Mortality Prediction in Hospitalized COPD Patients Based on FEV1/FVC Severity Staging
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Statistical Analysis
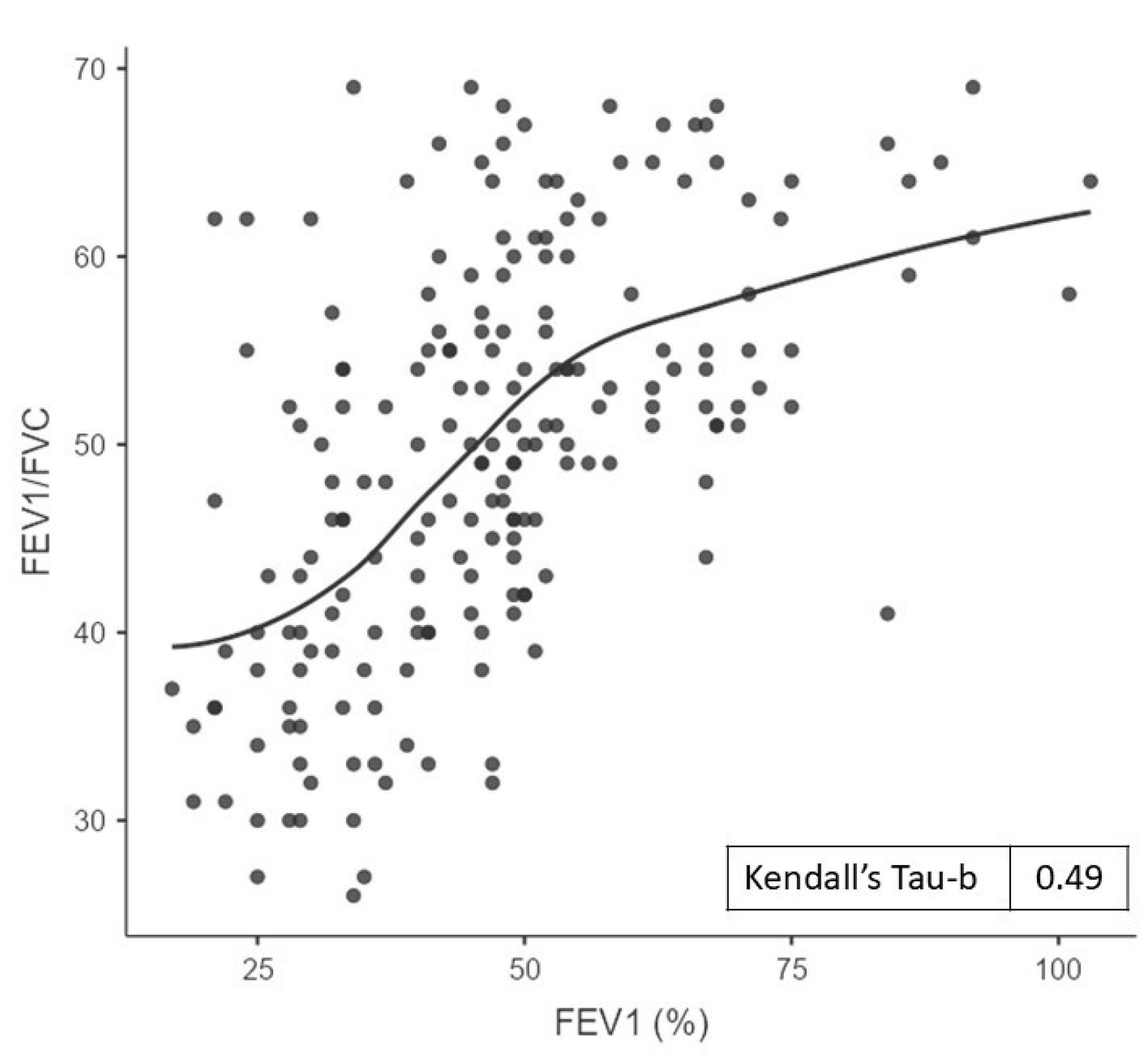
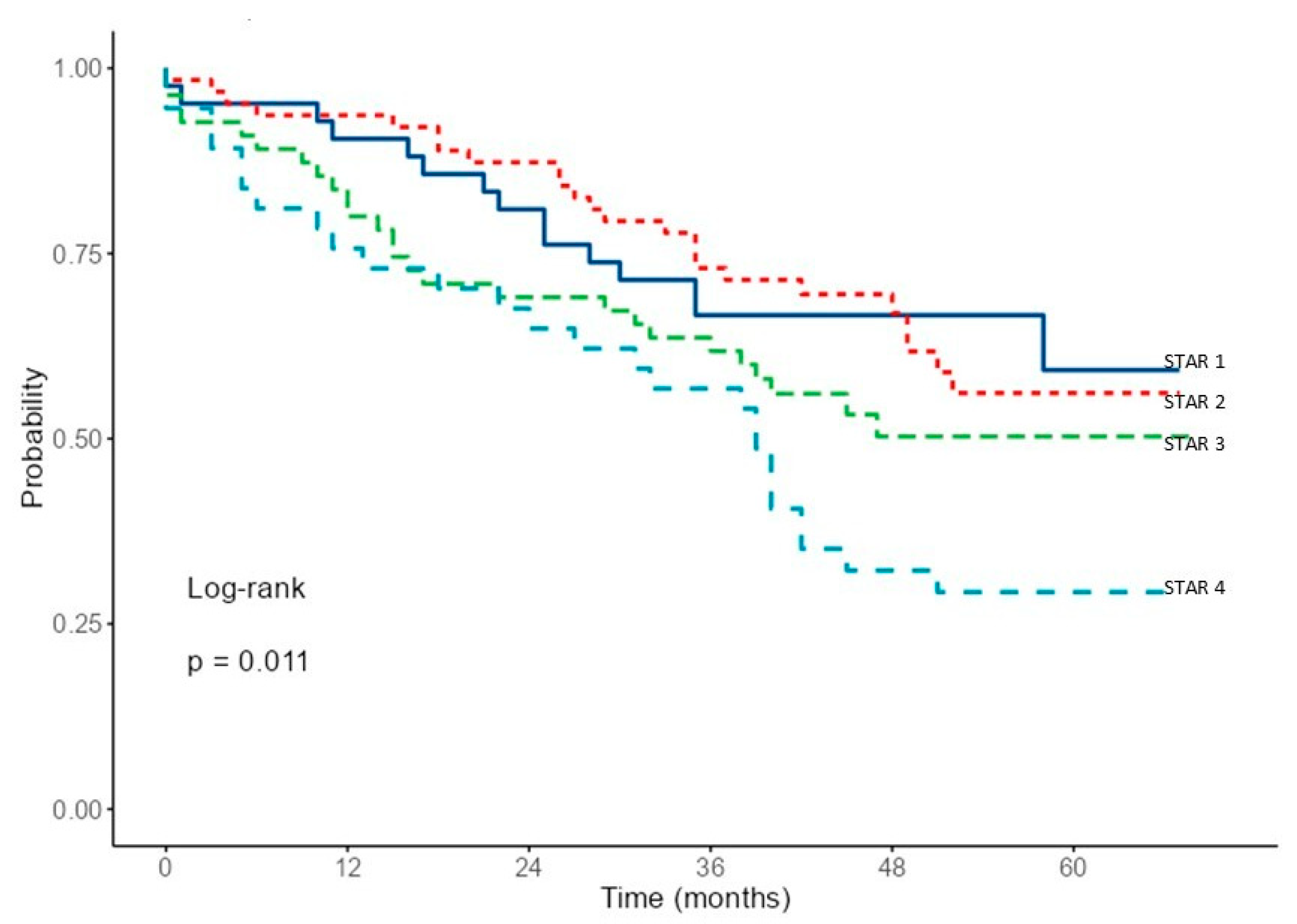
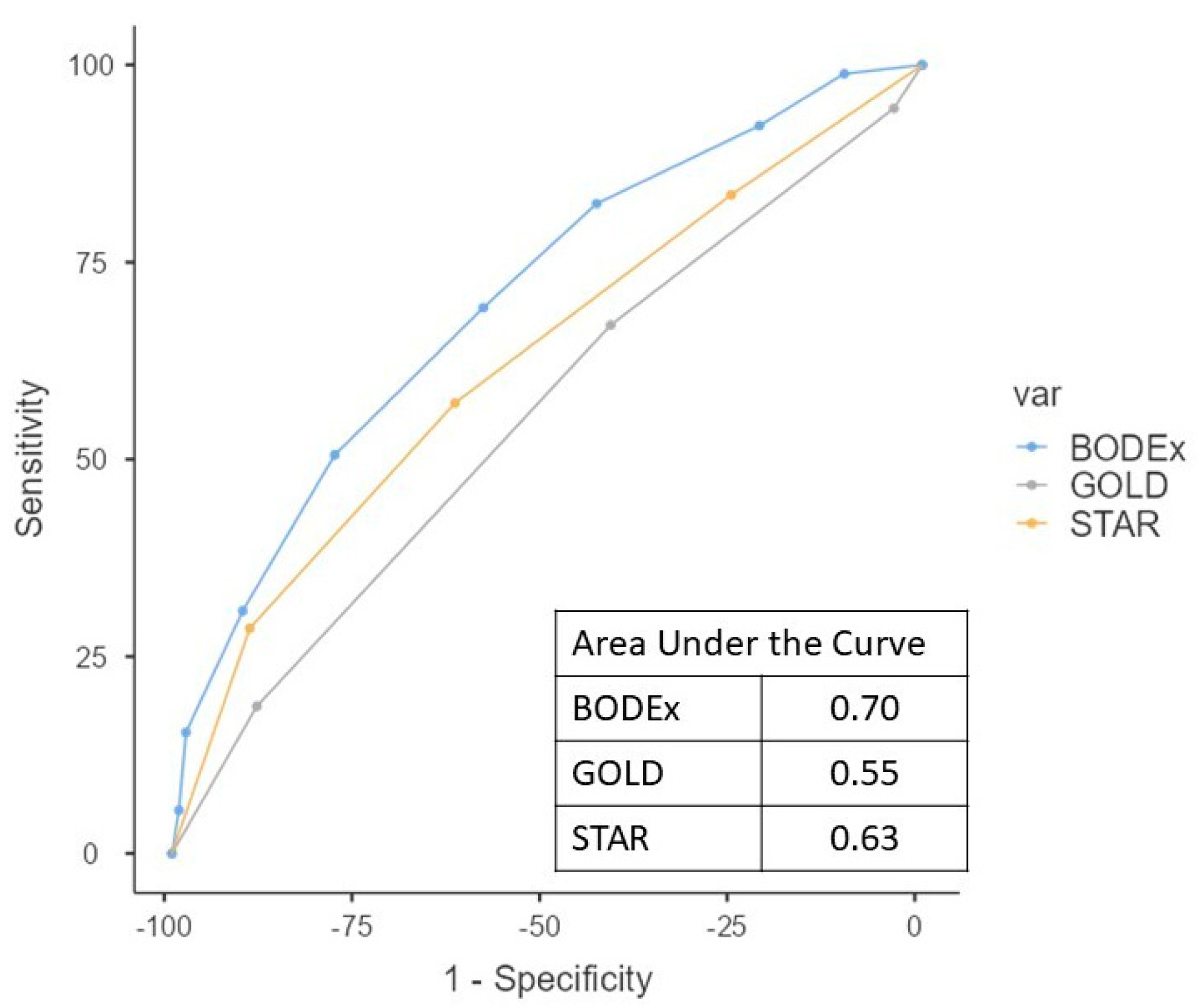
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COPD | Chronic Obstructive Pulmonary Disease |
| GOLD | The Global Initiative for Chronic Obstructive Lung Disease |
| FEV1 | Forced Expiratory Volume in One Second |
| FVC | Forced Vital Capacity |
| STAR | Staging of Airflow Obstruction by Ratio |
References
- 2025 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease. Available online: https://goldcopd.org/2025-gold-report/ (accessed on 27 August 2025).
- Baugh, A.D.; Shiboski, S.; Hansel, N.N.; Ortega, V.; Barjaktarevic, I.; Barr, R.G.; Bowler, R.; Comellas, A.P.; Cooper, C.B.; Couper, D.; et al. Reconsidering the utility of race-specific lung function prediction equations. Am. J. Respir. Crit. Care Med. 2022, 205, 819–829. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Nakhmani, A.; Fortis, S.; Strand, M.J.; Silverman, E.K.; Sciurba, F.C.; Bodduluri, S. FEV1/FVC severity stages for chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2023, 208, 676–684. [Google Scholar] [CrossRef]
- Calverley, P.M.A. A Rising STAR in Chronic Obstructive Pulmonary Disease or more deckchair rearrangement? Am. J. Respir. Crit. Care Med. 2024, 210, 1285–1287. [Google Scholar] [CrossRef]
- Backman, H.; Vanfleteren, L.E.G.W.; Mannino, D.M.; Ekström, M. Severity of airflow obstruction based on FEV1/FVC versus FEV1 percent predicted in the general U.S. population. Am. J. Respir. Crit. Care Med. 2024, 210, 1308–1316. [Google Scholar] [CrossRef]
- Bertels, X.; Riemann, S.; Vauterin, D.; Lahousse, L.; Brusselle, G.G. All-cause mortality of staging of airflow obstruction by ratio-categorized patients with chronic obstructive pulmonary disease among the general population. Am. J. Respir. Crit. Care Med. 2024, 210, 1374–1376. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Nakhmani, A.; Fortis, S.; Strand, M.J.; Silverman, E.K.; Wilson, C.G.; Sciurba, F.C.; Bodduluri, S. STAR has better discrimination for mortality than ERS/ATS Chronic Obstructive Pulmonary Disease severity classification. Am. J. Respir. Crit. Care Med. 2024, 210, 1376–1379. [Google Scholar] [CrossRef]
- Buchardt, S.T.E.; Weinreich, U.M.; Lindgren, F.L.; Lauridsen, M.D.; Karlsen, J.H.; Kragholm, K.; Torp-Pedersen, C.; Jacobsen, P.A. Patient characteristics and mortality across diagnostic settings in COPD. Respir. Med. 2024, 234, 107843. [Google Scholar] [CrossRef]
- Bestall, J.C.; Paul, E.A.; Garrod, R.; Garnham, R.; Jones, P.W.; Wedzicha, J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef]
- Soler-Cataluña, J.J.; Martínez-García, M.A.; Sánchez, L.S.; Tordera, M.P.; Sánchez, P.R. Severe exacerbations and BODE index: Two independent risk factors for death in male COPD patients. Respir. Med. 2009, 103, 692–699. [Google Scholar] [CrossRef]
- Ondeck, N.T.; Bohl, D.D.; Bovonratwet, P.; McLynn, R.P.; Cui, J.J.; Shultz, B.N.; Lukasiewicz, A.M.; Grauer, J.N. Discriminative ability of commonly used indices to predict adverse outcomes after poster lumbar fusion: A comparison of demographics, ASA, the modified Charlson Comorbidity Index, and the modified Frailty Index. Spine J. 2018, 18, 44–52. [Google Scholar] [CrossRef]
- Whittaker, H.; Rothnie, K.J.; Quint, J.K. Cause-specific mortality in COPD subpopulations: A cohort study of 339 647 people in England. Thorax 2024, 79, 202–208. [Google Scholar] [CrossRef]
- Singh, D.; Stockley, R.; Anzueto, A.; Agusti, A.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Han, M.K.; Martinez, F.J.; de Oca, M.M.; et al. GOLD Science Committee recommendations for the use of pre- and post-bronchodilator spirometry for the diagnosis of COPD. Eur. Respir. J. 2025, 65, 2401603. [Google Scholar] [CrossRef]
- Smith, B.M.; Hoffman, E.A.; Basner, R.C.; Kawut, S.M.; Kalhan, R.; Barr, R.G. Not all measures of hyperinflation are created equal: Lung structure and clinical correlates of gas trapping and hyperexpansion in COPD: The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study. Chest 2014, 145, 1305–1315. [Google Scholar] [CrossRef]
- Neder, J.A. Is this really a new START in Chronic Obstructive Pulmonary Disease? Am. J. Respir. Crit. Care Med. 2024, 209, 339–340. [Google Scholar] [CrossRef]
- Ambrosino, P.; Vitacca, M.; Marcuccio, G.; Spanevello, A.; Ambrosino, N.; Maniscalco, M. A Comparison of GOLD and STAR severity stages in individuals with COPD undergoing pulmonary rehabilitation. Chest 2025, 167, 387–401. [Google Scholar] [CrossRef]
- Gedebjerg, A.; Szépligeti, S.K.; Wackerhausen, L.H.; Horváth-Puhó, E.; Dahl, R.; Hansen, J.G.; Sørensen, H.T.; Nørgaard, M.; Lange, P.; Thomsen, R.W. Prediction of mortality in patients with chronic obstructive pulmonary disease with the new Global Initiative for Chronic Obstructive Lung Disease 2017 classification: A cohort study. Lancet Respir. Med. 2018, 6, 204–212. [Google Scholar] [CrossRef]
- Brat, K.; Svoboda, M.; Zatloukal, J.; Plutinsky, M.; Volakova, E.; Popelkova, P.; Novotna, B.; Dvorak, T.; Koblizek, V. Prognostic properties of the GOLD 2023 classification system. Int. J. Chron. Obstruct. Pulmon. Dis. 2023, 18, 661–667. [Google Scholar] [CrossRef]
- Huang, K.; Tang, X.; Chu, X.; Niu, H.; Li, W.; Zheng, Z.; Peng, Y.; Lei, J.; Li, Y.; Li, B.; et al. Comparison of STAR and GOLD in assessing disease severity among high-risk and COPD patients: Evidence from Enjoying Breathing Program in China. Int. J. Chron. Obstruct. Pulmon. Dis. 2024, 19, 2751–2762. [Google Scholar] [CrossRef]
- Kotlyarov, S. The role of multidimensional indices for mortality prediction in Chronic Obstructive Pulmonary Disease. Diagnostics 2023, 13, 1344. [Google Scholar] [CrossRef]
- Garcia-Pachon, E.; Padilla-Navas, I. Contribution of anemia to multidimensional indices for predicting mortality in hospitalized patients with Chronic Obstructive Pulmonary Disease (COPD). Cureus 2024, 16, e72126. [Google Scholar] [CrossRef]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 6, 2101499. [Google Scholar] [CrossRef] [PubMed]
| Total | Survivors | Deceased | p Value | |
|---|---|---|---|---|
| Number of patients | 197 | 106 | 91 | |
| Female, n (%) | 45 | 34 (75%) | 11 (25%) | |
| Male, n (%) | 152 | 72 (47%) | 80 (53%) | |
| Age (years) | 73 ± 10 | 70 ± 9 | 77 ± 9 | <0.001 |
| Current smokers, n (%) | 80 (41%) | 49 (61%) | 31 (39%) | 0.08 |
| Pack-years | 58 ± 26 | 53 ± 23 | 63 ± 28 | 0.006 |
| FVC % predicted | 73 ± 20 | 74 ± 19 | 72 ± 20 | 0.34 |
| FEV1% predicted | 47 ± 17 | 49 ± 16 | 45 ± 18 | 0.08 |
| FEV1/FVC, % | 50 ± 11 | 52 ± 10 | 48 ± 11 | 0.007 |
| Dyspnea (mMRC scale) | 2.2 ± 0.9 | 1.9 ± 0.9 | 2.5 ± 0.8 | <0.001 |
| Severe exacerbations in the past 5 years, n | 1.03 ± 1.54 | 0.65 ± 1.26 | 1.46 ± 1.73 | <0.001 |
| BODEx | 3.7 ± 2.0 | 3.0 ± 1.8 | 4.5 ± 1.9 | <0.001 |
| GOLD | 2.7 ± 0.8 | 2.7 ± 0.7 | 2.8 ± 0.8 | 0.20 |
| STAR | 2.4 ± 1.0 | 2.2 ± 0.9 | 2.7 ± 1.1 | 0.001 |
| STAR Grade | 1 | 2 | 3 | 4 | p Value |
|---|---|---|---|---|---|
| Number and percentage | 42 (21%) | 63 (32%) | 55 (28%) | 37 (19%) | |
| Age, years | 70 ± 11 | 74 ± 10 | 73 ± 11 | 74 ± 8 | 0.29 |
| Severe exacerbations, n | 0.67 ± 1.07 | 0.76 ± 1.30 | 1.13 ± 1.63 | 1.73 ± 2.04 | 0.025 |
| Dyspnea, mMRC | 1.8 ± 0.9 | 2.0 ± 0.9 | 2.2 ± 0.9 | 2.8 ± 0.6 | <0.001 |
| FEV1, % of reference | 58 ± 18 | 53 ± 15 | 44 ± 11 | 31 ± 8 | <0.001 |
| FEV1/FVC, % | 64 ± 3 | 54 ± 3 | 45 ± 3 | 34 ± 4 | <0.001 |
| Body mass index | 31 ± 7 | 28 ± 6 | 26 ± 5 | 24 ± 4 | <0.001 |
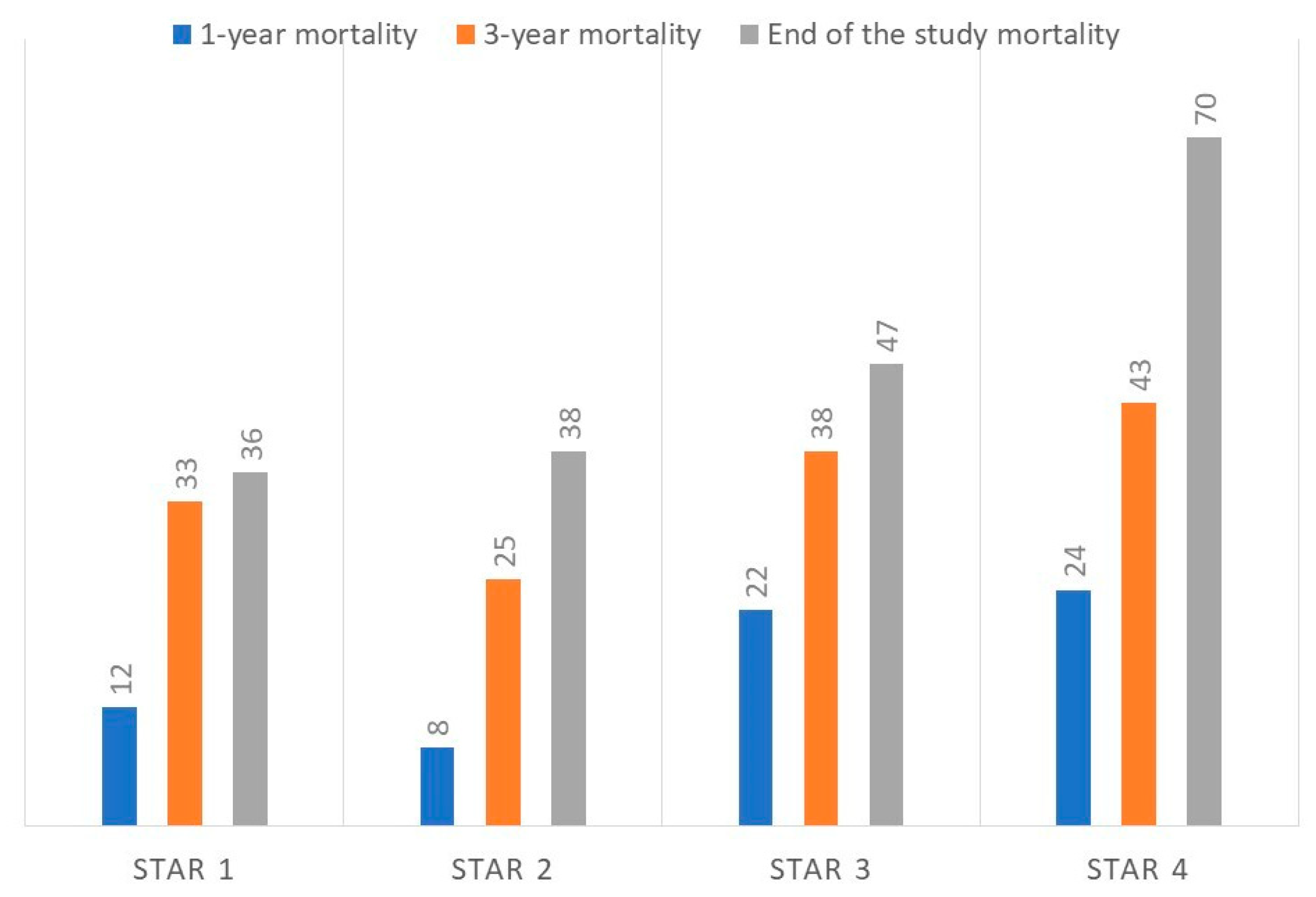
| 1-year mortality | 37/5 (12%) | 58/5 (8%) | 43/12 (22%) | 28/9 (24%) | 0.07 |
| 3-year mortality | 28/14 (33%) | 47/16 (25%) | 34/21 (38%) | 21/16 (43%) | 0.27 |
| Mortality at the end of the study | 27/15 (36%) | 39/24 (38%) | 29/26 (47%) | 11/26 (70%) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Pachon, E.; Zamora-Molina, L.; Baeza-Martinez, C.; Ruiz-Alcaraz, S.; Bordallo-Vazquez, P.; Perez-Remacho, F.J.; Ibarra-Macia, A.; Galan-Negrillo, M.; Grau-Delgado, J. Mortality Prediction in Hospitalized COPD Patients Based on FEV1/FVC Severity Staging. J. Clin. Med. 2025, 14, 7766. https://doi.org/10.3390/jcm14217766
Garcia-Pachon E, Zamora-Molina L, Baeza-Martinez C, Ruiz-Alcaraz S, Bordallo-Vazquez P, Perez-Remacho FJ, Ibarra-Macia A, Galan-Negrillo M, Grau-Delgado J. Mortality Prediction in Hospitalized COPD Patients Based on FEV1/FVC Severity Staging. Journal of Clinical Medicine. 2025; 14(21):7766. https://doi.org/10.3390/jcm14217766
Chicago/Turabian StyleGarcia-Pachon, Eduardo, Lucia Zamora-Molina, Carlos Baeza-Martinez, Sandra Ruiz-Alcaraz, Paula Bordallo-Vazquez, Francisco J. Perez-Remacho, Ana Ibarra-Macia, Marta Galan-Negrillo, and Justo Grau-Delgado. 2025. "Mortality Prediction in Hospitalized COPD Patients Based on FEV1/FVC Severity Staging" Journal of Clinical Medicine 14, no. 21: 7766. https://doi.org/10.3390/jcm14217766
APA StyleGarcia-Pachon, E., Zamora-Molina, L., Baeza-Martinez, C., Ruiz-Alcaraz, S., Bordallo-Vazquez, P., Perez-Remacho, F. J., Ibarra-Macia, A., Galan-Negrillo, M., & Grau-Delgado, J. (2025). Mortality Prediction in Hospitalized COPD Patients Based on FEV1/FVC Severity Staging. Journal of Clinical Medicine, 14(21), 7766. https://doi.org/10.3390/jcm14217766






