Sex Differences in Prognostic Markers: Exploring Outcome Variability After Mechanical Thrombectomy in Large Vessel Occlusion Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Image Acquisition and Analysis
2.3. Clinical and Demographic Data
2.4. Statistical Analysis
3. Results
3.1. Clinical and Demographic Data
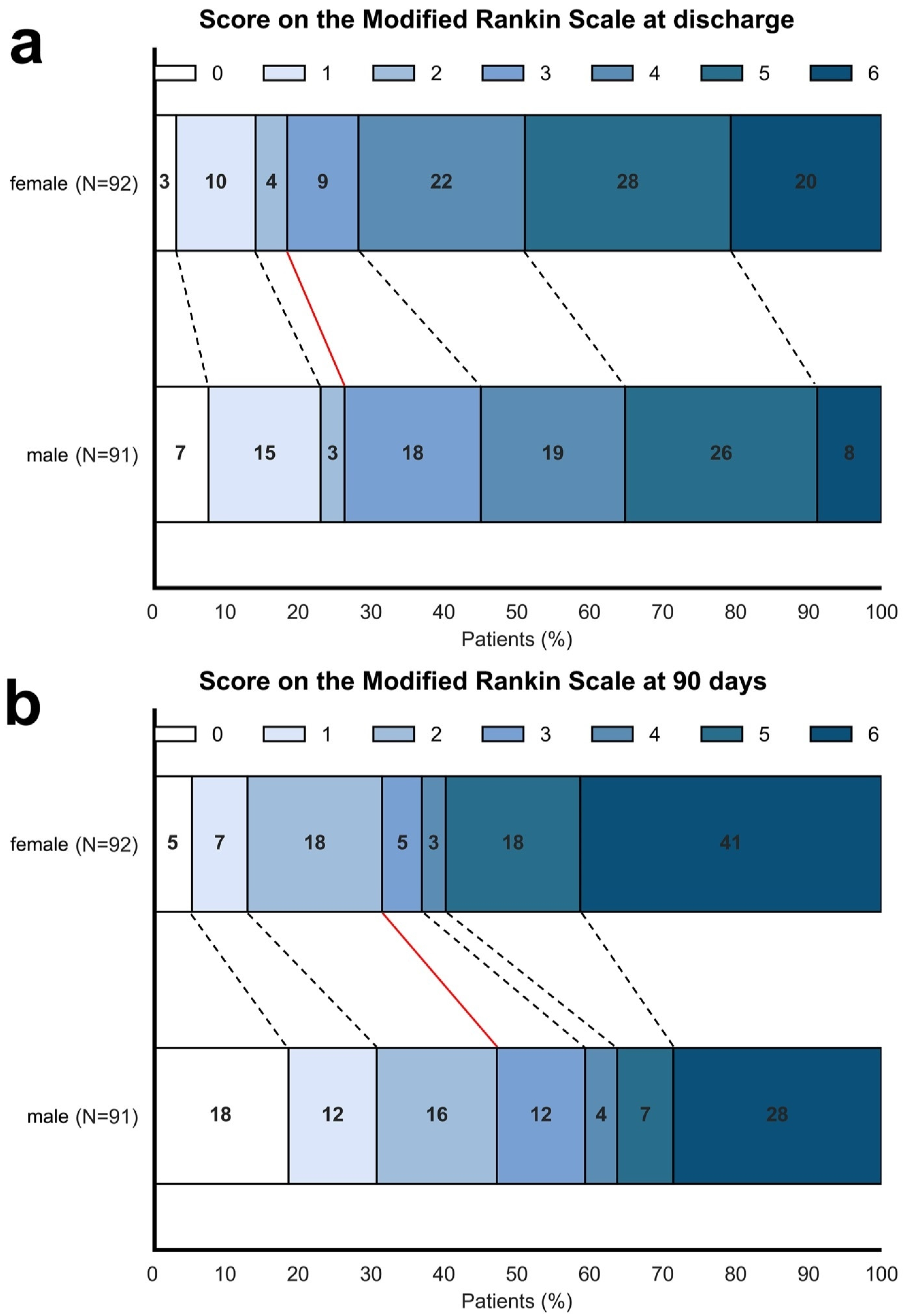
3.2. Association Between Sex and Functional Outcome
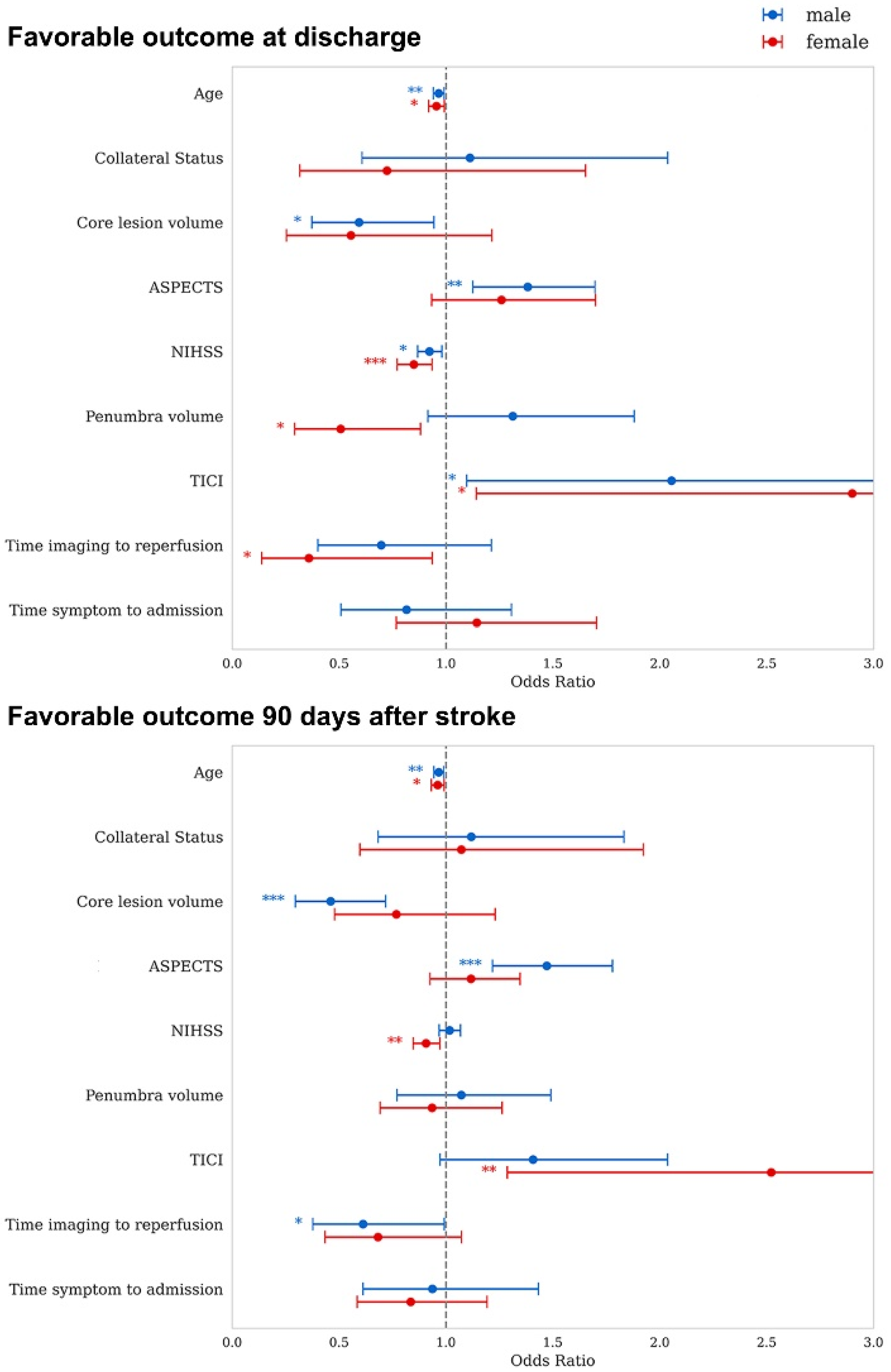
3.3. Sex Dependent Associations of Prognostic Markers with Outcome
4. Discussion
4.1. Study Aim and Key Findings
4.2. Association Between Sex and Functional Outcome
4.3. Sex Dependent Associations of Prognostic Markers with Outcome
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LVO | Large vessel occlusion |
| MT | Mechanical thrombectomy |
| ASPECTS | Alberta stroke program early CT score |
| NIHSS | National Institutes of Health Stroke Scale |
| mRS | Modified Rankin scale |
| RCT | Randomized controlled trials |
| NCCT | Non-enhanced cranial CT |
| CBF | Cerebral blood flow |
| Tmax | Time-to-maximum of the tissue residue function |
| MCA | Middle cerebral artery |
| TICI | Thrombolysis in cerebral infarction |
| OR | Odds ratio |
| CI | Confidence interval |
| IQR | Interquartile range |
| SD | Standard deviation |
| ICA | Internal carotid artery |
References
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.J.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.L.M.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Groot, A.E.; Treurniet, K.M.; Jansen, I.G.H.; Lingsma, H.F.; Hinsenveld, W.; van de Graaf, R.A.; Roozenbeek, B.; Willems, H.C.; Schonewille, W.J.; Marquering, H.A.; et al. Endovascular treatment in older adults with acute ischemic stroke in the MR CLEAN Registry. Neurology 2020, 95, e131–e139. [Google Scholar] [CrossRef]
- Almekhlafi, M.A.; Davalos, A.; Bonafe, A.; Chapot, R.; Gralla, J.; Pereira, V.M.; Goyal, M. STAR Registry Investigators Impact of age and baseline NIHSS scores on clinical outcomes in the mechanical thrombectomy using solitaire FR in acute ischemic stroke study. AJNR Am. J. Neuroradiol. 2014, 35, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Yu, Z.; Jia, B.; Xian, Y.; Ren, Z.; Mo, D.; Ma, N.; Gao, F.; Tong, X.; Shi, X.; et al. Time to Endovascular Reperfusion and Outcome in Acute Ischemic Stroke: A Nationwide Prospective Registry in China. Clin. Neuroradiol. 2022, 32, 997–1009. [Google Scholar] [CrossRef]
- Alexandre, A.M.; Monforte, M.; Brunetti, V.; Scarcia, L.; Cirillo, L.; Zini, A.; Scala, I.; Nardelli, V.; Arbia, F.; Arbia, G.; et al. Baseline clinical and neuroradiological predictors of outcome in patients with large ischemic core undergoing mechanical thrombectomy: A retrospective multicenter study. Int. J. Stroke 2024, 19, 779–788. [Google Scholar] [CrossRef]
- Marburg, M.; Rudolf, L.F.; Matthis, C.; Neumann, A.; Schareck, C.; Schacht, H.; Schulz, R.; Machner, B.; Schramm, P.; Royl, G.; et al. The lesion core extent modulates the impact of early perfusion mismatch imaging on outcome variability after thrombectomy in stroke. Front. Neurol. 2024, 15, 1366240. [Google Scholar] [CrossRef] [PubMed]
- Seners, P.; Oppenheim, C.; Turc, G.; Albucher, J.-F.; Guenego, A.; Raposo, N.; Christensen, S.; Calvière, L.; Viguier, A.; Darcourt, J.; et al. Perfusion Imaging and Clinical Outcome in Acute Ischemic Stroke with Large Core. Ann. Neurol. 2021, 90, 417–427. [Google Scholar] [CrossRef]
- Gensicke, H.; Al-Ajlan, F.; Fladt, J.; Campbell, B.C.V.; Majoie, C.B.L.M.; Bracard, S.; Hill, M.D.; Muir, K.W.; Demchuk, A.; San Román, L.; et al. Comparison of Three Scores of Collateral Status for Their Association with Clinical Outcome: The HERMES Collaboration. Stroke 2022, 53, 3548–3556. [Google Scholar] [CrossRef]
- Broocks, G.; Meyer, L.; Elsayed, S.; McDonough, R.; Bechstein, M.; Faizy, T.D.; Sporns, P.; Schön, G.; Minnerup, J.; Kniep, H.C.; et al. Association Between Net Water Uptake and Functional Outcome in Patients with Low ASPECTS Brain Lesions: Results From the I-LAST Study. Neurology 2023, 100, e954–e963. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.J.; Rudolf, L.F.; Schramm, P.; Frontzkowski, L.; Marburg, M.; Matthis, C.; Schacht, H.; Fiehler, J.; Thomalla, G.; Hummel, F.C.; et al. Preserved Corticospinal Tract Revealed by Acute Perfusion Imaging Relates to Better Outcome After Thrombectomy in Stroke. Stroke 2023, 54, 3081–3089. [Google Scholar] [CrossRef]
- Koch, P.J.; Frey, B.M.; Backhaus, W.; Petersen, N.; Girard, G.; Wróbel, P.P.; Braaß, H.; Bönstrup, M.; Kunkel Genannt Bode, L.; Cheng, B.; et al. Neurotransmitter-informed connectivity maps and their application for outcome inference after stroke. Brain 2025, awaf185. [Google Scholar] [CrossRef] [PubMed]
- de Ridder, I.R.; Fransen, P.S.S.; Beumer, D.; Berkhemer, O.A.; van den Berg, L.A.; Wermer, M.J.; Lingsma, H.; van Zwam, W.H.; Roos, Y.B.; van Oostenbrugge, R.J.; et al. Is Intra-Arterial Treatment for Acute Ischemic Stroke Less Effective in Women than in Men? Interv. Neurol. 2016, 5, 174–178. [Google Scholar] [CrossRef]
- Uchida, K.; Yoshimura, S.; Sakai, N.; Yamagami, H.; Morimoto, T. Sex Differences in Management and Outcomes of Acute Ischemic Stroke with Large Vessel Occlusion. Stroke 2019, 50, 1915–1918. [Google Scholar] [CrossRef]
- Madsen, T.E.; DeCroce-Movson, E.; Hemendinger, M.; McTaggart, R.A.; Yaghi, S.; Cutting, S.; Furie, K.L.; Saad, A.; Siket, M.S.; Jayaraman, M. V Sex differences in 90-day outcomes after mechanical thrombectomy for acute ischemic stroke. J. Neurointerv. Surg. 2019, 11, 221–225. [Google Scholar] [CrossRef]
- Dmytriw, A.A.; Ku, J.C.; Yang, V.X.D.; Hui, N.; Uchida, K.; Morimoto, T.; Spears, J.; Marotta, T.R.; Diestro, J.D.B. Do outcomes between women and men differ after endovascular thrombectomy? a meta-analysis. Am. J. Neuroradiol. 2021, 42, 910–915. [Google Scholar] [CrossRef]
- Fifi, J.T.; Nguyen, T.N.; Song, S.; Sharrief, A.; Pujara, D.K.; Shaker, F.; Fournier, L.E.; Jones, E.M.; Lechtenberg, C.G.; Slavin, S.J.; et al. Sex differences in endovascular thrombectomy outcomes in large vessel occlusion: A propensity-matched analysis from the SELECT study. J. Neurointerv. Surg. 2023, 15, 105–112. [Google Scholar] [CrossRef]
- Wróbel, D.; Wrona, P.; Homa, T.; Jakobschy, K.; Wrona, G.; Sawczyńska, K.; Giełczyński, M.; Popiela, T.; Słowik, A.; Turaj, W. Sex Alters the Effect of Perfusion Deficits on Functional Outcome in Patients with Acute Ischemic Stroke Undergoing Mechanical Thrombectomy. Cerebrovasc. Dis. 2024, 54, 165–174. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Wang, J.; Zhang, F.; Xiao, L.; Fang, Y.; Yan, D.; Xu, G.; Liu, C.; Huang, Z.; et al. Sex differences in outcomes after endovascular treatment of patients with vertebrobasilar artery occlusion. Eur. Stroke J. 2023, 8, 566–574. [Google Scholar] [CrossRef]
- Lagebrant, C.; Ramgren, B.; Hassani Espili, A.; Marañon, A.; Kremer, C. Sex Differences in Collateral Circulation and Outcome After Mechanical Thrombectomy in Acute Ischemic Stroke. Front. Neurol. 2022, 13, 878759. [Google Scholar] [CrossRef] [PubMed]
- Romoli, M.; Banerjee, S.; Cordici, F.; Lobotesis, K.; Longoni, M.; Lafe, E.; Casetta, I.; Katsanos, A.H.; Palaiodimou, L.; Zini, A.; et al. Impact of Sex on Thrombectomy Outcomes in Ischemic Stroke: A Propensity Score-Matched Study, Systematic Review, and Meta-Analysis. Stroke Vasc. Interv. Neurol. 2024, 4, e001196. [Google Scholar] [CrossRef]
- Chalos, V.; De Ridder, I.R.; Lingsma, H.F.; Brown, S.; Van Oostenbrugge, R.J.; Goyal, M.; Campbell, B.C.V.; Muir, K.W.; Guillemin, F.; Bracard, S.; et al. Does Sex Modify the Effect of Endovascular Treatment for Ischemic Stroke?: A Subgroup Analysis of 7 Randomized Trials. Stroke 2019, 50, 2413–2419. [Google Scholar] [CrossRef]
- Ciardi, C.; Cirio, J.J.; Scrivano, E.V.; Bleise, C.D.; Lylyk, I.; Lylyk, P. Sex-Related Differences after Endovascular Treatment of Acute Ischemic Stroke in the “Real World”. J. Stroke Cerebrovasc. Dis. 2020, 29, 105240. [Google Scholar] [CrossRef]
- Carvalho, A.; Cunha, A.; Gregório, T.; Paredes, L.; Costa, H.; Veloso, M.; Castro, S.; Ribeiro, M.; Barros, P.J.G. Is the Efficacy of Endovascular Treatment for Acute Ischemic Stroke Sex-Related. Interv. Neurol. 2018, 7, 42–47. [Google Scholar] [CrossRef]
- Abdalkader, M.; Ning, S.; Qureshi, M.M.; Haussen, D.C.; Strbian, D.; Nagel, S.; Demeestere, J.; Puetz, V.; Mohammaden, M.H.; Olive Gadea, M.; et al. Sex Differences in Outcomes of Late-Window Endovascular Stroke Therapy. Stroke 2024, 55, 278–287. [Google Scholar] [CrossRef]
- Huo, X.; Sun, D.; Raynald; Jia, B.; Tong, X.; Wang, A.; Ma, N.; Gao, F.; Mo, D.; Nguyen, T.N.; et al. Sex differences in outcomes of endovascular therapy for acute vertebrobasilar occlusion: Data from ANGEL-ACT Registry. J. Neurol. 2024, 271, 1376–1384. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, X.; Kwan, A.T.H.; Mofatteh, M.; Nguyen, T.N.; Zhou, S.; Wei, H.; Dmytriw, A.A.; Regenhardt, R.W.; Yan, Z.; et al. Sex Differences in Outcomes after Endovascular Thrombectomy for Patients with Acute Ischemic Stroke. Eur. Neurol. 2024, 87, 113–121. [Google Scholar] [CrossRef]
- Sun, D.; Raynald; Huo, X.; Jia, B.; Tong, X.; Ma, G.; Wang, A.; Ma, N.; Gao, F.; Mo, D.; et al. Sex-Related Differences in Outcomes of Endovascular Treatment for Anterior Circulation Large Vessel Occlusion. Stroke 2023, 54, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Regenhardt, R.W.; Turner, A.C.; Hirsch, J.A.; Young, M.J.; Alotaibi, N.M.; Stapleton, C.J.; Patel, A.B.; Leslie-Mazwi, T.M.; Rost, N.S.; Etherton, M.R. Sex-specific differences in presentations and determinants of outcomes after endovascular thrombectomy for large vessel occlusion stroke. J. Neurol. 2022, 269, 307–315. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Sarraj, A.; Hassan, A.E.; Grotta, J.; Sitton, C.; Cutter, G.; Cai, C.; Chen, P.R.; Imam, B.; Pujara, D.; Arora, A.; et al. Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT): A Prospective, Multicenter Cohort Study of Imaging Selection. Ann. Neurol. 2020, 87, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, D.; Volny, O.; Cimflova, P.; Svub, K.; Dvornikova, K.; Bar, M. The importance of CT perfusion for diagnosis and treatment of ischemic stroke in anterior circulation. J. Integr. Neurosci. 2022, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Miteff, F.; Levi, C.R.; Bateman, G.A.; Spratt, N.; McElduff, P.; Parsons, M.W. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain 2009, 132, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Deb-Chatterji, M.; Schlemm, E.; Flottmann, F.; Meyer, L.; Alegiani, A.; Brekenfeld, C.; Fiehler, J.; Gerloff, C.; Thomalla, G.; Gerloff, C.; et al. Sex Differences in Outcome After Thrombectomy for Acute Ischemic Stroke are Explained by Confounding Factors. Clin. Neuroradiol. 2021, 31, 1101–1109. [Google Scholar] [CrossRef]
- Casetta, I.; Fainardi, E.; Pracucci, G.; Saia, V.; Sallustio, F.; da Ros, V.; Nappini, S.; Nencini, P.; Bigliardi, G.; Vinci, S.; et al. Sex differences in outcome after thrombectomy for acute ischemic stroke. A propensity score-matched study. Eur. Stroke J. 2022, 7, 151–157. [Google Scholar] [CrossRef]
- Demeestere, J.; Christensen, S.; Mlynash, M.; Federau, C.; Albers, G.W.; Lemmens, R.; Lansberg, M.G. Effect of Sex on Clinical Outcome and Imaging after Endovascular Treatment of Large-Vessel Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105468. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Saczynski, J.S.; Gore, J.M.; Goldberg, R.J. Age and sex differences in duration of prehospital delay in patients with acute myocardial infarction: A systematic review. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 82–92. [Google Scholar] [CrossRef]
- Bonkhoff, A.K.; Schirmer, M.D.; Bretzner, M.; Hong, S.; Regenhardt, R.W.; Brudfors, M.; Donahue, K.L.; Nardin, M.J.; Dalca, A.V.; Giese, A.-K.; et al. Outcome after acute ischemic stroke is linked to sex-specific lesion patterns. Nat. Commun. 2021, 12, 3289. [Google Scholar] [CrossRef] [PubMed]
| Variable | n = 183 | Male (n = 91) | Female (n = 92) | p-Value |
|---|---|---|---|---|
| Age (±SD) in yrs | 72 ± 13 | 70 ± 13 | 75 ± 12 | * 0.005 |
| Median NIHSS (IQR) | 16 (11–18) | 15 (11.5–18) | 16 (11–18) | 0.623 |
| Median ASPECTS (IQR) | 7 (6–9) | 7 (5.5–8) | 8 (6.75–9) | 0.086 |
| Core lesion (±SD) (in % of whole brain vol.) | 7 (5) | 6 (4) | 7 (6) | 0.366 |
| Core lesion (±SD) in mL | 70 (50) | 70 (45) | 70 (55) | 0.981 |
| Median collateral status (IQR) | 2 (2–3) | 2 (2–2) | 2 (2–3) | 0.155 |
| Time symptom to admission (min ± SD) (n = 118) | 96 (60) | 92 (59) | 99 (68) | 0.554 |
| Time imaging to reperfusion (min ± SD) (n = 170) | 114 (50) | 109 (43) | 122 (56) | 0.111 |
| Number (proportion%) of patients with | ||||
| affection of the right hemisphere | 93 (51) | 47 (52) | 46 (50) | 0.825 |
| IV thrombolysis received | 125 (68) | 63 (69) | 62 (67) | 0.791 |
| Successful recanalization (≥TICI 2b) | 113 (62) | 61 (67) | 52 (57) | 0.145 |
| Vessel occlusion in CT angiography | ||||
| Proximal ICA | 50 (27) | 29 (32) | 21 (23) | 0.186 |
| Distal ICA | 15 (8) | 9 (10) | 6 (7) | 0.422 |
| Distal ICA/carotid T | 22 (12) | 12 (13) | 10 (11) | 0.652 |
| M1 | 101 (55) | 46 (51) | 55 (60) | 0.181 |
| Diagnosis of | ||||
| Arterial hypertension | 120 (66) | 59 (65) | 61 (66) | 0.836 |
| Diabetes mellitus | 40 (22) | 18 (20) | 22 (24) | 0.615 |
| Hypercholesterolemia | 46 (25) | 23 (25) | 23 (25) | 0.967 |
| Atrial fibrillation | 80 (44) | 32 (35) | 48 (52) | * 0.021 |
| History of ischemic stroke | 17 (9) | 6 (7) | 11 (12) | 0.214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schacht, H.; Neumann, A.; Petersen, N.; Ehm, L.M.; Marburg, M.; Matthis, C.; Jensen-Kondering, U.; Schramm, P.; Minnerup, J.; Royl, G.; et al. Sex Differences in Prognostic Markers: Exploring Outcome Variability After Mechanical Thrombectomy in Large Vessel Occlusion Stroke. J. Clin. Med. 2025, 14, 7855. https://doi.org/10.3390/jcm14217855
Schacht H, Neumann A, Petersen N, Ehm LM, Marburg M, Matthis C, Jensen-Kondering U, Schramm P, Minnerup J, Royl G, et al. Sex Differences in Prognostic Markers: Exploring Outcome Variability After Mechanical Thrombectomy in Large Vessel Occlusion Stroke. Journal of Clinical Medicine. 2025; 14(21):7855. https://doi.org/10.3390/jcm14217855
Chicago/Turabian StyleSchacht, Hannes, Alexander Neumann, Nora Petersen, Lis Merrit Ehm, Maria Marburg, Christine Matthis, Ulf Jensen-Kondering, Peter Schramm, Jens Minnerup, Georg Royl, and et al. 2025. "Sex Differences in Prognostic Markers: Exploring Outcome Variability After Mechanical Thrombectomy in Large Vessel Occlusion Stroke" Journal of Clinical Medicine 14, no. 21: 7855. https://doi.org/10.3390/jcm14217855
APA StyleSchacht, H., Neumann, A., Petersen, N., Ehm, L. M., Marburg, M., Matthis, C., Jensen-Kondering, U., Schramm, P., Minnerup, J., Royl, G., & Koch, P. J. (2025). Sex Differences in Prognostic Markers: Exploring Outcome Variability After Mechanical Thrombectomy in Large Vessel Occlusion Stroke. Journal of Clinical Medicine, 14(21), 7855. https://doi.org/10.3390/jcm14217855






