Hydrogel-Based Delivery Systems for Non-Opioid Analgesics: Advances, Challenges, and Clinical Prospects
Abstract
1. Introduction
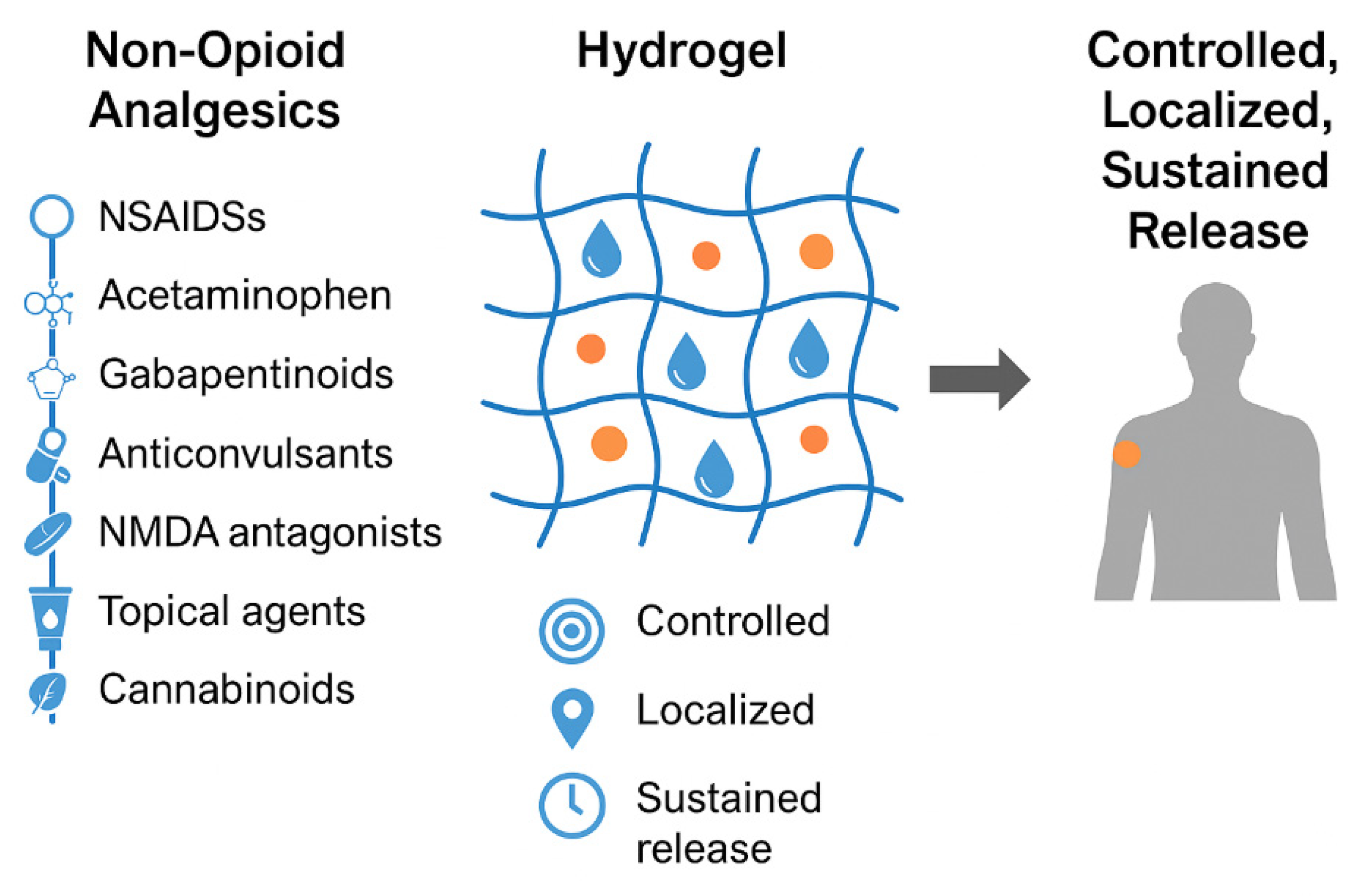
2. Non-Opioid Analgesic Drugs
2.1. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
2.2. Acetaminophen (Paracetamol)
2.3. Gabapentinoids
2.4. Antidepressants
2.5. Anticonvulsants
2.6. NMDA Receptor Antagonists
2.7. Topical Agents
2.8. Cannabinoids
3. Fundamentals and Advanced Concepts in Hydrogel Technologies
3.1. Definition, Basic Principles, and Characteristics of Hydrogels
3.1.1. Definition
3.1.2. Basic Principles
3.2. Key Characteristics
3.2.1. Physicochemical Properties
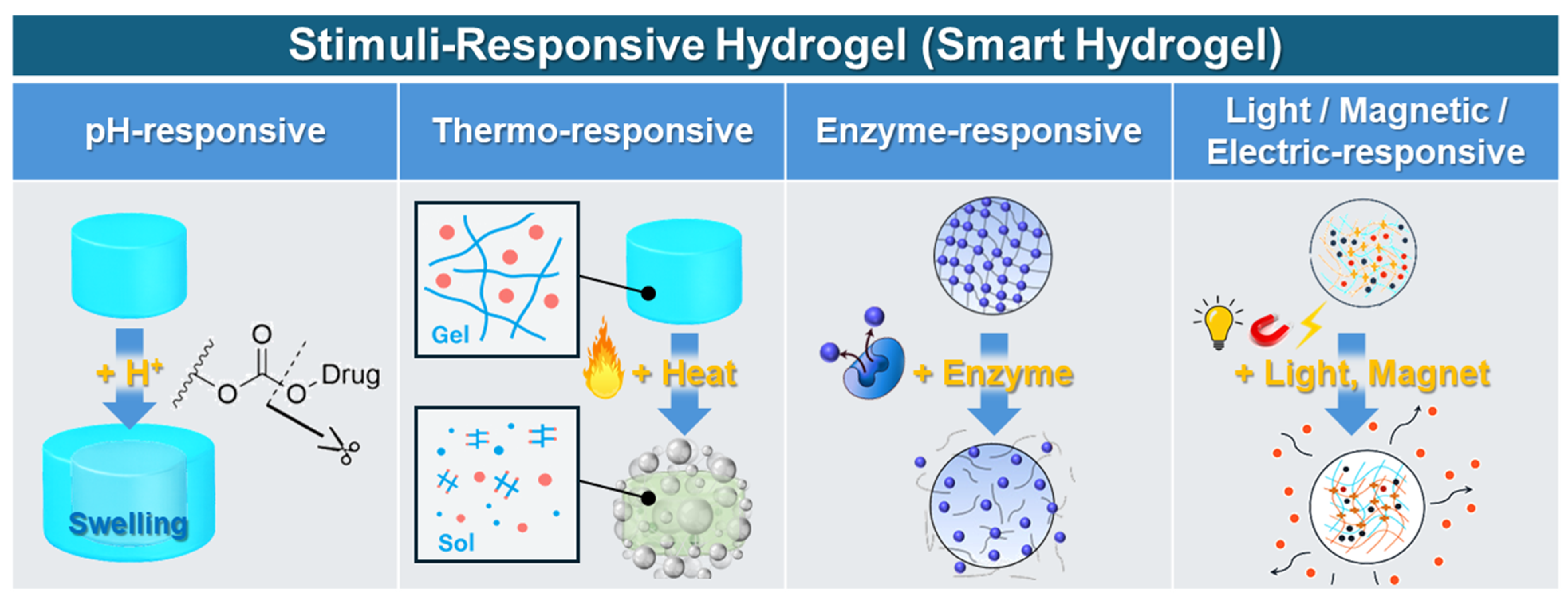
3.2.2. Stimulus-Responsive Behaviors (Smart Hydrogels)
3.3. Types of Hydrogels
3.3.1. Natural Hydrogels
3.3.2. Synthetic Hydrogels
3.3.3. Hybrid Hydrogels
3.4. Advanced Functional Hydrogel Designs
3.4.1. Stimulus-Responsive Hydrogels
3.4.2. Self-Healing Hydrogels
3.4.3. Mechanically Robust Hydrogels
3.4.4. Hybrid and Nanocomposite Hydrogels
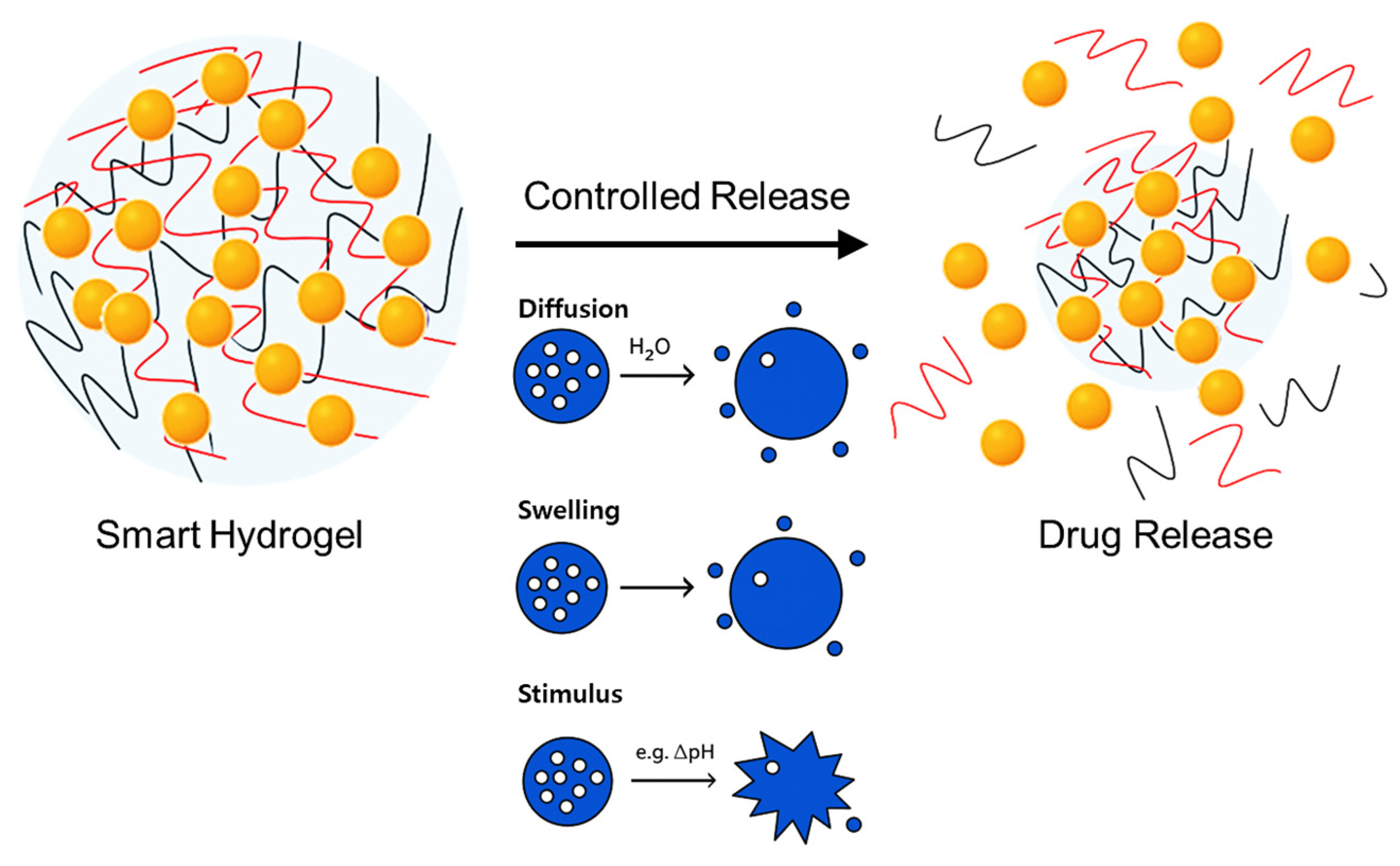
3.5. Drug Encapsulation and Release Mechanisms in Hydrogels
3.5.1. Encapsulation Principles
3.5.2. Drug Release Mechanisms
3.6. Clinical Implications of Hydrogel-Based Sustained Release for Non-Opioid Analgesics
3.6.1. Clinical Benefits of Hydrogel-Based Delivery Systems
3.6.2. Clinical Examples and Recent Advances
3.7. Hydrogel-Based Delivery of Non-Opioid Analgesics
3.7.1. NSAID-Loaded Hydrogels
3.7.2. Gabapentinoid-Loaded Hydrogels
3.7.3. Antidepressant-Loaded Hydrogels
3.7.4. Ketamine-Loaded Hydrogels
3.7.5. Topical Analgesic-Loaded Hydrogels
3.7.6. Cannabinoid-Loaded Hydrogels
4. Challenges and Innovations in Hydrogel Non-Opioid Analgesics Systems
4.1. Optimizing Drug Loading and Sustained Release
4.2. Biocompatibility and Safety Concerns
4.3. Mechanical Stability in Dynamic Environments
4.4. 3D-Printed and Customized Hydrogel Systems
4.5. Translational and Manufacturing Challenges
Regulatory Landscape of FDA-Approved Hydrogel Analgesic Systems
4.6. Sustainability and Ethical Considerations
4.7. Interdisciplinary Collaboration and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Viscusi, E.R.; Epelde, F.; Ruiz, L.J.R.; Trillo-Calvo, E. Present and Future of Pharmacological Management for Acute Moderate-to-Severe Postoperative, Traumatic, or Musculoskeletal Pain in Europe: A Narrative Review. Pain Ther. 2024, 13, 1351–1376. [Google Scholar] [CrossRef]
- Rech, M.A.; Griggs, C.; Lovett, S.; Motov, S. Acute pain management in the Emergency Department: Use of multimodal and non-opioid analgesic treatment strategies. Am. J. Emerg. Med. 2022, 58, 57–65. [Google Scholar] [CrossRef]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Doleman, B.; Mathiesen, O.; Sutton, A.J.; Cooper, N.J.; Lund, J.N.; Williams, J.P. Non-opioid analgesics for the prevention of chronic postsurgical pain: A systematic review and network meta-analysis. Br. J. Anaesth. 2023, 130, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, G.; Jiang, R.; Luo, Y.; Zuo, Y.; Liu, J. Development of Non-opioid Analgesics Targeting Two-pore Domain Potassium Channels. Curr. Neuropharmacol. 2022, 20, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.; Chen, J.; Alzarah, M.; Wallace, M. Non-opioid Analgesics and Emerging Therapies. Cancer Treat. Res. 2021, 182, 125–142. [Google Scholar]
- Zhao, H.; Yang, S.; Wang, H.; Zhang, H.; An, Y. Non-opioid analgesics as adjuvants to opioid for pain management in adult patients in the ICU: A systematic review and meta-analysis. J. Crit. Care 2019, 54, 136–144. [Google Scholar] [CrossRef]
- Martinez, V.; Beloeil, H.; Marret, E.; Fletcher, D.; Ravaud, P.; Trinquart, L. Non-opioid analgesics in adults after major surgery: Systematic review with network meta-analysis of randomized trials. Br. J. Anaesth. 2017, 118, 22–31. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, X.; Xu, H.; Jiao, P.; Zhao, L.X.; Su, G. Nanomaterial-Based Drug Delivery Systems for Pain Treatment and Relief: From the Delivery of a Single Drug to Co-Delivery of Multiple Therapeutics. Pharmaceutics 2023, 15, 2309. [Google Scholar] [CrossRef]
- Kolliopoulos, V.; Mikos, A.G. Decellularized extracellular matrix as a drug delivery carrier. J. Control. Release 2025, 382, 113661. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, W.; Hu, L.; Yi, X.; Tang, F. Application of Hydrogels as Sustained-Release Drug Carriers in Bone Defect Repair. Polymers 2022, 14, 4906. [Google Scholar] [CrossRef]
- Vegad, U.; Patel, M.; Khunt, D.; Zupančič, O.; Chauhan, S.; Paudel, A. pH stimuli-responsive hydrogels from non-cellulosic biopolymers for drug delivery. Front. Bioeng. Biotechnol. 2023, 11, 1270364. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Xin, Y.; Wu, X.; Ao, Q. Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering. Polymers 2022, 14, 2184. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhang, L.; Dou, X.; Bai, R.; Wang, H.; Deng, J.; Zhang, Y.; Sun, Q.; Li, Q.; Wang, X.; et al. Mechanically Robust Hydrogels Facilitating Bone Regeneration through Epigenetic Modulation. Adv. Sci. 2022, 9, e2203734. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Abla, K.K.; Domiati, S.; Elmaradny, H. Superiority of microemulsion-based hydrogel for non-steroidal anti-inflammatory drug transdermal delivery: A comparative safety and anti-nociceptive efficacy study. Int. J. Pharm. 2022, 622, 121830. [Google Scholar] [CrossRef]
- Shahid, M.; Subhan, F.; Ahmad, N.; Sewell, R.D.E. Efficacy of a topical gabapentin gel in a cisplatin paradigm of chemotherapy-induced peripheral neuropathy. BMC Pharmacol. Toxicol. 2019, 20, 51. [Google Scholar] [CrossRef]
- Shahid, M.; Subhan, F.; Ahmad, N.; Ali, G.; Akbar, S.; Fawad, K.; Sewell, R.D. Topical gabapentin gel alleviates allodynia and hyperalgesia in the chronic sciatic nerve constriction injury neuropathic pain model. Eur. J. Pain 2017, 21, 668–680. [Google Scholar] [CrossRef]
- Mazaleuskaya, L.L.; Muzykantov, V.R.; FitzGerald, G.A. Nanotherapeutic-directed approaches to analgesia. Trends Pharmacol. Sci. 2021, 42, 527–550. [Google Scholar] [CrossRef]
- Babaie, S.; Taghvimi, A.; Hong, J.H.; Hamishehkar, H.; An, S.; Kim, K.H. Recent advances in pain management based on nanoparticle technologies. J. Nanobiotechnology 2022, 20, 290. [Google Scholar] [CrossRef]
- Thompson, T.; Whiter, F.; Gallop, K.; Veronese, N.; Solmi, M.; Newton, P.; Stubbs, B. NMDA receptor antagonists and pain relief: A meta-analysis of experimental trials. Neurology 2019, 92, e1652–e1662. [Google Scholar] [CrossRef]
- Hawkey, C.J. COX-1 and COX-2 inhibitors. Best. Pract. Res. Clin. Gastroenterol. 2001, 15, 801–820. [Google Scholar] [CrossRef]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Antiinflamm Antiallergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef]
- Ohashi, N.; Kohno, T. Analgesic Effect of Acetaminophen: A Review of Known and Novel Mechanisms of Action. Front. Pharmacol. 2020, 11, 580289. [Google Scholar] [CrossRef]
- Aminoshariae, A.; Khan, A. Acetaminophen: Old drug, new issues. J. Endod. 2015, 41, 588–593. [Google Scholar] [CrossRef]
- Trinh, L.T.; Lim, S.; Lee, H.J.; Kim, I.T. Development of Efficient Sodium Alginate/Polysuccinimide-Based Hydrogels as Biodegradable Acetaminophen Delivery Systems. Gels 2023, 9, 980. [Google Scholar] [CrossRef] [PubMed]
- Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Ristić, I.; Cakić, S.; Petrović, S.D. Temperature-Sensitive Hydrogels as Carriers for Modulated Delivery of Acetaminophen. Gels 2023, 9, 684. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Dickenson, A.H. Mechanisms of the gabapentinoids and α 2 δ-1 calcium channel subunit in neuropathic pain. Pharmacol. Res. Perspect. 2016, 4, e00205. [Google Scholar] [CrossRef] [PubMed]
- Chincholkar, M. Analgesic mechanisms of gabapentinoids and effects in experimental pain models: A narrative review. Br. J. Anaesth. 2018, 120, 1315–1334. [Google Scholar] [CrossRef]
- Rose, M.A.; Kam, P.C. Gabapentin: Pharmacology and its use in pain management. Anaesthesia 2002, 57, 451–462. [Google Scholar] [CrossRef]
- Meaadi, J.; Obara, I.; Eldabe, S.; Nazar, H. The safety and efficacy of gabapentinoids in the management of neuropathic pain: A systematic review with meta-analysis of randomised controlled trials. Int. J. Clin. Pharm. 2023, 45, 556–565. [Google Scholar] [CrossRef]
- Martin, C.J.; Alcock, N.; Hiom, S.; Birchall, J.C. Development and Evaluation of Topical Gabapentin Formulations. Pharmaceutics 2017, 9, 31. [Google Scholar] [CrossRef]
- Sinha, S.; Gabriel, V.A.; Arora, R.K.; Shin, W.; Scott, J.; Bharadia, S.K.; Verly, M.; Rahmani, W.M.; Nickerson, D.A.; Fraulin, F.O.; et al. Interventions for postburn pruritus. Cochrane Database Syst. Rev. 2024, 6, Cd013468. [Google Scholar] [CrossRef]
- Hannaman, M.R.; Fitts, D.A.; Doss, R.M.; Weinstein, D.E.; Bryant, J.L. The refined biomimetic NeuroDigm GEL™ model of neuropathic pain in a mature rat. F1000Research 2016, 5, 2516. [Google Scholar] [CrossRef]
- McQuay, H.J.; Tramèr, M.; Nye, B.A.; Carroll, D.; Wiffen, P.J.; Moore, R.A. A systematic review of antidepressants in neuropathic pain. Pain 1996, 68, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Saarto, T.; Wiffen, P.J. Antidepressants for neuropathic pain. Cochrane Database Syst. Rev. 2007, 2007, Cd005454. [Google Scholar] [CrossRef] [PubMed]
- Obata, H. Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int. J. Mol. Sci. 2017, 18, 2483. [Google Scholar] [CrossRef] [PubMed]
- Casolaro, M.; Casolaro, I. Polyelectrolyte Hydrogel Platforms for the Delivery of Antidepressant Drugs. Gels 2016, 2, 24. [Google Scholar] [CrossRef]
- Zeng, J.; Su, P.; Li, F.; Yun, Y.; Liang, H.; Qu, K.; Fan, Y.; Zhang, M.; Song, J.; Yao, Y.; et al. An Injectable Hydrogel for Treatment of Chronic Neuropathic Pain. Macromol. Biosci. 2022, 22, e2100529. [Google Scholar] [CrossRef]
- Jensen, T.S. Anticonvulsants in neuropathic pain: Rationale and clinical evidence. Eur. J. Pain 2002, 6, 61–68. [Google Scholar] [CrossRef]
- Tremont-Lukats, I.W.; Megeff, C.; Backonja, M.M. Anticonvulsants for neuropathic pain syndromes: Mechanisms of action and place in therapy. Drugs 2000, 60, 1029–1052. [Google Scholar] [CrossRef]
- Covington, E.C. Anticonvulsants for neuropathic pain and detoxification. Clevel. Clin. J. Med. 1998, 65 (Suppl. S1), SI21–SI29, discussion SI45-27. [Google Scholar] [CrossRef]
- Qu, J.; Xie, K.; Chen, S.; He, X.; Wang, Y.; Chamberlin, M.; Zhao, X.; Zhu, G.; Xu, C.; Shi, P. Multifunctional hydrogel electronics for closed-loop antiepileptic treatment. Sci. Adv. 2024, 10, eadq9207. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Niesters, M.; Martini, C.; Dahan, A. Ketamine for chronic pain: Risks and benefits. Br. J. Clin. Pharmacol. 2014, 77, 357–367. [Google Scholar] [CrossRef]
- Kavalali, E.T.; Monteggia, L.M. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am. J. Psychiatry 2012, 169, 1150–1156. [Google Scholar] [CrossRef]
- Gao, M.; Rejaei, D.; Liu, H. Ketamine use in current clinical practice. Acta Pharmacol. Sin. 2016, 37, 865–872. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, P.; Jiang, Y.; Li, L.; Ju, F.; Cheng, Q.; Zhou, Y.L.; Zhou, Y. Sustained-Release Esketamine Based Nanoparticle-Hydrogel Delivery System for Neuropathic Pain Management. Int. J. Nanomed. 2023, 18, 1131–1143. [Google Scholar] [CrossRef]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef]
- Burks, T.F.; Buck, S.H.; Miller, M.S. Mechanisms of depletion of substance P by capsaicin. Fed. Proc. 1985, 44, 2531–2534. [Google Scholar]
- Anand, P.; Bley, K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Wickenden, A.D.; Chaplan, S.R. Sodium channel blockers for the treatment of neuropathic pain. Neurotherapeutics 2009, 6, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Hohmann, M.S.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef]
- Nair, A.B.; Vaka, S.R.; Gupta, S.; Repka, M.A.; Murthy, S.N. In vitro and in vivo evaluation of a hydrogel-based prototype transdermal patch system of alfuzosin hydrochloride. Pharm. Dev. Technol. 2012, 17, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.G.; Rosenfeld, M.J. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med. J. 2013, 4, e0022. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T. Cannabis for Neuropathic Pain in Multiple Sclerosis-High Expectations, Poor Data. Front. Pharmacol. 2019, 10, 1239. [Google Scholar] [CrossRef]
- Jylkkä, J.; Hupli, A.; Nikolaeva, A.; Alanen, S.; Back, A.E.; Lindqvist, S.; Krabbe, A.; Lavie-Ajayi, M.; Kantonen, O. The holistic effects of medical cannabis compared to opioids on pain experience in Finnish patients with chronic pain. J. Cannabis Res. 2023, 5, 38. [Google Scholar] [CrossRef]
- Bhaskar, A.; Bell, A.; Boivin, M.; Briques, W.; Brown, M.; Clarke, H.; Cyr, C.; Eisenberg, E.; de Oliveira Silva, R.F.; Frohlich, E.; et al. Consensus recommendations on dosing and administration of medical cannabis to treat chronic pain: Results of a modified Delphi process. J. Cannabis Res. 2021, 3, 22. [Google Scholar] [CrossRef]
- Raina, N.; Pahwa, R.; Bhattacharya, J.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M.; Oliveira, S.M.R.; Dolma, K.G.; Rahmatullah, M.; Wilairatana, P.; et al. Drug Delivery Strategies and Biomedical Significance of Hydrogels: Translational Considerations. Pharmaceutics 2022, 14, 574. [Google Scholar] [CrossRef]
- Demisli, S.; Galani, E.; Goulielmaki, M.; Kyrilis, F.L.; Ilić, T.; Hamdi, F.; Crevar, M.; Kastritis, P.L.; Pletsa, V.; Nallet, F.; et al. Encapsulation of cannabidiol in oil-in-water nanoemulsions and nanoemulsion-filled hydrogels: A structure and biological assessment study. J. Colloid Interface Sci. 2023, 634, 300–313. [Google Scholar] [CrossRef]
- Reddy, T.S.; Zomer, R.; Mantri, N. Nanoformulations as a strategy to overcome the delivery limitations of cannabinoids. Phytother. Res. 2023, 37, 1526–1538. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- Podhorská, B.; Chylíková-Krumbholcová, E.; Dvořáková, J.; Šlouf, M.; Kobera, L.; Pop-Georgievski, O.; Frejková, M.; Proks, V.; Janoušková, O.; Filipová, M.; et al. Soft Hydrogels with Double Porosity Modified with RGDS for Tissue Engineering. Macromol. Biosci. 2024, 24, e2300266. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Horkay, F.; Basser, P.J. Hydrogel composite mimics biological tissues. Soft Matter 2022, 18, 4414–4426. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Lee, M.; Bao, Z. Mechanically tunable conductive interpenetrating network hydrogels that mimic the elastic moduli of biological tissue. Nat. Commun. 2018, 9, 2740. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, X.; Liu, J.; Zhang, H.; Fu, D. Advances in the application of natural/synthetic hybrid hydrogels in tissue engineering and delivery systems: A comprehensive review. Int. J. Pharm. 2025, 672, 125323. [Google Scholar] [CrossRef]
- Li, C.S.; Xu, Y.; Li, J.; Qin, S.H.; Huang, S.W.; Chen, X.M.; Luo, Y.; Gao, C.T.; Xiao, J.H. Ultramodern natural and synthetic polymer hydrogel scaffolds for articular cartilage repair and regeneration. Biomed. Eng. Online 2025, 24, 13. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Lavrentev, F.V.; Shilovskikh, V.V.; Alabusheva, V.S.; Yurova, V.Y.; Nikitina, A.A.; Ulasevich, S.A.; Skorb, E.V. Diffusion-Limited Processes in Hydrogels with Chosen Applications from Drug Delivery to Electronic Components. Molecules 2023, 28, 5931. [Google Scholar] [CrossRef] [PubMed]
- DeLong, S.A.; Moon, J.J.; West, J.L. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials 2005, 26, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; di Marco, G.; Iudin, D.; Viola, M.; van Nostrum, C.F.; van Ravensteijn, B.G.P.; Vermonden, T. Stimuli-Responsive Hydrogels: The Dynamic Smart Biomaterials of Tomorrow. Macromolecules 2023, 56, 8377–8392. [Google Scholar] [CrossRef]
- Liu, L.; Yao, W.; Rao, Y.; Lu, X.; Gao, J. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef]
- Tanga, S.; Aucamp, M.; Ramburrun, P. Injectable Thermoresponsive Hydrogels for Cancer Therapy: Challenges and Prospects. Gels 2023, 9, 418. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.; Kan, C.W.; Wang, W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 11658. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Z.; Wang, H.; Song, Z.; Yu, D.; Li, G.; Liu, X.; Liu, W. Progress in Research on Metal Ion Crosslinking Alginate-Based Gels. Gels 2024, 11, 16. [Google Scholar] [CrossRef]
- Savić Gajić, I.M.; Savić, I.M.; Svirčev, Z. Preparation and Characterization of Alginate Hydrogels with High Water-Retaining Capacity. Polymers 2023, 15, 2592. [Google Scholar] [CrossRef]
- Guo, F.; Du, Y.; Wang, Y.; Wang, M.; Wang, L.; Yu, N.; Luo, S.; Wu, F.; Yang, G. Targeted drug delivery systems for matrix metalloproteinase-responsive anoparticles in tumor cells: A review. Int. J. Biol. Macromol. 2024, 257, 128658. [Google Scholar] [CrossRef]
- Cook, A.B.; Palange, A.; Schlich, M.; Bellotti, E.; Brahmachari, S.; di Francesco, M.; Decuzzi, P. Matrix metalloproteinase responsive hydrogel microplates for programmed killing of invasive tumour cells. RSC Appl. Polym. 2023, 1, 19–29. [Google Scholar] [CrossRef]
- Xing, Y.; Zeng, B.; Yang, W. Light responsive hydrogels for controlled drug delivery. Front. Bioeng. Biotechnol. 2022, 10, 1075670. [Google Scholar] [CrossRef] [PubMed]
- Su, R.S.; Galas, R.J., Jr.; Lin, C.Y.; Liu, J.C. Redox-Responsive Resilin-Like Hydrogels for Tissue Engineering and Drug Delivery Applications. Macromol. Biosci. 2019, 19, e1900122. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Neofytou, E.; Cahill, T.J., 3rd; Beygui, R.E.; Zare, R.N. Drug release from electric-field-responsive nanoparticles. ACS Nano 2012, 6, 227–233. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide hydrogels: Functionalization, construction and served as scaffold for tissue engineering. Carbohydr. Polym. 2022, 278, 118952. [Google Scholar] [CrossRef]
- Xiang, Q.; Hao, Y.; Xia, Z.; Liao, M.; Rao, X.; Lao, S.; He, Q.; Ma, C.; Liao, W. Biomedical Applications and Nutritional Value of Specific Food-Derived Polysaccharide-Based Hydrogels. Adv. Nutr. 2024, 15, 100309. [Google Scholar] [CrossRef]
- Neves, M.I.; Araújo, M.; Moroni, L.; da Silva, R.M.P.; Barrias, C.C. Glycosaminoglycan-Inspired Biomaterials for the Development of Bioactive Hydrogel Networks. Molecules 2020, 25, 978. [Google Scholar] [CrossRef]
- Tsanaktsidou, E.; Kammona, O.; Kiparissides, C. Recent Developments in Hyaluronic Acid-Based Hydrogels for Cartilage Tissue Engineering Applications. Polymers 2022, 14, 839. [Google Scholar] [CrossRef]
- Kapusta, O.; Jarosz, A.; Stadnik, K.; Giannakoudakis, D.A.; Barczyński, B.; Barczak, M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. Int. J. Mol. Sci. 2023, 24, 2191. [Google Scholar] [CrossRef]
- Zöller, K.; To, D.; Bernkop-Schnürch, A. Biomedical applications of functional hydrogels: Innovative developments, relevant clinical trials and advanced products. Biomaterials 2025, 312, 122718. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.-L.; Li, J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) That Releases Doxorubicin-Encapsulated Micelles as a Smart Drug Delivery System. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Coyle, R.C.; Richards, D.J.; Berry, C.L.; Barrs, R.W.; Biggs, J.; James Chou, C.; Trusk, T.C.; Mei, Y. Development of peptide-functionalized synthetic hydrogel microarrays for stem cell and tissue engineering applications. Acta Biomater. 2016, 45, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.; Agnelli, S.; Sartore, L. Designing Viscoelastic Gelatin-PEG Macroporous Hybrid Hydrogel with Anisotropic Morphology and Mechanical Properties for Tissue Engineering Application. Micro 2023, 3, 434–457. [Google Scholar] [CrossRef]
- Baishya, G.; Parasar, B.; Limboo, M.; Kumar, R.; Dutta, A.; Hussain, A.; Phukan, M.M.; Saikia, D. Advancements in nanocomposite hydrogels: A comprehensive review of biomedical applications. Discov. Mater. 2024, 4, 40. [Google Scholar] [CrossRef]
- Chen, W.; Ming, Y.; Wang, M.; Huang, M.; Liu, H.; Huang, Y.; Huang, Z.; Qing, L.; Wang, Q.; Jia, B. Nanocomposite Hydrogels in Regenerative Medicine: Applications and Challenges. Macromol. Rapid Commun. 2023, 44, e2300128. [Google Scholar] [CrossRef]
- Cai, M.H.; Chen, X.Y.; Fu, L.Q.; Du, W.L.; Yang, X.; Mou, X.Z.; Hu, P.Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, Y.; Ge, Y.; Raza, F.; Li, S.; Zafar, H.; Wu, Y.; Paiva-Santos, A.C.; Yu, C.; Sun, M.; et al. pH-Sensitive Peptide Hydrogels as a Combination Drug Delivery System for Cancer Treatment. Pharmaceutics 2022, 14, 652. [Google Scholar] [CrossRef]
- Kang Derwent, J.J.; Mieler, W.F. Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans. Am. Ophthalmol. Soc. 2008, 106, 206–213, discussion 213-204. [Google Scholar]
- Sarwar, S.; Bashir, S.; Asim, M.H.; Ikram, F.; Ahmed, A.; Omema, U.; Asif, A.; Chaudhry, A.A.; Hu, Y.; Ustundag, C.B. In-depth drug delivery to tumoral soft tissues via pH responsive hydrogel. RSC Adv. 2022, 12, 31402–31411. [Google Scholar] [CrossRef]
- Ying, R.; Huang, W.C.; Mao, X. Synthesis of Agarose-Based Multistimuli-Responsive Hydrogel Dressing for Accelerated Wound Healing. ACS Biomater. Sci. Eng. 2022, 8, 293–302. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Kruczkowska, W.; Gałęziewska, J.; Grabowska, K.; Liese, G.; Buczek, P.; Kłosiński, K.K.; Kciuk, M.; Pasieka, Z.; Kałuzińska-Kołat, Ż.; Kołat, D. Biomedical Trends in Stimuli-Responsive Hydrogels with Emphasis on Chitosan-Based Formulations. Gels 2024, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Rumon, M.M.H.; Akib, A.A.; Sultana, F.; Moniruzzaman, M.; Niloy, M.S.; Shakil, M.S.; Roy, C.K. Self-Healing Hydrogels: Development, Biomedical Applications, and Challenges. Polymers 2022, 14, 4539. [Google Scholar] [CrossRef]
- Weng, L.; Gouldstone, A.; Wu, Y.; Chen, W. Mechanically strong double network photocrosslinked hydrogels from N,N-dimethylacrylamide and glycidyl methacrylated hyaluronan. Biomaterials 2008, 29, 2153–2163. [Google Scholar] [CrossRef]
- Dhand, A.P.; Galarraga, J.H.; Burdick, J.A. Enhancing Biopolymer Hydrogel Functionality through Interpenetrating Networks. Trends Biotechnol. 2021, 39, 519–538. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of double network hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Y.; Xiang, Y.; Zhang, S.; Guo, X. Application of smart hydrogel materials in cartilage injury repair: A systematic review and meta-analysis. J. Biomater. Appl. 2024, 39, 96–116. [Google Scholar] [CrossRef]
- Lin, H.; Yin, C.; Mo, A.; Hong, G. Applications of Hydrogel with Special Physical Properties in Bone and Cartilage Regeneration. Materials 2021, 14, 235. [Google Scholar] [CrossRef]
- Kaur, K.; Murphy, C.M. Advances in the Development of Nano-Engineered Mechanically Robust Hydrogels for Minimally Invasive Treatment of Bone Defects. Gels 2023, 9, 809. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Ahmad, N.; Bukhari, S.N.A.; Hussain, M.A.; Ejaz, H.; Munir, M.U.; Amjad, M.W. Nanoparticles incorporated hydrogels for delivery of antimicrobial agents: Developments and trends. RSC Adv. 2024, 14, 13535–13564. [Google Scholar] [CrossRef]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle-hydrogel superstructures for biomedical applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Faidra Angelerou, M.G.; Markus, R.; Paraskevopoulou, V.; Foralosso, R.; Clarke, P.; Alvarez, C.V.; Chenlo, M.; Johnson, L.; Rutland, C.; Allen, S.; et al. Mechanistic investigations into the encapsulation and release of small molecules and proteins from a supramolecular nucleoside gel in vitro and in vivo. J. Control. Release 2020, 317, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Luna, V.H.; González-Reynoso, O. Encapsulation of Biological Agents in Hydrogels for Therapeutic Applications. Gels 2018, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef]
- Shang, Q.; Su, Y.; Leslie, F.; Sun, M.; Wang, F. Advances in peptide-drug conjugate-based supramolecular hydrogel systems for local drug delivery. Med. Drug Discov. 2022, 14, 100125. [Google Scholar] [CrossRef]
- Hsu, X.L.; Wu, L.C.; Hsieh, J.Y.; Huang, Y.Y. Nanoparticle-Hydrogel Composite Drug Delivery System for Potential Ocular Applications. Polymers 2021, 13, 642. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Toews, P.; Bates, J. Influence of drug and polymer molecular weight on release kinetics from HEMA and HPMA hydrogels. Sci. Rep. 2023, 13, 16685. [Google Scholar] [CrossRef]
- Croitoru, C.; Roata, I.C.; Pascu, A.; Stanciu, E.M. Diffusion and Controlled Release in Physically Crosslinked Poly (Vinyl Alcohol)/Iota-Carrageenan Hydrogel Blends. Polymers 2020, 12, 1544. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, Z. Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications. Adv. Sci. 2023, 10, e2303326. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Bae, Y.H.; Okano, T. Hydrogels: Swelling, drug loading, and release. Pharm. Res. 1992, 9, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef]
- Yang, X.; Pan, Z.; Choudhury, M.R.; Yuan, Z.; Anifowose, A.; Yu, B.; Wang, W.; Wang, B. Making smart drugs smarter: The importance of linker chemistry in targeted drug delivery. Med. Res. Rev. 2020, 40, 2682–2713. [Google Scholar] [CrossRef]
- Xin, H.; Maruf, D.S.A.A.; Akin-Ige, F.; Amin, S. Stimuli-responsive hydrogels for skin wound healing and regeneration. Emergent Mater. 2024, 8, 1339–1356. [Google Scholar] [CrossRef]
- Kumi, M.; Ejeromedoghene, O.; Sudane, W.D.; Zhang, Z. Unlocking the biological response of smart Stimuli-Responsive hydrogels and their application in biological systems. Eur. Polym. J. 2024, 209, 112906. [Google Scholar] [CrossRef]
- Grindy, S.; Gil, D.; Suhardi, J.; Fan, Y.; Moore, K.; Hugard, S.; Leape, C.; Randolph, M.; Asik, M.D.; Muratoglu, O.; et al. Hydrogel device for analgesic drugs with in-situ loading and polymerization. J. Control. Release 2023, 361, 20–28. [Google Scholar] [CrossRef]
- Steverink, J.G.; van Tol, F.R.; Oosterman, B.J.; Vermonden, T.; Verlaan, J.-J.; Malda, J.; Piluso, S. Robust gelatin hydrogels for local sustained release of bupivacaine following spinal surgery. Acta Biomater. 2022, 146, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Musuc, A.M.; Mititelu, M.; Chelu, M. Hydrogel for Sustained Delivery of Therapeutic Agents. Gels 2024, 10, 717. [Google Scholar] [CrossRef] [PubMed]
- Drosopoulou, K.; Kosheleva, R.I.; Ofrydopoulou, A.; Tsoupras, A.; Mitropoulos, A. Topical and Transdermal Delivery of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) for Inflammation and Pain: Current Trends and Future Directions in Delivery Systems. Processes 2025, 13, 907. [Google Scholar] [CrossRef]
- Omidian, H.; Wilson, R.L. Long-Acting Gel Formulations: Advancing Drug Delivery across Diverse Therapeutic Areas. Pharmaceuticals 2024. [Google Scholar] [CrossRef]
- Karthikeyan, E.; Sivaneswari, S. Advancements in Transdermal Drug Delivery Systems: Enhancing Medicine with Pain-Free and Controlled Drug Release. Intell. Pharm. 2025, 3, 277–295. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Kim, M.; Kim, S.; Lee, K.K.; Choi, H. Advanced Hydrogel Systems for Local Anesthetic Delivery: Toward Prolonged and Targeted Pain Relief. Gels 2024, 17, 131. [Google Scholar] [CrossRef]
- Bajaj, S.; Whiteman, A.; Brandner, B. Transdermal drug delivery in pain management. Contin. Educ. Anaesth. Crit. Care Pain 2011, 11, 39–43. [Google Scholar] [CrossRef]
- Shah, D.K.; Ghosh, S.; More, N.; Choppadandi, M.; Sinha, M.; Srivalliputtur, S.B.; Velayutham, R.; Kapusetti, G. ECM-mimetic, NSAIDs loaded thermo-responsive, immunomodulatory hydrogel for rheumatoid arthritis treatment. BMC Biotechnol. 2024, 24, 26. [Google Scholar] [CrossRef]
- Barthel, H.R.; Haselwood, D.; Longley, S., 3rd; Gold, M.S.; Altman, R.D. Randomized controlled trial of diclofenac sodium gel in knee osteoarthritis. Semin. Arthritis Rheum. 2009, 39, 203–212. [Google Scholar] [CrossRef]
- Altman, R.D.; Dreiser, R.L.; Fisher, C.L.; Chase, W.F.; Dreher, D.S.; Zacher, J. Diclofenac sodium gel in patients with primary hand osteoarthritis: A randomized, double-blind, placebo-controlled trial. J. Rheumatol. 2009, 36, 1991–1999. [Google Scholar] [CrossRef]
- Pușcașu, C.; Zanfirescu, A.; Negreș, S. Recent Progress in Gels for Neuropathic Pain. Gels 2023, 9, 417. [Google Scholar] [CrossRef]
- Gammaitoni, A.; Gallagher, R.M.; Welz-Bosna, M. Topical ketamine gel: Possible role in treating neuropathic pain. Pain Med. 2000, 1, 97–100. [Google Scholar] [CrossRef]
- Karami, T.; Ghobadi, E.; Akrami, M.; Haririan, I. Fabrication of a Controlled-Release Core-Shell Floating Tablet of Ketamine Hydrochloride Using a 3D Printing Technique for Management of Refractory Depressions and Chronic Pain. Polymers 2024, 16, 746. [Google Scholar] [CrossRef] [PubMed]
- Culp, C.; Kim, H.K.; Abdi, S. Ketamine Use for Cancer and Chronic Pain Management. Front. Pharmacol. 2020, 11, 599721. [Google Scholar] [CrossRef] [PubMed]
- Haley, R.M.; von Recum, H.A. Localized and targeted delivery of NSAIDs for treatment of inflammation: A review. Exp. Biol. Med. 2019, 244, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv Sci 2024, 11, e2306152. [Google Scholar] [CrossRef]
- Rodrigues, R.F.; Nunes, J.B.; Agostini, S.B.; dos Santos, P.F.; Cancino-Bernardi, J.; Placido, R.V.; Moraes, T.R.; Freitas, J.T.; Pereira, G.R.; Carvalho, F.C.; et al. Preclinical Evaluation of Polymeric Nanocomposite Containing Pregabalin for Sustained Release as Potential Therapy for Neuropathic Pain. Polymers 2021, 13, 3837. [Google Scholar] [CrossRef]
- Lv, Z.; Dong, C.; Zhang, T.; Zhang, S. Hydrogels in Spinal Cord Injury Repair: A Review. Front. Bioeng. Biotechnol. 2022, 10, 931800. [Google Scholar] [CrossRef]
- Liu, J.; Du, C.; Huang, W.; Lei, Y. Injectable smart stimuli-responsive hydrogels: Pioneering advancements in biomedical applications. Biomater. Sci. 2023, 12, 8–56. [Google Scholar] [CrossRef]
- Pan, Z.-c.; Liu, G.; Liao, J.-x.; Zhang, W.-j.; Liu, X.-p. The therapeutic application of hydrogels in chronic pain. J. Drug Deliv. Sci. Technol. 2025, 107, 106829. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef]
- Abd Ellah, N.H.; Helmy, A.M.; Tammam, O.Y.; El-Sherif, M.W.; Abouelmagd, S.A. Dual-responsive in situ gelling polymer matrix for tunable ketamine general anesthesia in experimental animals. Int. J. Pharm. 2024, 652, 123820. [Google Scholar] [CrossRef]
- Peppin, J.F.; Pappagallo, M. Capsaicinoids in the treatment of neuropathic pain: A review. Ther. Adv. Neurol. Disord. 2014, 7, 22–32. [Google Scholar] [CrossRef]
- Gaul, C.; Resch, S. Application of the capsaicin 8% cutaneous patch in neuropathic pain of the head and face: A case series. Cephalalgia 2015, 35, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Cruccu, G. Topical Treatment of Peripheral Neuropathic Pain: Applying the Evidence. J. Pain Symptom Manag. 2017, 53, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Saher, T.; Manzoor, R.; Abbas, K.; Mudassir, J.; Wazir, M.A.; Ali, E.; Ahmad Siddique, F.; Rasul, A.; Qadir, M.I.; Aleem, A.; et al. Analgesic and Anti-Inflammatory Properties of Two Hydrogel Formulations Comprising Polyherbal Extract. J. Pain Res. 2022, 15, 1203–1219. [Google Scholar] [CrossRef]
- Hammell, D.C.; Zhang, L.P.; Ma, F.; Abshire, S.M.; McIlwrath, S.L.; Stinchcomb, A.L.; Westlund, K.N. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur. J. Pain 2016, 20, 936–948. [Google Scholar] [CrossRef]
- Qi, J.; Zheng, Z.; Hu, L.; Wang, H.; Tang, B.; Lin, L. Development and characterization of cannabidiol-loaded alginate copper hydrogel for repairing open bone defects in vitro. Colloids Surf. B Biointerfaces 2022, 212, 112339. [Google Scholar] [CrossRef]
- Abraham, A.D.; Leung, E.J.Y.; Wong, B.A.; Rivera, Z.M.G.; Kruse, L.C.; Clark, J.J.; Land, B.B. Orally consumed cannabinoids provide long-lasting relief of allodynia in a mouse model of chronic neuropathic pain. Neuropsychopharmacology 2020, 45, 1105–1114. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Ouyang, J.; Karniadakis, G.E. Controlled release of entrapped nanoparticles from thermoresponsive hydrogels with tunable network characteristics. Soft Matter 2020, 16, 4756–4766. [Google Scholar] [CrossRef]
- Penn, M.J.; Hennessy, M.G. Optimal loading of hydrogel-based drug-delivery systems. Appl. Math. Model. 2022, 112, 649–668. [Google Scholar] [CrossRef]
- Ma, X.; Sekhar, K.P.C.; Zhang, P.; Cui, J. Advances in stimuli-responsive injectable hydrogels for biomedical applications. Biomater. Sci. 2024, 12, 5468–5480. [Google Scholar] [CrossRef]
- Liu, W.; Tawakol, A.P.; Rudeen, K.M.; Mieler, W.F.; Kang-Mieler, J.J. Treatment Efficacy and Biocompatibility of a Biodegradable Aflibercept-Loaded Microsphere-Hydrogel Drug Delivery System. Transl. Vis. Sci. Technol. 2020, 9, 13. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, Y.; Zhou, F.; Hu, Y.; Zhao, J.; Liu, Z.; Zhai, Q.; Qi, S.; Zhang, Z.; Chen, L. Bio-functional hydrogel with antibacterial and anti-inflammatory dual properties to combat with burn wound infection. Bioeng. Transl. Med. 2023, 8, e10373. [Google Scholar] [CrossRef] [PubMed]
- Lyu, B.; Zhang, Y.; Ren, J.; Gao, D.; Zhou, Y.; Wang, Y.; Ma, J. High mechanical properties nanocomposite hydrogel achieved based on montmorillonite and tailored microgel suspensions reinforcing polyacryamide networks. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133566. [Google Scholar] [CrossRef]
- Rial, R.; Soltero, J.F.A.; Verdes, P.V.; Liu, Z.; Ruso, J.M. Mechanical Properties of Composite Hydrogels for Tissue Engineering. Curr. Top. Med. Chem. 2018, 18, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Shen, Y.; Xu, L.; Pan, H.; Shen, N.; Li, K.; Ni, K. Preparation and characterization of dual-network interpenetrating structure hydrogels with shape memory and self-healing properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128061. [Google Scholar] [CrossRef]
- Panteli, P.A.; Patrickios, C.S. Multiply Interpenetrating Polymer Networks: Preparation, Mechanical Properties, and Applications. Gels 2019, 5, 36. [Google Scholar] [CrossRef]
- Agrawal, A.; Hussain, C.M. 3D-Printed Hydrogel for Diverse Applications: A Review. Gels 2023, 9, 960. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, M.; Chong, C.M.; Lin, D.; Chen, S.; Zhen, Y.; Ding, H.; Zhong, H.J. Recent Advances in the 3D Printing of Conductive Hydrogels for Sensor Applications: A Review. Polymers 2024, 16, 2131. [Google Scholar] [CrossRef]
- Hatami, H.; Mojahedian, M.M.; Kesharwani, P.; Sahebkar, A. Advancing personalized medicine with 3D printed combination drug therapies: A comprehensive review of application in various conditions. Eur. Polym. J. 2024, 215, 113245. [Google Scholar] [CrossRef]
- Alzoubi, L.; Aljabali, A.A.A.; Tambuwala, M.M. Empowering Precision Medicine: The Impact of 3D Printing on Personalized Therapeutic. AAPS PharmSciTech 2023, 24, 228. [Google Scholar] [CrossRef]
- El-Tanani, M.; Satyam, S.M.; Rabbani, S.A.; El-Tanani, Y.; Aljabali, A.A.A.; Al Faouri, I.; Rehman, A. Revolutionizing Drug Delivery: The Impact of Advanced Materials Science and Technology on Precision Medicine. Pharmaceutics 2025, 17, 375. [Google Scholar] [CrossRef]
- Han, Y.; Cao, Y.; Lei, H. Dynamic Covalent Hydrogels: Strong yet Dynamic. Gels 2022, 8, 577. [Google Scholar] [CrossRef]
- Domokos, A.; Nagy, B.; Szilágyi, B.; Marosi, G.; Nagy, Z.K. Integrated Continuous Pharmaceutical Technologies—A Review. Org. Process Res. Dev. 2021, 25, 721–739. [Google Scholar] [CrossRef]
- Sun, W.; Wu, W.; Dong, X.; Yu, G. Frontier and hot topics in the application of hydrogel in the biomedical field: A bibliometric analysis based on CiteSpace. J. Biol. Eng. 2024, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, L.; Fu, Y. Nanotechnology-based ocular drug delivery systems: Recent advances and future prospects. J. Nanobiotechnology 2023, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Boyer, B.; Huber, K.; Zimlichman, E.; Saunders, R.; McClellan, M.; Kahn, C.; Noach, R.; Salzberg, C. Advancing the future of equitable access to health care: Recommendations from international health care leaders. Health Aff. Sch. 2024, 2, qxae094. [Google Scholar] [CrossRef] [PubMed]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef]
- Ullah, A.; Kim, D.Y.; Lim, S.I.; Lim, H.-R. Hydrogel-Based Biointerfaces: Recent Advances, Challenges, and Future Directions in Human–Machine Integration. Gels 2025, 11, 232. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and Advances in Stimuli-Responsive Hydrogels and Their Applications: A Review. Gels 2025, 11, 30. [Google Scholar] [CrossRef]
- Choi, H.; Choi, W.-S.; Jeong, J.-O. A Review of Advanced Hydrogel Applications for Tissue Engineering and Drug Delivery Systems as Biomaterials. Gels 2024, 10, 693. [Google Scholar] [CrossRef]
| Drug Class | Representative Agents | Mechanism of Action | Clinical Applications | Limitations |
|---|---|---|---|---|
| NSAIDs | Ibuprofen, Diclofenac | COX inhibition → ↓ prostaglandin synthesis | Inflammation, osteoarthritis, postoperative pain | GI irritation, renal toxicity |
| Acetaminophen | Paracetamol | Central COX inhibition, serotonergic/endocannabinoid modulation | Mild–moderate pain, fever | Hepatotoxicity (high dose) |
| Anticonvulsants | Gabapentin, Pregabalin | Bind α2δ subunit of calcium channels | Neuropathic pain, fibromyalgia | Sedation, dizziness |
| Antidepressants | Amitriptyline, Duloxetine | Modulate serotonin & norepinephrine pathways | Neuropathic pain, CRPS | CV risks, dry mouth, sedation |
| Anticonvulsants | Carbamazepine, Lamotrigine | Na+ channel inhibition, GABA enhancement | Neuropathic pain, trigeminal neuralgia | Cognitive side effects |
| NMDA antagonists | Ketamine | Block NMDA receptor → ↓ central sensitization | Refractory pain, CRPS | Psychomimetic effects |
| Topical agents | Capsaicin, Lidocaine | Substance P depletion/Na+ channel block | Localized neuropathic pain | Local irritation |
| Cannabinoids | THC, CBD | CB1/CB2 receptor modulation | Neuropathic pain, MS spasticity | Cognitive/psychoactive effects |
| Type | Examples | Advantages | Limitations |
|---|---|---|---|
| Natural | Alginate, Chitosan, Hyaluronic acid, Collagen | Biocompatible, ECM-like, degradable | Mechanical weakness, batch variability |
| Synthetic | PEG, PVA, PLGA, PNIPAAm | Tunable mechanics, reproducibility | Lack of bioactivity |
| Hybrid | Gelatin-PEG, Nanocomposites | Combines strengths of natural/synthetic | Complex synthesis |
| Stimulus | Representative Materials | Mechanism | Clinical Application |
|---|---|---|---|
| pH-responsive | Poly(acrylic acid), Chitosan | Ionization-driven swelling | Tumor, inflammatory tissue |
| Thermo-responsive | PNIPAAm, Pluronic F127 | LCST sol–gel transition | Injectable depots |
| Enzyme-responsive | MMP-cleavable hydrogels | Enzyme-triggered degradation | Cancer, wound healing |
| Light-responsive | Photocrosslinkable gels | Controlled release under irradiation | On-demand drug delivery |
| Redox-responsive | Disulfide-linked gels | Cleaved by GSH | Intracellular delivery |
| Magnetic/electric | Magnetic NP gels, Conductive polymers | External field control | Targeted therapy |
| Mechanism | Description | Example | Advantages | Limitations |
|---|---|---|---|---|
| Diffusion-controlled | Drug release via concentration gradient | NSAID hydrogels | Simple, predictable | Burst release |
| Swelling-controlled | Polymer expansion drives release | PNIPAAm gels | Sustained release | Depends on swelling rate |
| Chemically controlled | Covalent linkages degrade | Enzyme-responsive gels | Precise targeting | Complex synthesis |
| Stimulus-responsive | pH, temp, magnetic, etc. | Ketamine gels | On-demand release | Need external trigger |
| Drug | Formulation | Model/Condition | Outcome |
|---|---|---|---|
| Diclofenac | Hydrogel patch | Osteoarthritis (clinical) | Pain reduction, ↓ GI side effects |
| Gabapentin | Hydrogel gel | Neuropathic pain (preclinical) | Sustained analgesia, ↓ sedation |
| Amitriptyline | Injectable hydrogel | Neuropathic pain (preclinical) | Prolonged effect, ↓ systemic toxicity |
| Ketamine | Injectable hydrogel | CRPS, chronic pain | Localized relief, ↓ psychomimetic effects |
| Lidocaine | Hydrogel patch | Neuropathic pain | Effective local anesthesia |
| CBD | Alginate-copper hydrogel | Arthritis, bone defect | Reduced inflammation, sustained effect |
| Challenge | Limitation | Current Strategy | Future Direction |
|---|---|---|---|
| Drug loading | Low encapsulation efficiency | Nanoparticle integration, chemical conjugation | Personalized optimization |
| Biocompatibility | Immune reaction, cytotoxicity | Natural polymers, biofunctionalization | Long-term safety trials |
| Mechanical stability | Fragile under stress | Double-network, IPN, nanocomposites | Smart adaptive hydrogels |
| Manufacturing | Reproducibility, scalability | GMP, continuous manufacturing | Automated 3D-printing |
| Ethics & access | Sustainability, fairness | Biodegradable materials | Equitable access policies |
| Delivery Platform | Mechanism/Carrier type | Release Characteristics | Advantages | Limitations |
|---|---|---|---|---|
| Hydrogels | 3D polymer networks | Sustained, localized | Biocompatible, tunable, minimally invasive | Mechanical weakness |
| Lipid nanoparticles | Lipid core–shell carriers | Rapid or burst release | High permeability, suitable for lipophilic drugs | Stability and aggregation issues |
| Microneedles | Transdermal micro-protrusion | Pulsatile or rapid onset | Painless delivery, self-administration | Limited drug load, potential irritation |
| Polymeric depots | PLGA-based injectable systems | Long-term sustained release | Clinically approved (DepoFoam, etc.) | Limited for hydrophilic drugs |
| Focus Area | Current Progress | Future Outlook |
|---|---|---|
| 3D printing | Patient-specific hydrogel structures | Personalized medicine platforms |
| Self-healing gels | Injectable, stress-resistant hydrogels | Long-term implants, robotics |
| Nanocomposite hydrogels | Drug co-delivery + tissue regeneration | Theranostic platforms |
| Regulatory science | Preclinical trials, limited approvals | Streamlined FDA/EMA pathways |
| Interdisciplinary collaboration | Material scientists + clinicians | Translational consortia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.K.; Jeong, W.; Chae, M. Hydrogel-Based Delivery Systems for Non-Opioid Analgesics: Advances, Challenges, and Clinical Prospects. J. Clin. Med. 2025, 14, 7768. https://doi.org/10.3390/jcm14217768
Lee KK, Jeong W, Chae M. Hydrogel-Based Delivery Systems for Non-Opioid Analgesics: Advances, Challenges, and Clinical Prospects. Journal of Clinical Medicine. 2025; 14(21):7768. https://doi.org/10.3390/jcm14217768
Chicago/Turabian StyleLee, Kyung Kwan, Wonwoo Jeong, and Minsuk Chae. 2025. "Hydrogel-Based Delivery Systems for Non-Opioid Analgesics: Advances, Challenges, and Clinical Prospects" Journal of Clinical Medicine 14, no. 21: 7768. https://doi.org/10.3390/jcm14217768
APA StyleLee, K. K., Jeong, W., & Chae, M. (2025). Hydrogel-Based Delivery Systems for Non-Opioid Analgesics: Advances, Challenges, and Clinical Prospects. Journal of Clinical Medicine, 14(21), 7768. https://doi.org/10.3390/jcm14217768






