Abstract
Background: Acute Respiratory Distress Syndrome (ARDS) was first described in 1967 by Ashbaugh et al. as a severe acute hypoxemic respiratory failure with reduced lung compliance, representing a common end-path of severe pulmonary endothelial inflammation from diverse etiologies. Since then, several definitions for the adult syndrome have been proposed, culminating in the 2024 “New Global Definition” (Berlin 2.0). In pediatrics, dedicated criteria (pediatric ARDS, PARDS) have been established over the past decade, with the most recent update published by the Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) in 2023. Methods: We performed a narrative literature review of consensus statements and key studies defining ARDS in adult and pediatric (non-neonatal) populations. Primary sources included the full Berlin 2.0 and PALICC-2 documents, supplemented by PubMed, Embase, and society guidelines. Definitions were compared across major diagnostic domains: timing of onset, imaging requirements, oxygenation thresholds, inclusion of patients with chronic comorbidities, ventilatory support modalities, and applicability in resource-limited settings. Results: Both definitions show convergence in incorporating non-invasive oxygenation indices and adaptability to resource-limited contexts. Key distinctions include the use of the Oxygenation Index (OI) or Oxygen Saturation Index (OSI) in invasively ventilated pediatric patients—metrics that integrate mean airway pressure and correlate more strongly than PaO2/FIO2 with short-term outcomes—and PALICC-2’s explicit inclusion of patients with chronic lung disease or cyanotic congenital heart disease when acute deterioration is documented. Imaging criteria differ, with Berlin 2.0 requiring bilateral opacities (and permitting lung ultrasound) versus PALICC-2’s acceptance of unilateral findings. Conclusions: Berlin 2.0 and PALICC-2 represent substantial progress toward globally applicable ARDS definitions, but physiologic and structural differences remain. These distinctions have prognostic and research implications, and harmonization will be critical to improve cross-age comparability, optimize clinical trial design, and ultimately enhance patient outcomes.
1. Introduction
Acute Respiratory Distress Syndrome (ARDS) is a life-threatening condition characterized by acute severe hypoxemic respiratory failure secondary to non-cardiogenic pulmonary edema, first described in 1967 by Ashbaugh et al. [1]. Since then, its clinical definition has undergone multiple revisions to improve diagnostic accuracy and standardization for both clinical care and research (Figure 1). The most widely adopted adult definition, the Berlin definition, was established in 2012 [2,3], but its limitations [4]—particularly in resource-limited settings lacking chest radiography, arterial blood gas analysis, or mechanical ventilation—led to adaptations such as the Kigali modification [5].
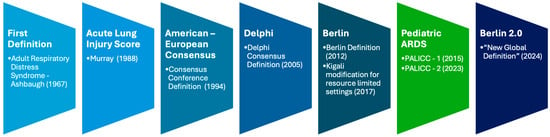
Figure 1.
ARDS definitions timeline [1,2,5,6,7,8].
Recently, the wider use of non-invasive oxygenation indices [9,10,11,12,13], High-Flow Nasal Oxygen (HFNO), especially during COVID-19 [14,15,16], and lung ultrasound have challenged the Berlin criteria and prompted calls to include these modalities in ARDS definitions [17,18,19,20,21]. To address these shifts in practice, a global consensus conference in 2021 produced the 2024 “New Global Definition” (Berlin 2.0) [6], expanding diagnostic inclusivity while maintaining comparability with previous definitions.
Pediatric ARDS (PARDS) presents additional challenges due to age-specific physiology, epidemiology, and management considerations. The first pediatric-specific definition, proposed by the Pediatric Acute Lung Injury Consensus Conference (PALICC) Group in 2015 [7,22,23], was followed by an international description of PARDS incidence and epidemiology [24]. Subsequent years saw advances in pathobiology, lung-protective strategies, and supportive technologies [25,26,27], alongside variability in adoption and implementation across settings, with implications for outcomes [28,29,30]. The updated PALICC-2 criteria [8] incorporate physiological refinements, explicit guidance for patients with chronic comorbidities, and adaptations for resource-limited environments, while also integrating advances in digital monitoring, automated data capture, and multicenter data-sharing platforms.
Despite this progress, important discrepancies remain between adult and pediatric ARDS definitions—most notably in oxygenation thresholds, imaging criteria, and the scope of patients they encompass. Understanding these differences is essential not only for accurate diagnosis and prognostication, but also for harmonizing research, facilitating cross-age comparisons, helping improving patient outcomes. This manuscript compares Berlin 2.0 and PALICC-2 across key diagnostic domains, evaluating their strengths, weaknesses, and implications for future research and clinical practice.
2. Materials and Methods
We conducted a literature review to identify and compare the most recent consensus definitions of ARDS in adults and pediatric patients. Searches were performed in MEDLINE/PubMed, Embase, Web of Science Core Collection, Scopus, and the Cochrane Library for records from 1 January 1967 to 31 August 2025. Full search strings and key words are listed in Supplementary File S1. We included peer-reviewed guidelines, consensus statements, systematic reviews, and original studies that proposed, evaluated, or compared ARDS definitions (adult or pediatric). We excluded non-human studies, non-peer-reviewed sources, single-patient case reports without discussion of diagnostic criteria, and non-English/Spanish articles.
The 2024 “New Global Definition” (Berlin 2.0) for adult ARDS [6] and the 2023 Second Pediatric Acute Lung Injury Consensus Conference definition [8] were selected as the primary reference frameworks. Full-text documents for both definitions were reviewed in detail, focusing on their diagnostic domains, inclusion and exclusion criteria, and adaptations for resource-limited settings.
Comparative analysis was structured around oxygenation metrics, imaging features, cardiac criteria, patient population inclusivity, and diagnostic pathways according to type of respiratory support. Additional literature was reviewed to contextualize prognostic relevance, particularly of the oxygenation index (OI) in pediatric populations, and to identify strengths, weaknesses, and potential areas for harmonization. Findings were synthesized into summary tables and narrative sections to facilitate interpretation for both clinical and research applications.
3. Results
Key similarities and differences between the two aforementioned definitions emerged across multiple diagnostic domains, as detailed below.
- Oxygenation criteria
In adults, the primary metric for defining hypoxemia is the arterial oxygen tension to inspired oxygen fraction ratio (PaO2/FIO2, in mmHg). Severity categories for intubated patients are: mild (>200–≤300), moderate (≤200–>100), and severe (≤100). When arterial blood gases are unavailable, the SpO2/FIO2 ratio can be used, with corresponding thresholds: mild (>235–≤315), moderate (≤235–>148), and severe (≤148). Patients on Non-Invasive Mechanical Ventilation (NIV), CPAP or HFNO therapy (≥30 L/min) qualify for ARDS criteria when the PaO2/FIO2 < 300 mmHg or SpO2/FIO2 < 315 (if SpO2 < 97%). The latest update includes an additional category for resource-limited settings where neither arterial blood gas, HFNO, NIV, and/or mechanical ventilation are routinely available. In this case, an SpO2/FIO2 ≤ 315 is indicative to trigger an ARDS ventilatory approach.
In pediatric patients, PALICC-2 uses the Oxygenation Index (OI) (OI = mean airway pressure (MAP) (cmH2O) × FIO2/PaO2 (mmHg)) or the OSI (OSI = MAP × FIO2/SpO2) as the preferred metrics when invasive ventilation is in place. PARDS is defined when the OI ≥ 4 or OSI ≥ 5, with only two severity groups: mild/moderate: OI < 16 or OSI <12, and severe: OI ≥ 16 or OSI ≥ 12. Patients on NIV support are categorized as follows: mild/moderate: PaO2/FIO2 > 100 or SpO2/FIO2 > 150; severe: PaO2/FIO2, ≤100 or SpO2/FIO2 ≤ 150. Additionally, PALICC-2 includes two categories for patients with “Possible PARDS” (Nasal CPAP/NIV or HFNO (≥1.5 L/kg/min or ≥30 L/min) with PaO2/FIO2, ≤300 or SpO2/FIO2 ≤ 250) and “At risk for PARDS” (Any interface with and oxygen supplementation to maintain SpO2 ≥ 88% but not meeting definition for PARDS or possible PARDS) provided that the respiratory failure is not justified solely from airway obstruction (e.g., asthma, bronchospasm). These differences are summarized in Table 1.

Table 1.
Oxygenation criteria differences between Berlin 2.0 and PALICC-2 definitions.
- Imaging criteria
Berlin 2.0 requires the presence of bilateral opacities on chest radiograph, Computed Tomography (CT), or lung ultrasound (LUS), not fully explained by effusions, collapse, or nodules. PALICC-2, in contrast, accepts unilateral or bilateral opacities, recognizing that focal disease patterns are more common in children. Imaging modalities in PALICC-2 are limited to radiography or CT, whereas lung ultrasound is not included. The inclusion of LUS in adult criteria may expand diagnostic access in resource-limited settings but introduces potential operator-dependent variability.
- Hemodynamic criteria
In adults, ARDS diagnosis requires that respiratory failure is not fully explained by cardiac failure or fluid overload, and objective assessment (e.g., echocardiography) should be used when available to exclude the predominance of a hydrostatic edema. In pediatrics, left ventricular dysfunction or cyanotic congenital heart disease do not preclude a PARDS diagnosis, as long as there is a known precipitating acute pulmonary insult and a documented functional deterioration from baseline oxygenation status. Both definitions acknowledge the frequent overlapping of respiratory and hemodynamic pathophysiologic features and propose more rational and clinically adequate criteria than past definitions.
- Other diagnostic considerations
Both definitions require onset within one week of a known clinical insult or new/worsening respiratory symptoms, and both allow clinical judgment when precise timing is unclear. Both accept a range of underlying etiologies, but PALICC-2 is explicit in including chronic pulmonary or cardiac conditions when criteria for acute deterioration are met. Additionally, as previously commented, both definitions now incorporate considerations for resource-limited settings, adjusting oxygenation thresholds when advanced monitoring is not available.
Neither the Berlin nor the PALICC definitions incorporate sex-specific criteria. However, observational studies have identified gender-related disparities in ARDS management [31]. Women, especially those of shorter stature, are less likely to receive low-tidal-volume ventilation because predicted body weight is derived from height and sex, and errors in height estimation disproportionately affect them [32,33]. Although ARDS incidence appears higher in men and adjusted mortality is generally similar, some analyses suggest worse outcomes among women with severe ARDS [31]. Pregnancy introduces additional physiologic considerations [34], but pregnant patients remain systematically excluded from major ARDS trials, leaving an important evidence gap.
4. Discussion
The updated Berlin 2.0 and PALICC-2 definitions represent the most current consensus frameworks for diagnosing ARDS in adults and children, respectively, reflecting substantial advances in physiologic understanding, monitoring capabilities, and adaptability to diverse clinical environments. While both share common structural elements—such as onset criteria, inclusivity of multiple etiologies, and adaptations for low-resource settings—they diverge in key domains that have direct implications for prognosis, research comparability, and bedside applicability (Figure 2). Central to this divergence is the choice of oxygenation metrics, with Berlin 2.0 retaining the PaO2/FIO2, ratio as its primary tool, and PALICC-2 prioritizing the Oxygenation Index for invasively ventilated patients. The following discussion examines some of the limitations of current criteria, the prognostic role of OI in pediatric populations comparing the operational strengths and limitations of each definition.
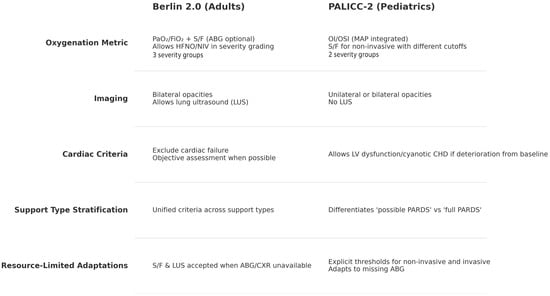
Figure 2.
Main differences between Berlin 2.0 and PALICC-2 diagnostic criteria.
- Oxygenation criteria in the ARDS definition
As mentioned, oxygenation criteria represented by the PaO2/FIO2, as a categorization of the deterioration in lung function, remain the cornerstone of the ARDS definition in adults. In favor is the abundance of data coming from the numerous clinical trials using this criterion. There are however several unsolved shortcomings. The relationship between PaO2 and FIO2 is non-linear, especially at lower levels of FIO2, limiting its validity when assessed in non-standardized conditions as is the case in the Berlin definition. PaO2/FIO2, can vary greatly depending on the FIO2 used as most of the lung regions in ARDS function as low ventilation to perfusion ratio units. The ratio also changes in response to time spent on mechanical ventilation and ventilator settings, particularly PEEP. Although a minimum PEEP of 5 cmH2O is mandated in the Berlin definition, the clinical heterogeneity in ventilatory strategies and PEEP titration limits the reliability of this criterion. Furthermore, the use of SpO2 instead of PaO2 increases this shortcoming with a potential larger impact in resource limited environments. Therefore, future revisions of oxygenation criteria, especially in the adult population, should consider a standardized assessment, for instance as the one proposed by Villar et al. [35] who demonstrated that the assessment of ARDS criteria combining a PEEP of 10 cmH2O and an FIO2 ≥ 0.5, 24 after initiation of mechanical ventilation was more closely related to ARDS severity than non-standardized assessments.
- Relevance of Oxygenation Index (OI) in Mechanically Ventilated Pediatric Patients
Multiple studies have demonstrated that serial measurements of OI during the early course of PARDS provide superior prognostic accuracy compared to a single baseline assessment [23,36,37]. In particular, OI measured at 24–72 h after diagnosis has been shown to refine mortality risk stratification, with the 24-h value emerging as a simple and highly accurate predictor of 30-day survival [38,39]. In pediatric oncology patients with PARDS, for example, each incremental increase in OI at 24 h was independently associated with a higher hazard of death at 30 days, with pragmatic thresholds of OI ≥ 10 or PaO2/FIO2 ≤ 150 identifying mortality rates of approximately 50%, compared to ~10% in patients below these cutoffs [40]. Similar trends have been reported in general PICU cohorts, where OI correlates more strongly than PaO2/FIO2 with illness severity, multi-organ dysfunction, and the need for advanced support such as ECMO [41,42].
The non-invasive surrogate OSI, which substitutes SpO2 for PaO2, has been shown to reproduce much of OI’s prognostic capability when arterial blood gases are unavailable [43,44]. OSI-based severity categories align well with OI and can be monitored continuously at the bedside, though they inherit the limitations of pulse oximetry—including reduced accuracy at high saturations, in low perfusion states, or in patients with darker skin pigmentation [45,46,47].
Beyond short-term mortality, OI trajectories during the first week of illness have been associated with other clinically relevant outcomes, including ventilator-free days and the resolution of PARDS [48,49]. A persistently elevated or rising OI despite optimized ventilatory management signals refractory disease and is often used to prompt evaluation for rescue therapies such as ECMO. While there is no universally accepted OI threshold for initiating ECMO in pediatrics, many centers consider referral when OI remains high despite optimal lung-protective strategies, with decisions guided by the broader clinical context [50,51].
Evidence linking OI directly to long-term functional or neurocognitive outcomes is less robust. Survivors of PARDS, particularly those requiring prolonged mechanical ventilation, may experience deficits in physical function, health-related quality of life, and neurodevelopment, but these sequelae appear to result from a complex interplay of factors—including the severity and duration of hypoxemia, sedation exposure, delirium, immobility, and systemic inflammation—rather than OI alone [52,53,54]. Current data are insufficient to establish specific OI thresholds that predict neurologic injury, highlighting an important gap for future research.
Therefore, OI—and its non-invasive counterpart OSI—provides a more granular and prognostically relevant measure of respiratory failure severity in mechanically ventilated children than PaO2/FIO2 alone (Figure 3). Incorporating serial OI measurements into routine practice can improve early risk stratification, guide escalation of support, and inform decisions about rescue interventions. However, its role as a predictor of long-term morbidity, particularly neurocognitive outcomes, remains incompletely defined and warrants further investigation.
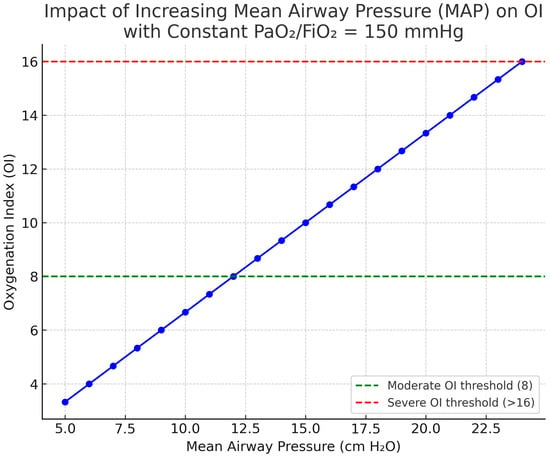
Figure 3.
Influence of mean airway pressure (MAP) on the oxygenation index (OI). For a given PaO2/FIO2 (e.g., 150 mmHg for this example), MAP increments raise OI, reflecting the ventilatory burden required to sustain oxygenation. Thus, OI increases linearly together with MAP increments despite a constant PaO2/FIO2 of 150 mmHg. Abbreviations: MAP, mean airway pressure; OI, oxygenation index; OSI, oxygen saturation index; FiO2, fraction of inspired oxygen. OI = (MAP × FIO2 × 100)/PaO2; OSI is the saturation-based analogue using SpO2 instead of PaO2. Image generated using ChatGPT 5.0.
- Strengths and weaknesses of Berlin 2.0 and PALICC-2
Both Berlin 2.0 and PALICC-2 preserve the one-week onset criterion, accept a broad range of underlying etiologies, and incorporate diagnostic pathways suitable for resource-limited settings. They also acknowledge the role of SpO2/FIO2 when arterial blood gases are unavailable, although they differ in the precise thresholds and in the clinical scenarios where this substitution is appropriate.
Berlin 2.0 stands out for its broad operational scope. By explicitly including patients on high-flow nasal oxygen and non-invasive ventilation within its severity classification, and by allowing the use of lung ultrasound as an imaging alternative, it greatly enhances applicability in diverse healthcare contexts. The definition also offers distinct diagnostic tracks for intubated, non-intubated, and low-resource settings, enabling flexibility in environments where advanced monitoring or imaging may not be available. This global implementability is further strengthened by the accommodation of oxygenation ratios and LUS when Arterial Blood Gas (ABG) or chest radiography are lacking, as well as by the removal of fixed PEEP requirements in resource-limited contexts. Importantly, Berlin 2.0 retains the PaO2/FIO2 metric and its established severity cutoffs, preserving continuity with decades of prior research and facilitating longitudinal comparisons.
However, these strengths come with some limitations. The requirement for bilateral opacities on imaging may inadvertently exclude clinically relevant cases with unilateral involvement [3], which would be considered under the pediatric criteria. While the inclusion of LUS expands diagnostic accessibility, it introduces operator-dependent variability [55], meaning that accuracy is strongly influenced by the skill and training of the clinician performing the scan. The reliance on SpO2/FIO2 ratios also carries the inherent biases and inaccuracies of pulse oximetry, particularly in patients with darker skin pigmentation, at high saturation levels (SpO2 > 97%), or in states of poor perfusion—circumstances in which ABG confirmation is advisable when important management decisions are at stake [45,46,47]. Furthermore, the prognostic value of Berlin 2.0’s severity classification in non-intubated patients remains uncertain and will require validation in prospective studies.
PALICC-2, in contrast, derives much of its strength from the physiological depth of its primary oxygenation metrics. Including mean airway pressure in OI and OSI captures both hypoxemia severity and the ventilatory burden needed to sustain it. This nuance makes OI and OSI more closely aligned with the pathophysiology of pediatric respiratory failure and more predictive of outcomes in invasively ventilated children. PALICC-2’s diagnostic structure further differentiates between “possible PARDS” in patients receiving high-flow or nasal interfaces and “full PARDS” in those on CPAP, BiPAP, or invasive ventilation, preventing the conflation of physiologically distinct groups. The explicit inclusion of patients with chronic pulmonary disease or cyanotic congenital heart disease, provided there is an acute deterioration from baseline, broadens clinical applicability and reflects the realities of pediatric intensive care. The acceptance of unilateral opacities in imaging is similarly pragmatic, acknowledging that focal patterns are common in children [56]. In adults the limitations of the imaging criteria are progressively being under debate. First the low sensitivity of chest radiographs [57] and the fact that an initial unilateral involvement can rapidly progress to bilateral infiltrates may miss an early diagnosis of ARDS. Second, severe respiratory failure with unilateral lung involvement shares many of the pathophysiologic, treatment and outcome characteristics of ARDS.
Nevertheless, PALICC-2 is not without challenges. The use of OI and OSI requires accurate, standardized measurement of mean airway pressure, and differences in ventilator management across centers may affect these values independent of the patient’s underlying lung injury. Like Berlin 2.0, PALICC-2’s reliance on SpO2 in OSI and SpO2/FIO2 carries the limitations of pulse oximetry [45,46,47], which can compromise accuracy in certain patient populations and clinical situations. The absence of lung ultrasound from its imaging criteria avoids the pitfalls of operator variability but removes a potentially valuable tool in contexts where chest radiography or CT are not feasible. Finally, the non-equivalence of severity thresholds and primary oxygenation metrics compared to Berlin 2.0 complicates direct cross-age epidemiological comparisons and the synthesis of data in multicenter or mixed-age research.
Therefore, Berlin 2.0 offers a globally adaptable framework that balances innovation with continuity, while PALICC-2 provides a physiologically targeted approach that may more accurately reflect disease severity in children. Both definitions make meaningful contributions toward aligning ARDS diagnosis with modern clinical practice, but each carries trade-offs between inclusivity, operational complexity, diagnostic precision, and comparability across age groups. Addressing these differences, especially in the context of collaborative research, remains an important challenge ahead.
- Gender considerations:
While current ARDS definitions are gender-neutral, their implementation may perpetuate bias, particularly in ventilatory management. Systematic measurement of height, integration of predicted body weight calculators into clinical workflows, and tailored protocols for pregnancy could mitigate these disparities. Future research should prespecify sex-stratified analyses and actively address the evidence gap in pregnant patients to ensure equitable diagnosis, assessment, and management of ARDS.
- Key sources of heterogeneity and reporting standards
Recently proposed consensus definitions have advanced a harmonized conceptual model of ARDS and broadened diagnostic inclusivity, but clinical adoption still requires prospective evidence of reliability and validity. Therefore, we propose a minimum validation framework comprising (i) standardized imaging reporting with inter-rater agreement; (ii) predefined HFNO settings; (iii) oxygenation metrics listed with well-defined ventilatory context (PEEP and MAP) and, when feasible, sensitivity analyses using OI/OSI, P/FP (i.e., PaO2/FIO2 adjusted for PEEP) [58], or mechanical power (MP)—an estimate of energy delivered by the ventilator per unit time [59].
To curb heterogeneity, we recommend structured chest radiograph/LUS interpretation (with double reading and competency statements), and an HFNO reporting set—device/interface, flow (L·min−1 and L·kg−1·min−1 in pediatrics), FIO2, cannula fit, patient position, sampling timing, and concurrent NIV/CPAP. Mechanical power has biological plausibility and prognostic validation, but methodologic nuances and pediatric data gaps argue for reporting it as a research metric rather than a diagnostic criterion at this stage. Thus, we believe that a transparent, standardized reporting will support reproducibility and the validation needed for safe implementation across adult and pediatric populations.
5. Conclusions
Current ARDS definitions for adults and children represent a substantial evolution towards frameworks that are more adaptable, physiologically informed, and globally applicable. Both aim to balance diagnostic precision with flexibility across a range of clinical contexts, yet their effective implementation will depend on local resources, training, and the capacity to collect standardized data. Future progress will hinge on harmonizing adult and pediatric criteria to facilitate cross-age research, validating prognostic tools like the oxygenation index in diverse populations, and integrating novel modalities—such as advanced imaging and non-invasive monitoring—without compromising feasibility in low-resource environments. Large-scale, prospective studies linking diagnostic criteria to both short- and long-term outcomes will be essential, not only to refine severity classification but also to ensure that definitions remain aligned with evolving evidence, technology, gender disparities and the overarching goal of improving survival and quality of life for patients with ARDS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14217644/s1, File S1: Keywords used during literature search.
Author Contributions
Conceptualization, P.G.-P. and F.S.-S.; methodology, P.G.-P.; writing—original draft preparation, P.G.-P.; writing—review and editing, P.G.-P. and F.S.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABG | Arterial Blood Gas |
| ARDS | Acute Respiratory Distress Syndrome |
| BiPAP | Bilevel Positive Airway Pressure |
| CPAP | Continuous Positive Airway Pressure |
| CT | Computed Tomography |
| CXR | Chest Radiography |
| ECMO | Extracorporeal Membrane Oxygenation |
| HFNO | High-Flow Nasal Oxygen |
| LUS | Lung Ultrasound |
| MAP | Mean Airway Pressure |
| NIV | Non-Invasive Ventilation |
| OI | Oxygenation Index |
| OSI | Oxygen Saturation Index |
| PALICC | Pediatric Acute Lung Injury Consensus Conference |
| PARDS | Pediatric Acute Respiratory Distress Syndrome |
| PEEP | Positive End-Expiratory Pressure |
| SpO2 | Pulse Oximetry |
References
- Ashbaugh, D.G.; Bigelow, D.B.; Petty, T.L.; Levine, B.E. Acute respiratory distress in adults. Lancet 1967, 2, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Taylor Thompson, B.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. ARDS Definition Task Force. Acute respiratory distress syndrome: The Berlin definition. J. Am. Med. Assoc. 2012, 307, 2526–2533. [Google Scholar]
- Ferguson, N.D.; Fan, E.; Camporota, L.; Antonelli, M.; Anzueto, A.; Beale, R.; Brochard, L.; Brower, R.; Esteban, A.; Gattinoni, L.; et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012, 38, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.J.; Dünser, M.W.; Dondorp, A.M.; Adhikari, N.K.; Iyer, S.; Kwizera, A.; Lubell, Y.; Papali, A.; Pisani, L.; Riviello, E.D.; et al. Current challenges in the management of sepsis in ICUs in resource-poor settings and suggestions for the future. Intensive Care Med. 2017, 43, 612–624. [Google Scholar] [CrossRef]
- Riviello, E.D.; Kiviri, W.; Twagirumugabe, T.; Mueller, A.; Banner-Goodspeed, V.M.; Officer, L.; Novack, V.; Mutumwinka, M.; Talmor, D.S.; Fowler, R.A. Hospital incidence and outcomes of the acute respiratory distress syndrome using the Kigali modification of the Berlin definition. Am. J. Respir. Crit. Care Med. 2016, 193, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Arabi, Y.; Arroliga, A.C.; Bernard, G.; Bersten, A.D.; Brochard, L.J.; Calfee, C.S.; Combes, A.; Daniel, B.M.; Ferguson, N.D.; et al. A New Global Definition of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2024, 209, 37–47. [Google Scholar] [CrossRef]
- Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: Consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr. Crit. Care Med. 2015, 16, 428–439. [Google Scholar] [CrossRef]
- Emeriaud, G.; López-Fernández, Y.M.; Iyer, N.P.; Bembea, M.M.; Agulnik, A.; Barbaro, R.P.; Baudin, F.; Bhalla, A.; De Carvalho, W.B.; Carroll, C.L.; et al. Executive Summary of the Second International Guidelines for the Diagnosis and Management of Pediatric Acute Respiratory Distress Syndrome (PALICC-2). Pediatr. Crit. Care Med. 2023, 24, 143–168. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Hayden, D.L.; Schoenfeld, D.A.; Ware, L.B.; National Institutes of Health National Heart Lung Blood Institute ARDS Network. Comparison of the SpO2/FIO2 ratio the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007, 132, 410–417. [Google Scholar] [CrossRef]
- Brown, S.M.; Grissom, C.K.; Moss, M.; Rice, T.W.; Schoenfeld, D.; Hou, P.C.; Thompson, B.T.; Brower, R.G. Nonlinear imputation of PaO2/FIO2 from SpO2/FIO2 among patients with acute respiratory distress syndrome. Chest 2016, 150, 307–313. [Google Scholar] [CrossRef]
- Vercesi, V.; Pisani, L.; van Tongeren, P.S.; Lagrand, W.K.; Leopold, S.J.; Huson, M.M.; Henwood, P.C.; Walden, A.; Smit, M.; Riviello, E.D.; et al. External confirmation exploration of the Kigali modification for diagnosing moderate or severe ARDS. Intensive Care Med. 2018, 44, 523–524. [Google Scholar] [CrossRef]
- Moss, M.; Huang, D.T.; Brower, R.G.; Ferguson, N.D.; Ginde, A.A.; Gong, M.N.; Grissom, C.K.; Gundel, S.; Hayden, D.; Hite, R.D.; et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N. Engl. J. Med. 2019, 380, 1997–2008. [Google Scholar]
- Wick, K.D.; Matthay, M.A.; Ware, L.B. Pulse oximetry for the diagnosis and management of acute respiratory distress syndrome. Lancet Respir. Med. 2022, 10, 1086–1098. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Tonetti, T.; Navalesi, P.; Nava, S.; Antonelli, M.; Pesenti, A.; Grasselli, G.; Grieco, D.L.; Menga, L.S.; Pisani, L.; et al. High flow nasal oxygen for severe hypoxemia: Oxygenation response and outcome in COVID-19 patients. Am. J. Respir. Crit. Care Med. 2022, 205, 431–439. [Google Scholar] [CrossRef]
- Gershengorn, H.B.; Hu, Y.; Chen, J.T.; Hsieh, S.J.; Dong, J.; Gong, M.N.; Chan, C.W. The impact of high-flow nasal cannula use on patient mortality and the availability of mechanical ventilators in COVID-19. Ann. Am. Thorac. Soc. 2021, 18, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Calligaro, G.L.; Lalla, U.; Audley, G.; Gina, P.; Miller, M.G.; Mendelson, M.; Dlamini, S.; Wasserman, S.; Meintjes, G.; Peter, J.; et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: A multi-centre prospective observational study. EClinicalMedicine 2020, 28, 100570. [Google Scholar] [CrossRef]
- Ware, L.B. Go with the flow: Expanding the definition of acute respiratory distress syndrome to include high-flow nasal oxygen. Am. J. Respir. Crit. Care Med. 2022, 205, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Thompson, B.T.; Ware, L.B. The Berlin definition of acute respiratory distress syndrome: Should patients receiving high-flow nasal oxygen be included? Lancet Respir. Med. 2021, 9, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Wooten, W.M.; Shaffer, L.E.T.; Hamilton, L.A. Bedside ultrasound versus chest radiography for detection of pulmonary edema: A prospective cohort study. J. Ultrasound Med. 2019, 38, 967–973. [Google Scholar] [CrossRef]
- Sachdev, A.; Khatri, A.; Saxena, K.K.; Gupta, D.; Gupta, N.; Menon, G.R. Chest sonography versus chest radiograph in children admitted to paediatric intensive care—A prospective study. Trop. Dr. 2021, 51, 296–301. [Google Scholar] [CrossRef]
- Smit, M.R.; Hagens, L.A.; Heijnen, N.F.L.; Pisani, L.; Cherpanath, T.G.V.; Dongelmans, D.A.; de Grooth, H.-J.S.; Pierrakos, C.; Tuinman, P.R.; Zimatore, C.; et al. Lung ultrasound prediction model for acute respiratory distress syndrome: A multicenter prospective observational study. Am. J. Respir. Crit. Care Med. 2023, 207, 1591–1601. [Google Scholar] [CrossRef]
- Thomas, N.J.; Jouvet, P.; Willson, D. Acute lung injury in children—Kids really aren’t just little adults. Pediatr. Crit. Care Med. 2013, 14, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Khemani, R.G.; Smith, L.S.; Zimmerman, J.J.; Erickson, S.; Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: Definition, incidence, and epidemiology: Proceedings from the pediatric acute lung injury consensus conference. Pediatr. Crit. Care Med. 2015, 16, S23–S40. [Google Scholar] [CrossRef]
- Khemani, R.G.; Smith, L.; Lopez-Fernandez, Y.M.; Kwok, J.; Morzov, R.; Klein, M.J.; Yehya, N.; Willson, D.; Kneyber, M.C.; Lillie, J.; et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): An international, observational study. Lancet Respir. Med. 2019, 7, 115–128. [Google Scholar] [CrossRef]
- Amato, M.B.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.; Mercat, A.; et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Brochard, L.; Slutsky, A.; Pesenti, A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am. J. Respir. Crit. Care Med. 2017, 195, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Rehder, K.J.; Williford, L.; Cheifetz, I.M.; Turner, D.A. Use of high flow nasal cannula in critically ill infants, children, and adults: A critical review of the literature. Intensive Care Med. 2013, 39, 247–257. [Google Scholar] [CrossRef]
- Rowan, C.M.; Klein, M.J.; Hsing, D.D.; Dahmer, M.K.; Spinella, P.C.; Emeriaud, G.; Hassinger, A.B.; Piñeres-Olave, B.E.; Flori, H.R.; Haileselassie, B.; et al. Early use of adjunctive therapies for pediatric acute respiratory distress syndrome: A PARDIE study. Am. J. Respir. Crit. Care Med. 2020, 201, 1389–1397. [Google Scholar] [CrossRef]
- Khemani, R.G.; Parvathaneni, K.; Yehya, N.; Bhalla, A.K.; Thomas, N.J.; Newth, C.J. PEEP lower than the ARDS network protocol is associated with higher pediatric ARDS mortality. Am. J. Respir. Crit. Care Med. 2018, 198, 77–89. [Google Scholar] [CrossRef]
- Bhalla, A.K.M.; Klein, M.J.; Emeriaud, G.; Lopez-Fernandez, Y.M.; Napolitano, N.R.-N.; Fernandez, A.; Al-Subu, A.M.; Gedeit, R.; Shein, S.L.; Nofziger, R.; et al. Adherence to lung-protective ventilation principles in pediatric acute respiratory distress syndrome: A pediatric acute respiratory distress syndrome incidence and epidemiology study. Crit. Care Med. 2021, 49, 1779–1789. [Google Scholar] [CrossRef]
- McNicholas, B.A.; Madotto, F.; Pham, T.; Rezoagli, E.; Masterson, C.H.; Horie, S.; Bellani, G.; Brochard, L.; Laffey, J.G. Demographics, management and outcome of females and males with acute respiratory distress syndrome in the LUNG SAFE prospective cohort study. Eur. Respir. J. 2019, 54, 1900609. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Hyzy, R.C. Short people got no reason: Gender, height, and disparities in the management of acute lung injury. Crit. Care 2011, 15, 1010. [Google Scholar] [CrossRef]
- Swart, P.; Nijbroek, S.G.L.H.; Paulus, F.; Neto, A.S.; Schultz, M.J. Sex Differences in Use of Low Tidal Volume Ventilation in COVID-19—Insights From the PRoVENT–COVID Study. Front. Med. 2022, 8, 780005. [Google Scholar] [CrossRef]
- Osmundo, G.S., Jr.; Paganotti, C.F.; da Costa, R.A.; Silva, T.H.D.S.; Bombonati, P.C.; Malbouisson, L.M.S.; Francisco, R.P.V. Prone Positioning: A Safe and Effective Procedure in Pregnant Women Presenting with Severe Acute Respiratory Distress Syndrome. Vaccines 2022, 10, 2182. [Google Scholar] [CrossRef]
- Villar, J.; Mora-Ordoñez, J.M.; Soler, J.A.; Mosteiro, F.; Vidal, A.; Ambrós, A.; Fernández, L.; Murcia, I.; Civantos, B.; Romera, M.A.; et al. The PANDORA Study: Prevalence and Outcome of Acute Hypoxemic Respiratory Failure in the Pre-COVID-19 Era. Crit Care Explor. 2022, 4, e0684. [Google Scholar] [CrossRef]
- Yehya, N.; Thomas, N.J.; Khemani, R.G. Risk Stratification Using Oxygenation in the First 24 Hours of Pediatric Acute Respiratory Distress Syndrome. Crit Care Med. 2018, 46, 619–624. [Google Scholar] [CrossRef]
- Wong, J.J.-M.; Phan, H.P.; Phumeetham, S.; Ong, J.S.M.; Chor, Y.K.; Qian, S.; Samransamruajkit, R.; Anantasit, N.; Gan, C.S.; Xu, F.; et al. Risk Stratification in Pediatric Acute Respiratory Distress Syndrome: A Multicenter Observational Study. Crit Care Med. 2017, 45, 1820–1828. [Google Scholar] [CrossRef]
- Pisani, L.; Roozeman, J.-P.; Simonis, F.D.; Giangregorio, A.; van der Hoeven, S.M.; Schouten, L.R.; Horn, J.; Neto, A.S.; Festic, E.; Dondorp, A.M.; et al. Risk stratification using SpO2/FiO2 and PEEP at initial ARDS diagnosis and after 24 h in patients with moderate or severe ARDS. Ann Intensive Care. 2017, 7, 108. [Google Scholar] [CrossRef]
- Rowan, C.M.; Hege, K.M.; Speicher, R.H.; Goodman, M.; Perkins, S.M.; Slaven, J.E.; Westenkirchner, D.F.; Haut, P.R.; Nitu, M.E. Oxygenation index predicts mortality in pediatric stem cell transplant recipients requiring mechanical ventilation. Pediatr Transplant. 2012, 16, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, L.; Pei, Y.; Huang, H.; Chen, C.; Tang, W.; Jiang, X.; Li, Y. The Association Between Oxygenation Status at 24 h After Diagnosis of Pulmonary Acute Respiratory Distress Syndrome and the 30-Day Mortality among Pediatric Oncological Patients. Front. Pediatr. 2022, 10, 805264. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, D.; McCrindle, B.W.; Nakagawa, S.; Bohn, D. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am. J. Respir. Crit. Care Med. 2005, 172, 206–211. [Google Scholar] [CrossRef]
- Khemani, R.G.; Markovitz, B.P.; Curley, M.A.Q. Characteristics of children intubated and mechanically ventilated in 16 PICUs. Chest 2009, 136, 765–771. [Google Scholar] [CrossRef]
- Batchinsky, A.I.; Wendorff, D.B.; Jones, J.B.; Beely, B.R.; Roberts, T.; Choi, J.H.P.; Harea, G.B.; Cancio, L.C.; Davis, M.; Cannon, J.; et al. Noninvasive SpO2/FiO2 ratio as surrogate for PaO2/FiO2 ratio during simulated prolonged field care and ground and high-altitude evacuation. J. Trauma Acute Care Surg. 2020, 89 (Suppl. 2), S126–S131. [Google Scholar] [CrossRef]
- Reddy, M.; Kulkarni, M.; Kanakalakshmi, S.T.; Shenoy, L.; KrishnaBhat, R.R. Non-invasive SpO2/FiO2 ratio (SFR) as surrogate for PaO2/FiO2 ratio (PFR): A scoping review. J. Crit. Care Med. 2025, 11, 221–232. [Google Scholar] [CrossRef]
- Sjoding, M.W.; Dickson, R.P.; Iwashyna, T.J.; Gay, S.E.; Valley, T.S. Racial bias in pulse oximetry measurement. N. Engl. J. Med. 2020, 383, 2477–2478. [Google Scholar] [CrossRef]
- Bickler, P.E.; Feiner, J.R.; Severinghaus, J.W. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiology 2005, 102, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Jubran, A. Pulse oximetry. Crit. Care 2015, 19, 272. [Google Scholar] [CrossRef]
- Yehya, N.; Thomas, N.J. Disassociating lung mechanics and oxygenation in pediatric acute respiratory distress syndrome. Crit. Care Med. 2017, 45, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Newth, C.J.; Sward, K.A.; Khemani, R.G.; Page, K.; Meert, K.L.; Carcillo, J.A.; Shanley, T.P.; Moler, F.W.; Pollack, M.M.; Dalton, H.J.; et al. Variability in usual care mechanical ventilation for pediatric acute respiratory distress syndrome: Time for a Decision Support Protocol? Pediatr. Crit. Care Med. 2017, 18, e521–e529. [Google Scholar] [CrossRef] [PubMed]
- Zabrocki, L.A.; Brogan, T.V.; Statler, K.D.; Poss, W.B.; Rollins, M.D.; Bratton, S.L. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit. Care Med. 2011, 39, 364–370. [Google Scholar] [CrossRef]
- Dalton, H.J.; Reeder, R.; Garcia-Filion, P.; Holubkov, R.; Berg, R.A.; Zuppa, A.; Moler, F.W.; Shanley, T.; Pollack, M.M.; Newth, C.; et al. Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am. J. Respir. Crit. Care Med. 2017, 196, 762–771. [Google Scholar] [CrossRef]
- Manning, J.C.; Pinto, N.P.; Rennick, J.E.; Colville, G.; Curley, M.A.Q. Conceptualizing post–intensive care syndrome in children—The PICS-p framework. Pediatr. Crit. Care Med. 2018, 19, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Knoester, H.; Bronner, M.B.; Bos, A.P. Surviving pediatric intensive care: Physical outcome after 3 months. Intensive Care Med. 2008, 34, 1076–1082. [Google Scholar] [CrossRef]
- Als, L.C.; Picouto, M.D.; Hau, S.M.; Nadel, S.; Cooper, M.; Wray, J. Mental and physical well-being following admission to pediatric intensive care. Pediatr. Crit. Care Med. 2015, 16, e141–e149. [Google Scholar] [CrossRef]
- Mojoli, F.; Bouhemad, B.; Mongodi, S.; Lichtenstein, D. Lung ultrasound for critically ill patients. Am. J. Respir. Crit. Care Med. 2019, 199, 701–714. [Google Scholar] [CrossRef]
- De Luca, D.; Piastra, M.; Chidini, G.; Tissieres, P.; Calderini, E.; Essouri, S.; Villanueva, A.M.; Allende, A.V.; Pons-Odena, M.; Respiratory Section of the European Society for Pediatric Neonatal Intensive Care (ESPNIC); et al. The use of the Berlin definition for acute respiratory distress syndrome during infancy and early childhood: Multicenter evaluation and expert consensus. Intensive Care Med. 2013, 39, 2083–2091. [Google Scholar] [CrossRef]
- Bardají-Carrillo, M.; Martín-Fernández, M.; López-Herrero, R.; Priede-Vimbela, J.M.; Arroyo-Hernantes, I.; Cobo-Zubia, R.; Prieto-Utrera, R.; Gómez-Sánchez, E.; Villar, J.; Tamayo, E.; et al. Chest radiographs in acute respiratory distress syndrome: An Achilles’ heel of the Berlin criteria? Front. Med. 2025, 12, 1554752. [Google Scholar] [CrossRef]
- Palanidurai, S.; Phua, J.; Chan, Y.H.; Mukhopadhyay, A. P/FP ratio: Incorporation of PEEP into the PaO2/FiO2 ratio for prognostication and classification of acute respiratory distress syndrome. Ann. Intensive Care 2021, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Tonetti, T.; Cressoni, M.; Cadringher, P.; Herrmann, P.; Moerer, O.; Protti, A.; Gotti, M.; Chiurazzi, C.; Carlesso, E.; et al. Ventilator-related causes of lung injury: The mechanical power. Intensive Care Med. 2016, 42, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).