Impact of Intramedullary Implants on Metallic Element Homeostasis in Children with Forearm Fractures
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants of the Study
2.2. Research Material
2.3. Elemental Analysis
2.4. Statistical Analysis
3. Results
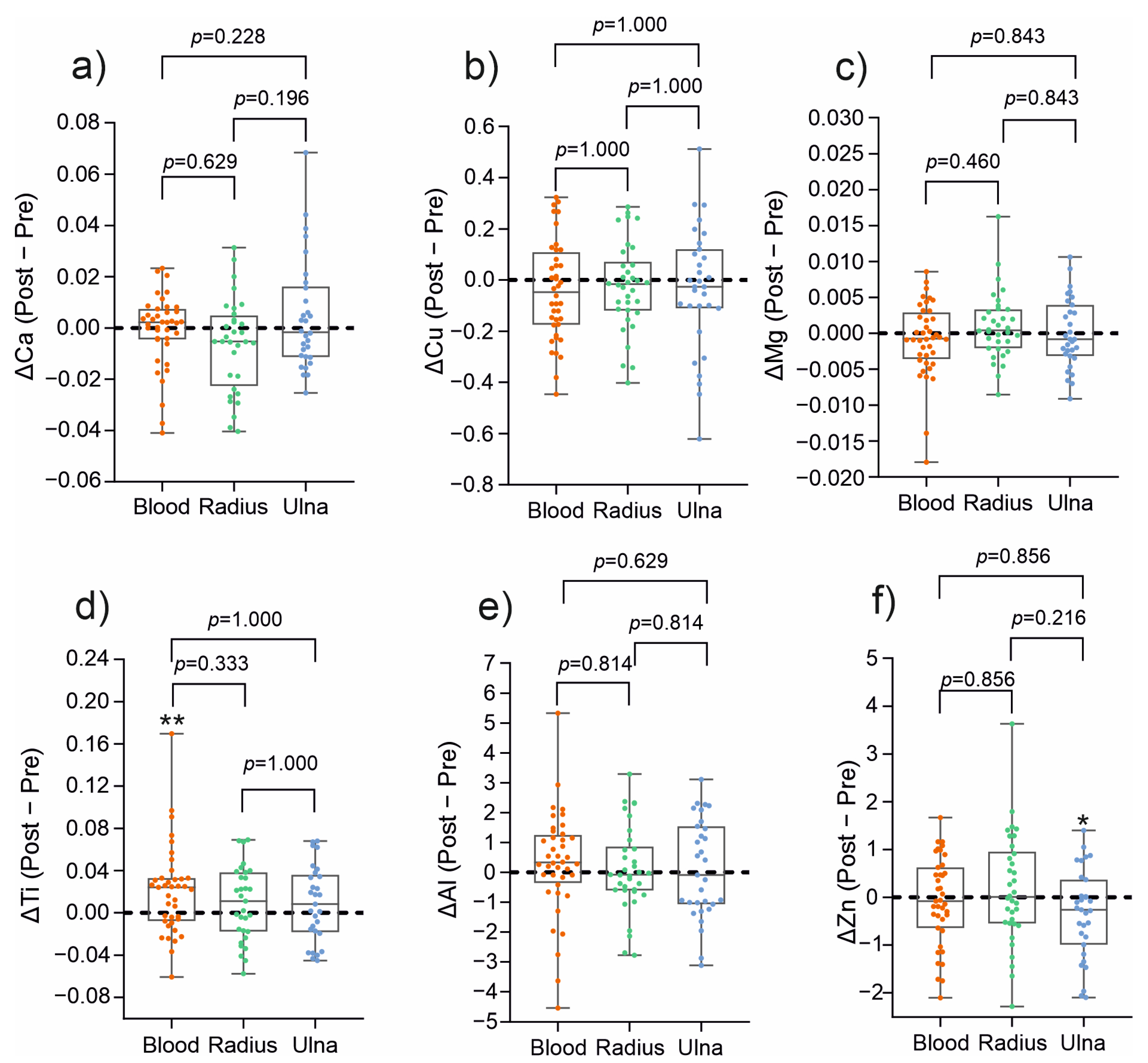
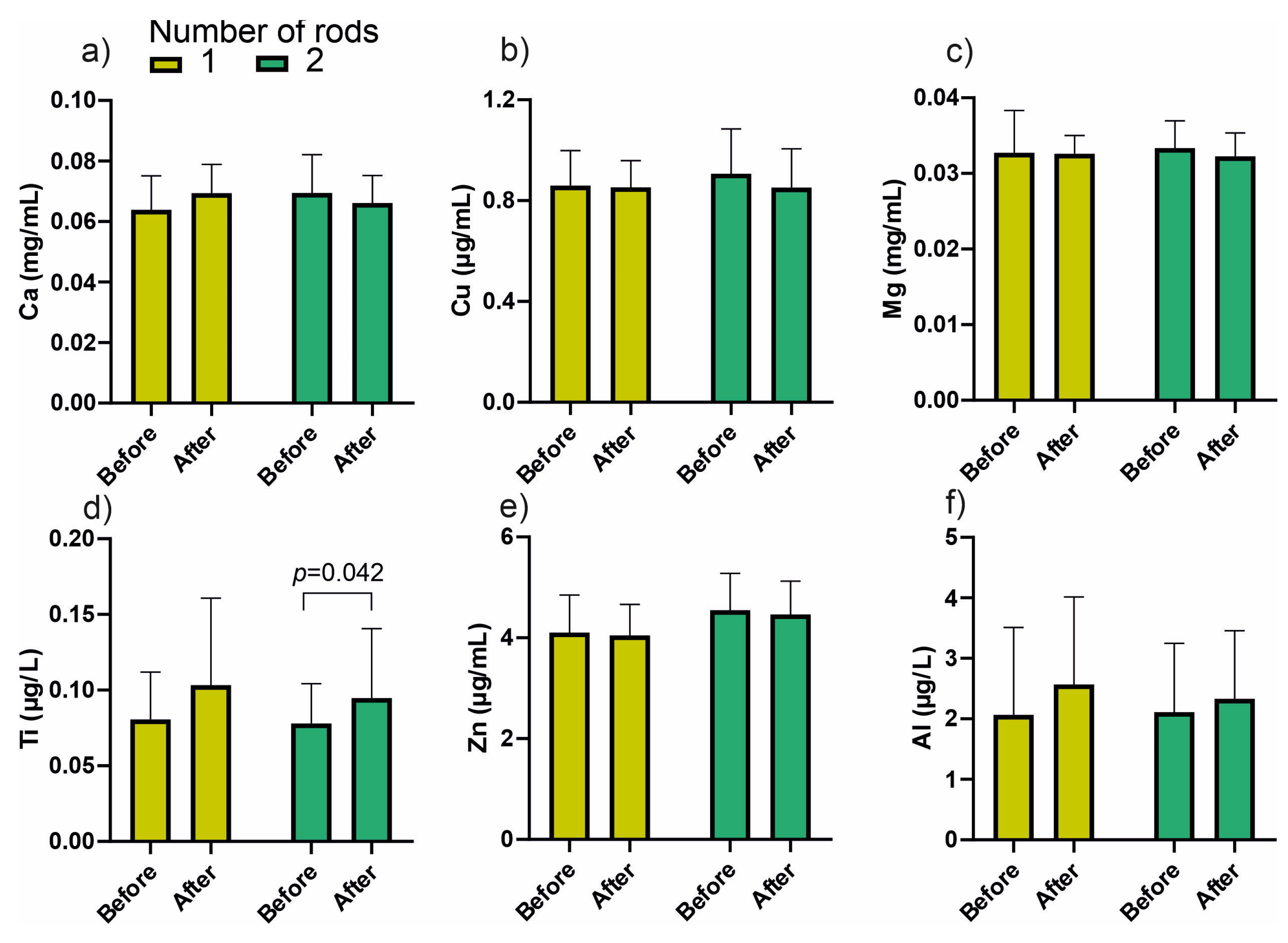
3.1. Changes in Metal Ion Content in the Material from the Medullary Cavity and Blood
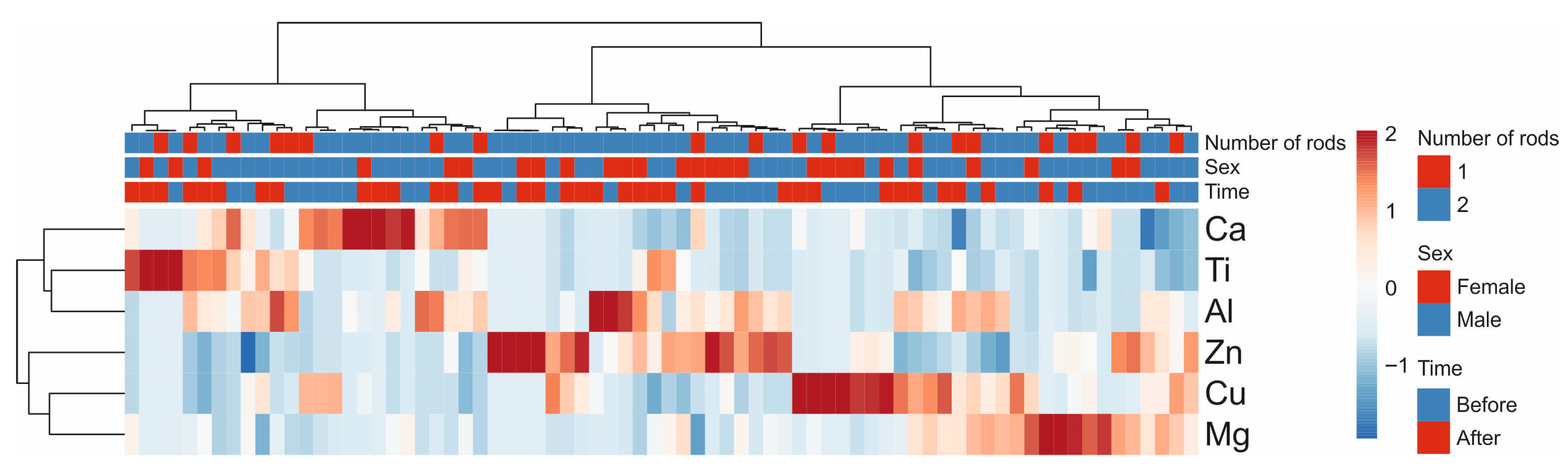
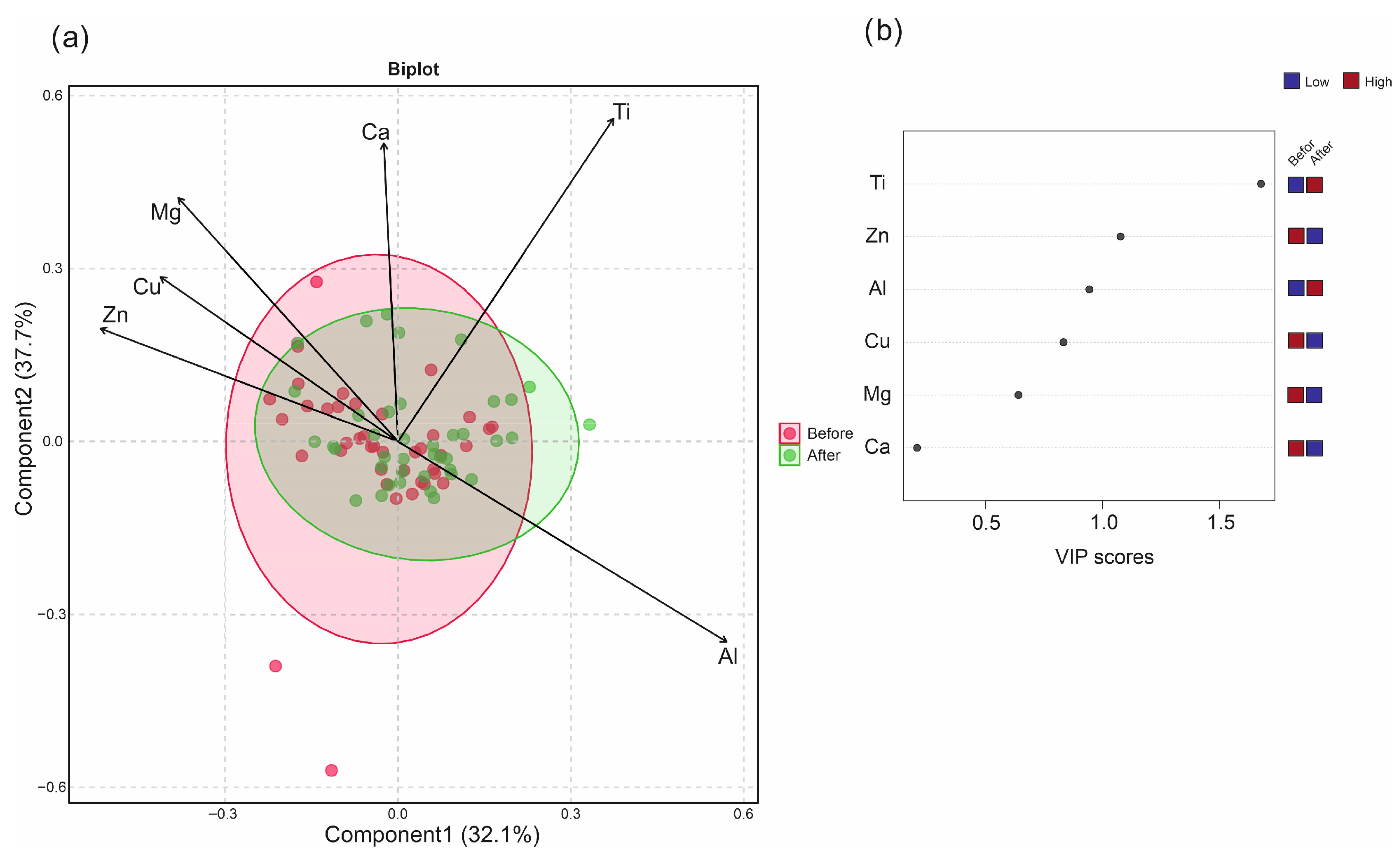
3.2. Multivariate Analysis of Metallic Elements Concentration in Blood Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Synder, M. Złamania Kości u Dzieci. Diagnostyka, Leczenie, Odrębności; Klinika Ortopedii i Ortopedii Dziecięcej UM w Łodzi: Łódź, Poland, 2019. [Google Scholar]
- Beaty, J.H.; Kasser, J.R. Rockwood and Wilkins’ Fracture in Children, 6th ed.; Lippincott Williams & Wilkins (LWW): Philadelphia, PA, USA, 2006. [Google Scholar]
- Patel, A.; Li, L.; Anand, A. Systematic Review: Functional Outcomes and Complications of Intramedullary Nailing versus Plate Fixation for Both-Bone Diaphyseal Forearm Fractures in Children. Injury 2014, 45, 1135–1143. [Google Scholar] [CrossRef]
- Sinikumpu, J.-J.; Pokka, T.; Serlo, W. The Changing Pattern of Pediatric Both-Bone Forearm Shaft Fractures among 86,000 Children from 1997 to 2009. Eur. J. Pediatr. Surg. 2013, 23, 289–296. [Google Scholar] [CrossRef]
- Kapila, R.; Sharma, R.; Chugh, A.; Goyal, M. Evaluation of Clinical Outcomes of Management of Paediatric Bone Forearm Fractures Using Titanium Elastic Nailing System: A Prospective Study of 50 Cases. J. Clin. Diagn. Res. 2016, 10, RC12–RC15. [Google Scholar] [CrossRef] [PubMed]
- Lascombes, P.; Haumont, T.; Journeau, P. Use and Abuse of Flexible Intramedullary Nailing in Children and Adolescents. J. Pediatr. Orthop. 2006, 26, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Schmittenbecher, P.P. State-of-the-Art Treatment of Forearm Shaft Fractures. Injury 2005, 36 (Suppl. S1), S25–S34. [Google Scholar] [CrossRef]
- Sinikumpu, J.-J.; Serlo, W. The Shaft Fractures of the Radius and Ulna in Children: Current Concepts. J. Pediatr. Orthop. B 2015, 24, 200–206. [Google Scholar] [CrossRef]
- Allen, J.D.; Murr, K.; Albitar, F.; Jacobs, C.; Moghadamian, E.S.; Muchow, R. Titanium Elastic Nailing Has Superior Value to Plate Fixation of Midshaft Femur Fractures in Children 5 to 11 Years. J. Pediatr. Orthop. 2018, 38, e111–e117. [Google Scholar] [CrossRef]
- Berger, P.; De Graaf, J.S.; Leemans, R. The Use of Elastic Intramedullary Nailing in the Stabilisation of Paediatric Fractures. Injury 2005, 36, 1217–1220. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium Dioxide Nanoparticles: A Review of Current Toxicological Data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, F. Correlation between Peripheric Blood Markers and Surgical Invasiveness during Humeral Shaft Fracture Osteosynthesis in Young and Middle-Aged Patients. Diagnostics 2024, 14, 1112. [Google Scholar] [CrossRef]
- IARC. Surgical Implants and Other Foreign Bodies; IARC: Lyon, France, 1999; ISBN 978-92-832-1274-4. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data Using Principal Component Analysis and Heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Price, C.T.; Langford, J.R.; Liporace, F.A. Essential Nutrients for Bone Health and a Review of Their Availability in the Average North American Diet. Open Orthop. J. 2012, 6, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, S.M.; Wilkinson, J.M.; Ferner, R.E. Systemic Toxicity Related to Metal Hip Prostheses. Clin. Toxicol. 2014, 52, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Badhe, R.V.; Akinfosile, O.; Bijukumar, D.; Barba, M.; Mathew, M.T. Systemic Toxicity Eliciting Metal Ion Levels from Metallic Implants and Orthopedic Devices—A Mini Review. Toxicol. Lett. 2021, 350, 213–224. [Google Scholar] [CrossRef]
- Karpouzos, A.; Diamantis, E.; Farmaki, P.; Savvanis, S.; Troupis, T. Nutritional Aspects of Bone Health and Fracture Healing. J. Osteoporos. 2017, 2017, 4218472. [Google Scholar] [CrossRef]
- Zhong, Q.; Pan, X.; Chen, Y.; Lian, Q.; Gao, J.; Xu, Y.; Wang, J.; Shi, Z.; Cheng, H. Prosthetic Metals: Release, Metabolism and Toxicity. IJN 2024, 19, 5245–5267. [Google Scholar] [CrossRef]
- Swiatkowska, I.; Martin, N.G.; Henckel, J.; Apthorp, H.; Hamshere, J.; Hart, A.J. Blood and Plasma Titanium Levels Associated with Well-Functioning Hip Implants. J. Trace Elem. Med. Biol. 2020, 57, 9–17. [Google Scholar] [CrossRef]
- Danielewicz, A.; Wójciak, M.; Sawicki, J.; Dresler, S.; Sowa, I.; Latalski, M. Comparison of Different Surgical Systems for Treatment of Early-Onset Scoliosis in the Context of Release of Titanium Ions. Spine 2021, 46, E594–E601. [Google Scholar] [CrossRef]
- Tamagawa, S.; Nojiri, H.; Sato, T.; Matsukawa, T.; Homma, Y.; Takano, H.; Ishijima, M. Comparison of Blood Metal Ion Levels in Scoliosis Patients Following Instrumented Spinal Fusion with Cobalt-Chromium and Titanium Alloy Rods. Sci. Rep. 2025, 15, 16549. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M.; Szwerc, W.; Strzemski, M.; Wichłacz, Z.; Sawicki, J.; Kocjan, R.; Latalski, M.; Sowa, I. Optimization of High-Resolution Continuum Source Graphite Furnace Atomic Absorption Spectrometry for Direct Analysis of Selected Trace Elements in Whole Blood Samples. Talanta 2017, 165, 351–356. [Google Scholar] [CrossRef]
- Li, Y.; Graham, C.K.; Robbins, C.; Caird, M.S.; Farley, F.A. Elevated Serum Titanium Levels in Children with Early Onset Scoliosis Treated With Growth-Friendly Instrumentation. J. Pediatr. Orthop. 2020, 40, e420–e423. [Google Scholar] [CrossRef] [PubMed]
- Witzleb, W.-C.; Ziegler, J.; Krummenauer, F.; Neumeister, V.; Guenther, K.-P. Exposure to Chromium, Cobalt and Molybdenum from Metal-on-Metal Total Hip Replacement and Hip Resurfacing Arthroplasty. Acta Orthop. 2006, 77, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Luetzner, J.; Krummenauer, F.; Lengel, A.M.; Ziegler, J.; Witzleb, W.-C. Serum Metal Ion Exposure after Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 2007, 461, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Reiner, T.; Sorbi, R.; Müller, M.; Nees, T.; Kretzer, J.P.; Rickert, M.; Moradi, B. Blood Metal Ion Release After Primary Total Knee Arthroplasty: A Prospective Study. Orthop. Surg. 2020, 12, 396–403. [Google Scholar] [CrossRef]
- Reiner, T.; Bader, N.; Panzram, B.; Bülhoff, M.; Omlor, G.; Kretzer, J.P.; Raiss, P.; Zeifang, F. In Vivo Blood Metal Ion Levels in Patients after Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2019, 28, 539–546. [Google Scholar] [CrossRef]
- Hanawa, T. Metal Ion Release from Metal Implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Savarino, L.; Stea, S.; Granchi, D.; Visentin, M.; Ciapetti, G.; Donati, M.E.; Rollo, G.; Zinghi, G.; Pizzoferrato, A.; Montanaro, L.; et al. Sister Chromatid Exchanges and Ion Release in Patients Wearing Fracture Fixation Devices. J. Biomed. Mater. Res. 2000, 50, 21–26. [Google Scholar] [CrossRef]
- Cundy, P.J.; Antoniou, G.; Freeman, B.J.C.; Cundy, W.J. Persistently Raised Serum Titanium Levels After Spinal Instrumentation in Children. Spine 2022, 47, 1241–1247. [Google Scholar] [CrossRef]
- Cundy, T.P.; Antoniou, G.; Sutherland, L.M.; Freeman, B.J.C.; Cundy, P.J. Serum Titanium, Niobium, and Aluminum Levels After Instrumented Spinal Arthrodesis in Children. Spine 2013, 38, 564–570. [Google Scholar] [CrossRef]
- Alberghina, F.; McManus, R.; Keogh, C.; Turner, H.; Moore, D.; Noël, J.; Kennedy, J.; Kiely, P. The Evaluation of Serum Metal Ion Levels and Metallosis in Graduated Patients with Magnetically Controlled Growing Rods. J. Pediatr. Orthop. 2024, 44, 43–48. [Google Scholar] [CrossRef]
- Mathew, S.E.; Xie, Y.; Bagheri, L.; Claton, L.E.; Chu, L.; Badreldin, A.; Abdel, M.P.; van Wijnen, A.J.; Haft, G.F.; Milbrandt, T.A.; et al. Are Serum Ion Levels Elevated in Pediatric Patients with Metal Implants? J. Pediatr. Orthop. 2022, 42, 162. [Google Scholar] [CrossRef]
- McGarry, S.; Morgan, S.J.; Grosskreuz, R.M.; Williams, A.E.; Smith, W.R. Serum Titanium Levels in Individuals Undergoing Intramedullary Femoral Nailing with a Titanium Implant. J. Trauma Acute Care Surg. 2008, 64, 430–433. [Google Scholar] [CrossRef]
- Cundy, W.J.; Mascarenhas, A.R.; Antoniou, G.; Freeman, B.J.C.; Cundy, P.J. Local and Systemic Metal Ion Release Occurs Intraoperatively during Correction and Instrumented Spinal Fusion for Scoliosis. J. Child. Orthop. 2015, 9, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Tanoğlu, O.; Say, F.; Yücens, M.; Alemdaroğlu, K.B.; İltar, S.; Aydoğan, N.H. Titanium Alloy Intramedullary Nails and Plates Affect Serum Metal Ion Levels within the Fracture Healing Period. Biol. Trace Elem. Res. 2020, 196, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Patton, M.S.; Lyon, T.D.B.; Ashcroft, G.P. Levels of Systemic Metal Ions in Patients with Intramedullary Nails. Acta Orthop. 2008, 79, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Sansone, V.; Pagani, D.; Melato, M. The Effects on Bone Cells of Metal Ions Released from Orthopaedic Implants. A Review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef]
- Jiang, W.; Wan, Y.; Cui, P.; Ning, X. Intermediate-Term Trends in Serum Levels of Metal Ions after Hip Resurfacing Arthroplasty. J. Orthop. Surg. Res. 2015, 10, 188. [Google Scholar] [CrossRef]
- Sargeant, A.; Goswami, T. Hip Implants—Paper VI—Ion Concentrations. Mater. Des. 2007, 28, 155–171. [Google Scholar] [CrossRef]
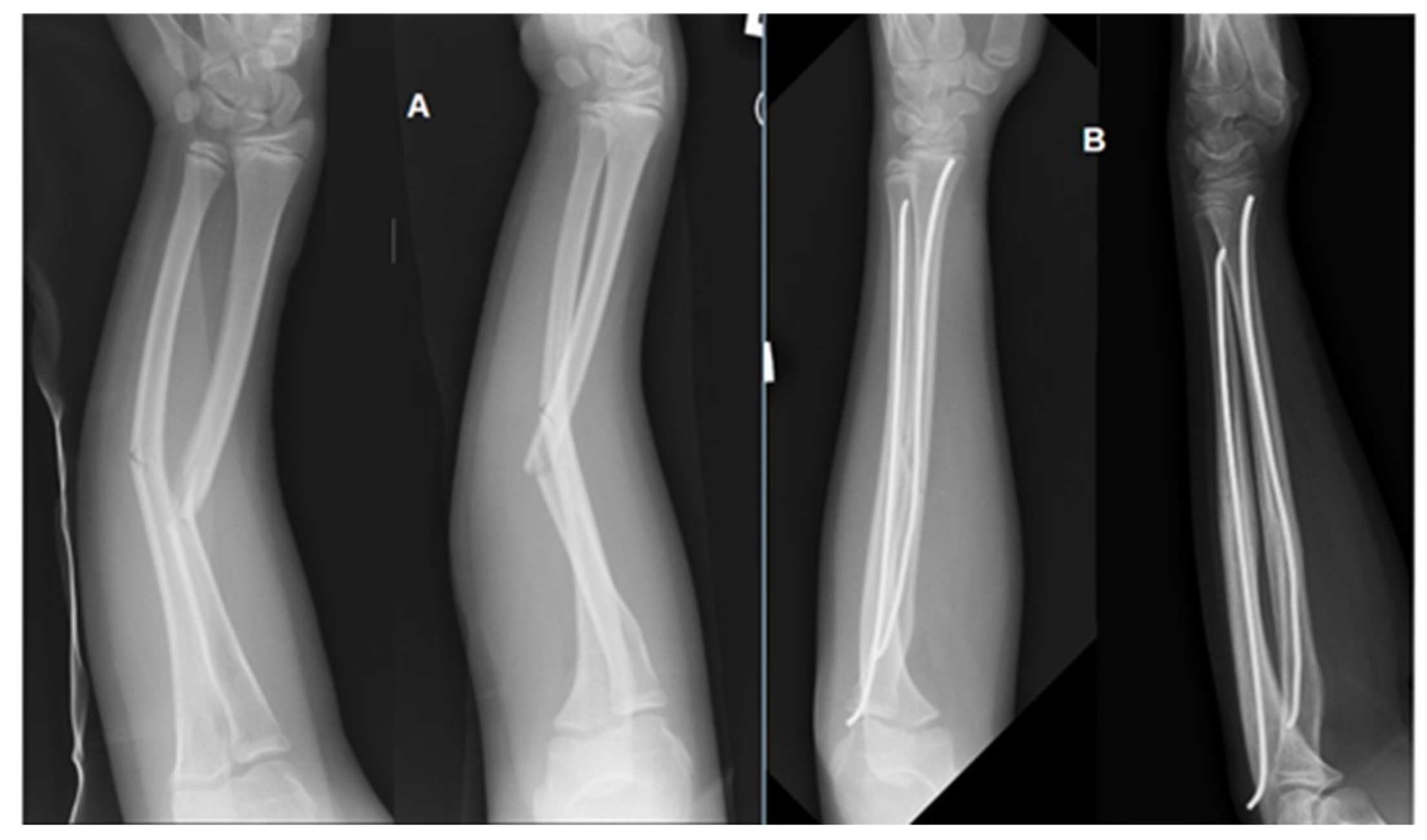
| Characteristic | Total (n = 40) | Girls (n = 14) | Boys (n = 26) |
|---|---|---|---|
| Sex, n (%) | 14 (35%) | 26 (65%) | |
| Age at first surgery, years (mean ± SD) | 9.7 ± 3.1 | ||
| Distribution of fracture types among patients | |||
| Ulna fracture only | 4 | 1 | 3 |
| Radius fracture only | 8 | 2 | 6 |
| Both bones | 28 | 11 | 17 |
| Element | Stabilized Ulna | Stabilized Radius | Blood | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | p-Value | Before | After | p-Value | Before | After | p-Value | |
| Ca | 0.067 ± 0.001 | 0.071 ± 0.018 | 0.282 | 0.077 ± 0.003 | 0.069 ± 0.009 | 0.056 | 0.068 ± 0.012 | 0.067 ± 0.009 | 0.713 |
| Cu | 0.866 ± 0.026 | 0.831 ± 0.030 | 0.434 | 0.856 ± 0.144 | 0.830 ± 0.121 | 0.395 | 0.892 ± 0.166 | 0.852 ± 0.140 | 0.198 |
| Mg | 0.032 ± 0.003 | 0.032 ± 0.004 | 0.972 | 0.031 ± 0.004 | 0.032 ± 0.003 | 0.223 | 0.033 ± 0.004 | 0.032 ± 0.003 | 0.332 |
| Zn | 4.476 ± 0.734 | 4.135 ± 0.570 | 0.049 | 4.026 ± 0.786 | 4.187 ± 0.803 | 0.422 | 4.408 ± 0.748 | 4.335 ± 0.667 | 0.621 |
| Ti | 0.089 ± 0.041 | 0.091 ± 0.035 | 0.764 | 0.079 ± 0.023 | 0.087 ± 0.027 | 0.170 | 0.079 ± 0.028 | 0.097 ± 0.487 | 0.006 |
| Al | 2.354 ± 1.235 | 2.466 ± 0.198 | 0.709 | 2.242 ± 1.223 | 2.327 ± 1.088 | 0.732 | 2.099 ± 1.220 | 2.402 ± 1.218 | 0.278 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowa, K.; Danielewicz, A.; Wójciak, M.; Sawicki, J.; Dresler, S.; Warda, K.; Latalski, M.; Sowa, I. Impact of Intramedullary Implants on Metallic Element Homeostasis in Children with Forearm Fractures. J. Clin. Med. 2025, 14, 7829. https://doi.org/10.3390/jcm14217829
Sowa K, Danielewicz A, Wójciak M, Sawicki J, Dresler S, Warda K, Latalski M, Sowa I. Impact of Intramedullary Implants on Metallic Element Homeostasis in Children with Forearm Fractures. Journal of Clinical Medicine. 2025; 14(21):7829. https://doi.org/10.3390/jcm14217829
Chicago/Turabian StyleSowa, Kacper, Anna Danielewicz, Magdalena Wójciak, Jan Sawicki, Sławomir Dresler, Katarzyna Warda, Michał Latalski, and Ireneusz Sowa. 2025. "Impact of Intramedullary Implants on Metallic Element Homeostasis in Children with Forearm Fractures" Journal of Clinical Medicine 14, no. 21: 7829. https://doi.org/10.3390/jcm14217829
APA StyleSowa, K., Danielewicz, A., Wójciak, M., Sawicki, J., Dresler, S., Warda, K., Latalski, M., & Sowa, I. (2025). Impact of Intramedullary Implants on Metallic Element Homeostasis in Children with Forearm Fractures. Journal of Clinical Medicine, 14(21), 7829. https://doi.org/10.3390/jcm14217829







