Tacrolimus–Sirolimus Combined Exposure and Acute Rejection in Kidney Transplant Recipients Undergoing Early Conversion to Sirolimus: A Multicenter Retrospective Cohort Threshold Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Definitions
2.3. Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Characteristics
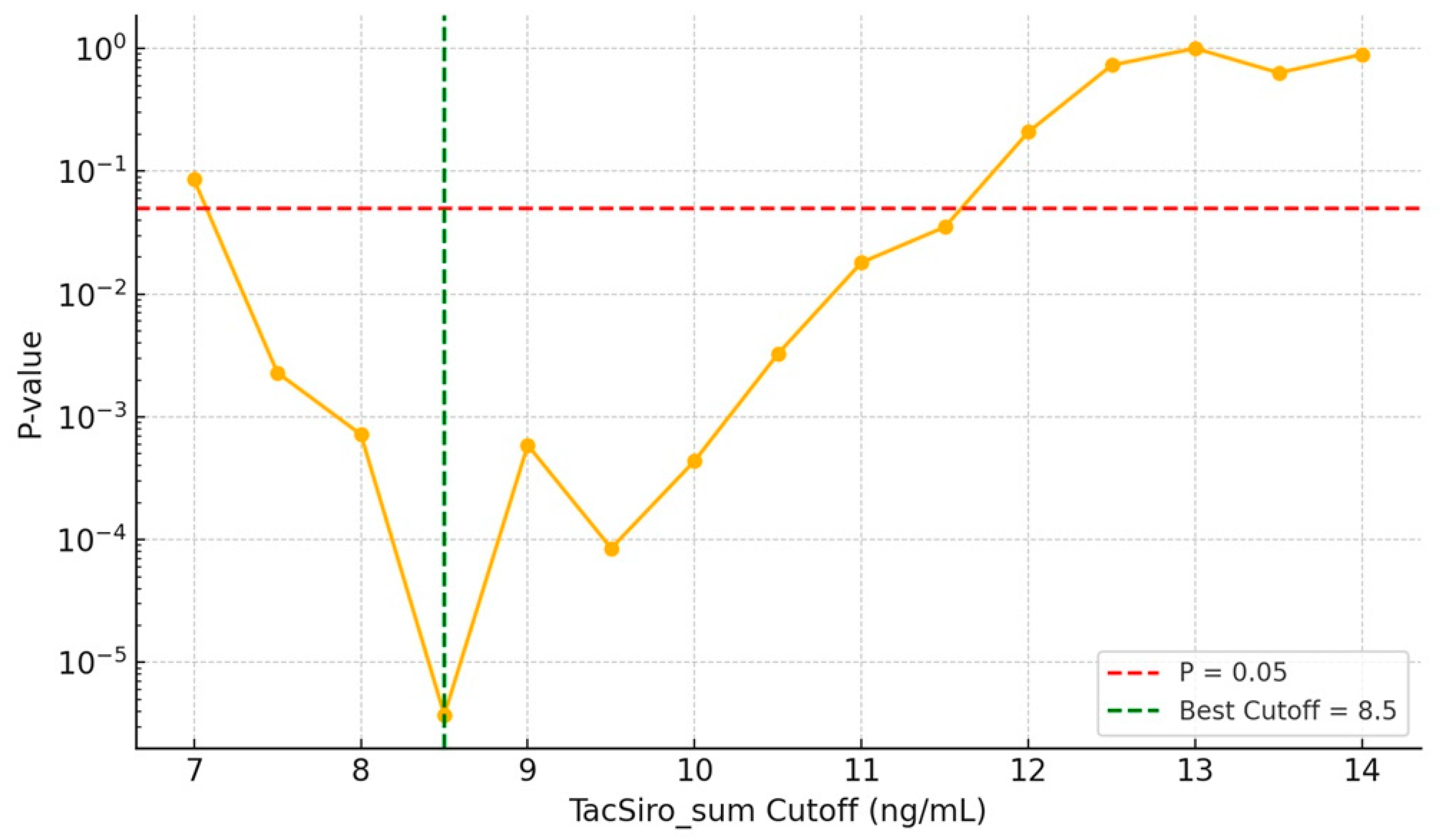
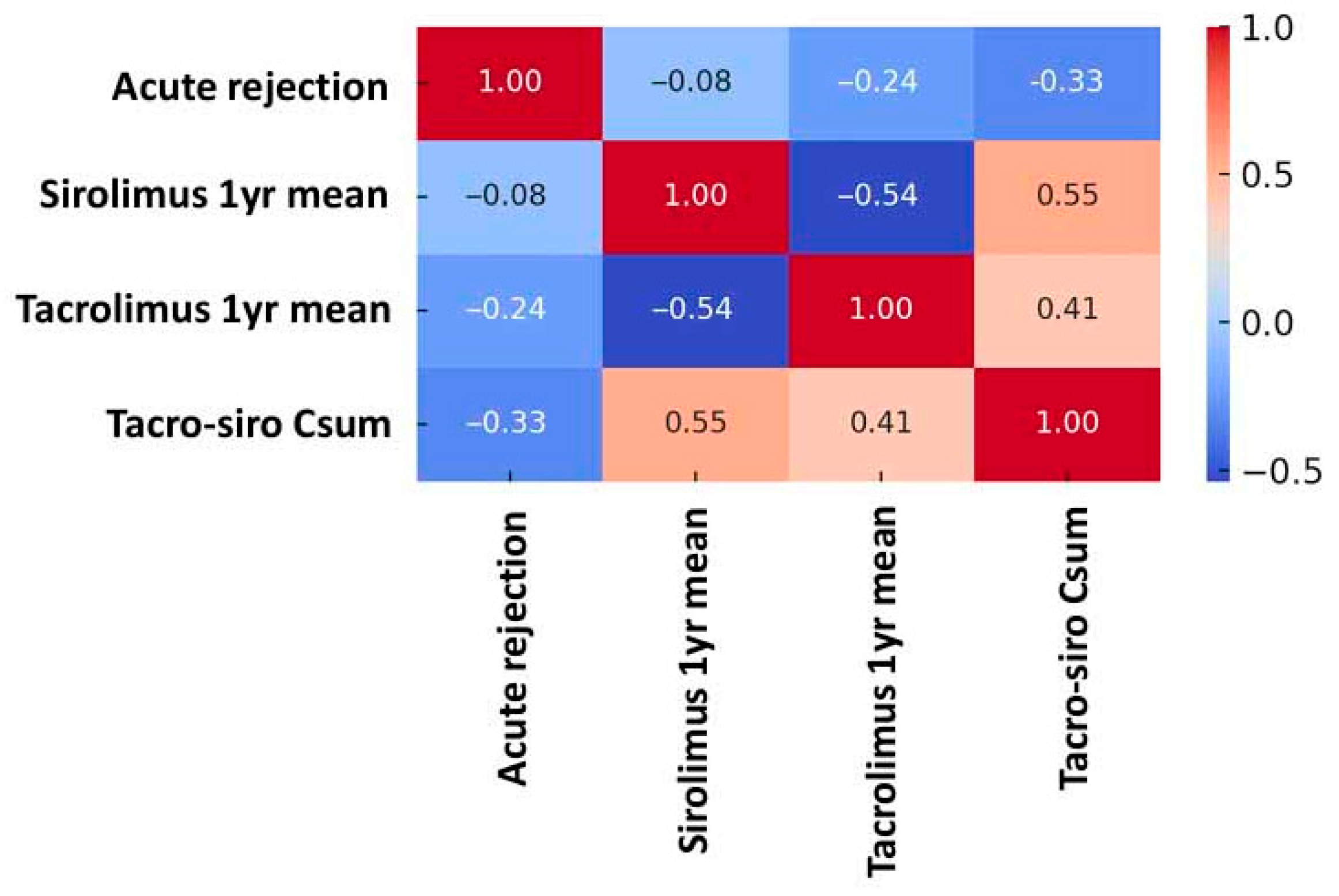
3.2. Risk of Acute Rejection by Combined Drug Exposure
3.3. Renal Function and Infection Outcomes by Treatment Strategy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitko, S.; Klinger, M.; Salmela, K.; Wlodarczyk, Z.; Tyden, G.; Senatorski, G.; Ostrowski, M.; Fauchald, P.; Kokot, F.; Stefoni, S.; et al. Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: Results of the Atlas study. Transplantation 2005, 80, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.S.; Rein, J.L. The Many Faces of Calcineurin Inhibitor Toxicity-What the FK? Adv. Chronic Kidney Dis. 2020, 27, 56–66. [Google Scholar] [CrossRef]
- Budde, K.; Prashar, R.; Haller, H.; Rial, M.C.; Kamar, N.; Agarwal, A.; de Fijter, J.W.; Rostaing, L.; Berger, S.P.; Djamali, A.; et al. Conversion from Calcineurin Inhibitor- to Belatacept-Based Maintenance Immunosuppression in Renal Transplant Recipients: A Randomized Phase 3b Trial. J. Am. Soc. Nephrol. 2021, 32, 3252–3264. [Google Scholar] [CrossRef] [PubMed]
- Karpe, K.M.; Talaulikar, G.S.; Walters, G.D. Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients. Cochrane Database Syst. Rev. 2017, 7, Cd006750. [Google Scholar] [CrossRef]
- Cuadrado-Payán, E.; Diekmann, F.; Cucchiari, D. Medical Aspects of mTOR Inhibition in Kidney Transplantation. Int. J. Mol. Sci. 2022, 23, 7707. [Google Scholar] [CrossRef]
- Shihab, F.; Qazi, Y.; Mulgaonkar, S.; McCague, K.; Patel, D.; Peddi, V.R.; Shaffer, D. Association of Clinical Events with Everolimus Exposure in Kidney Transplant Patients Receiving Low Doses of Tacrolimus. Am. J. Transplant. 2017, 17, 2363–2371. [Google Scholar] [CrossRef]
- Mjörnstedt, L.; Sørensen, S.S.; von zur Mühlen, B.; Jespersen, B.; Hansen, J.M.; Bistrup, C.; Andersson, H.; Gustafsson, B.; Undset, L.H.; Fagertun, H.; et al. Improved Renal Function After Early Conversion from a Calcineurin Inhibitor to Everolimus: A Randomized Trial in Kidney Transplantation. Am. J. Transplant. 2012, 12, 2744–2753. [Google Scholar] [CrossRef]
- Cortazar, F.; Molnar, M.Z.; Isakova, T.; Czira, M.E.; Kovesdy, C.P.; Roth, D.; Mucsi, I.; Wolf, M. Clinical outcomes in kidney transplant recipients receiving long-term therapy with inhibitors of the mammalian target of rapamycin. Am. J. Transplant. 2012, 12, 379–387. [Google Scholar] [CrossRef]
- Manzia, T.M.; Carmellini, M.; Todeschini, P.; Secchi, A.; Sandrini, S.; Minetti, E.; Furian, L.; Spagnoletti, G.; Pisani, F.; Piredda, G.B.; et al. A 3-month, Multicenter, Randomized, Open-label Study to Evaluate the Impact on Wound Healing of the Early (. Delayed) Introduction of Everolimus in De Novo Kidney Transplant Recipients, With a Follow-up Evaluation at 12 Months After Transplant (NEVERWOUND Study). Transplantation 2020, 104, 374–386. [Google Scholar] [CrossRef]
- Ueno, P.; Felipe, C.; Ferreira, A.; Cristelli, M.; Viana, L.; Mansur, J.; Basso, G.; Hannun, P.; Aguiar, W.; Tedesco Silva, H., Jr.; et al. Wound Healing Complications in Kidney Transplant Recipients Receiving Everolimus. Transplantation 2017, 101, 844–850. [Google Scholar] [CrossRef]
- Han, A.; Jo, A.J.; Kwon, H.; Kim, Y.H.; Lee, J.; Huh, K.H.; Lee, K.W.; Park, J.B.; Jang, E.; Park, S.C.; et al. Optimum tacrolimus trough levels for enhanced graft survival and safety in kidney transplantation: A retrospective multicenter real-world evidence study. Int. J. Surg. 2024, 110, 6711–6722. [Google Scholar] [CrossRef]
- Kim, J.M.; Kwon, H.E.; Han, A.; Ko, Y.; Shin, S.; Kim, Y.H.; Lee, K.W.; Park, J.B.; Kwon, H.; Min, S. Risk Prediction and Management of BKPyV-DNAemia in Kidney Transplant Recipients: A Multicenter Analysis of Immunosuppressive Strategies. Transpl. Int. 2025, 38, 14738. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.P.; Sommerer, C.; Witzke, O.; Tedesco, H.; Chadban, S.; Mulgaonkar, S.; Qazi, Y.; de Fijter, J.W.; Oppenheimer, F.; Cruzado, J.M.; et al. Two-year outcomes in de novo renal transplant recipients receiving everolimus-facilitated calcineurin inhibitor reduction regimen from the TRANSFORM study. Am. J. Transplant. 2019, 19, 3018–3034. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.H.; Lee, J.G.; Ha, J.; Oh, C.K.; Ju, M.K.; Kim, C.D.; Cho, H.R.; Jung, C.W.; Lim, B.J.; Kim, Y.S. De novo low-dose sirolimus versus mycophenolate mofetil in combination with extended-release tacrolimus in kidney transplant recipients: A multicentre, open-label, randomized, controlled, non-inferiority trial. Nephrol. Dial. Transplant. 2017, 32, 1415–1424. [Google Scholar] [CrossRef]
- Zou, Z.Y.; Dai, L.R.; Hou, Y.B.; Yu, C.Z.; Chen, R.J.; Chen, Y.Y.; Liu, B.; Shi, H.B.; Gong, N.Q.; Chen, Z.S.; et al. Sirolimus in combination with low-dose extended-release tacrolimus in kidney transplant recipients. Front. Med. 2023, 10, 1281939. [Google Scholar] [CrossRef]
- Fantus, D.; Rogers, N.M.; Grahammer, F.; Huber, T.B.; Thomson, A.W. Roles of mTOR complexes in the kidney: Implications for renal disease and transplantation. Nat. Rev. Nephrol. 2016, 12, 587–609. [Google Scholar] [CrossRef]
- Rangan, G.K. Sirolimus-associated proteinuria and renal dysfunction. Drug Saf. 2006, 29, 1153–1161. [Google Scholar] [CrossRef]
- A, M. Immunosuppressive Agents; American College of Clinical Pharmacy: Lenexa, KS, USA, 2011. [Google Scholar]
- Halloran, P.F. Immunosuppressive drugs for kidney transplantation. N. Engl. J. Med. 2004, 351, 2715–2729. [Google Scholar] [CrossRef]
- Hernández, D.; Hernández, D.; Martínez, D.; Martínez, D.; Gutiérrez, E.; Gutiérrez, E.; López, V.; López, V.; Gutiérrez, C.; Gutiérrez, C.; et al. Clinical evidence on the use of anti-mTOR drugs in renal transplantation. Nefrología 2011, 31, 27–34. [Google Scholar] [CrossRef]
- Mallat, S.G.; Tanios, B.Y.; Itani, H.S.; Lotfi, T.; McMullan, C.; Gabardi, S.; Akl, E.A.; Azzi, J.R. CMV and BKPyV Infections in Renal Transplant Recipients Receiving an mTOR Inhibitor-Based Regimen Versus a CNI-Based Regimen: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. Clin. J. Am. Soc. Nephrol. 2017, 12, 1321–1336. [Google Scholar] [CrossRef]
- Shen, C.L.; Wu, B.S.; Lien, T.J.; Yang, A.H.; Yang, C.Y. BK Polyomavirus Nephropathy in Kidney Transplantation: Balancing Rejection and Infection. Viruses 2021, 13, 487. [Google Scholar] [CrossRef]
- Davis, S.; Gralla, J.; Klem, P.; Tong, S.; Wedermyer, G.; Freed, B.; Wiseman, A.; Cooper, J.E. Lower tacrolimus exposure and time in therapeutic range increase the risk of de novo donor-specific antibodies in the first year of kidney transplantation. Am. J. Transplant. 2018, 18, 907–915. [Google Scholar] [CrossRef]
- Larsson, P.; Englund, B.; Ekberg, J.; Felldin, M.; Broecker, V.; Mjornstedt, L.; Baid-Agrawal, S. Difficult-to-Treat Rejections in Kidney Transplant Recipients: Our Experience with Everolimus-Based Quadruple Maintenance Therapy. J. Clin. Med. 2023, 12, 6667. [Google Scholar] [CrossRef] [PubMed]


| Variables | Total (N = 8027) | Standard a (N = 7791) | Early Conversion b (N = 236) | p-Value |
|---|---|---|---|---|
| Mean age, years | 45.5 ± 13.9 | 45.4 ± 13.9 | 49.2 ± 12.2 | <0.001 |
| Male sex | 4650 (57.9%) | 4510 (57.9%) | 140 (59.3%) | 0.70 |
| Body mass index, kg/m2 | 23.0 ± 28.8 | 23.0 ± 29.2 | 22.7 ± 3.8 | 0.44 |
| Diabetes | 1651 (20.6%) | 1586 (20.4%) | 65 (27.5%) | 0.009 |
| Pre-transplant PRA class I, % | 13.2 ± 25.8 | 13.0 ± 25.7 | 17.5 ± 29.2 | 0.061 |
| Pre-transplant PRA class II, % | 12.2 ± 25.4 | 12.1 ± 25.2 | 15.8 ± 28.1 | 0.1 |
| HLA mismatch | 3.3 ± 1.5 | 3.3 ± 1.5 | 3.4 ± 1.4 | 0.26 |
| Desensitization | 171 (14.6%) | 140 (18.0%) | 31 (13.1%) | 0.54 |
| Donor age, years | 44.5 ± 13.3 | 44.5 ± 13.3 | 45.8 ± 12.1 | 0.11 |
| Preemptive transplantation | 1454 (18.1%) | 1403 (18.0%) | 51 (21.6%) | 0.18 |
| Induction | ||||
| No induction | 273 (3.4%) | 258 (3.3%) | 15 (6.4%) | <0.001 |
| Basiliximab | 6151 (76.6%) | 6001 (77.0%) | 150 (63.6%) | <0.001 |
| Anti-thymocyte globulin | 1601 (20.0%) | 1530 (19.7%) | 71 (30.1%) | <0.001 |
| ABO incompatible | 1184 (14.8%) | 1154 (14.8%) | 30 (12.7%) | 0.42 |
| Pre-transplant B flow, positive | 261 (3.3%) | 250 (3.2%) | 11 (4.7%) | 0.33 |
| Pre-transplant T flow, positive | 212 (2.6%) | 207 (2.7%) | 5 (2.1%) | 0.69 |
| Pre-transplant DSA, positive | 916 (11.4%) | 880 (11.3%) | 36 (15.3%) | 0.08 |
| BK virus at 3 months post-transplant, positive | 733 (9.1%) | 693 (8.9%) | 40 (16.9%) | <0.001 |
| Variables | Total (N = 1180) | Standard Group (N = 944) | Early Conversion (N = 236) | SMD |
|---|---|---|---|---|
| Mean age, years | 48.53 ± 13.68 | 48.38 ± 14.02 | 49.16 ± 12.21 | 0.39 |
| Male sex | 681 (57.7%) | 541 (57.3%) | 140 (59.3%) | 0.04 |
| Body mass index, kg/m2 | 22.51 ± 3.76 | 22.48 ± 3.76 | 22.66 ± 3.76 | 0.04 |
| Diabetes | 297 (25.2%) | 232 (24.6%) | 65 (27.5%) | 0.06 |
| Pre-transplant PRA class I, % | 17.40 ± 29.3 | 17.38 ± 29.3 | 17.47 ± 29.2 | 0.02 |
| Pre-transplant PRA class II, % | 15.18 ± 28.0 | 15.08 ± 28.0 | 15.77 ± 28.1 | 0.02 |
| HLA mismatch | 3.25 ± 1.51 | 3.21 ± 1.52 | 3.38 ± 1.45 | |
| Desensitization | 171 (14.5%) | 140 (20.2%) | 31 (13.1%) | 0.07 |
| Donor age, years | 45.30 ± 12.87 | 45.19 ± 13.07 | 45.78 ± 12.05 | 0.04 |
| Preemptive transplantation | 242 (20.5%) | 191 (20.2%) | 51 (21.6%) | 0.03 |
| Induction | 0.05 | |||
| No induction | 22 (1.9%) | 7 (0.7%) | 15 (6.4%) | - |
| Basiliximab | 850 (72.0%) | 700 (74.2%) | 150 (63.6%) | - |
| Anti-thymocyte globulin | 279 (23.6%) | 209 (22.1%) | 70 (29.7%) | - |
| ABO incompatible | 170 (14.4%) | 140 (14.8%) | 30 (12.7%) | 0.06 |
| Pre-transplant B flow, positive | 80 (6.8%) | 69 (7.3%) | 11 (4.7%) | 0.01 |
| Pre-transplant T flow, positive | 37 (3.1%) | 32 (3.4%) | 5 (2.1%) | 0.06 |
| Pre-transplant DSA, positive | 137 (11.6%) | 108 (11.4%) | 29 (12.3%) | 0.04 |
| BK virus at 3 months post-transplant, positive | 230 (19.5%) | 190 (20.1%) | 40 (16.9%) | 0.06 |
| Rejection at 3–12 months post-transplant | 45 (7.5%) | 27 (2.9%) | 18 (7.6%) | 0.21/ 0.001 a |
| Variables | Total (N = 8027) | Standard (N = 7791) | Early Conversion (N = 236) | p-Value |
|---|---|---|---|---|
| Infection-related admission | 200 (16.9%) | 146 (15.5%) | 54 (22.9%) | 0.009 |
| PCP | 26 (2.2%) | 22 (2.3%) | 4 (1.7%) | 0.729 |
| BK viremia (3–12 months) | 74 (6.3%) | 64 (6.8%) | 10 (4.2%) | 0.155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.; Ko, Y.; Kim, J.-M.; Kwon, H.E.; Kim, Y.H.; Shin, S.; Jung, J.H.; Kwon, H. Tacrolimus–Sirolimus Combined Exposure and Acute Rejection in Kidney Transplant Recipients Undergoing Early Conversion to Sirolimus: A Multicenter Retrospective Cohort Threshold Analysis. J. Clin. Med. 2025, 14, 7808. https://doi.org/10.3390/jcm14217808
Choi B, Ko Y, Kim J-M, Kwon HE, Kim YH, Shin S, Jung JH, Kwon H. Tacrolimus–Sirolimus Combined Exposure and Acute Rejection in Kidney Transplant Recipients Undergoing Early Conversion to Sirolimus: A Multicenter Retrospective Cohort Threshold Analysis. Journal of Clinical Medicine. 2025; 14(21):7808. https://doi.org/10.3390/jcm14217808
Chicago/Turabian StyleChoi, Byunghyun, Youngmin Ko, Jin-Myung Kim, Hye Eun Kwon, Young Hoon Kim, Sung Shin, Joo Hee Jung, and Hyunwook Kwon. 2025. "Tacrolimus–Sirolimus Combined Exposure and Acute Rejection in Kidney Transplant Recipients Undergoing Early Conversion to Sirolimus: A Multicenter Retrospective Cohort Threshold Analysis" Journal of Clinical Medicine 14, no. 21: 7808. https://doi.org/10.3390/jcm14217808
APA StyleChoi, B., Ko, Y., Kim, J.-M., Kwon, H. E., Kim, Y. H., Shin, S., Jung, J. H., & Kwon, H. (2025). Tacrolimus–Sirolimus Combined Exposure and Acute Rejection in Kidney Transplant Recipients Undergoing Early Conversion to Sirolimus: A Multicenter Retrospective Cohort Threshold Analysis. Journal of Clinical Medicine, 14(21), 7808. https://doi.org/10.3390/jcm14217808






