Takotsubo Cardiomyopathy and Stressed Heart Morphology: Molecular, Hemodynamic, and Imaging Intersections
Abstract
1. Introduction
2. Methods
3. Results
3.1. Overview of Literature Findings
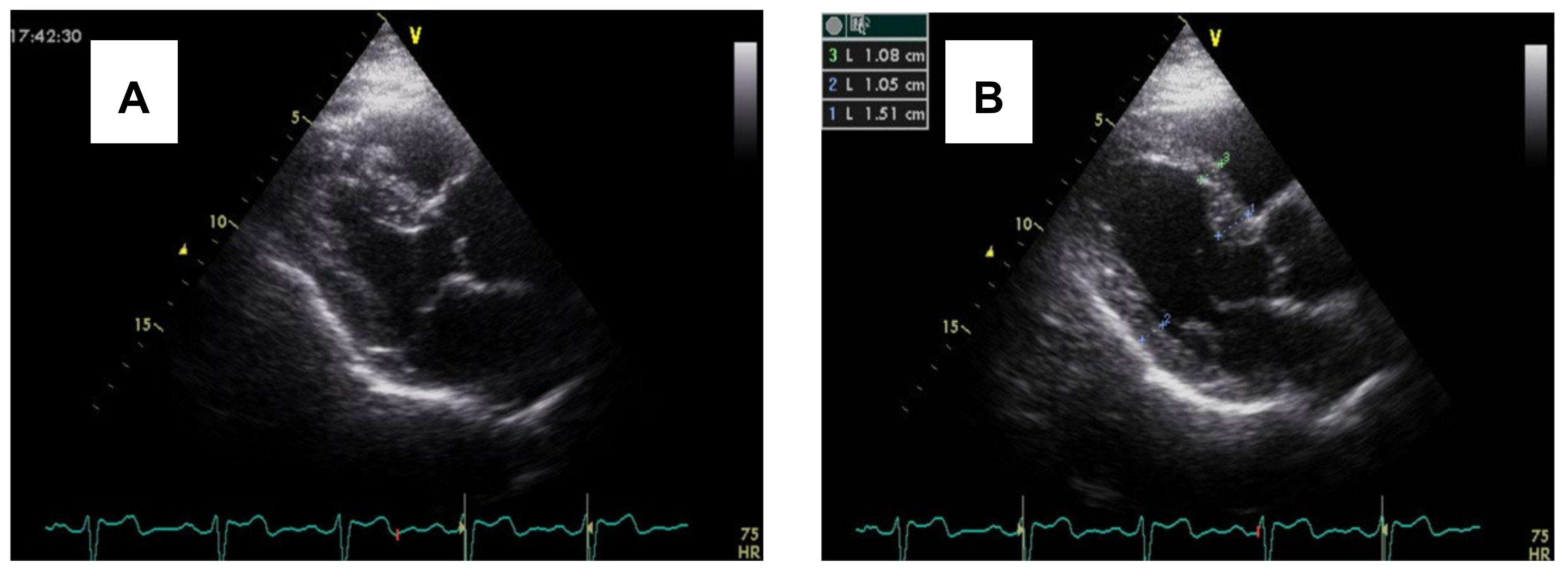
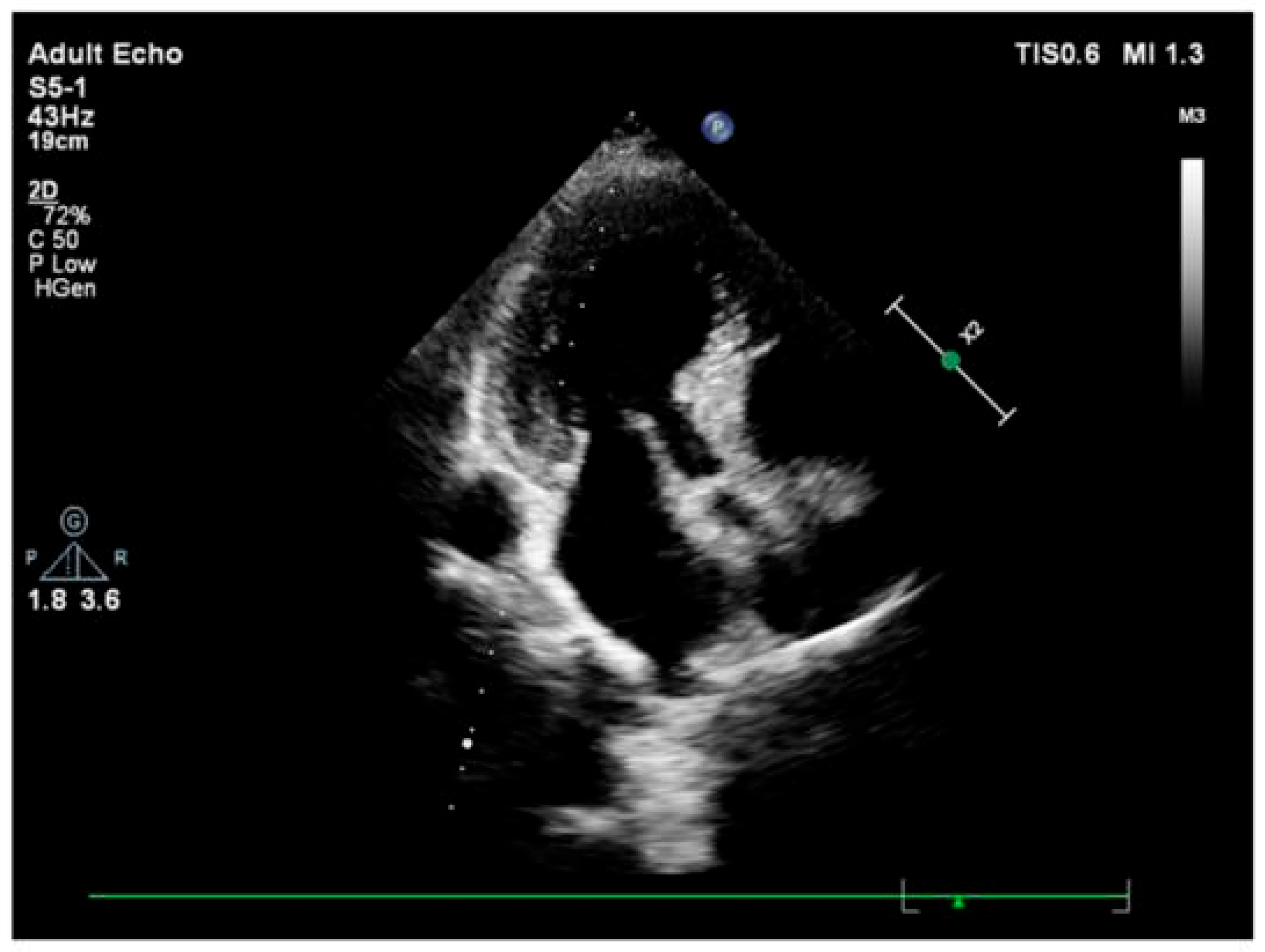
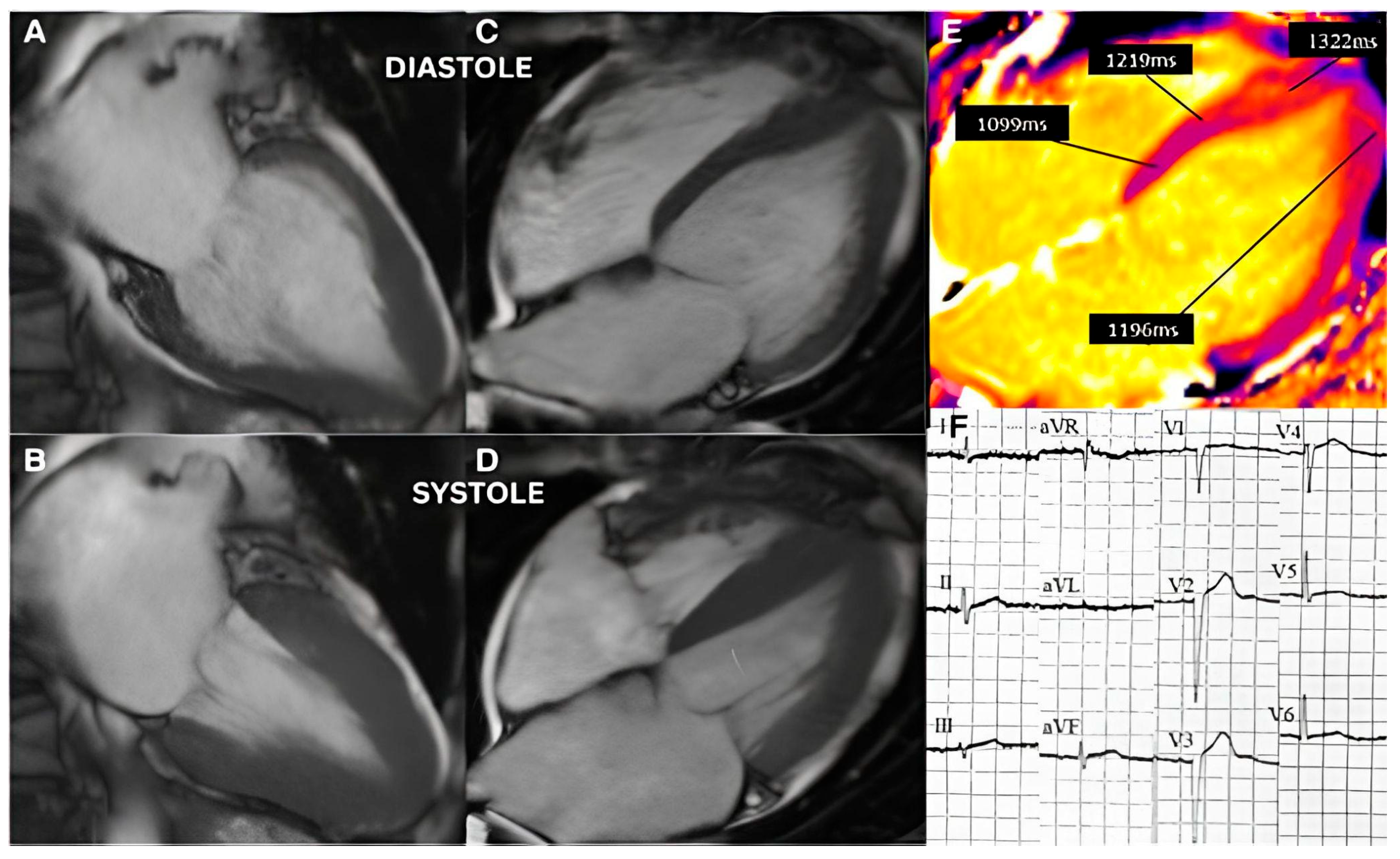
3.2. Imaging and Hemodynamic Observations
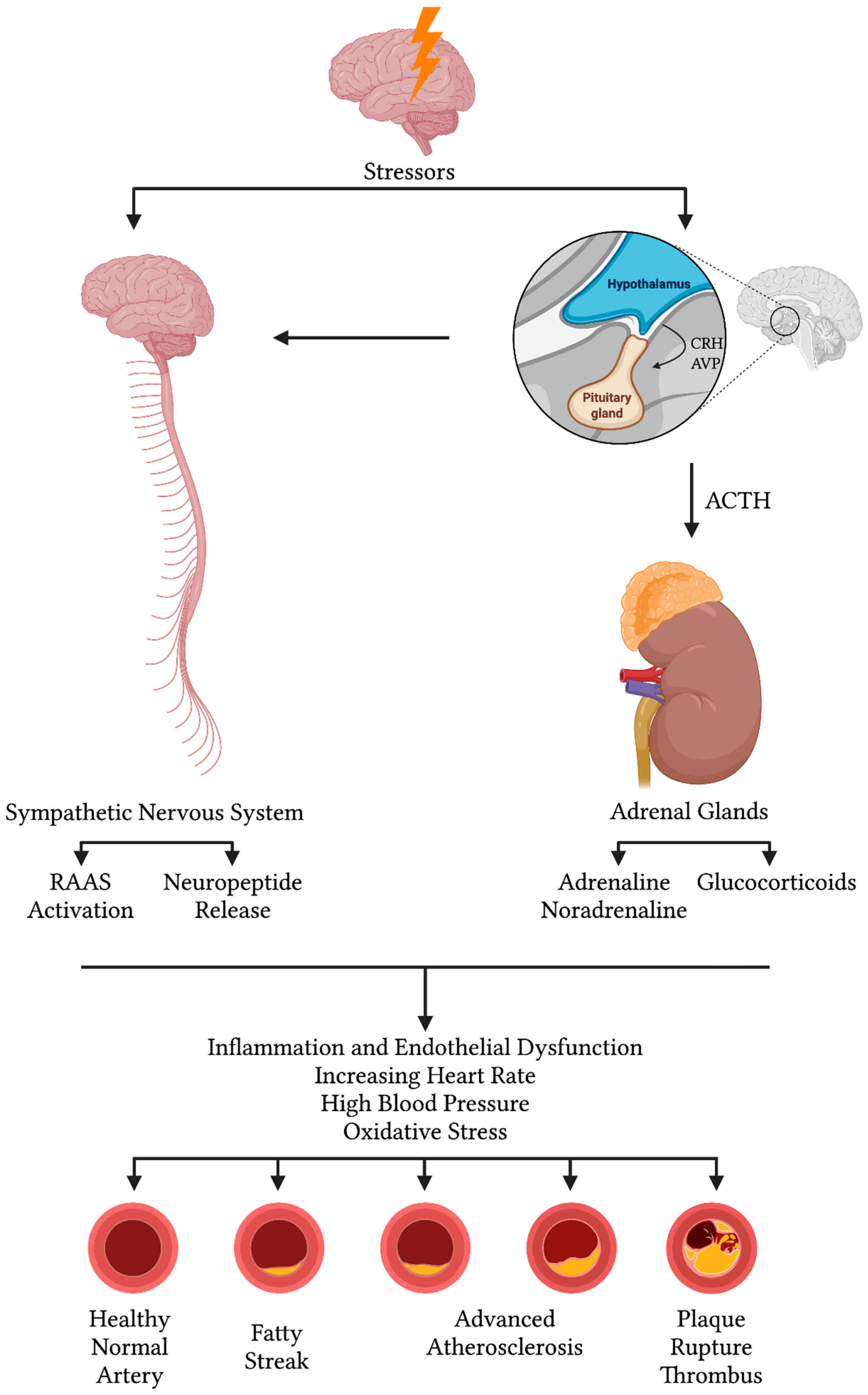
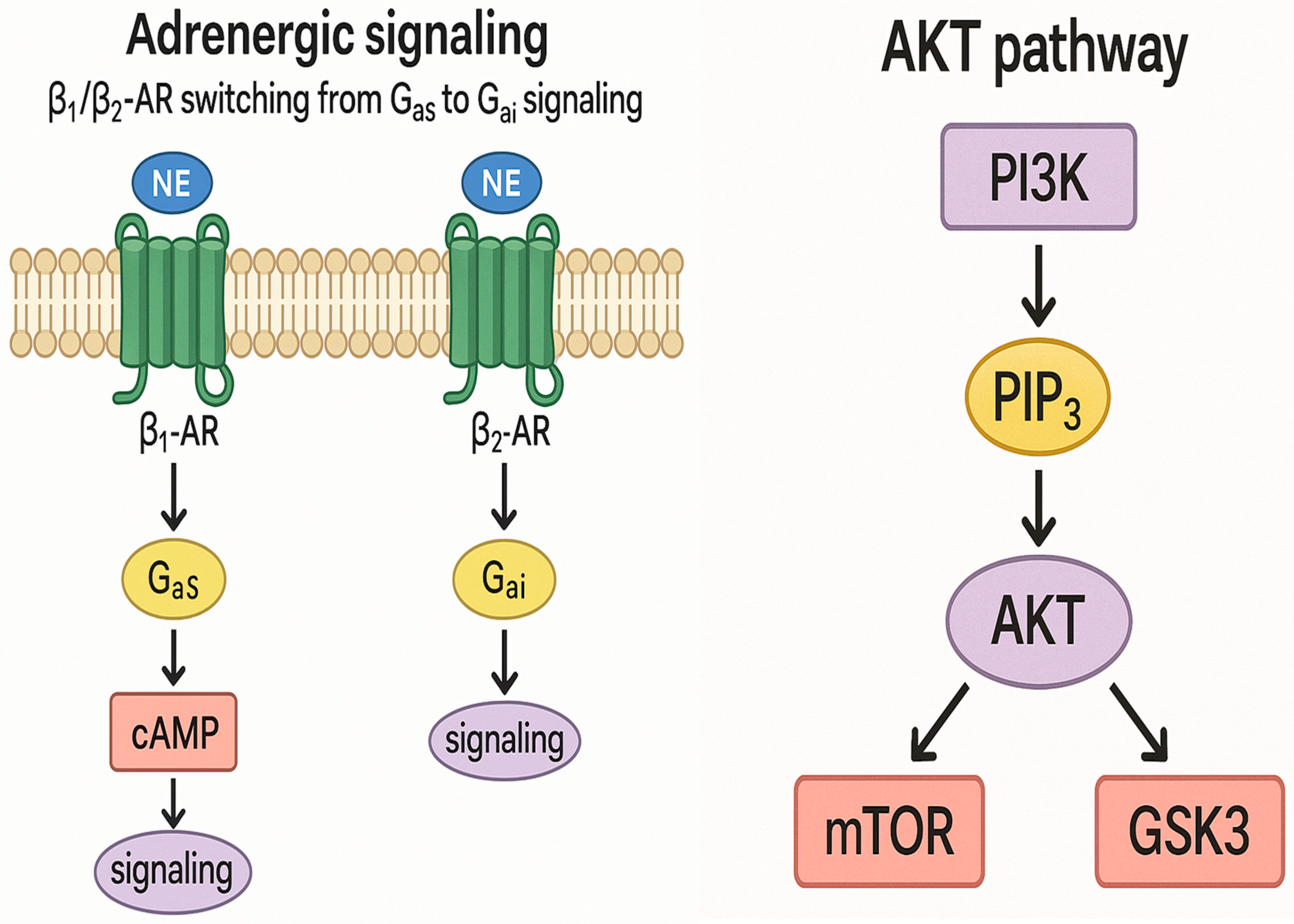
3.3. Molecular and Neurohormonal Mechanisms
3.4. Integration of Acute and Chronic Stress Effects
4. Discussion
4.1. Repeated Acute Stress, Takotsubo Cardiomyopathy, and Stressed Heart Morphology
4.2. Perfusion Abnormalities and Segmental Remodeling Under Cumulative Chronic Stress
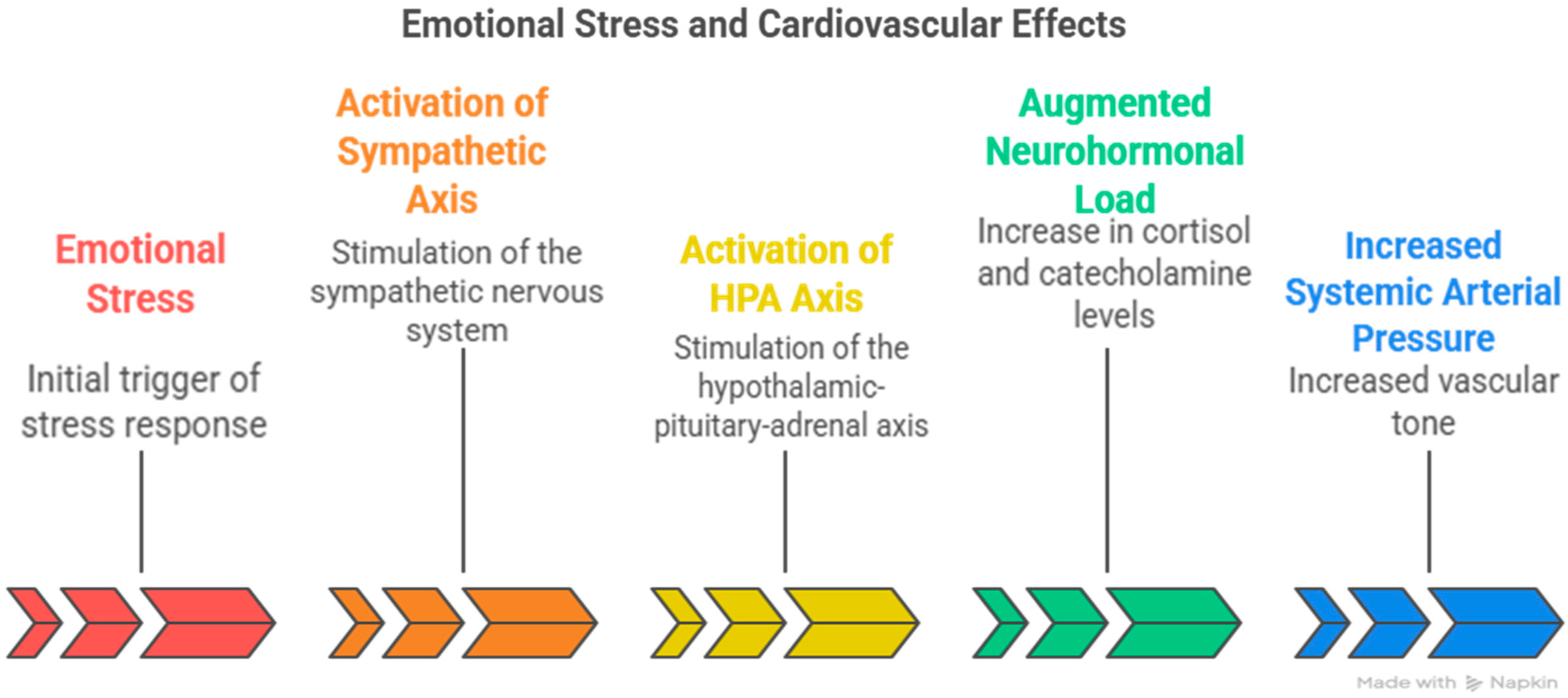
4.3. Acute Versus Chronic Stress Effect on the Heart and Blood Hemodynamics
4.4. Molecular Mechanisms in Takotsubo Cardiomyopathy
4.5. Stressed Heart Morphology and Takotsubo Cardiomyopathy Pathophysiological Correlation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoganathan, T.; Perez-Liva, M.; Balvay, D.; Le Gall, M.; Lallemand, A.; Certain, A.; Autret, G.; Mokrani, Y.; Guillonneau, F.; Bruce, J.; et al. Acute Stress Induces Long-Term Metabolic, Functional, and Structural Remodeling of the Heart. Nat. Commun. 2023, 14, 3835. [Google Scholar] [CrossRef]
- Dawson, D.K. Acute Stress-Induced (Takotsubo) Cardiomyopathy. Heart 2018, 104, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, A.; Giudice, C.D.; Vecchio, G.E.D.; Correra, A.; Maratea, A.C.; Grieco, M.; Amata, A.; Quagliariello, V.; Maurea, N.; Proietti, R.; et al. Takotsubo Syndrome and Oxidative Stress: Physiopathological Linkage and Future Perspectives. Antioxidants 2025, 14, 522. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.S.; Sarvet, A.L.; Meyers, J.L.; Saha, T.D.; Ruan, W.J.; Stohl, M.; Grant, B.F. Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry 2018, 75, 336. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef]
- Yalçin, F.; Yalçin, H.; Abraham, M.R.; Abraham, T.P. Ultimate Phases of Hypertensive Heart Disease and Stressed Heart Morphology by Conventional and Novel Cardiac Imaging. Am. J. Cardiovasc. Dis. 2021, 11, 628. [Google Scholar]
- Yalçin, F.; Yiǧit, F.; Erol, T.; Baltali, M.; Korkmaz, M.E.; Müderrisoǧu, H. Effect of Dobutamine Stress on Basal Septal Tissue Dynamics in Hypertensive Patients with Basal Septal Hypertrophy. J. Hum. Hypertens. 2006, 20, 628–630. [Google Scholar] [CrossRef]
- Fefer, P.; Chelvanathan, A.; Dick, A.J.; Teitelbaum, E.J.; Strauss, B.H.; Cohen, E.A. Takotsubo Cardiomyopathy and Left Ventricular Outflow Tract Obstruction. J. Intervent. Cardiol. 2009, 22, 444–452. [Google Scholar] [CrossRef]
- Kawaji, T.; Shiomi, H.; Morimoto, T.; Tazaki, J.; Imai, M.; Saito, N.; Makiyama, T.; Shizuta, S.; Ono, K.; Kimura, T. Clinical Impact of Left Ventricular Outflow Tract Obstruction in Takotsubo Cardiomyopathy. Circ. J. Off. J. Jpn. Circ. Soc. 2015, 79, 839–846. [Google Scholar] [CrossRef]
- Yalçin, F.; Muderrisoglu, H.; Korkmaz, M.E.; Ozin, B.; Baltali, M.; Yigit, F. The Effect of Dobutamine Stress on Left Ventricular Outflow Tract Gradients in Hypertensive Patients with Basal Septal Hypertrophy. Angiology 2004, 55, 295–301. [Google Scholar] [CrossRef]
- Dearing, C.; Sanford, E.; Olmstead, N.; Morano, R.; Wulsin, L.; Myers, B. Sex-Specific Cardiac Remodeling in Aged Rats after Adolescent Chronic Stress: Associations with Endocrine and Metabolic Factors. Biol. Sex Differ. 2024, 15, 65. [Google Scholar] [CrossRef]
- Elliott, B.M.; Faraday, M.M.; Grunberg, N.E. Repeated Acute Stress Alters Heart Morphometry in Male and Female Rats Differently. Stress 2003, 6, 63–70. [Google Scholar] [CrossRef]
- Baltabaeva, A.; Marciniak, M.; Bijnens, B.; Moggridge, J.; He, F.J.; Antonios, T.F.; MacGregor, G.A.; Sutherland, G.R. Regional Left Ventricular Deformation and Geometry Analysis Provides Insights in Myocardial Remodelling in Mild to Moderate Hypertension. Eur. J. Echocardiogr. 2008, 9, 501–508. [Google Scholar] [CrossRef][Green Version]
- Durante, A.; Mazzapicchi, A.; Baiardo Redaelli, M. Systemic and Cardiac Microvascular Dysfunction in Hypertension. Int. J. Mol. Sci. 2024, 25, 13294. [Google Scholar] [CrossRef]
- Yalçin, F.; Yalçin, H.; Abraham, R.; Abraham, T.P. Hemodynamic Stress and Microscopic Remodeling. Int. J. Cardiol. Cardiovasc. Risk Prev. 2021, 11, 200115. [Google Scholar] [CrossRef]
- Smaardijk, V.R.; Maas, A.H.E.M.; Lodder, P.; Kop, W.J.; Mommersteeg, P.M.C. Sex and Gender-Stratified Risks of Psychological Factors for Adverse Clinical Outcomes in Patients with Ischemic Heart Disease: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2020, 302, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, F.; Abraham, M.R.; Garcia, M.J. Stress and Heart in Remodeling Process: Multiple Stressors at the Same Time Kill. J. Clin. Med. 2024, 13, 2597. [Google Scholar] [CrossRef] [PubMed]
- Sadler, D.B.; Aurigemma, G.P.; Williams, D.W.; Reda, D.J.; Materson, B.J.; Gottdiener, J.S. Systolic Function in Hypertensive Men with Concentric Remodeling. Hypertension 1997, 30, 777–781. [Google Scholar] [CrossRef]
- Çağatay, B.; Yalçin, F.; Kıraç, A.; Küçükler, N.; Abraham, M.R. The Science Behind Stress: From Theory to Clinic, Is Basal Septal Hypertrophy the Missing Link between Hypertension and Takotsubo Cardiomyopathy? Stresses 2024, 4, 330–341. [Google Scholar] [CrossRef]
- Yalçin, F.; Topaloglu, C.; Kuçukler, N.; Ofgeli, M.; Abraham, T.P. Could Early Septal Involvement in the Remodeling Process Be Related to the Advance Hypertensive Heart Disease? IJC Heart Vasc. 2015, 7, 141–145. [Google Scholar] [CrossRef][Green Version]
- Yalçin, F.; Yalçin, H.; Seyfeli, E.; Akgul, F. Stress-Induced Hypercontractility in Patients with Hypertension: An Interesting Imaging Finding. Int. J. Cardiol. 2010, 143, e1–e3. [Google Scholar] [CrossRef]
- Wachtell, K.; Gerdts, E.; Palmieri, V.; Olsen, M.H.; Nieminen, M.S.; Papademetriou, V.; Boman, K.; Dahlöf, B.; Aurigemma, G.P.; Rokkedal, J.E.; et al. In-Treatment Midwall and Endocardial Fractional Shortening Predict Cardiovascular Outcome in Hypertensive Patients with Preserved Baseline Systolic Ventricular Function: The Losartan Intervention for Endpoint Reduction Study. J. Hypertens. 2010, 28, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, K.R.; Reul, J.M.H.M. Mineralocorticoid and Glucocorticoid Receptor-Mediated Control of Genomic Responses to Stress in the Brain. Stress Amst. Neth. 2018, 21, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Torpy, J.M.; Burke, A.E.; Glass, R.M. Acute Emotional Stress and the Heart. JAMA 2007, 298, 360. [Google Scholar] [CrossRef] [PubMed]
- Fauvel, J.P.; Cerutti, C.; Quelin, P.; Laville, M.; Gustin, M.P.; Paultre, C.Z.; Ducher, M. Mental Stress-Induced Increase in Blood Pressure Is Not Related to Baroreflex Sensitivity in Middle-Aged Healthy Men. Hypertension 2000, 35, 887–891. [Google Scholar] [CrossRef]
- Herd, J.A. Cardiovascular Response to Stress. Physiol. Rev. 1991, 71, 305–330. [Google Scholar] [CrossRef]
- Ring, C.; Burns, V.E.; Carroll, D. Shifting Hemodynamics of Blood Pressure Control during Prolonged Mental Stress. Psychophysiology 2002, 39, 585–590. [Google Scholar] [CrossRef]
- Yalçin, F.; Yalçin, H.; Küçükler, N.; Arslan, S.; Akkuş, O.; Kurtul, A.; Abraham, M.R. Basal Septal Hypertrophy as the Early Imaging Biomarker for Adaptive Phase of Remodeling Prior to Heart Failure. J. Clin. Med. 2021, 11, 75. [Google Scholar] [CrossRef]
- Banerjee, A.; Locke-Winter, C.; Rogers, K.B.; Mitchell, M.B.; Brew, E.C.; Cairns, C.B.; Bensard, D.D.; Harken, A.H. Preconditioning against Myocardial Dysfunction after Ischemia and Reperfusion by an Alpha 1-Adrenergic Mechanism. Circ. Res. 1993, 73, 656–670. [Google Scholar] [CrossRef]
- Benini, R.; Oliveira, L.A.; Gomes-de-Souza, L.; Santos, A.; Casula, L.C.; Crestani, C.C. Influence of Strain on Expression and Habituation of Autonomic and Cardiovascular Responses to Restraint Stress in Rats. Physiol. Behav. 2025, 290, 114781. [Google Scholar] [CrossRef]
- Hearse, D.J.; Sutherland, F.J. Catecholamines and Preconditioning: Studies of Contraction and Function in Isolated Rat Hearts. Am. J. Physiol.-Heart Circ. Physiol. 1999, 277, H136–H143. [Google Scholar] [CrossRef]
- Lagraauw, H.M.; Kuiper, J.; Bot, I. Acute and Chronic Psychological Stress as Risk Factors for Cardiovascular Disease: Insights Gained from Epidemiological, Clinical and Experimental Studies. Brain Behav. Immun. 2015, 50, 18–30. [Google Scholar] [CrossRef]
- Paur, H.; Wright, P.T.; Sikkel, M.B.; Tranter, M.H.; Mansfield, C.; O’Gara, P.; Stuckey, D.J.; Nikolaev, V.O.; Diakonov, I.; Pannell, L.; et al. High Levels of Circulating Epinephrine Trigger Apical Cardiodepression in a Β2-Adrenergic Receptor/Gi-Dependent Manner: A New Model of Takotsubo Cardiomyopathy. Circulation 2012, 126, 697–706. [Google Scholar] [CrossRef]
- Mao, S.; Luo, X.; Li, Y.; He, C.; Huang, F.; Su, C. Role of PI3K/AKT/mTOR Pathway Associated Oxidative Stress and Cardiac Dysfunction in Takotsubo Syndrome. Curr. Neurovasc. Res. 2020, 17, 35–43. [Google Scholar] [CrossRef]
- Ueyama, T.; Hano, T.; Kasamatsu, K.; Yamamoto, K.; Tsuruo, Y.; Nishio, I. Estrogen Attenuates the Emotional Stress-Induced Cardiac Responses in the Animal Model of Tako-Tsubo (Ampulla) Cardiomyopathy. J. Cardiovasc. Pharmacol. 2003, 42, S117–S120. [Google Scholar] [CrossRef]
- Verna, E.; Provasoli, S.; Ghiringhelli, S.; Morandi, F.; Salerno-Uriarte, J. Abnormal Coronary Vasoreactivity in Transient Left Ventricular Apical Ballooning (Tako-Tsubo) Syndrome. Int. J. Cardiol. 2018, 250, 4–10. [Google Scholar] [CrossRef]
- Lyon, A.R.; Rees, P.S.; Prasad, S.; Poole-Wilson, P.A.; Harding, S.E. Stress (Takotsubo) Cardiomyopathy—A Novel Pathophysiological Hypothesis to Explain Catecholamine-Induced Acute Myocardial Stunning. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Scally, C.; Abbas, H.; Ahearn, T.; Srinivasan, J.; Mezincescu, A.; Rudd, A.; Spath, N.; Yucel-Finn, A.; Yuecel, R.; Oldroyd, K.; et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2019, 139, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, S.W.; Windenburg, D.C.; Lesser, J.R.; Maron, M.S.; Hauser, R.G.; Lesser, J.N.; Haas, T.S.; Hodges, J.S.; Maron, B.J. Natural History and Expansive Clinical Profile of Stress (Tako-Tsubo) Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 55, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; Parodi, G.; Greco, C.; Antoniucci, D.; Brenner, R.; Bossone, E.; Cacciotti, L.; Capucci, A.; Citro, R.; Delmas, C.; et al. Comorbidities Frequency in Takotsubo Syndrome: An International Collaborative Systematic Review Including 1109 Patients. Am. J. Med. 2015, 128, 654.e11–654.e19. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, J.; Xu, Y.; Teng, C.; Lu, X.; Wang, Y.; Zuo, X.; Li, Q.; Huang, Z.; Ma, J.; et al. Conventional Cardiovascular Risk Factors Associated with Takotsubo Cardiomyopathy: A Comprehensive Review. Clin. Cardiol. 2021, 44, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, S.W. Cardiogenic Shock Complicating Takotsubo Events. JACC Heart Fail. 2018, 6, 937–939. [Google Scholar] [CrossRef] [PubMed]
- Seyani, C.; Adam, R.; Gant, D.; Shambrook, J.; Flett, A. A Dynamic Variant of Takotsubo Cardiomyopathy Mimicking Apical Hypertrophic Cardiomyopathy: A Case Report. Eur. Heart J. Case Rep. 2025, 9, ytae432. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, F.; Yalcin, H.; Abraham, M.R.; Abraham, T.P. Stressed Heart Morphology: A Specific Location for Variety of Stress Stimuli. J. Hypertens. 2022, 40, e79. [Google Scholar] [CrossRef]
- Yalçin, F.; Abraham, R.; Abraham, T.P. Basal Septal Hypertrophy: Extremely Sensitive Region to Variety of Stress Stimuli and Stressed Heart Morphology. J. Hypertens. 2022, 40, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, F.; Yalçin, H.; Abraham, T.P. Exercise Hypertension Should Be Recalled in Basal Septal Hypertrophy as the Early Imaging Biomarker in Patients with Stressed Heart Morphology. Blood Press. Monit. 2020, 25, 118–119. [Google Scholar] [CrossRef]
- Yalçin, F.; Çağatay, B.; Küçükler, N.; Abraham, T.P. Geomeric and Functional Aspects in Hypertension and Takotsubo: Importance of Basal Septal Hypertrophy. Eur. J. Prev. Cardiol. 2023, 30, 1996–1997. [Google Scholar] [CrossRef]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.C.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral Features of Myocardial Stunning Due to Sudden Emotional Stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef]
- Wang, J.; Shi, X.; Ma, C.; Zheng, H.; Xiao, J.; Bian, H.; Ma, Z.; Gong, L. Visit-to-Visit Blood Pressure Variability Is a Risk Factor for All-Cause Mortality and Cardiovascular Disease: A Systematic Review and Meta-Analysis. J. Hypertens. 2017, 35, 10–17. [Google Scholar] [CrossRef]
- Yalcin, F.; Melek, I.; Mutlu, T. Stressed Heart Morphology and Neurologic Stress Score Effect Beyond Hemodynamic Stress on Focal Geometry. J. Hypertens. 2022, 40, e79. [Google Scholar] [CrossRef]
- Yalcin, F.; Kucukler, N.; Cingolani, O.; Mbiyangandu, B.; Sorensen, L.L.; Pinheiro, A.C.; Abraham, M.R.; Abraham, T.P. Intracavitary Gradients in Mice with Early Regional Remodeling at the Compensatory Hyperactive Stage Prior to Lv Tissue Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 1585. [Google Scholar] [CrossRef]
- Merli, E.; Sutcliffe, S.; Gori, M.; Sutherland, G. Tako-Tsubo Cardiomyopathy: New Insights into the Possible Underlying Pathophysiology. Eur. J. Echocardiogr. 2006, 7, 53–61. [Google Scholar] [CrossRef]
- Abuarqoub, A.; Garis, R.; Shaaban, H.; Khaddash, I.; Shamoon, F. Takotsubo Cardiomyopathy with Basal Hypertrophy and Outflow Obstruction in a Patient with Bowel Ischemia. Int. J. Crit. Illn. Inj. Sci. 2018, 8, 44–47. [Google Scholar] [CrossRef]
- Yalçin, F.; Muderrisoǧlu, H. Tako-Tsubo Cardiomyopathy May Be Associated with Cardiac Geometric Features as Observed in Hypertensive Heart Disease. Int. J. Cardiol. 2009, 135, 251–252. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, O.A.A.; Cagatay, B.; Kucukler, N.; Yalcin, F.; Garcia, M.J. Takotsubo Cardiomyopathy and Stressed Heart Morphology: Molecular, Hemodynamic, and Imaging Intersections. J. Clin. Med. 2025, 14, 7638. https://doi.org/10.3390/jcm14217638
Mahmoud OAA, Cagatay B, Kucukler N, Yalcin F, Garcia MJ. Takotsubo Cardiomyopathy and Stressed Heart Morphology: Molecular, Hemodynamic, and Imaging Intersections. Journal of Clinical Medicine. 2025; 14(21):7638. https://doi.org/10.3390/jcm14217638
Chicago/Turabian StyleMahmoud, Omar Atef Abdelhamid, Boran Cagatay, Nagehan Kucukler, Fatih Yalcin, and Mario J. Garcia. 2025. "Takotsubo Cardiomyopathy and Stressed Heart Morphology: Molecular, Hemodynamic, and Imaging Intersections" Journal of Clinical Medicine 14, no. 21: 7638. https://doi.org/10.3390/jcm14217638
APA StyleMahmoud, O. A. A., Cagatay, B., Kucukler, N., Yalcin, F., & Garcia, M. J. (2025). Takotsubo Cardiomyopathy and Stressed Heart Morphology: Molecular, Hemodynamic, and Imaging Intersections. Journal of Clinical Medicine, 14(21), 7638. https://doi.org/10.3390/jcm14217638





