 | 11/5.7 | 1.000 | |||
| -Density (mixed) | 0/0 | 0.186 | 7/3.5 | 0.499 | 15/6.2 | 0.604 | |||
| Postoperative complications | |||||||||
| -Seizure | 0/0 | 1.000 | 2/9.5 | 0.117 | 2/6.9 | 1.000 | |||
| -Bleeding requiring re-operation | 1/16.7 | 0.080 | 3/9.1 | 0.059 | 6/33.3 | <0.001 | |||
| -Pneumonia | 0/0 | 1.000 | 1/12.5 | 0.211 | 4/13.8 | 0.054 | |||
| -Pulmonary embolism | 0/0 | 1.000 | 0/0 | 1.000 | 1/25 | 0.193 | |||
| -Myocardial infarction | 0/0 | 1.000 | 0/0 | 1.000 | 1/33.3 | 0.148 | |||
| -Stroke | 0/0 | 1.000 | 0/0 | 1.000 | 1/50 | 0.101 | |||
| First recurrence | 0/0 | 1.000 | 1/1.7 | 0.704 | 3/5.9 | 1.000 | |||
| Second recurrence | 0/0 | 1.000 | 1/9.1 | 0.279 | 2/50 | 0.014 | |||
| Third recurrence | 0/0 | 1.000 | 1/100 | 0.029 | 1/100 | 0.052 | |||
| (B) | |||||||||
| Multivariate Analysis | |||||||||
| Age Groups (Years) | |||||||||
| 18–64 (n = 147) | 65–79 (n = 347) | ≥80 (n = 385) | |||||||
| Predictors | aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | aOR | 95% CI | p-Value |
| Age | NA | NA | 0.151 | ||||||
| Charlson comorbidity index | 3.3 | 0.8–13.2 | 0.092 | NA | NA | 0.173 | |||
| ASA status | NA | NA | 0.433 | NA | NA | 0.255 | |||
| Epilepsy | NA | NA | 0.078 | ||||||
| Diabetes mellitus | NA | NA | 0.104 | ||||||
| Atrial fibrillation | 2.9 | 1.1–7.8 | 0.031 | ||||||
| Prior AAT | NA | NA | 0.257 | ||||||
| Operative technique | NA | NA | 0.056 | ||||||
| mRS at admission (mRS > 3) | 9.6 | 1.1–83.6 | 0.041 | ||||||
| Preoperative altered state of consciousness | NA | NA | 0.756 | ||||||
| Preoperative aphasia | NA | NA | 0.074 | ||||||
| Preoperative hemiplegia | NA | NA | 0.486 | ||||||
| Preoperative GCS score ≤ 7 | 8.4 | 1.1–63.9 | 0.040 | ||||||
| Postoperative bleeding requiring re-operation | NA | NA | 0.111 | NA | NA | 0.331 | NA | NA | 0.129 |
| Postoperative pneumonia | 15.4 | 4.8–49.8 | <0.001 | ||||||
| Second recurrence | NA | NA | 0.060 | ||||||
| Third recurrence | NA | NA | 0.999 | NA | NA | 0.097 | |||
| (A) | |||||||||
| Variables | Age Groups (Years) | ||||||||
| 18–64 (n = 147) | 65–79 (n = 347) | ≥80 (n = 385) | |||||||
| Univariate Analysis | |||||||||
| Mean ± SD | p-Value | Mean ± SD | p-Value | Mean ± SD | p-Value | ||||
| Age (mean ± SD) | 58.5 ± 7.2 | 0.038 | 74.2 ± 4.3 | 0.012 | 85.3 ± 3.7 | 0.004 | |||
| Median (IQR) | p-value | Median (IQR) | p-value | Median (IQR) | p-value | ||||
| Charlson comorbidity index (median, IQR) | 2 (2) | 0.075 | 5 (3) | <0.001 | 5 (2) | <0.001 | |||
| ASA status (median, IQR) | 3 (0) | <0.001 | 3 (0) | <0.001 | 3 (0) | <0.001 | |||
| n/% | p-value | n/% | p-value | n/% | p-value | ||||
| Sex (female) | 9/25.7 | 0.049 | 24/23.8 | 0.242 | 73/51 | 0.459 | |||
| Recent head trauma | 11/12.1 | 0.467 | 70/27.6 | 0.687 | 181/56.2 | 0.038 | |||
| Epilepsy | 4/36.4 | 0.052 | 9/60 | 0.009 | 13/86.7 | 0.015 | |||
| Dementia | 0/0 | 1.000 | 25/61 | <0.001 | 72/76.6 | <0.001 | |||
| Arterial hypertension | 9/13.4 | 0.818 | 72/28.7 | 0.792 | 161/56.5 | 0.081 | |||
| Diabetes mellitus | 4/25 | 0.248 | 35/42.7 | 0.001 | 65/63.1 | 0.029 | |||
| Chronic kidney disease | 1/50 | 0.266 | 14/50 | 0.010 | 41/65.1 | 0.054 | |||
| Atrial fibrillation | 0/0 | 0.634 | 27/38.6 | 0.038 | 67/55.8 | 0.659 | |||
| Chronic heart disease | 2/16.7 | 1.000 | 23/34.8 | 0.224 | 58/60.4 | 0.156 | |||
| Cerebral vascular/transient ischemic accident | 2/18.2 | 1.000 | 21/44.7 | 0.009 | 36/52.2 | 0.791 | |||
| Operative technique (one burr hole) | 11/11 | 0.211 | 70/30.2 | 0.110 | 135/51.3 | 0.243 | |||
| Year of surgery | - | 0.833 | - | 0.045 | - | 0.089 | |||
| CSDH location (bilateral) | 5/17.9 | 0.766 | 14/18.4 | 0.043 | 45/57 | 0.530 | |||
| Prior antiplatelet/anticoagulant therapy | 7/18.9 | 0.416 | 61/33.3 | 0.031 | 143/57.2 | 0.070 | |||
| Perioperative AAT short stop and heparin bridging | NA | NA | 4/80 | 0.024 | 3/75 | 6.27 | |||
| No perioperative stop of AAT | 0/0 | 1.000 | 1/12.5 | 0.450 | 6/85.7 | 0.129 | |||
| At admission: mRS > 3 | 17/47.2 | <0.001 | 90/68.7 | <0.001 | 194/78.5 | <0.001 | |||
| Preoperative symptoms and signs | |||||||||
| -Nausea | 3/20 | 0.696 | 7/33.3 | 0.620 | 6/46.2 | 0.779 | |||
| -Headache | 6/6.6 | 0.001 | 17/13.8 | <0.001 | 43/44.3 | 0.034 | |||
| -Dizziness | 1/4 | 0.128 | 9/17.6 | 0.091 | 17/37 | 0.018 | |||
| -Change in behavior | 5/29.4 | 0.071 | 22/40 | 0.049 | 38/64.4 | 0.089 | |||
| -Cognitive deterioration | 9/23.1 | 0.106 | 47/41.2 | <0.001 | 94/63.5 | 0.003 | |||
| -Gait disorder | 11/20 | 0.147 | 51/31.7 | 0.191 | 116/52.5 | 0.606 | |||
| -Seizure < 24 h | 3/23.1 | 0.400 | 12/48 | 0.035 | 10/71.4 | 0.275 | |||
| -Neurological deficits | 15/20.3 | 0.058 | 72/33.8 | 0.005 | 145/56 | 0.231 | |||
| -Altered state of consciousness | 16/33.3 | <0.001 | 67/42.7 | <0.001 | 135/65.9 | <0.001 | |||
| -GCS score ≤ 7 | 1/50 | 0.235 | 6/100 | <0.001 | 5/100 | 0.064 | |||
| -Aphasia | 9/31 | 0.008 | 37/33 | 0.202 | 89/64.5 | 0.002 | |||
| -Dysarthria | 2/33.3 | 0.205 | 12/44.4 | 0.073 | 34/63 | 0.185 | |||
| -Hemiparesis | 14/22.2 | 0.030 | 64/34.2 | 0.008 | 130/54.9 | 0.601 | |||
| -Hemiplegia | 0/0 | 1.000 | 3/100 | 0.022 | 4/66.7 | 0.690 | |||
| -Sensitive deficit | 5/21.7 | 0.327 | 8/22.2 | 0.442 | 22/51.2 | 0.747 | |||
| Preoperative CT-based severity criteria | |||||||||
| -Hematoma thickness (mean ± SD in mm) | 23.1 ± 8.9 | 0.836 | 24.1 ± 9.8 | 0.220 | 28.1 ± 15 | 0.057 | |||
| -Midline shift > 10 mm | 8/16 | 0.616 | 30/33.7 | 0.337 | 44/57.1 | 0.794 | |||
| -Presence of septations | 9/14.5 | 1.000 | 56/31.8 | 0.377 | 108/55.7 | 0.912 | |||
| -Density (mixed) | 12/15.6 | 0.623 | 68/34.3 | 0.018 | 142/58.7 | 0.053 | |||
| Postoperative complications | |||||||||
| -Seizure | 5/45.5 | 0.010 | 14/66.7 | <0.001 | 23/79.3 | 0.006 | |||
| -Bleeding requiring re-operation | 1/16.7 | 1.000 | 15/45.5 | 0.026 | 22/75.9 | 0.019 | |||
| -Pneumonia | 0/0 | 1.000 | 7/87.5 | <0.001 | 15/83.3 | 0.013 | |||
| -Pulmonary embolism | 0/0 | 1.000 | 1/100 | 0.282 | 4/100 | 0.127 | |||
| -Myocardial infarction | 0/0 | 1.000 | 0/0 | 1.000 | 3/100 | 0.252 | |||
| -Stroke | 1/100 | 0.143 | 0/0 | 1.000 | 2/100 | 0.502 | |||
| (B) | |||||||||
| Multivariate Analysis | |||||||||
| Predictors | aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | aOR | 95% CI | p-Value |
| Age | NA | NA | 0.314 | NA | NA | 0.771 | NA | NA | 0.105 |
| Sex (female) | 4.3 | 1.1–17.4 | 0.040 | ||||||
| Charlson comorbidity index | NA | NA | 0.470 | 1.3 | 1–1.6 | 0.036 | NA | NA | 0.697 |
| ASA status | 10.6 | 1.8–63 | 0.010 | NA | NA | 0.194 | 2 | 1.6–3.5 | 0.014 |
| Recent head trauma | NA | NA | 0.358 | ||||||
| Epilepsy | 11.4 | 1.3–101.5 | 0.029 | NA | NA | 0.542 | 19.1 | 1.5–242.3 | 0.023 |
| Dementia | 4.5 | 1.3–15.2 | 0.016 | 3.3 | 1.5–7.3 | 0.003 | |||
| Arterial hypertension | NA | NA | 0.246 | ||||||
| Diabetes mellitus | NA | NA | 0.593 | NA | NA | 0.277 | |||
| Chronic kidney disease | NA | NA | 0.680 | NA | NA | 0.189 | |||
| Atrial fibrillation | NA | NA | 0.609 | ||||||
| Cerebral vascular/transient ischemic accident | NA | NA | 0.782 | ||||||
| Year of surgery | NA | NA | 0.948 | NA | NA | 0.689 | |||
| OP technique (burr hole) | |||||||||
| CSDH location (bilateral) | 0.3 | 0.1–0.8 | 0.016 | ||||||
| Prior antiplatelet/anticoagulant therapy | NA | NA | 0.265 | NA | NA | 0.401 | |||
| Perioperative AAT short stop and heparin bridging | NA | NA | 0.999 | ||||||
| mRS at admission (mRS > 3) | 49.5 | 8.3–293.6 | <0.001 | 79.5 | 28.3–223 | <0.001 | 47.8 | 20.1–113.6 | <0.001 |
| Preoperative symptoms and signs | |||||||||
| -Headache | NA | NA | 0.214 | NA | NA | 0.287 | NA | NA | 0.668 |
| -Dizziness | NA | NA | 0.869 | NA | NA | 0.076 | |||
| -Change in behavior | NA | NA | 0.085 | NA | NA | 0.098 | NA | NA | 0.784 |
| -Cognitive deterioration | NA | NA | 0.891 | NA | NA | 0.475 | |||
| -Seizure < 24 h | NA | NA | 0.119 | ||||||
| -Neurological deficits | NA | NA | 0.334 | NA | NA | 0.737 | |||
| -Altered state of consciousness | NA | NA | 0.195 | NA | NA | 0.951 | 2 | 1.1–3.8 | 0.035 |
| -GCS score ≤ 7 | NA | NA | 0.377 | NA | NA | 0.309 | |||
| -Aphasia | NA | NA | 0.927 | NA | NA | 0.090 | |||
| -Dysarthria | NA | NA | 0.484 | ||||||
| -Hemiparesis | NA | NA | 0.792 | NA | NA | 0.660 | |||
| -Hemiplegia | NA | NA | 0.441 | ||||||
| Hematoma thickness | NA | NA | 0.711 | ||||||
| CSDH CT-density (mixed) | NA | NA | 0.807 | NA | NA | 0.158 | |||
| Postoperative complications | |||||||||
| -Seizure | 29.5 | 2.8–311.7 | 0.005 | NA | NA | 0.132 | NA | NA | 0.277 |
| -Bleeding requiring re-operation | NA | NA | 0.062 | 7.2 | 1.7–30.8 | 0.007 | |||
| -Pneumonia | 38.8 | 2.2–695.3 | 0.013 | NA | NA | 0.420 | |||
| Logistic Regression | Bootstrapped Logistic Regression | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | B | aOR | 95%CI | p-Value | Bias | SE | p-Value | Bca 95% CI |
| Age | 0.044 | NA | NA | 0.314 | 2.435 | 12.846 | 0.427 | −4–25.9 |
| Sex (female) | 1.600 | 4.3 | 1.1–17.4 | 0.040 | 30.941 | 153.816 | 0.047 | −19.6–330.3 |
| Charlson comorbidity index | 0.192 | NA | NA | 0.470 | 7.378 | 50.768 | 0.425 | −21.2–80.4 |
| ASA status | 2.233 | 10.6 | 1.8–63 | 0.010 | 56.365 | 206.594 | 0.002 | 1.1–414.7 |
| Epilepsy | 3.100 | 11.4 | 1.3–101.5 | 0.029 | 108.019 | 453.970 | 0.008 | −3.2–955.2 |
| mRS at admission (mRS > 3) | 4.931 | 49.5 | 8.3–293.6 | <0.001 | 131.308 | 537.116 | 0.001 | 4–971.5 |
| Preoperative Headache | −0.307 | NA | NA | 0.214 | 6.239 | 150.200 | 0.536 | −143.7–204.2 |
| Preoperative change in behavior | 2.173 | NA | NA | 0.085 | 68.720 | 278.639 | 0.019 | −0.5–528.7 |
| Preoperative neurological deficits | −2.061 | NA | NA | 0.334 | −70.445 | 383.031 | 0.071 | −633.8–46.7 |
| Preoperative altered state of consciousness | 0.974 | NA | NA | 0.195 | 14.840 | 143.402 | 0.299 | −137–276.8 |
| Preoperative aphasia | −0.536 | NA | NA | 0.927 | −22.385 | 150.895 | 0.443 | −307.6–86.3 |
| Preoperative hemiparesis | 0.528 | NA | NA | 0.792 | 32.000 | 173.635 | 0.386 | −35.2–361.7 |
| Postoperative seizure | 3.311 | 29.5 | 2.8–311.7 | 0.005 | 73.073 | 276.964 | 0.002 | 0.5–614.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmanian, S.; Rodemerk, J.; Al-Rubaiey, S.; Ahmadzai, M.; Timner, E.; Schock, L.; Dinger, T.F.; Gembruch, O.; Jabbarli, R.; Dammann, P.; et al. Age-Related Comparative Study of In-Hospital Mortality, Functional Outcome, and Recurrence in a Large Cohort of Patients Surgically Treated for Chronic Subdural Hematoma. J. Clin. Med. 2025, 14, 7856. https://doi.org/10.3390/jcm14217856
Salmanian S, Rodemerk J, Al-Rubaiey S, Ahmadzai M, Timner E, Schock L, Dinger TF, Gembruch O, Jabbarli R, Dammann P, et al. Age-Related Comparative Study of In-Hospital Mortality, Functional Outcome, and Recurrence in a Large Cohort of Patients Surgically Treated for Chronic Subdural Hematoma. Journal of Clinical Medicine. 2025; 14(21):7856. https://doi.org/10.3390/jcm14217856
Chicago/Turabian StyleSalmanian, Schahin, Jan Rodemerk, Sali Al-Rubaiey, Madiha Ahmadzai, Elias Timner, Lisa Schock, Thiemo Florin Dinger, Oliver Gembruch, Ramazan Jabbarli, Philipp Dammann, and et al. 2025. "Age-Related Comparative Study of In-Hospital Mortality, Functional Outcome, and Recurrence in a Large Cohort of Patients Surgically Treated for Chronic Subdural Hematoma" Journal of Clinical Medicine 14, no. 21: 7856. https://doi.org/10.3390/jcm14217856
APA StyleSalmanian, S., Rodemerk, J., Al-Rubaiey, S., Ahmadzai, M., Timner, E., Schock, L., Dinger, T. F., Gembruch, O., Jabbarli, R., Dammann, P., Sure, U., & Chihi, M. (2025). Age-Related Comparative Study of In-Hospital Mortality, Functional Outcome, and Recurrence in a Large Cohort of Patients Surgically Treated for Chronic Subdural Hematoma. Journal of Clinical Medicine, 14(21), 7856. https://doi.org/10.3390/jcm14217856







