Enhancing Safety in Refractive Surgery: A Pilot Evaluation of In Vivo Confocal Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Data
2.3. IVCM (HRT2-RCM)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCVA | Best-Corrected Visual Acuity |
| Femto-LASIK | Femtosecond Laser-Assisted In Situ Keratomileusis |
| HSK | Herpes Simplex Keratitis |
| ICL | Implantable Collamer Lens (Phakic Intraocular Lens) |
| IVCM | In Vivo Confocal Microscopy |
| OCT | Optical Coherence Tomography |
| OD | Oculus Dexter (Right Eye) |
| OS | Oculus Sinister (Left Eye) |
| PTK | Phototherapeutic Keratectomy |
| SMILE | Small Incision Lenticule Extraction |
| TSPK | Thygeson’s Superficial Punctate Keratitis |
References
- Ang, M.; Gatinel, D.; Reinstein, D.Z.; Mertens, E.; Alió del Barrio, J.L.; Alió, J.L. Refractive Surgery beyond 2020. Eye 2021, 35, 362–382. [Google Scholar] [CrossRef] [PubMed]
- Miraftab, M.; Hashemi, H.; Aghamirsalim, M.; Fayyaz, S.; Asgari, S. Matched Comparison of Corneal Higher Order Aberrations Induced by SMILE to Femtosecond Assisted LASIK and to PRK in Correcting Moderate and High Myopia: 3.00 Mm vs. 6.00 Mm. BMC Ophthalmol. 2021, 21, 216. [Google Scholar] [CrossRef]
- Shah, R. History and Results; Indications and Contraindications of SMILE Compared with LASIK. Asia-Pac. J. Ophthalmol. 2019, 8, 371. [Google Scholar] [CrossRef]
- Breyer, D.R.; Beckers, L.; Hagen, P.; Kaymak, H.; Klabe, K.; Auffarth, G.U.; Kretz, F.T.A. Comparison of Long-Term Results with Small Incision Refractive Lenticule Extraction (ReLEX SMILE) vs. Femto-LASIK. Klin. Monatsblatter Fur Augenheilkd. 2019, 236, 1201–1207. [Google Scholar]
- Li, J.; Huang, Y.; Song, Y.; Xu, Y.; Zhang, Y.; Wen, J.; Wang, Z.; Zhang, F. Epithelial remodeling associated with corneal power and corneal higher-order aberrations after ray-tracing guided FS-LASIK for myopia. Photodiagnosis Photodyn. Ther. 2025, 56, 105230. [Google Scholar] [CrossRef] [PubMed]
- Pazo, E.E.; Huang, H.; Fan, Q.; Zhang, C.; Yue, Y.; Yang, L.; Xu, L.; Moore, J.E.; He, W. Intense Pulse Light for Treating Post-LASIK Refractory Dry Eye. Photobiomodul. Photomed. Laser Surg. 2021, 39, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Yalçınkaya, G.; Yıldız, B.K.; Çakır, İ.; Yıldırım, Y.; Demirok, A. Evaluation of Peripapillary-Macular Microvascularity and Choroidal Vascularity Index after Refractive Surgery. Photodiagnosis Photodyn. Ther. 2022, 37, 102714. [Google Scholar] [CrossRef]
- Greenstein, S.A.; Hersh, P.S. Update on corneal crosslinking for keratoconus and corneal ectasia. Curr. Opin. Ophthalmol. 2024, 35, 273–277. [Google Scholar] [CrossRef]
- Jain, N.; Murthy, S.; Rathi, V.M.; Das, S.; Jakati, S.; Mishra, D.K.; Vemuganti, G.K.; Ramappa, M. Corneal Pathology. In Ocular Pathology: Basics to Clinico-Pathologic Correlation; Vemuganti, G.K., Murthy, S., Mishra, D.K., Eds.; Springer Nature: Singapore, 2025; pp. 149–187. ISBN 978-981-96-8614-8. [Google Scholar]
- Dumitrache, M. Ophthalmological Pathology and Management in Eye Disease: Cornea. In Clinical Ophthalmology: A Guide to Diagnosis and Treatment; Dumitrache, M., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 139–197. ISBN 978-3-031-68453-1. [Google Scholar]
- Ibrahim, R.; Kumar, P.; Aranjani, J.M. Microbial keratitis in the age of resistance: Unlocking the therapeutic potential of phage therapy. Graefe’s Arch Clin. Exp. Ophthalmol. 2025. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K.; Lalgudi, V.G.; Kundu, G.; Mimouni, M.; Liu, H.; Jhanji, V.; Prakash, G.; Roy, A.S.; Shetty, R.; et al. Role of Artificial Intelligence, Machine Learning and Deep Learning Models in Corneal Disorders—A Narrative Review. J. Français D’ophtalmol. 2024, 47, 104242. [Google Scholar] [CrossRef]
- Chiang, J.C.B.; Roy, M.; Kim, J.; Markoulli, M.; Krishnan, A.V. In-Vivo Corneal Confocal Microscopy: Imaging Analysis, Biological Insights and Future Directions. Commun. Biol. 2023, 6, 652. [Google Scholar] [CrossRef]
- Cañadas, P.; Alberquilla García-Velasco, M.; Hernández Verdejo, J.L.; Teus, M.A. Update on Corneal Confocal Microscopy Imaging. Diagnostics 2022, 13, 46. [Google Scholar] [CrossRef]
- Martin, R. Cornea and Anterior Eye Assessment with Slit Lamp Biomicroscopy, Specular Microscopy, Confocal Microscopy, and Ultrasound Biomicroscopy. Indian J. Ophthalmol. 2018, 66, 195–201. [Google Scholar] [CrossRef]
- Alvani, A.; Hashemi, H.; Pakravan, M.; Mahbod, M.; Seyedian, M.A.; Amanzadeh, K.; Khabazkhoob, M.; Jafarzadehpur, E.; Fotouhi, A. Post-LASIK Ectasia Versus Keratoconus: An In Vivo Confocal Microscopy Study. Cornea 2020, 39, 1006. [Google Scholar] [CrossRef]
- Kobayashi, A.; Sugiyama, K. In Vivo Corneal Confocal Microscopic Findings of Palisades of Vogt and Its Underlying Limbal Stroma. Cornea 2005, 24, 435–437. [Google Scholar] [CrossRef]
- Chao, C.; Tajbakhsh, Z.; Stapleton, F.; Mobeen, R.; Madigan, M.C.; Jalbert, I.; Briggs, N.; Golebiowski, B. Corneal Epithelial Dendritic Cells, Tear Neuropeptides and Corneal Nerves Continue to Be Affected More than 12 Months after LASIK. Acta Ophthalmol. 2023, 101, e302–e314. [Google Scholar] [CrossRef] [PubMed]
- El Zarif, M.; Abdul Jawad, K.; Alió, J.L.; Makdissy, N.; De Miguel, M.P. In Vivo Confocal Microscopy Evaluation of Infiltrated Immune Cells in Corneal Stroma Treated with Cell Therapy in Advanced Keratoconus. J. Ophthalmic Inflamm. Infect. 2024, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Hovakimyan, M.; Falke, K.; Stahnke, T.; Guthoff, R.; Witt, M.; Wree, A.; Stachs, O. Morphological Analysis of Quiescent and Activated Keratocytes: A Review of Ex Vivo and in Vivo Findings. Curr. Eye Res. 2014, 39, 1129–1144. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Peterson, T.; Ungricht, E.; McCabe, S.; Ronquillo, Y.C.; Brooks, B.; Towne, F.; Hoopes, P. Thygeson Superficial Punctate Keratitis: A Clinical and Immunologic Review. Eye Contact Lens 2022, 48, 232–238. [Google Scholar] [CrossRef]
- Thygeson, P. Superficial Punctate Keratitis. JAMA 1950, 144, 1544–1549. [Google Scholar] [CrossRef]
- Mandal, N.; Yeung, S.N.; Tadrous, C.; Iovieno, A. Thygeson’s Superficial Punctate Keratitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 1837–1841. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Wei, Z.; Cao, K.; Su, G.; Hamrah, P.; Labbe, A.; Liang, Q. Characteristics of Toxic Keratopathy, an In Vivo Confocal Microscopy Study. Trans. Vis. Sci. Technol. 2021, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- You, J.Y.; Botelho, P.J. Corneal In Vivo Confocal Microscopy: Clinical Applications. Rhode Isl. Med. J. 2016, 99, 30–33. [Google Scholar]
- Naquin, E.R.; Garg, R.; Chen, W.J.; Karmakar, E.; Prasad, A.; Mandadi, S.; Depala, K.; Gopianand, J.S.; Gnana-Prakasam, J.P. Iron: More than Meets the Eye. Nutrients 2025, 17, 2964. [Google Scholar] [CrossRef]
- Al Bdour, M.; Alsaadi, A.; Almaazmi, A.; Alryalat, S.A. Clinical Review of Cornea: Part I. In Ophthalmology Board and FRCS Part 2 Exams: Eye Yield Clinical; Alryalat, S.A., Ed.; Springer Nature: Singapore, 2025; pp. 257–277. ISBN 978-981-96-1517-9. [Google Scholar]
- Rozitis, E.; Petsoglou, C.; Devasahayam, R.; Hau, S.; Lacorzana, J. Typical Manifestations of the Different Corneal Deposits by In Vivo Confocal Microscopy (IVCM): A Review. Clin. Exp. Ophthalmol. 2025, 53, 834–857. [Google Scholar] [CrossRef]
- Recchioni, A.; Barua, A.; Dominguez-Vicent, A. Enhancing Clinical Decision-Making in Complex Corneal Disorders: The Role of In-Vivo Confocal Microscopy. Life 2023, 13, 679. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Li, J.; Li, J.; Peng, H.; Wang, Q. In Vivo Confocal Microscopy of Sub-Basal Corneal Nerves and Corneal Densitometry after Three Kinds of Refractive Procedures for High Myopia. Int. Ophthalmol. 2023, 43, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Naujokaitis, T.; Auffarth, G.U.; Łabuz, G.; Kessler, L.J.; Khoramnia, R. Diagnostic Techniques to Increase the Safety of Phakic Intraocular Lenses. Diagnostics 2023, 13, 2503. [Google Scholar] [CrossRef]
- Gómez-Benlloch, A.; Widmer-Pintos, J.; Arnaldos-López, C. Punctiform and Polychromatic Pre-Descemet Corneal Dystrophy, a Rare Corneal Pathology. Clin. Exp. Optom. 2025, 108, 744–745. [Google Scholar] [CrossRef]
- Constantin, C. Corneal Dystrophies: Pathophysiological, Genetic, Clinical, and Therapeutic Considerations. Rom. J. Ophthalmol. 2021, 65, 104–108. [Google Scholar] [CrossRef]
- Eker, S.; Oflaz, A.B.; Bozkurt, B. Anterior Segment Swept Source Optical Coherence Tomography and In Vivo Confocal Microscopy Findings in a Case with Bleb-Like Epithelial Basal Membrane Dystrophy. Cornea 2023, 42, 1049–1051. [Google Scholar] [CrossRef]
- Ivarsen, A.; Fledelius, W.; Hjortdal, J.Ø. Three-Year Changes in Epithelial and Stromal Thickness after PRK or LASIK for High Myopia. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Chirapapaisan, C.; Muller, R.T.; Sahin, A.; Cruzat, A.; Cavalcanti, B.M.; Jamali, A.; Pavan-Langston, D.; Hamrah, P. Effect of Herpes Simplex Keratitis Scar Location on Bilateral Corneal Nerve Alterations: An in Vivo Confocal Microscopy Study. Br. J. Ophthalmol. 2022, 106, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Pniakowska, Z.; Jurowski, P.; Wierzbowska, J. The Role of Corneal Biomechanical Properties Assessment in Laser Vision Correction ? The Introduction: OphthaTherapy. Ther. Ophthalmol. 2022, 9, 183–186. [Google Scholar] [CrossRef]
- Liu, C.-F.; Lee, J.-S.; Sun, C.-C.; Lin, K.-K.; Hou, C.-H.; Yeung, L.; Peng, S.-Y. Correlation between Pigmented Arc and Epithelial Thickness (COPE) Study in Orthokeratology-Treated Patients Using OCT Measurements. Eye 2020, 34, 352–359. [Google Scholar] [CrossRef]
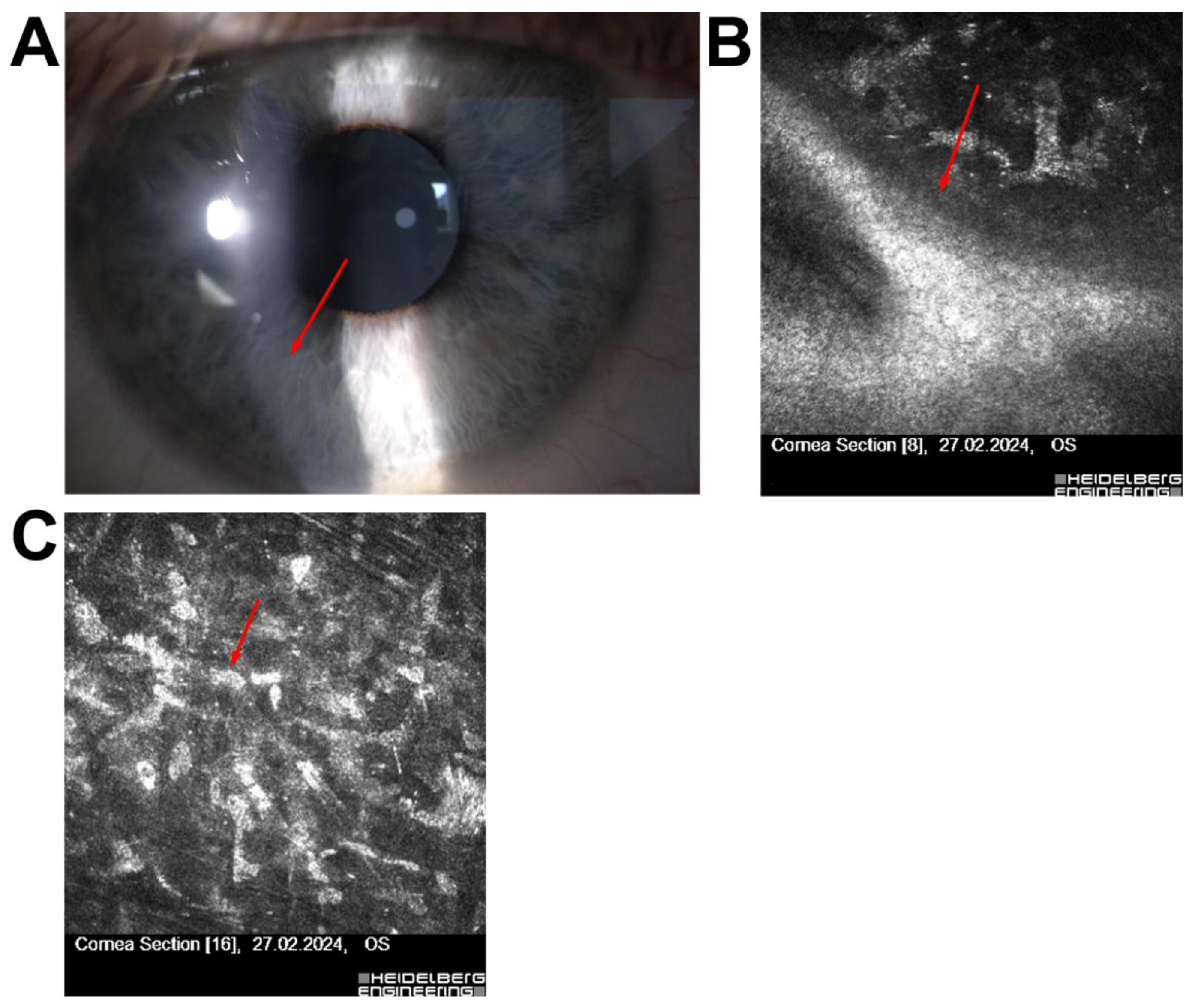

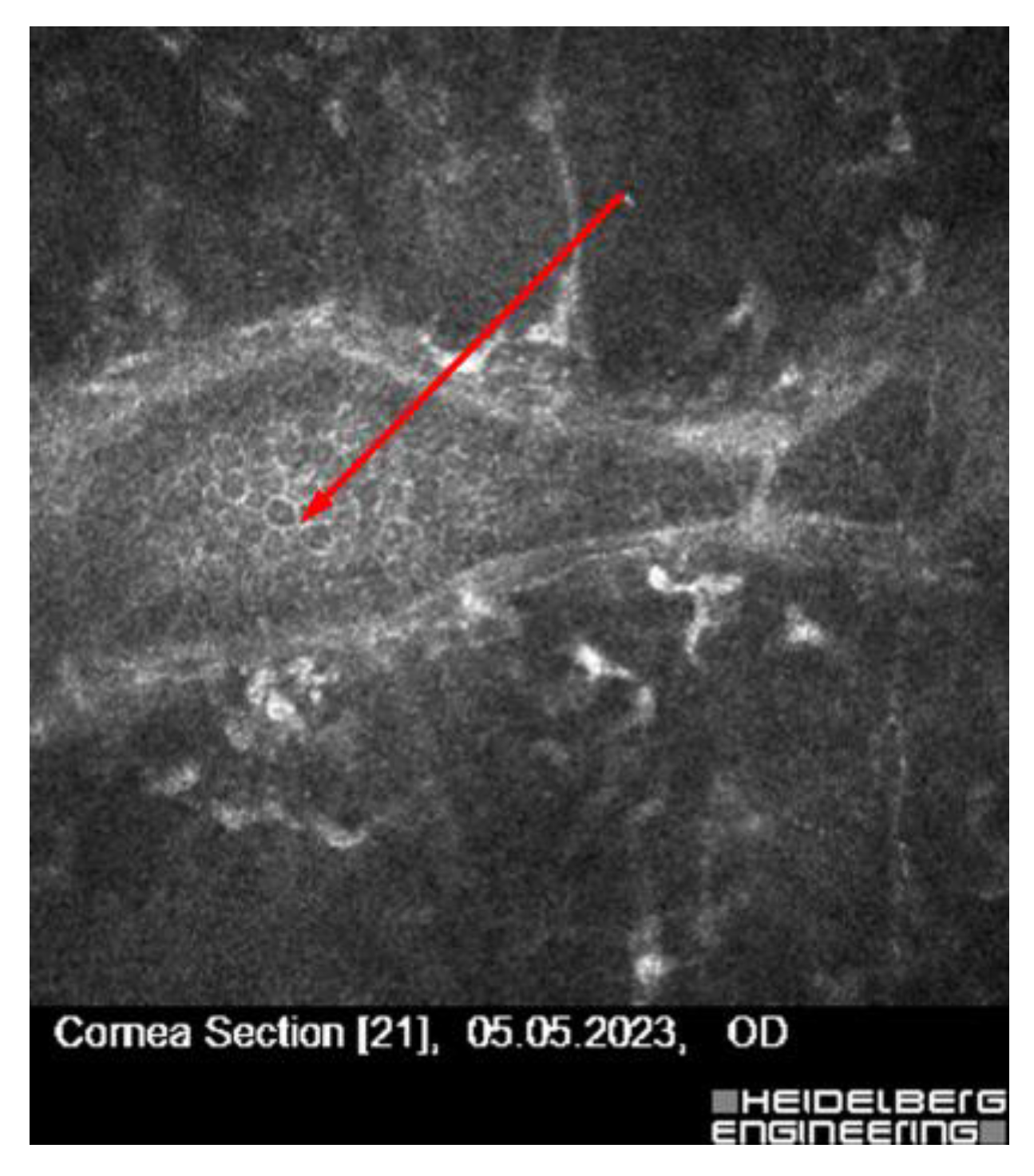
| Patient | Age/Sex | Clinical History | Confocal Microscopy Findings | Diagnosis | Clinical Decision/Outcome |
|---|---|---|---|---|---|
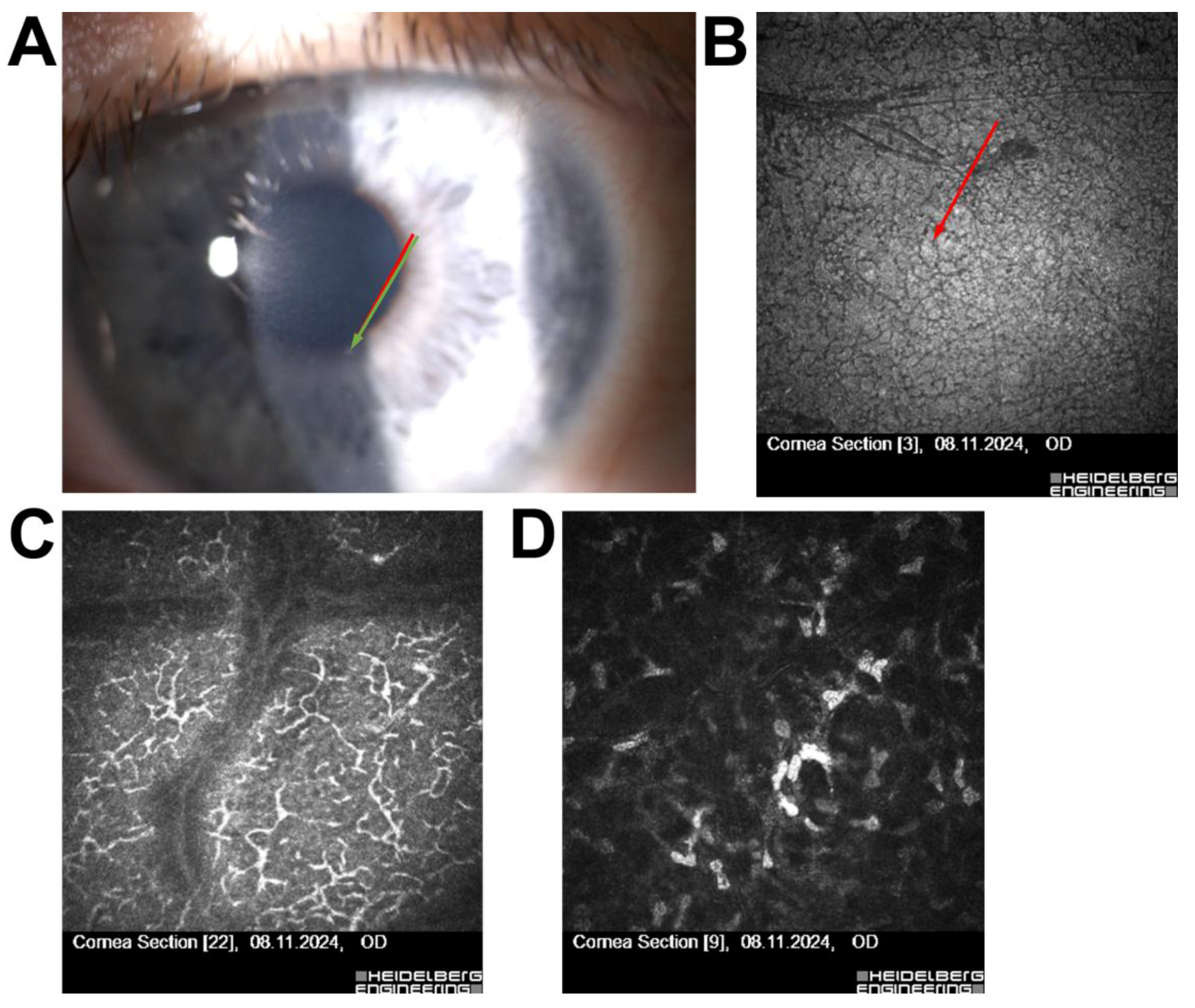
| 1 | 52/F | Long-term contact lens use | Subepithelial opacities | Thygeson’s superficial punctate keratitis | Disqualified from surgery |
| 2 | 30/F | High myopia, astigmatism | Hudson–Stähli line (iron deposition) | Benign corneal iron line | Disqualified due to ectasia risk |
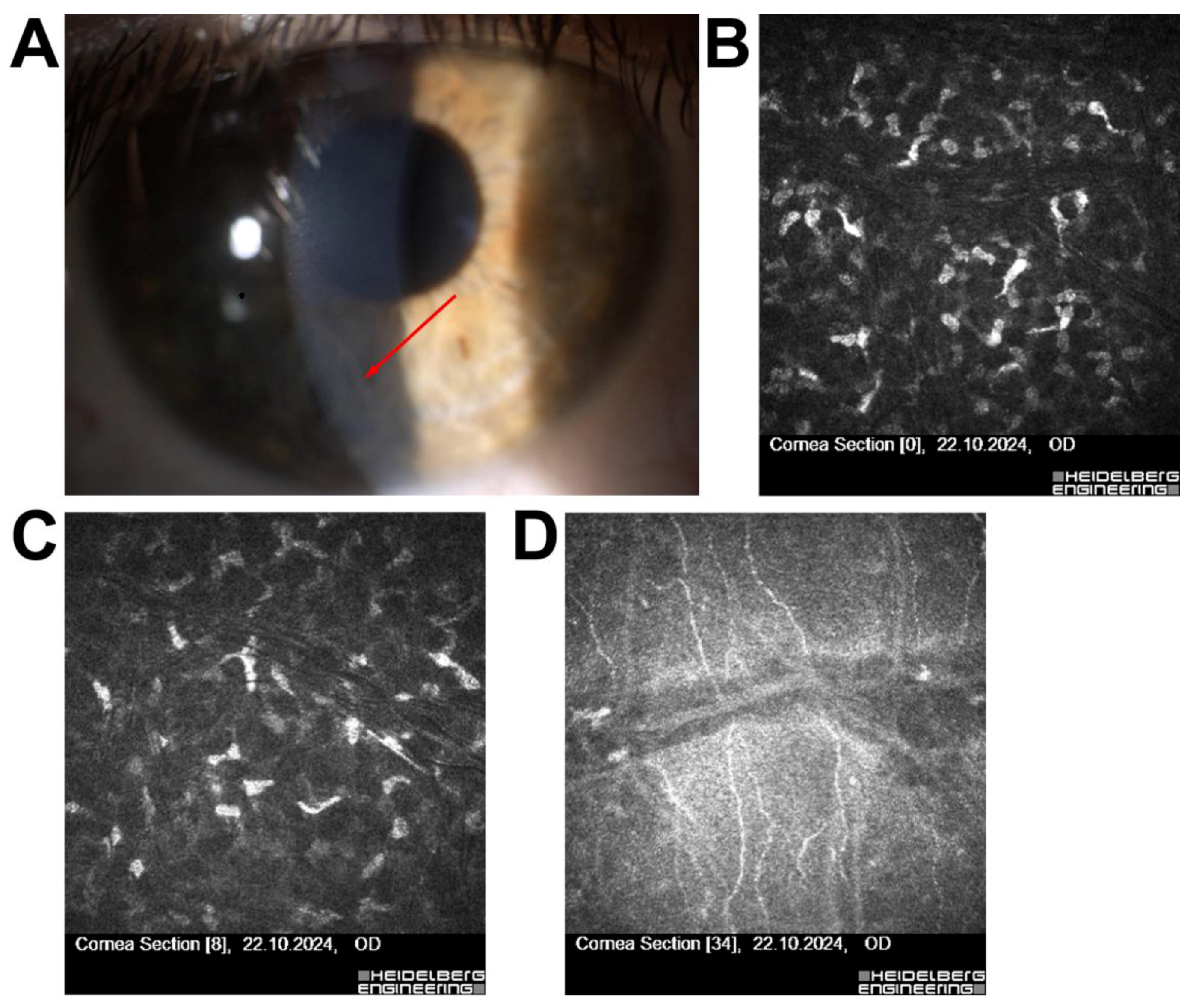
| 3 | 49/F | Corneal foreign body removal (remote) | No active inflammation | Low ectasia risk | Qualified for surgery |
| 4 | 29/M | Scheduled for ReLEx SMILE | Superficial corneal scar, no stromal/inflammatory changes | Scar without contraindications | Surgery completed without complications |
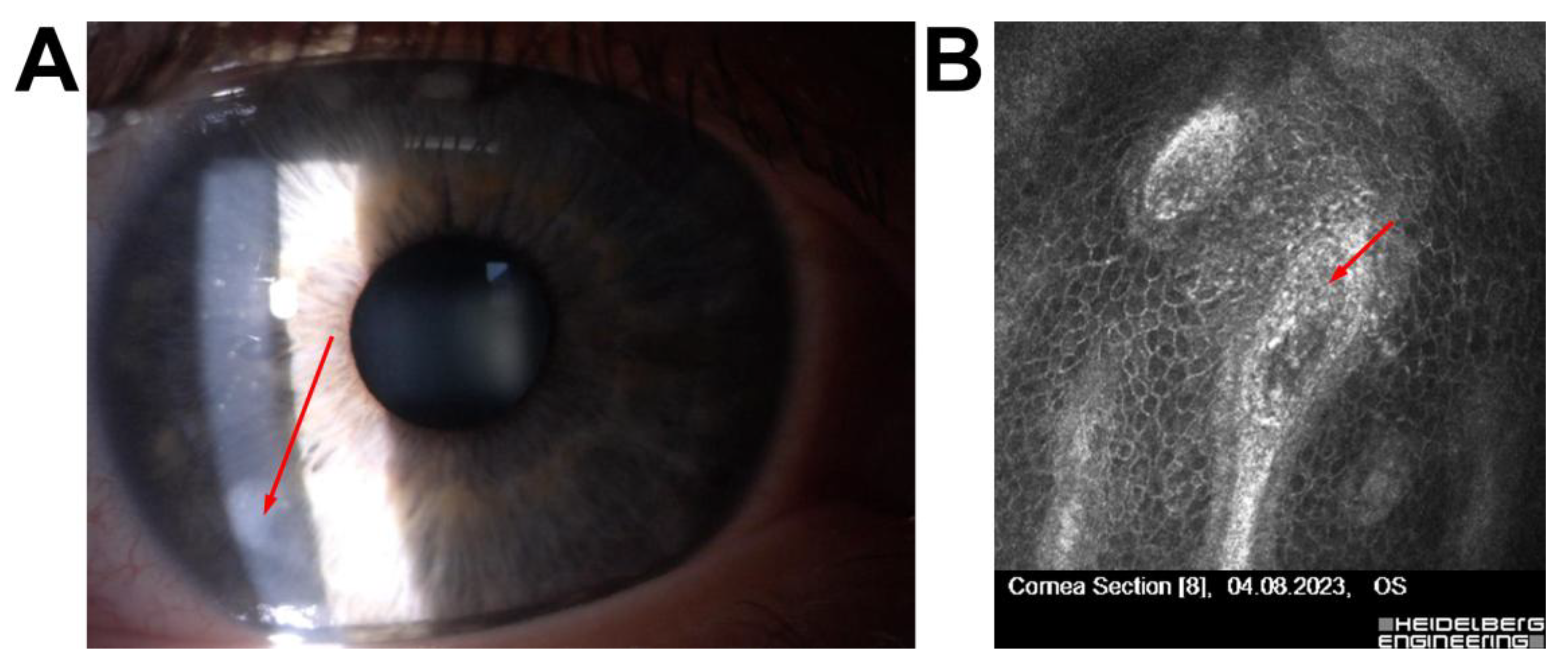
| 5 | 54/F | High myopia, low corneal thickness, large pupil | Pre-Descemet’s corneal dystrophy, no endotheliitis | Dystrophy | Qualified for phakic ICL implantation |
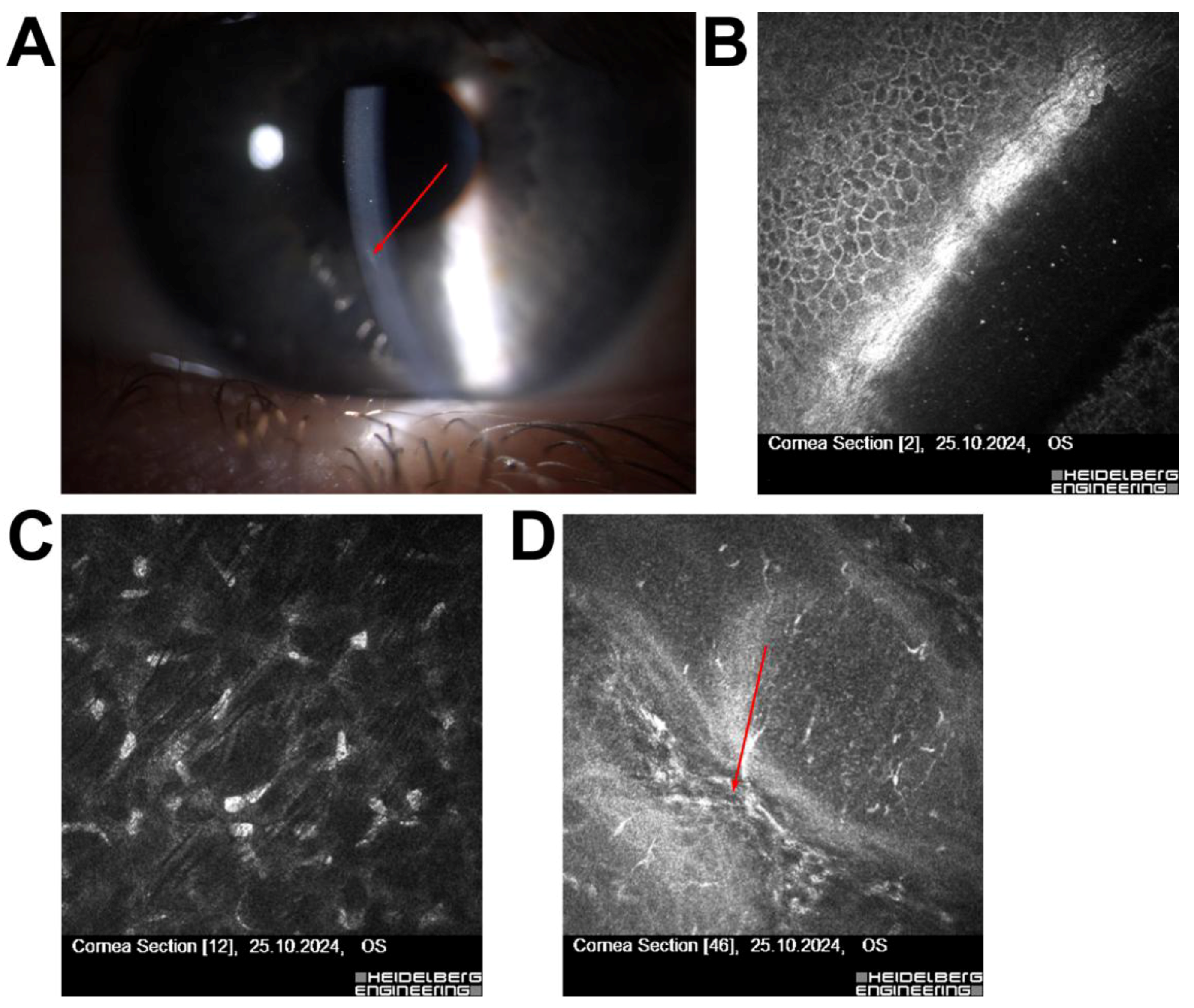
| 6 | 56/M | Post-Femto-LASIK (3 years), fluctuating vision | Features of Cogan’s dystrophy | Secondary Cogan’s dystrophy | Treated with PTK (20 µm), UCVA improved |
| 7 | 58/M | Asymptomatic, subepithelial lesion | Subclinical herpes simplex keratitis | Herpes simplex (subclinical) | Antiviral prophylaxis (acyclovir) before planned surgery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janiszewska-Bil, D.; Kijonka, M.; Kokot-Lesiuk, J.; Derhartunian, V.; Lyssek-Boroń, A.; Dobrowolski, D.; Wylęgała, E.; Grabarek, B.O.; Krysik, K. Enhancing Safety in Refractive Surgery: A Pilot Evaluation of In Vivo Confocal Microscopy. J. Clin. Med. 2025, 14, 7714. https://doi.org/10.3390/jcm14217714
Janiszewska-Bil D, Kijonka M, Kokot-Lesiuk J, Derhartunian V, Lyssek-Boroń A, Dobrowolski D, Wylęgała E, Grabarek BO, Krysik K. Enhancing Safety in Refractive Surgery: A Pilot Evaluation of In Vivo Confocal Microscopy. Journal of Clinical Medicine. 2025; 14(21):7714. https://doi.org/10.3390/jcm14217714
Chicago/Turabian StyleJaniszewska-Bil, Dominika, Magdalena Kijonka, Joanna Kokot-Lesiuk, Victor Derhartunian, Anita Lyssek-Boroń, Dariusz Dobrowolski, Edward Wylęgała, Beniamin Oskar Grabarek, and Katarzyna Krysik. 2025. "Enhancing Safety in Refractive Surgery: A Pilot Evaluation of In Vivo Confocal Microscopy" Journal of Clinical Medicine 14, no. 21: 7714. https://doi.org/10.3390/jcm14217714
APA StyleJaniszewska-Bil, D., Kijonka, M., Kokot-Lesiuk, J., Derhartunian, V., Lyssek-Boroń, A., Dobrowolski, D., Wylęgała, E., Grabarek, B. O., & Krysik, K. (2025). Enhancing Safety in Refractive Surgery: A Pilot Evaluation of In Vivo Confocal Microscopy. Journal of Clinical Medicine, 14(21), 7714. https://doi.org/10.3390/jcm14217714






