Evaluation of the Safety and Efficacy of Transcatheter Closure of Perimembranous Ventricular Septal Defects with a Single Device Type: A Single-Centre Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
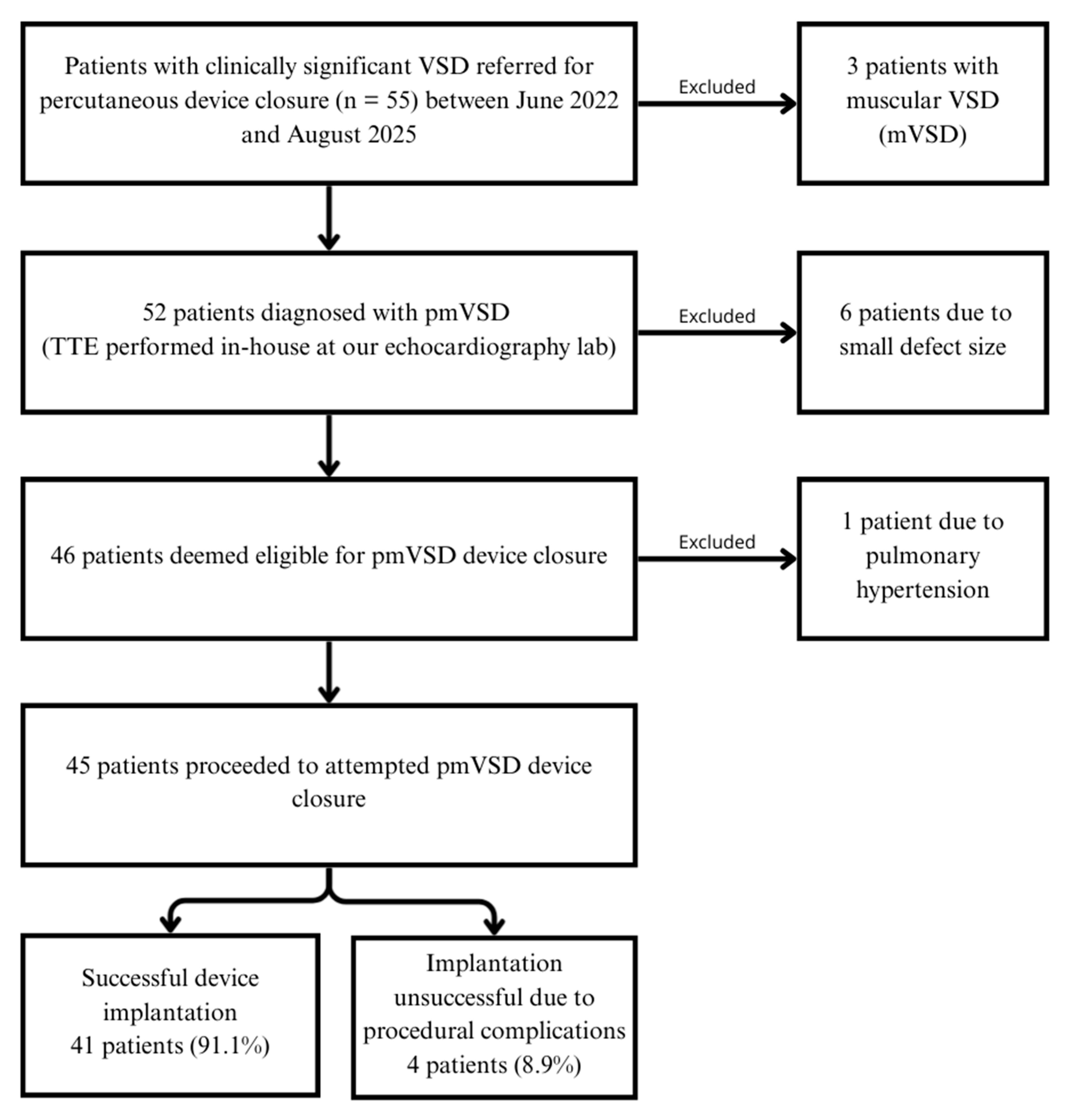
2.2. Patient Selection
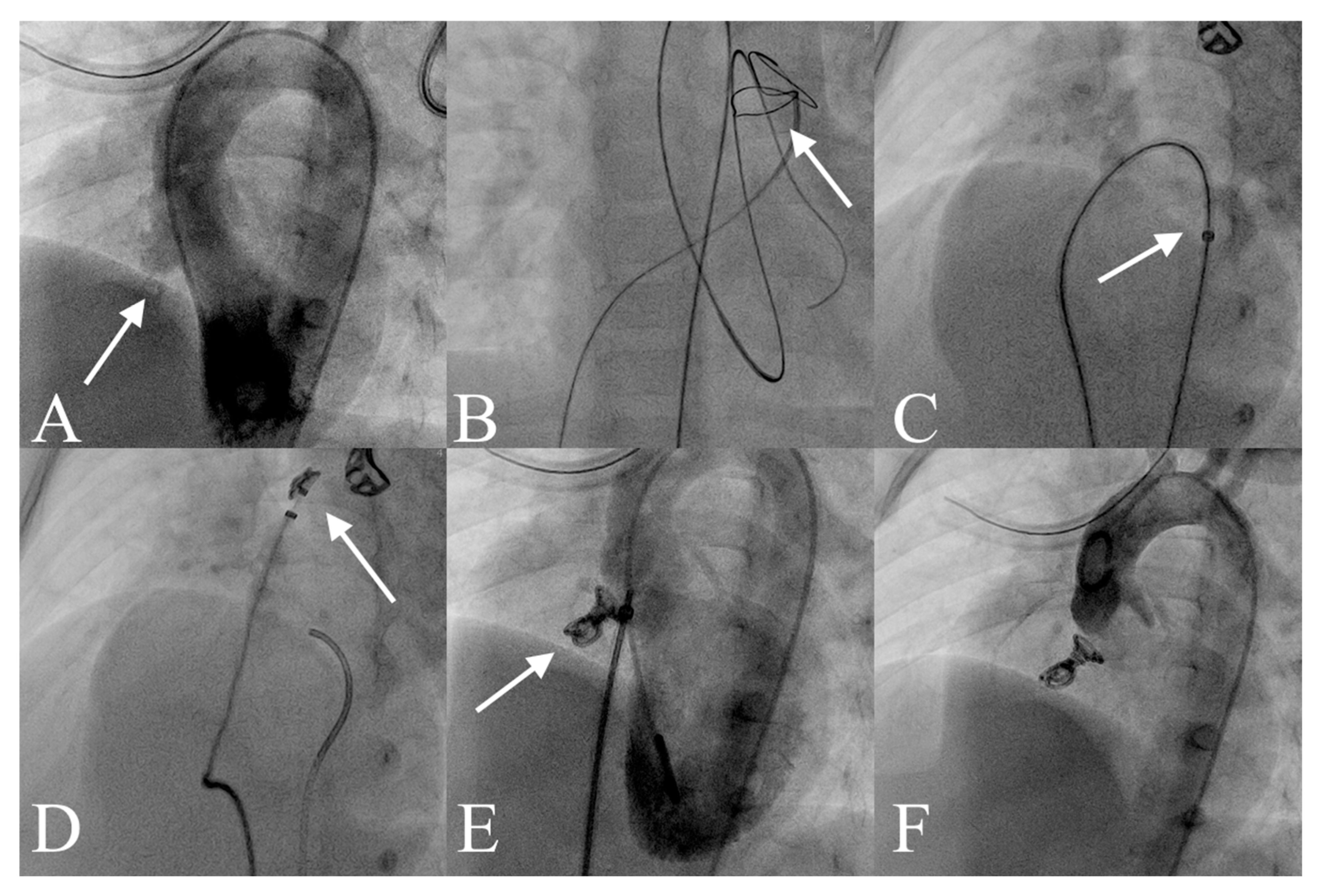
2.3. VSD Closure Protocol
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penny, D.J.; Vick, G.W., 3rd. Ventricular septal defect. Lancet 2011, 377, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.E.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Lopez, L.; Houyel, L.; Colan, S.D.; Anderson, R.H.; Béland, M.J.; Aiello, V.D.; Bailliard, F.; Cohen, M.S.; Jacobs, J.P.; Kurosawa, H.; et al. Classification of Ventricular Septal Defects for the Eleventh Iteration of the International Classification of Diseases-Striving for Consensus: A Report From the International Society for Nomenclature of Paediatric and Congenital Heart Disease. Ann. Thorac. Surg. 2018, 106, 1578–1589. [Google Scholar] [CrossRef]
- Scully, B.B.; Morales, D.L.S.; Zafar, F.; McKenzie, E.D.; Fraser, C.D.; Heinle, J.S. Current expectations for surgical repair of isolated ventricular septal defects. Ann. Thorac. Surg. 2010, 89, 544–549. [Google Scholar] [CrossRef]
- Lillehei, C.W.; Cohen, M.; Warden, H.E.; Ziegler, N.R.; Varco, R.L. The results of direct vision closure of ventricular septal defects in eight patients by means of controlled cross circulation. Surg. Gynecol. Obstet. 1955, 101, 446–466. [Google Scholar]
- Carminati, M.; Butera, G.; Chessa, M.; De Giovanni, J.; Fisher, G.; Gewillig, M.; Peuster, M.; Piechaud, J.F.; Santoro, G.; Sievert, H.; et al. Transcatheter closure of congenital ventricular septal defects: Results of the European Registry. Eur. Heart J. 2007, 28, 2361–2368. [Google Scholar] [CrossRef]
- Butera, G.; Carminati, M.; Chessa, M.; Piazza, L.; Micheletti, A.; Negura, D.G.; Abella, R.; Giamberti, A.; Frigiola, A. Transcatheter closure of perimembranous ventricular septal defects: Early and long-term results. J. Am. Coll. Cardiol. 2007, 50, 1189–1195. [Google Scholar] [CrossRef]
- Collins, N.J.; Benson, L.; Horlick, E. Late complete heart block in an adult patient undergoing percutaneous ventricular septal defect closure. J. Invasive Cardiol. 2008, 20, E200-E203. [Google Scholar]
- Yang, J.; Yang, L.; Yu, S.; Liu, J.; Zuo, J.; Chen, W.; Duan, W.; Zheng, Q.; Xu, X.; Li, J.; et al. Transcatheter versus surgical closure of perimembranous ventricular septal defects in children: A randomized controlled trial. J. Am. Coll. Cardiol. 2014, 63, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, K. Echocardiography for interventions in congenital heart diseases: Left to right shunt lesions. In Textbook of Echocardiography, 1st ed.; Amuthan, V., Parashar, S., Eds.; Jaypee Publishers: New Delhi, India, 2018; pp. 474–503. [Google Scholar]
- Haas, N.A.; Kock, L.; Bertram, H.; Boekenkamp, R.; De Wolf, D.; Ditkivskyy, I.; Freund, M.W.; Gewillig, M.; Happel, C.M.; Herberg, U.; et al. Interventional VSD-Closure with the Nit-Occlud® Le VSD-Coil in 110 Patients: Early and midterm results of the EUREVECO-Registry. Pediatr. Cardiol. 2017, 38, 215–227. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Phan, Q.T.; Dinh, L.H.; Tran, H.B.; Won, H.; Thottian, J.J.; Duc, D.D.; Quang, T.N.; Kim, S.W. Nit-Occlud Le VSD versus duct occluders for percutaneous perimembranous ventricular septal defect closure. Congenit. Heart Dis. 2018, 13, 584–593. [Google Scholar] [CrossRef]
- Mohammed, M.H.A.; Tamimi, O.; Al-Mutairi, M.; Alomrani, A. Outcome of ventricular septal defect closure with the Nit-Occlud® Le VSD-Coil: Single centre experience. Sudan J. Paediatr. 2022, 22, 172–178. [Google Scholar] [CrossRef]
- Ghaderian, M.; Shahsanaei, F.; Behdad, S.; Mozafari, S. Long-term Consequences of Ventricular Septal Defect Closure Using Nit-Occlud Le VSD Coil Device: A Systematic Review and Meta-Analysis. Heart Views 2022, 23, 93–99. [Google Scholar] [CrossRef]
- Houeijeh, A.; Godart, F.; Jalal, Z.; Ovaert, C.; Heitz, F.; Mauran, P.; Baruteau, A.E.; Guirguis, L.; Hadeed, K.; Baudelet, J.B.; et al. Transcatheter closure of a perimembranous ventricular septal defect with Nit-Occlud Lê VSD Coil: A French multicentre study. Arch. Cardiovasc. Dis. 2020, 113, 104–112. [Google Scholar] [CrossRef]
- Pettersen, M.D.; Du, W.; Skeens, M.E.; Humes, R.A. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: An echocardiographic study. J. Am. Soc. Echocardiogr. 2008, 21, 922–934. [Google Scholar] [CrossRef]
- Singhi, A.K.; Sivakumar, K. Echocardiographic Classification of Perimembranous Ventricular Septal Defect Guides Selection of the Occluder Design for Their Transcatheter Device Closure. J. Cardiovasc. Imaging 2021, 29, 316–326. [Google Scholar] [CrossRef]
- Le, T.P. Closing VSDs-PFM coil. In Percutaneous Interventions for Congenital Heart Disease; Sievert, H., Qureshi, S., Wilson, N., Hijazi, Z., Eds.; Informa Healthcare: London, UK, 2007; pp. 357–362. [Google Scholar]
- Chin, C.Y.; Chen, C.A.; Fu, C.M.; Hsu, J.Y.; Lin, H.C.; Chiu, S.-N.; Chang, Y.-M.; Lu, C.-W.; Chou, H.-W.; Huang, S.-C.; et al. Risk Factors of Long-Term Sequelae After Transcatheter Closure of Perimembranous Ventricular Septal Defect in Young Children. Circ. J. 2024, 88, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Gangopadhyay, D.; Goyal, N.; Murthy, S.; Nandi, D.; Bandyopadhyay, B.; Dutta, J. Transcatheter closure of ventricular septal defects in children less than 10 kg: Experience from a tertiary care referral hospital in Eastern India. Cardiol. Young 2022, 32, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, K.; Narin, N.; Ozdemir, R.; Oksuz, S.; Demircan, T.; Bagli, S.; Aktas, R.; Kecici, R.N.; Karadeniz, C. Closure of transcatheter ventricular septal defect using LifetechTM Konar-MF Occluder in children weighing less than 10 kilograms: Mid-term results, a tertiary single center experience. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4053–4059. [Google Scholar] [CrossRef] [PubMed]
- Odemis, E.; Saygi, M.; Guzeltas, A.; Tanidir, I.C.; Ergul, Y.; Ozyilmaz, I.; Bakir, I. Transcatheter closure of perimembranous ventricular septal defects using Nit-Occlud® Lê VSD coil: Early and mid-term results. Pediatr. Cardiol. 2014, 35, 817–823. [Google Scholar] [CrossRef]
- Gu, M.B.; Bai, Y.; Zhao, X.X.; Zheng, X.; Li, W.P.; Qin, Y.W. Transcatheter closure of postoperative residual perimembranous ventricular septal defects. Ann. Thorac. Surg. 2009, 88, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Bulut, M.O.; Küçük, M.; Ballı, Ş.; Çelebi, A. Treatment of severe haemolysis following Nit-Occlud Lê VSD coil implantation with Amplatzer Duct Occluder II. Turk Kardiyol. Dern. Ars. 2016, 44, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Promphan, W.; Wongwaitaweewong, K.; Eleena, A.; Roymanee, S.; Jarutach, J.; Buntharikpornpun, R.; Puttharak, S.; Patrakunwiwat, P.; Prachasilchai, P. Long-Term Outcomes Following Transcatheter Closure of Small Perimembranous Ventricular Septal Defects. Pediatr. Cardiol. 2025. [Google Scholar] [CrossRef]
- Shrestha, M.; Promphan, W.; Layangool, T.; Roymanee, S.; Wongwaitaweewong, K.; Prachasilchai, P.; Kirawittaya, T.; Sangtawesin, C.; Pattarakunwiwat, P. Feasibility and 1-year outcome of transcatheter closure of perimembranous ventricular septal defects with different devices. Catheter. Cardiovasc. Interv. 2019, 93, E30–E37. [Google Scholar] [CrossRef]
- Jiang, D.; Han, B.; Zhao, L.; Yi, Y.; Zhang, J.; Fan, Y.; Lv, J.; Wang, J.; Wang, Y. Transcatheter Device Closure of Perimembranous and Intracristal Ventricular Septal Defects in Children: Medium- and Long-Term Results. J. Am. Heart Assoc. 2021, 10, e020417. [Google Scholar] [CrossRef]
- Aksoy, S.; Cam, N.; Guney, M.R.; Gurkan, U.; Oz, D.; Poyraz, E.; Eksik, A.; Agirbasli, M. Myocardial ischemia in severe aortic regurgitation despite angiographically normal coronary arteries. Tohoku J. Exp. Med. 2012, 226, 69–73. [Google Scholar] [CrossRef] [PubMed]
| All Patients | Without Complications | With Complications | p-Value | |
|---|---|---|---|---|
| Age, years, median (IQR) | 2.69 (1.98–4.86) | 2.84 (2.01–4.91) | 2.64 (1.96–3.23) | 0.54 |
| Sex, females | 18 (40) | 14 (37.8) | 4 (50) | 0.69 |
| Weight, kg, median (IQR) | 14.2 (12–18) | 14.6 (12–18) | 13.85 (12.25–16.5) | 0.69 |
| Height, cm, median (IQR) | 92 (88–106) | 92 (88–106) | 92.5 (87.5–100.38) | 0.66 |
| pmVSD left side size, mm, median (IQR) | 7.2 (4.2–9.5) | 7.6 (4.5–9.6) | 5.25 (3.83–9.12) | 0.33 |
| VSD right side size, mm, median (IQR) | 3 (2.5–3.4) | 3 (2.5–3.4) | 3 (2.63–3.35) | 0.96 |
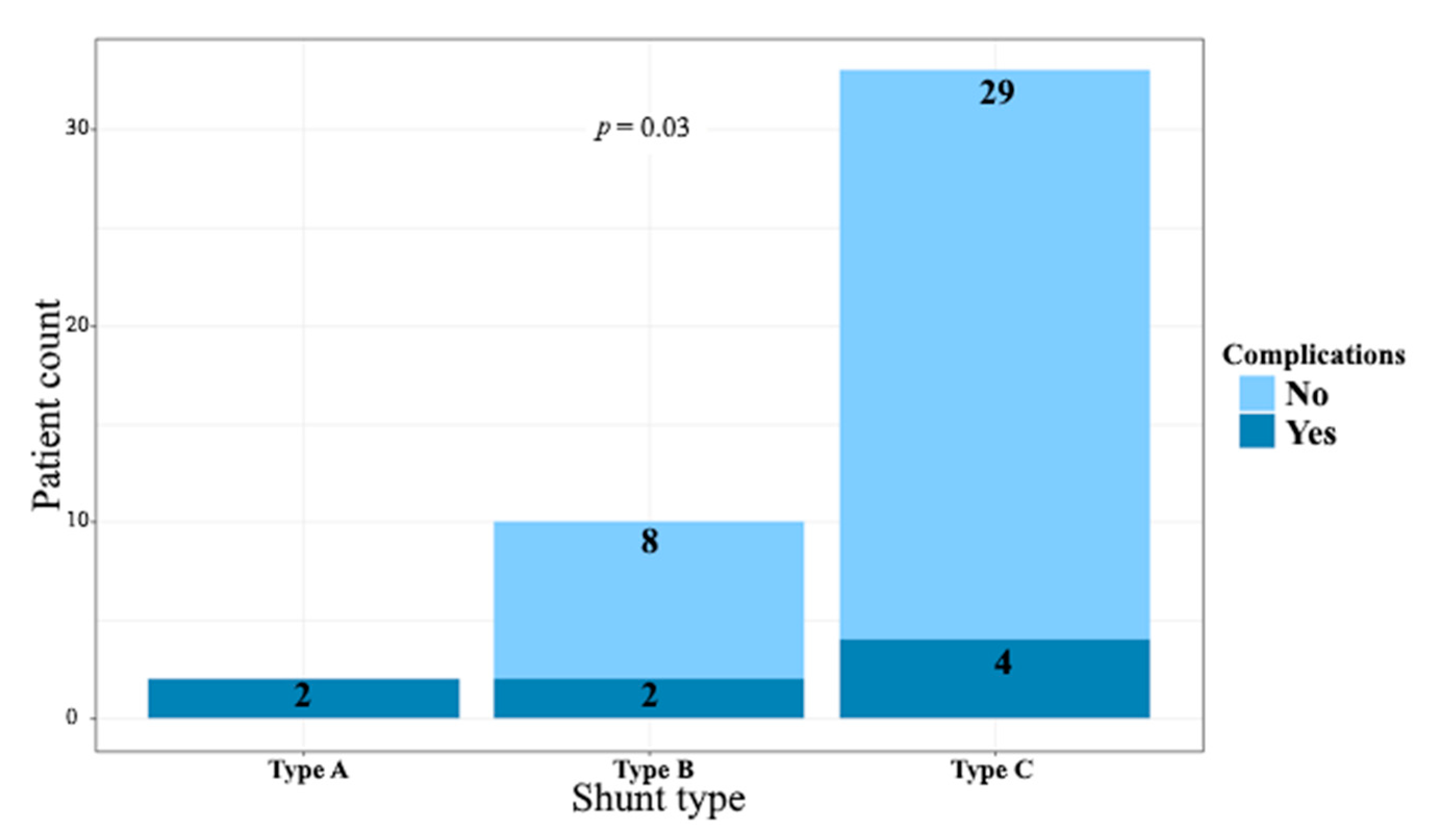
| Shunt type | ||||
| Type A | 2 (4.4) | 0 (0) | 2 (25) | 0.03 |
| Type B | 10 (22.2%) | 8 (21.6%) | 2 (25%) | |
| Type C | 33 (73.3%) | 29 (78.4%) | 4 (50%) | |
| Type D | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Multiple-exit pmVSD | 13 (29.5%) | 10 (27.8%) | 3 (37.5%) | 0.68 |
| Device Size | Successful Coil Implantation (41 Patients) |
|---|---|
| 8/6 | 16 (39) |
| 10/6 | 21 (51.2) |
| 12/6 | 4 (9.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weryński, P.; Trębacz, O.; Tarała, W.; Florek, P.; Podlewski, J.; Sabiniewicz, R. Evaluation of the Safety and Efficacy of Transcatheter Closure of Perimembranous Ventricular Septal Defects with a Single Device Type: A Single-Centre Experience. J. Clin. Med. 2025, 14, 7822. https://doi.org/10.3390/jcm14217822
Weryński P, Trębacz O, Tarała W, Florek P, Podlewski J, Sabiniewicz R. Evaluation of the Safety and Efficacy of Transcatheter Closure of Perimembranous Ventricular Septal Defects with a Single Device Type: A Single-Centre Experience. Journal of Clinical Medicine. 2025; 14(21):7822. https://doi.org/10.3390/jcm14217822
Chicago/Turabian StyleWeryński, Piotr, Oksana Trębacz, Wojciech Tarała, Patrycja Florek, Jacek Podlewski, and Robert Sabiniewicz. 2025. "Evaluation of the Safety and Efficacy of Transcatheter Closure of Perimembranous Ventricular Septal Defects with a Single Device Type: A Single-Centre Experience" Journal of Clinical Medicine 14, no. 21: 7822. https://doi.org/10.3390/jcm14217822
APA StyleWeryński, P., Trębacz, O., Tarała, W., Florek, P., Podlewski, J., & Sabiniewicz, R. (2025). Evaluation of the Safety and Efficacy of Transcatheter Closure of Perimembranous Ventricular Septal Defects with a Single Device Type: A Single-Centre Experience. Journal of Clinical Medicine, 14(21), 7822. https://doi.org/10.3390/jcm14217822






