Personalized Approaches to Patients with Intra-Abdominal Infections
Abstract
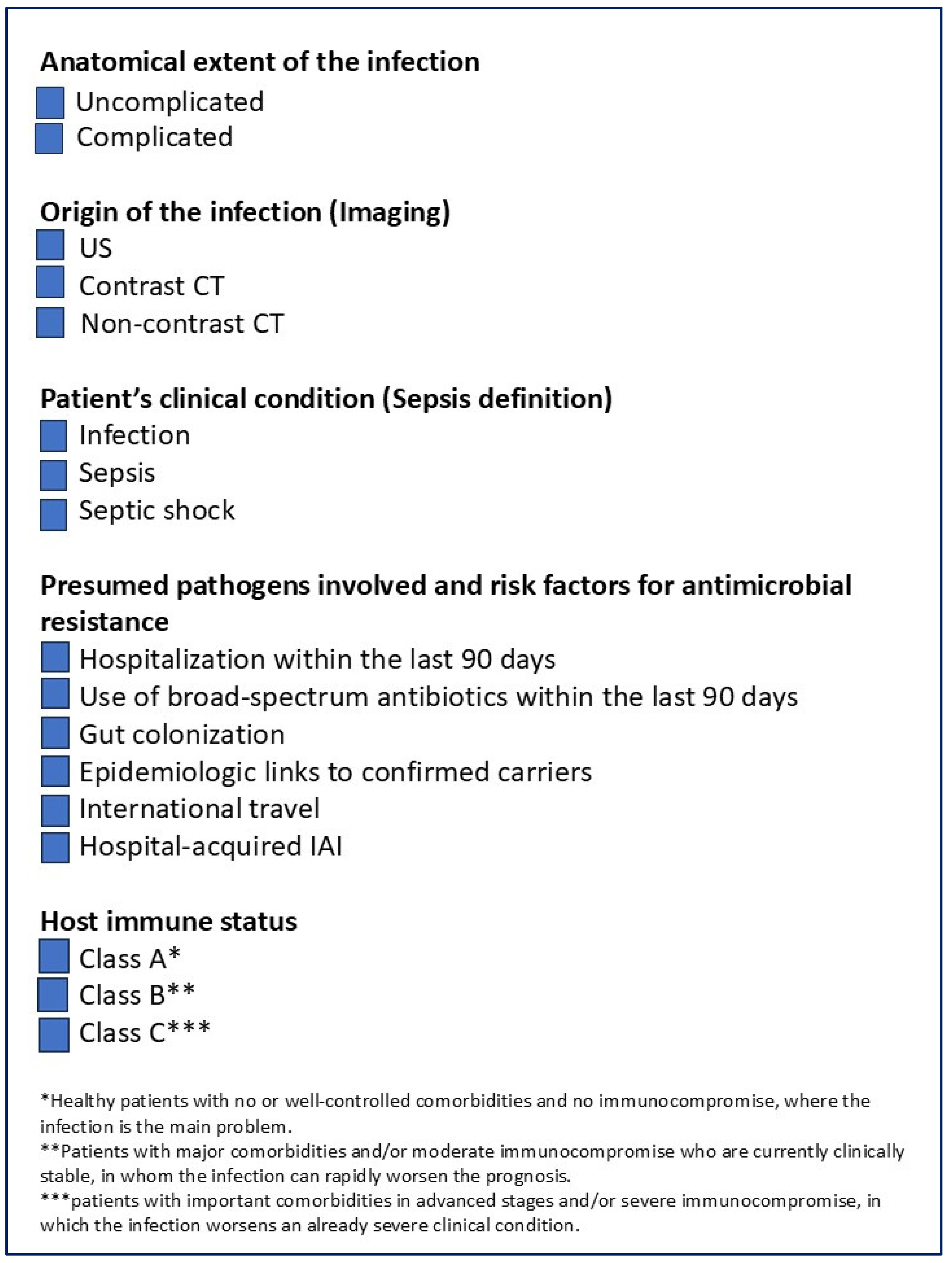
1. Introduction
- the anatomical extent of the infection,
- the origin of the infection,
- the patient’s clinical condition and
- the presumed pathogens involved and risk factors for antimicrobial resistance,
- the host’s immune status.
2. Materials and Methods
3. Factors to Assess for Optimizing Outcomes in Patients with cIAIs
3.1. Origin of Infection
3.2. Anatomical Extent of the Infection
3.3. Clinical Conditions
3.4. Presumed Pathogens Involved and Risk Factors for Antimicrobial Resistance (For the Correct Antimicrobial Selection)
- Multi-drug resistance (MDR): non-susceptibility to at least one agent in three or more antibiotic classes.
- Extensively drug-resistant (XDR): non-susceptibility to all but one or two antibiotic classes.
- Pan-drug resistance (PDR): non-susceptibility to all antibiotics in all classes.
- (1)
- hospitalization within the last 90 days,
- (2)
- use of broad-spectrum antibiotics for 5 days within the last 90 days,
- (3)
- gut colonization by ESBL within 90 days,
- (4)
- patients coming from healthcare settings with a high incidence of MDR bacteria (e.g., elderly people living in long-term facilities)
3.5. Host Immune Status
- Class A includes healthy patients with no or well-controlled comorbidities and no immunocompromise, where the infection is the main problem.
- Class B includes patients with major comorbidities and/or moderate immunocompromise who are currently clinically stable, in whom the infection can rapidly worsen the prognosis.
- Class C includes patients with important comorbidities in advanced stages and/or severe immunocompromise, in which the infection worsens an already severe clinical condition.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| cIAI | Complicated intra-abdominal infection |
| Cmax | Peak plasma concentration |
| CPE | Carbapenemase-producing Enterobacterales |
| CRE | Carbapenem-resistant Enterobacterales |
| CT | Computed tomography |
| ESBL | Extended-spectrum beta-lactamase |
| ESICM | European Society of Intensive Care Medicine |
| ESMID | European Society of Clinical Microbiology and Infectious Diseases |
| fT | Dosing interval |
| IAH | Intra-abdominal hypertension |
| IAI | Intra-abdominal infection |
| IAP | Intra-abdominal pressure |
| ICU | Intensive care unit |
| IDSA | Infectious Diseases Society of America |
| KPC | Klebsiella pneumoniae carbapenemase |
| MAP | Mean arterial pressure |
| MBLs | Metallo-beta-lactamases |
| MDR | Multi-drug resistance |
| MIC | Minimum inhibitory concentration |
| PCT | Procalcitonin |
| PD | Pharmacodynamic |
| PDR | Pan-drug resistance |
| PK | Pharmacokinetic |
| SOFA | Sequential Organ Failure Assessment |
| TDM | Therapeutic drug monitoring |
| US | Ultrasound |
| VRE | Vancomycin-resistant Enterococcus faecium |
| XDR | Extensively drug resistance |
| WISS | WSES complicated IAIs Score Study |
References
- Meng, R.; Guan, X.; Sun, L.; Fei, Z.; Li, Y.; Luo, M.; Ma, A.; Li, H. The efficacy and safety of eravacycline compared with current clinically common antibiotics in the treatment of adults with complicated intra-abdominal infections: A Bayesian network meta-analysis. Front. Med. 2022, 9, 935343. [Google Scholar] [CrossRef]
- Barie, P.S. Outcomes of surgical sepsis. Surg. Infect. 2018, 19, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Abu-Zidan, F.M.; Catena, F.; Griffiths, E.A.; Di Saverio, S.; Coimbra, R.; Ordoñez, C.A.; Leppaniemi, A.; Fraga, G.P.; Coccolini, F.; et al. Global validation of the WSES Sepsis Severity Score for patients with complicated intra-abdominal infections: A prospective multicentre study (WISS Study). World J. Emerg. Surg. 2015, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Sartelli, M.; Kirkpatrick, A.W. What do we mean by source control and what are we trying to accomplish with an open abdomen in severe complicated intra-abdominal sepsis? J. Trauma Acute Care Surg. 2024, 96, e39–e40. [Google Scholar] [CrossRef]
- Schuetz, P.; Beishuizen, A.; Broyles, M.; Ferrer, R.; Gavazzi, G.; Gluck, E.H.; González Del Castillo, J.; Jensen, J.U.; Kanizsai, P.L.; Kwa, A.L.H.; et al. Procalcitonin (PCT)-guided antibiotic stewardship: An international expert consensus on optimized clinical use. Clin. Chem. Lab. Med. 2019, 57, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef]
- Rud, B.; Vejborg, T.S.; Rappeport, E.D.; Reitsma, J.B.; Wille-Jørgensen, P. Computed tomography for diagnosis of acute appendicitis in adults. Cochrane Database Syst. Rev. 2019, 2019, CD009977. [Google Scholar] [CrossRef]
- Shaish, H.; Ream, J.; Huang, C.; Troost, J.; Gaur, S.; Chung, R.; Kim, S.; Patel, H.; Newhouse, J.H.; Khalatbari, S.; et al. Diagnostic Accuracy of Unenhanced Computed Tomography for Evaluation of Acute Abdominal Pain in the Emergency Department. JAMA Surg. 2023, 158, e231112. [Google Scholar] [CrossRef]
- Childs, D.D.; Lalwani, N.; Craven, T.; Arif, H.; Morgan, M.; Anderson, M.; Fulcher, A. A meta-analysis of the performance of ultrasound, hepatobiliary scintigraphy, CT and MRI in the diagnosis of acute cholecystitis. Abdom. Radiol. 2024, 49, 384–398. [Google Scholar] [CrossRef]
- Arruzza, E.; Milanese, S.; Li, L.S.K.; Dizon, J. Diagnostic accuracy of computed tomography and ultrasound for the diagnosis of acute appendicitis: A systematic review and meta-analysis. Radiography 2022, 28, 1127–1141. [Google Scholar] [CrossRef]
- Reitz, K.M.; Kennedy, J.; Li, S.R.; Handzel, R.; Tonetti, D.A.; Neal, M.D.; Zuckerbraun, B.S.; Hall, D.E.; Sperry, J.L.; Angus, D.C.; et al. Association between time to source control in sepsis and 90-day mortality. JAMA Surg. 2022, 157, 817–826. [Google Scholar] [CrossRef]
- Sartelli, M.; Catena, F.; Di Saverio, S.; Ansaloni, L.; Malangoni, M.; Moore, E.E.; Moore, F.A.; Ivatury, R.; Coimbra, R.; Leppaniemi, A.; et al. Current concept of abdominal sepsis: WSES position paper. World J. Emerg. Surg. 2014, 9, 22. [Google Scholar] [CrossRef]
- Sartelli, M.; Coccolini, F.; Kluger, Y.; Agastra, E.; Abu-Zidan, F.M.; Abbas, A.E.S.; Ansaloni, L.; Adesunkanmi, A.K.; Atanasov, B.; Augustin, G.; et al. WSES/GAIS/SIS-E/WSIS/AAST global clinical pathways for patients with intra-abdominal infections. World J. Emerg. Surg. 2021, 16, 49. [Google Scholar] [CrossRef]
- Andersen, B.R.; Kallehave, F.L.; Andersen, H.K. Antibiotics versus placebo for prevention of postoperative infection after appendicectomy. Cochrane Database Syst. Rev. 2005, 3, CD001439. [Google Scholar] [CrossRef]
- Mazeh, H.; Mizrahi, I.; Dior, U.; Simanovsky, N.; Shapiro, M.; Freund, H.R.; Eid, A. Role of antibiotic therapy in mild acute calculus cholecystitis: A prospective randomized controlled trial. World J. Surg. 2012, 36, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Regimbeau, J.M.; Fuks, D.; Pautrat, K.; Mauvais, F.; Haccart, V.; Msika, S.; Mathonnet, M.; Scotté, M.; Paquet, J.C.; Vons, C.; et al. Effect of postoperative antibiotic administration on postoperative infection following cholecystectomy for acute calculous cholecystitis: A randomized clinical trial. JAMA 2014, 312, 145–154. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Leite, R.M.; Seo, D.J.; Gomez-Eslava, B.; Hossain, S.; Lesegretain, A.; de Souza, A.V.; Bay, C.P.; Zilberstein, B.; Marchi, E.; Machado, R.B.; et al. Nonoperative vs. operative management of uncomplicated acute appendicitis: A systematic review and meta-analysis. JAMA Surg. 2022, 157, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, V.; Akl, E.A.; You, J.J.; Agarwal, A.; Shoucair, S.; Vandvik, P.O.; Agoritsas, T.; Heels-Ansdell, D.; Guyatt, G.H.; Tikkinen, K.A. Meta-analysis of antibiotics versus appendicectomy for non-perforated acute appendicitis. Br. J. Surg. 2016, 103, 656–667. [Google Scholar] [CrossRef]
- Salminen, P.; Paajanen, H.; Rautio, T.; Nordström, P.; Aarnio, M.; Rantanen, T.; Tuominen, R.; Hurme, S.; Virtanen, J.; Mecklin, J.P.; et al. Antibiotic therapy vs. appendectomy for treatment of uncomplicated acute appendicitis: The APPAC randomized clinical trial. JAMA 2015, 313, 2340–2348. [Google Scholar] [CrossRef]
- Yeh, D.D.; Vasileiou, G.; Qian, S.; Zhang, H.; Abdul Jawad, K.; Dodgion, C.; Lawless, R.; Rattan, R.; Pust, G.D.; Namias, N.; et al. Appendectomy versus nonoperative management of simple appendicitis: A post hoc analysis of an Eastern Association for the Surgery of Trauma multicenter study using a hierarchical ordinal scale. J. Trauma Acute Care Surg. 2022, 92, 1031–1038. [Google Scholar] [CrossRef]
- Lyu, Y.; Cheng, Y.; Wang, B.; Zhao, S.; Chen, L. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis: An up-to-date meta-analysis of randomized controlled trials. Surg. Endosc. 2018, 32, 4728–4741. [Google Scholar] [CrossRef] [PubMed]
- Chabok, A.; Påhlman, L.; Hjern, F.; Haapaniemi, S.; Smedh, K.; AVOD Study Group. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br. J. Surg. 2012, 99, 532–539. [Google Scholar]
- Mali, J.P.; Mentula, P.J.; Leppäniemi, A.K.; Sallinen, V.J. Symptomatic treatment for uncomplicated acute diverticulitis: A prospective cohort study. Dis. Colon Rectum. 2016, 59, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.; Ünlü, Ç.; de Korte, N.; van Dieren, S.; Stockmann, H.B.; Vrouenraets, B.C.; Consten, E.C.; van der Hoeven, J.A.; Eijsbouts, Q.A.; Faneyte, I.F.; et al. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br. J. Surg. 2017, 104, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Barie, P.S.; Kao, L.S.; Moody, M.; Sawyer, R.G. Infection or inflammation: Are uncomplicated acute appendicitis, acute cholecystitis, and acute diverticulitis infectious diseases? Surg. Infect. 2023, 24, 99–111. [Google Scholar] [CrossRef]
- van Dijk, S.T.; Daniels, L.; Ünlü, Ç.; de Korte, N.; van Dieren, S.; Stockmann, H.B.; Vrouenraets, B.C.; Consten, E.C.; van der Hoeven, J.A.; Eijsbouts, Q.A.; et al. Long-Term Effects of Omitting Antibiotics in Uncomplicated Acute Diverticulitis. Am. J. Gastroenterol. 2018, 113, 1045–1052. [Google Scholar] [CrossRef]
- Ra, J.H.; Rattan, R.; Patel, N.J.; Bhattacharya, B.; Butts, C.A.; Gupta, S.; Asfaw, S.H.; Como, J.J.; Sahr, S.M.; Bugaev, N. Duration of antimicrobial treatment for complicated intra-abdominal infections after definitive source control: A systematic review, meta-analysis, and practice management guideline from the Eastern Association for the Surgery of Trauma. J. Trauma Acute Care Surg. 2023, 95, 603–612. [Google Scholar] [CrossRef]
- Sawyer, R.G.; Claridge, J.A.; Nathens, A.B.; Rotstein, O.D.; Duane, T.M.; Evans, H.L.; Cook, C.H.; O’Neill, P.J.; Mazuski, J.E.; Askari, R.; et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N. Engl. J. Med. 2015, 372, 1996–2005. [Google Scholar] [CrossRef]
- Hassinger, T.E.; Guidry, C.A.; Rotstein, O.D.; Duane, T.M.; Evans, H.L.; Cook, C.H.; O’Neill, P.J.; Mazuski, J.E.; Askari, R.; Napolitano, L.M.; et al. Longer-duration antimicrobial therapy does not prevent treatment failure in high-risk patients with complicated intra-abdominal infections. Surg. Infect. 2017, 18, 659–663. [Google Scholar] [CrossRef]
- de Wijkerslooth, E.M.L.; Boerma, E.G.; van Rossem, C.C.; van Rosmalen, J.; Baeten, C.I.M.; Beverdam, F.H.; Bosmans, J.W.A.M.; Consten, E.C.J.; Dekker, J.W.T.; Emous, M.; et al. 2 days versus 5 days of postoperative antibiotics for complex appendicitis: A pragmatic, open-label, multicentre, non-inferiority randomised trial. Lancet 2023, 401, 366–376. [Google Scholar] [CrossRef]
- Montravers, P.; Tubach, F.; Lescot, T.; Veber, B.; Esposito-Farèse, M.; Seguin, P.; Paugam, C.; Lepape, A.; Meistelman, C.; Cousson, J.; et al. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: The DURAPOP randomised clinical trial. Int. Care Med. 2018, 44, 300–310. [Google Scholar] [CrossRef]
- Nobre, V.; Harbarth, S.; Graf, J.D.; Rohner, P.; Pugin, J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: A randomized trial. Am. J. Respir. Crit. Care Med. 2008, 177, 498–505. [Google Scholar] [CrossRef]
- Oliveira, C.F.; Botoni, F.A.; Oliveira, C.R.; Silva, C.B.; Pereira, H.A.; Serufo, J.C.; Nobre, V. Procalcitonin versus C-reactive protein for guiding antibiotic therapy in sepsis: A randomized trial. Crit. Care Med. 2013, 41, 2336–2343. [Google Scholar] [CrossRef]
- Qu, R.; Ji, Y.; Ling, Y.; Ye, C.Y.; Yang, S.M.; Liu, Y.Y.; Yang, R.Y.; Luo, Y.F.; Guo, Z. Procalcitonin is a good tool to guide duration of antibiotic therapy in patients with severe acute pancreatitis. A randomized prospective single-center controlled trial. Saudi Med. J. 2012, 33, 382–387. [Google Scholar] [PubMed]
- Schroeder, S.; Hochreiter, M.; Koehler, T.; Schweiger, A.M.; Bein, B.; Keck, F.S.; von Spiegel, T. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: Results of a prospective randomized study. Langenbecks Arch. Surg. 2009, 394, 221–226. [Google Scholar] [CrossRef]
- Shehabi, Y.; Sterba, M.; Garrett, P.M.; Rachakonda, K.S.; Stephens, D.; Harrigan, P.; Walker, A.; Bailey, M.J.; Johnson, B.; Millis, D.; et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2014, 190, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.S.; Huang, S.S.; Shyu, Y.C.; Lee, C.H.; Jwo, S.C.; Chen, P.J.; Chen, H.Y. A procalcitonin-based algorithm to guide antibiotic therapy in secondary peritonitis following emergency surgery: A prospective study with propensity score matching analysis. PLoS ONE 2014, 9, e90539. [Google Scholar] [CrossRef]
- Maseda, E.; Suarez-de-la-Rica, A.; Anillo, V.; Tamayo, E.; García-Bernedo, C.A.; Ramasco, F.; Villagran, M.J.; Maggi, G.; Gimenez, M.J.; Aguilar, L.; et al. Procalcitonin-guided therapy may reduce length of AB treatment in intensive care unit patients with secondary peritonitis: A multicenter retrospective study. J. Crit. Care 2015, 30, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Slieker, J.C.; Aellen, S.; Eggimann, P.; Guarnero, V.; Schäfer, M.; Demartines, N. Procalcitonin-Guided Antibiotics after Surgery for Peritonitis: A Randomized Controlled Study. Gastroenterol. Res. Pract. 2017, 2017, 3457614. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (SEPSIS-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Ho, V.P.; Kaafarani, H.; Rattan, R.; Namias, N.; Evans, H.; Zakrison, T.L. Sepsis 2019: What Surgeons Need to Know. Surg. Infect. 2020, 21, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Frutos-Vivar, F.; Ferguson, N.D.; Peñuelas, O.; Lorente, J.A.; Gordo, F.; Honrubia, T.; Algora, A.; Bustos, A.; García, G.; et al. Sepsis incidence and outcome: Contrasting the intensive care unit with the hospital ward. Crit. Care Med. 2007, 35, 1284–1289. [Google Scholar] [CrossRef]
- Rubio, I.; Osuchowski, M.F.; Shankar-Hari, M.; Skirecki, T.; Winkler, M.S.; Lachmann, G.; La Rosée, P.; Monneret, G.; Venet, F.; Bauer, M.; et al. Current gaps in sepsis immunology: New opportunities for translational research. Lancet Infect. Dis. 2019, 19, e422–e436. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock, 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Semler, M.W.; Self, W.H.; Wanderer, J.P.; Ehrenfeld, J.M.; Wang, L.; Byrne, D.W.; Stollings, J.L.; Kumar, A.B.; Hughes, C.G.; Hernandez, A.; et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N. Engl. J. Med. 2018, 378, 829–839. [Google Scholar] [CrossRef]
- Self, W.H.; Semler, M.W.; Wanderer, J.P.; Wang, L.; Byrne, D.W.; Collins, S.P.; Slovis, C.M.; Lindsell, C.J.; Ehrenfeld, J.M.; Siew, E.D.; et al. Balanced Crystalloids versus Saline in Noncritically Ill Adults. N. Engl. J. Med. 2018, 378, 819–828. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Coons, J.C.; Link, C.B.; Schmidhofer, M. Pharmacotherapy update on the use of vasopressors and inotropes in the intensive care unit. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 249–260. [Google Scholar] [CrossRef]
- Weinberger, J.; Rhee, C.; Klompas, M. A critical analysis of the literature on time-to-antibiotics in suspected sepsis. J. Infect. Dis. 2020, 222, S110–S118. [Google Scholar] [CrossRef]
- Nandhabalan, P.; Ioannou, N.; Meadows, C.; Wyncoll, D. Refractory septic shock: Our pragmatic approach. Crit. Care 2018, 22, 215. [Google Scholar] [CrossRef]
- Gordon, A.C.; Mason, A.J.; Thirunavukkarasu, N.; Perkins, G.D.; Cecconi, M.; Cepkova, M.; Pogson, D.G.; Aya, H.D.; Anjum, A.; Frazier, G.J.; et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA 2016, 316, 509–518. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; Van Regenmortel, N.; Saugel, B.; De Tavernier, B.; Van Gaal, P.J.; Joannes-Boyau, O.; Teboul, J.L.; Rice, T.W.; Mythen, M.; Monnet, X. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensive Care 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Antimicrobial delay and outcome in severe sepsis. Crit. Care Med. 2014, 42, e802. [Google Scholar] [CrossRef]
- Hranjec, T.; Rosenberger, L.H.; Swenson, B.; Metzger, R.; Flohr, T.R.; Politano, A.D.; Riccio, L.M.; Popovsky, K.A.; Sawyer, R.G. Aggressive versus conservative initiation of antimicrobial treatment in critically ill surgical patients with suspected intensive-care-unit-acquired infection: A quasi-experimental, before and after observational cohort study. Lancet Infect. Dis. 2012, 12, 774–780. [Google Scholar] [CrossRef]
- Seymour, C.W.; Gesten, F.; Prescott, H.C.; Friedrich, M.E.; Iwashyna, T.J.; Phillips, G.S.; Lemeshow, S.; Osborn, T.; Terry, K.M.; Levy, M.M. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N. Engl. J. Med. 2017, 376, 2235–2244. [Google Scholar] [CrossRef]
- Pea, F.; Viale, P.; Furlanut, M. Antimicrobial therapy in critically ill patients: A review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin. Pharmacokinet. 2005, 44, 1009–1034. [Google Scholar] [CrossRef]
- Yu, Z.; Pang, X.; Wu, X.; Shan, C.; Jiang, S. Clinical outcomes of prolonged infusion (extended infusion or continuous infusion) versus intermittent bolus of meropenem in severe infection: A meta-analysis. PLoS ONE 2018, 13, e0201667. [Google Scholar] [CrossRef]
- Rhodes, N.J.; Liu, J.; O’Donnell, J.N.; Dulhunty, J.M.; Abdul-Aziz, M.H.; Berko, P.Y.; Nadler, B.; Lipman, J.; Roberts, J.A. Prolonged Infusion Piperacillin-Tazobactam Decreases Mortality and Improves Outcomes in Severely Ill Patients: Results of a Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Voulgaris, G.L.; Maliaros, A.; Samonis, G.; Falagas, M.E. Prolonged versus short-term intravenous infusion of antipseudomonal beta-lactams for patients with sepsis: A systematic review and meta-analysis of randomised trials. Lancet Infect. Dis. 2018, 18, 108–120. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Zhou, Q.; Wang, Y.; Chen, L. Clinical outcomes with alternative dosing strategies for piperacillin/tazobactam: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0116769. [Google Scholar] [CrossRef]
- Leon, L.; Guerci, P.; Pape, E.; Thilly, N.; Luc, A.; Germain, A.; Butin-Druoton, A.L.; Losser, M.R.; Birckener, J.; Scala-Bertola, J.; et al. Serum and peritoneal exudate concentrations after high doses of β-lactams in critically ill patients with severe intra-abdominal infections: An observational prospective study. J. Antimicrob. Chemother. 2020, 75, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Pai Mangalore, R.; Peel, T.N.; Udy, A.A.; Peleg, A.Y. The clinical application of beta-lactam antibiotic therapeutic drug monitoring in the critical care setting. J. Antimicrob. Chemother. 2023, 78, 2395–2405. [Google Scholar] [CrossRef]
- Bloos, F.; Thomas-Rüddel, D.; Rüddel, H.; Engel, C.; Schwarzkopf, D.; Marshall, J.C.; Harbarth, S.; Simon, P.; Riessen, R.; Keh, D.; et al. Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: A prospective observational multi-center study. Crit. Care 2014, 18, R42. [Google Scholar] [CrossRef]
- Bloos, F.; Rüddel, H.; Thomas-Rüddel, D.; Schwarzkopf, D.; Pausch, C.; Harbarth, S.; Schreiber, T.; Gründling, M.; Marshall, J.; Simon, P.; et al. Effect of a multifaceted educational intervention for anti-infectious measures on sepsis mortality: A cluster randomized trial. Intensive Care Med. 2017, 43, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Azuhata, T.; Kinoshita, K.; Kawano, D.; Komatsu, T.; Sakurai, A.; Chiba, Y.; Tanjho, K. Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit. Care 2014, 18, R87. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, G.; Antonelli, M.; Deschepper, M.; Arvaniti, K.; Blot, K.; Brown, B.C.; de Lange, D.; De Waele, J.; Dikmen, Y.; Dimopoulos, G.; et al. Poor timing and failure of source control are risk factors for mortality in critically ill patients with secondary peritonitis. Intensive Care Med. 2022, 48, 1593–1606. [Google Scholar] [CrossRef] [PubMed]
- Rüddel, H.; Thomas-Rüddel, D.O.; Reinhart, K.; Bach, F.; Gerlach, H.; Lindner, M.; Marshall, J.C.; Simon, P.; Weiss, M.; Bloos, F.; et al. Adverse effects of delayed antimicrobial treatment and surgical source control in adults with sepsis: Results of a planned secondary analysis of a cluster-randomized controlled trial. Crit. Care 2022, 26, 51. [Google Scholar] [CrossRef]
- Haltmeier, T.; Falke, M.; Quaile, O.; Candinas, D.; Schnüriger, B. Damage-control surgery in patients with nontraumatic abdominal emergencies: A systematic review and meta-analysis. J. Trauma Acute Care Surg. 2022, 92, 1075–1085. [Google Scholar] [CrossRef]
- Weber, D.G.; Bendinelli, C.; Balogh, Z.J. Damage control surgery for abdominal emergencies. Br. J. Surg 2014, 101, e109–e118. [Google Scholar] [CrossRef]
- Kirkpatrick, A.W.; Coccolini, F.; Ansaloni, L.; Roberts, D.J.; Tolonen, M.; McKee, J.L.; Leppaniemi, A.; Faris, P.; Doig, C.J.; Catena, F.; et al. Closed or Open after Laparotomy (COOL) after Source Control for Severe Complicated Intra-Abdominal Sepsis Investigators. Closed or open after source control laparotomy for severe complicated intra-abdominal sepsis (the COOL trial): Study protocol for a randomized controlled trial. World J. Emerg. Surg. 2018, 13, 26. [Google Scholar]
- Blot, S.; De Waele, J.J.; Vogelaers, D. Essentials for selecting antimicrobial therapy for intra-abdominal infections. Drugs 2012, 72, e17–e32. [Google Scholar] [CrossRef]
- Montravers, P.; Grall, N.; Kantor, E.; Augustin, P.; Boussion, K.; Zappella, N. Microbiological profile of patients treated for postoperative peritonitis: Temporal trends 1999–2019. World J. Emerg. Surg. 2023, 18, 58. [Google Scholar] [CrossRef]
- Sartelli, M.; Tascini, C.; Coccolini, F.; Dellai, F.; Ansaloni, L.; Antonelli, M.; Bartoletti, M.; Bassetti, M.; Boncagni, F.; Carlini, M.; et al. Management of intra-abdominal infections: Recommendations by the Italian council for the optimization of antimicrobial use. World J. Emerg. Surg. 2024, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Blot, S.; Antonelli, M.; Arvaniti, K.; Blot, K.; Creagh-Brown, B.; de Lange, D.; De Waele, J.; Deschepper, M.; Dikmen, Y.; Dimopoulos, G.; et al. Epidemiology of intra-abdominal infection and sepsis in critically ill patients: “AbSeS”, a multinational observational cohort study and ESICM Trials Group Project. Intensive Care Med. 2019, 45, 1703–1717. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Giannella, M.; Trecarichi, E.M.; De Rosa, F.G.; Del Bono, V.; Bassetti, M.; Lewis, R.E.; Losito, A.R.; Corcione, S.; Saffioti, C.; Bartoletti, M.; et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: A prospective observational multicentre study. Clin. Microbiol. Infect. 2014, 20, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Weber, D.G.; Ruppé, E.; Bassetti, M.; Wright, B.J.; Ansaloni, L.; Catena, F.; Coccolini, F.; Abu-Zidan, F.M.; Coimbra, R.; et al. Antimicrobials: A global alliance for optimizing their rational use in intra-abdominal infections (AGORA). World J. Emerg. Surg. 2016, 11, 33. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Burns, K.; Rodríguez Baño, J.; Borg, M.; Daikos, G.; Dumpis, U.; Lucet, J.C.; Moro, M.L.; Tacconelli, E.; Simonsen, G.S.; et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: Guidance from the European Centre for Disease Prevention and Control. Antimicrob. Resist. Infect. Control 2017, 6, 113. [Google Scholar] [CrossRef]
- Morrissey, I.; Hackel, M.; Badal, R.; Bouchillon, S.; Hawser, S.; Biedenbach, D. A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals 2013, 6, 1335–1346. [Google Scholar] [CrossRef]
- Cain, S.E.; Kohn, J.; Bookstaver, P.B.; Albrecht, H.; Al-Hasan, M.N. Stratification of the impact of inappropriate empirical antimicrobial therapy for Gram-negative bloodstream infections by predicted prognosis. Antimicrob. Agents Chemother. 2015, 59, 245–250. [Google Scholar] [CrossRef]
- Karaiskos, I.; Giamarellou, H. Carbapenem-sparing strategies for ESBL producers: When and how. Antibiotics 2020, 9, 61. [Google Scholar] [CrossRef]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E. coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA 2018, 320, 984–994. [Google Scholar] [CrossRef]
- Henderson, A.; Paterson, D.L.; Chatfield, M.D.; Tambyah, P.A.; Lye, D.C.; De, P.P.; Lin, R.T.P.; Chew, K.L.; Yin, M.; Lee, T.H.; et al. Association Between Minimum Inhibitory Concentration, Beta-lactamase Genes and Mortality for Patients Treated With Piperacillin/Tazobactam or Meropenem From the MERINO Study. Clin. Infect. Dis. 2021, 73, e3842–e3850. [Google Scholar] [CrossRef]
- Gatti, M.; Viaggi, B.; Rossolini, G.M.; Pea, F.; Viale, P. An Evidence-Based Multidisciplinary Approach Focused at Creating Algorithms for Targeted Therapy of BSIs, cUTIs, and cIAIs Caused by Enterobacterales in Critically Ill Adult Patients. Infect. Drug Resist. 2021, 14, 2461–2498. [Google Scholar] [CrossRef]
- Heizmann, W.R.; Löschmann, P.A.; Eckmann, C.; von Eiff, C.; Bodmann, K.F.; Petrik, C. Clinical efficacy of tigecycline used as monotherapy or in combination regimens for complicated infections with documented involvement of multiresistant bacteria. Infection 2015, 43, 37–43. [Google Scholar] [CrossRef] [PubMed]
- McGovern, P.C.; Wible, M.; El-Tahtawy, A.; Biswas, P.; Meyer, R.D. All-cause mortality imbalance in the tigecycline phase 3 and 4 clinical trials. Int. J. Antimicrob. Agents 2013, 41, 463–467. [Google Scholar] [CrossRef]
- Dijkmans, A.C.; Zacarías, N.V.O.; Burggraaf, J.; Mouton, J.W.; Wilms, E.B.; van Nieuwkoop, C.; Touw, D.J.; Stevens, J.; Kamerling, I.M.C. Fosfomycin: Pharmacological, Clinical and Future Perspectives. Antibiotics 2017, 6, 24. [Google Scholar] [CrossRef]
- Solomkin, J.; Evans, D.; Slepavicius, A.; Lee, P.; Marsh, A.; Tsai, L.; Sutcliffe, J.A.; Horn, P. Assessing the Efficacy and Safety of Eravacycline vs Ertapenem in Complicated Intra-abdominal Infections in the Investigating Gram-Negative Infections Treated With Eravacycline (IGNITE 1) Trial: A Randomized Clinical Trial. JAMA Surg. 2017, 152, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.S.; Gardovskis, J.; Lawrence, K.; Montravers, P.; Sway, A.; Evans, D.; Tsai, L. IGNITE4: Results of a Phase 3, Randomized, Multicenter, Prospective Trial of Eravacycline vs Meropenem in the Treatment of Complicated Intraabdominal Infections. Clin. Infect. Dis. 2019, 69, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, A.L.V.; Gelfand, M.S.; Cleveland, K.O.; Saddler, K.; Sierra-Hoffman, M.A. A retrospective, multicentre evaluation of eravacycline utilisation in community and academic hospitals. J. Glob. Antimicrob. Resist. 2022, 29, 430–433. [Google Scholar] [CrossRef]
- Solomkin, J.; Hershberger, E.; Miller, B.; Popejoy, M.; Friedland, I.; Steenbergen, J.; Yoon, M.; Collins, S.; Yuan, G.; Barie, P.S. Ceftolozane/Tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: Results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin. Infect. Dis. 2015, 60, 1462–1471. [Google Scholar] [CrossRef]
- Mazuski, J.E.; Gasink, L.B.; Armstrong, J.; Broadhurst, H.; Stone, G.G.; Rank, D.; Llorens, L.; Newell, P.; Pachl, J. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: Results from a randomized, controlled, double-blind, phase 3 program. Clin. Infect. Dis. 2016, 62, 1380–1389. [Google Scholar] [CrossRef]
- Marino, A.; Maniaci, A.; Lentini, M.; Ronsivalle, S.; Nunnari, G.; Cocuzza, S.; Parisi, F.M.; Cacopardo, B.; Lavalle, S.; La Via, L. The Global Burden of Multidrug-Resistant Bacteria. Epidemiologia 2025, 5, 21. [Google Scholar]
- Giurazza, R.; Mazza, M.C.; Andini, R.; Sansone, P.; Pace, M.C.; Durante-Mangoni, E. Emerging Treatment Options for Multi-Drug-Resistant Bacterial Infections. Life 2021, 11, 519. [Google Scholar] [CrossRef]
- Motsch, J.; Murta de Oliveira, C.; Stus, V.; Köksal, I.; Lyulko, O.; Boucher, H.W.; Kaye, K.S.; File, T.M.; Brown, M.L.; Khan, I.; et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin. Infect. Dis. 2020, 70, 1799–1808. [Google Scholar] [CrossRef]
- Iregui, A.; Khan, Z.; Landman, D.; Quale, J. Activity of cefiderocol against Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii endemic to medical centers in New York City. Microb. Drug Resist. 2020, 26, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.M.; Tessier, J.M.; Sawyer, R.; Dellinger, E.P.; Miller, P.R.; Namias, N.; West, M.A.; Cook, C.H.; O’Neill, P.J.; Napolitano, L.; et al. Does Isolation of Enterococcus affect outcomes in intra-abdominal infections? Surg. Infect. 2017, 18, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Dupont, H.; Friggeri, A.; Touzeau, J.; Airapetian, N.; Tinturier, F.; Lobjoie, E.; Lorne, E.; Hijazi, M.; Régimbeau, J.M.; Mahjoub, Y. Enterococci increase the morbidity and mortality associated with severe intra-abdominal infections in elderly patients hospitalized in the intensive care unit. J. Antimicrob. Chemother. 2011, 66, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Barie, P.; Agnoletti, V.; Al-Hasan, M.N.; Ansaloni, L.; Biffl, W.; Buonomo, L.; Blot, S.; Cheadle, W.G.; Coimbra, R.; et al. Intra-abdominal infections survival guide: A position statement by the Global Alliance For Infections In Surgery. World J. Emerg. Surg. 2024, 19, 22. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, W.Q.; Chen, W.; Wei, T.; Wang, C.W.; Zhang, J.Y.; Zhang, Y.; Liang, T.B. Systematic Review and Meta-Analysis of the Efficacy of Appropriate Empiric Anti-Enterococcal Therapy for Intra-Abdominal Infection. Surg. Infect. 2021, 22, 131–143. [Google Scholar] [CrossRef]
- Bancke Laverde, B.L.; Maak, M.; Langheinrich, M.; Kersting, S.; Denz, A.; Krautz, C.; Weber, G.F.; Grützmann, R.; Brunner, M. The role of intraoperative swab during appendectomy in patients with uncomplicated and complicated appendicitis. Int. J. Color. Dis. 2023, 38, 272. [Google Scholar] [CrossRef]
- Davies, H.O.; Alkhamesi, N.A.; Dawson, P.M. Peritoneal fluid culture in appendicitis: Review in changing times. Int. J. Surg. 2010, 8, 426–429. [Google Scholar] [CrossRef]
- Bassetti, M.; Rello, J.; Blasi, F.; Goossens, H.; Sotgiu, G.; Tavoschi, L.; Zasowski, E.J.; Arber, M.R.; McCool, R.; Patterson, J.V.; et al. Systematic review of the impact of appropriate versus inappropriate initial antibiotic therapy on outcomes of patients with severe bacterial infections. Int. J. Antimicrob. Agents 2020, 56, 106184. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Vena, A.; Giacobbe, D.R.; Trucchi, C.; Ansaldi, F.; Antonelli, M.; Adamkova, V.; Alicino, C.; Almyroudi, M.P.; Atchade, E.; et al. Risk factors for intra-abdominal candidiasis in intensive care units: Results from EUCANDICU study. Infect. Dis. Ther. 2022, 11, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Lagunes, L.; Rey-Pérez, A.; Martín-Gómez, M.T.; Vena, A.; de Egea, V.; Muñoz, P.; Bouza, E.; Díaz-Martín, A.; Palacios-García, I.; Garnacho-Montero, J.; et al. Association between source control and mortality in 258 patients with intra-abdominal candidiasis: A retrospective multi-centric analysis comparing intensive care versus surgical wards in Spain. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 95–104. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Antonelli, M.; Cuenca-Estrella, M.; Dimopoulos, G.; Einav, S.; De Waele, J.J.; Garnacho-Montero, J.; Kanj, S.S.; Machado, F.R.; Montravers, P.; et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019, 45, 789–805. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Clancy, C.J. Abdominal candidiasis is a hidden reservoir of echinocandin resistance. Antimicrob. Agents Chemother. 2014, 58, 7601–7605. [Google Scholar] [CrossRef]
- Gioia, F.; Gomez-Lopez, A.; Alvarez, M.E.; Gomez-García de la Pedrosa, E.; Martín-Davila, P.; Cuenca-Estrella, M.; Moreno, S.; Fortun, J. Pharmacokinetics of echinocandins in suspected candida peritonitis: A potential risk for resistance. Int. J. Infect. Dis. 2020, 101, 24–28. [Google Scholar] [CrossRef]
- Welte, R.; Oberacher, H.; Gasperetti, T.; Pfisterer, H.; Griesmacher, A.; Santner, T.; Lass-Flörl, C.; Hörtnagl, C.; Leitner-Rupprich, S.; Aigner, M.; et al. Pharmacokinetics and antifungal activity of echinocandins in ascites fluid of critically ill patients. Antimicrob. Agents Chemother. 2021, 65, e0256520. [Google Scholar] [CrossRef]
- Lahmer, T.; Batres Baires, G.; Schmid, R.M.; Wiessner, J.R.; Ulrich, J.; Reichert, M.; Huber, W.; Sörgel, F.; Kinzig, M.; Rasch, S.; et al. Penetration of Isavuconazole in Ascites Fluid of Critically Ill Patients. J. Fungi 2021, 7, 376. [Google Scholar] [CrossRef]
- Maseda, E.; Martín-Loeches, I.; Zaragoza, R.; Pemán, J.; Fortún, J.; Grau, S.; Aguilar, G.; Varela, M.; Borges, M.; Giménez, M.J.; et al. Critical appraisal beyond clinical guidelines for intraabdominal candidiasis. Crit. Care 2023, 27, 382. [Google Scholar] [CrossRef]
- Rinaldi, M.; Bartoletti, M.; Bonazzetti, C.; Caroccia, N.; Gatti, M.; Tazza, B.; Horna, C.S.; Giannella, M.; Viale, P. Tolerability of pulsed high-dose L-AmB as pre-emptive therapy in patients at high risk for intra-abdominal candidiasis: A phase 2 study (LAMBDA study). Int. J. Antimicrob. Agents 2023, 62, 106998. [Google Scholar] [CrossRef]
- Samdani, T.; Pieracci, F.M.; Eachempati, S.R.; Benarroch-Gampel, J.; Weiss, A.; Pietanza, M.C.; Barie, P.S.; Nash, G.M. Colonic diverticulitis in chemotherapy patients: Should operative indications change? A retrospective cohort study. Int. J. Surg. 2014, 12, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Improta, M.; Sartelli, M.; Rasa, K.; Sawyer, R.; Coimbra, R.; Chiarugi, M.; Litvin, A.; Hardcastle, T.; Forfori, F.; et al. Acute abdomen in the immunocompromised patient: WSES, SIS-E, WSIS, AAST, and GAIS guidelines. World J. Emerg. Surg. 2021, 16, 40. [Google Scholar] [CrossRef]
- Mathew, A.; Shatila, M.; Lai, Z. Characteristics of appendicitis after immune checkpoint inhibitor therapy among cancer patients. J. Cancer Res. Clin. Oncol. 2023, 149, 4591–4599. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, D.; Cremonini, C.; Annunziata, E.; Catena, F.; Sartelli, M.; Kirkpatrick, A.W.; Musetti, S.; Strambi, S.; Chiarugi, M.; Coccolini, F.; et al. Acute diverticulitis in immunocompromised patients: Evidence from an international multicenter observational registry (Web-based International Register of Emergency Surgery and Trauma, Wires-T). Tech. Coloproctol. 2023, 27, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.S.; Cannom, R.R.; Abbas, M.A.; Etzioni, D. Diverticulitis in transplant patients and patients on chronic corticosteroid therapy: A systematic review. Dis. Colon Rectum 2010, 53, 1699–1707. [Google Scholar] [CrossRef]
- Oor, J.E.; Atema, J.J.; Boermeester, M.A.; Vrouenraets, B.C.; Ünlü, Ç. A systematic review of complicated diverticulitis in post-transplant patients. J. Gastrointest. Surg. 2014, 18, 2038–2046. [Google Scholar] [CrossRef]
- Coccolini, F.; Sartelli, M.; Sawyer, R.; Rasa, K.; Viaggi, B.; Abu-Zidan, F.; Soreide, K.; Hardcastle, T.; Gupta, D.; Bendinelli, C.; et al. Source control in emergency general surgery: WSES, GAIS, SIS-E., SIS-A guidelines. World J. Emerg. Surg. 2023, 18, 41. [Google Scholar] [CrossRef]
- Pilmis, B.; Weiss, E.; Scemla, A.; Le Monnier, A.; Grossi, P.A.; Slavin, M.A.; Van Delden, C.; Lortholary, O.; Paugam-Burtz, C.; Zahar, J.R. Multidrug-resistant Enterobacterales infections in abdominal solid organ transplantation. Clin. Microbiol. Infect. 2023, 29, 38–43. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartelli, M.; Coccolini, F.; Labricciosa, F.M.; Siquini, W.; Pipitone, G.; Palmieri, M.; Sbacco, V.; Vallicelli, C.; Marmorale, C.; Catena, F. Personalized Approaches to Patients with Intra-Abdominal Infections. J. Clin. Med. 2025, 14, 7774. https://doi.org/10.3390/jcm14217774
Sartelli M, Coccolini F, Labricciosa FM, Siquini W, Pipitone G, Palmieri M, Sbacco V, Vallicelli C, Marmorale C, Catena F. Personalized Approaches to Patients with Intra-Abdominal Infections. Journal of Clinical Medicine. 2025; 14(21):7774. https://doi.org/10.3390/jcm14217774
Chicago/Turabian StyleSartelli, Massimo, Federico Coccolini, Francesco M. Labricciosa, Walter Siquini, Giuseppe Pipitone, Miriam Palmieri, Valentina Sbacco, Carlo Vallicelli, Cristina Marmorale, and Fausto Catena. 2025. "Personalized Approaches to Patients with Intra-Abdominal Infections" Journal of Clinical Medicine 14, no. 21: 7774. https://doi.org/10.3390/jcm14217774
APA StyleSartelli, M., Coccolini, F., Labricciosa, F. M., Siquini, W., Pipitone, G., Palmieri, M., Sbacco, V., Vallicelli, C., Marmorale, C., & Catena, F. (2025). Personalized Approaches to Patients with Intra-Abdominal Infections. Journal of Clinical Medicine, 14(21), 7774. https://doi.org/10.3390/jcm14217774








