The Safety and Efficacy of Platelet-Rich Plasma in Enhancing Outcomes Following Circumcision in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Groups
2.2. Eligibility Criteria
2.3. PICO Strategy
2.4. Preparation of PRP
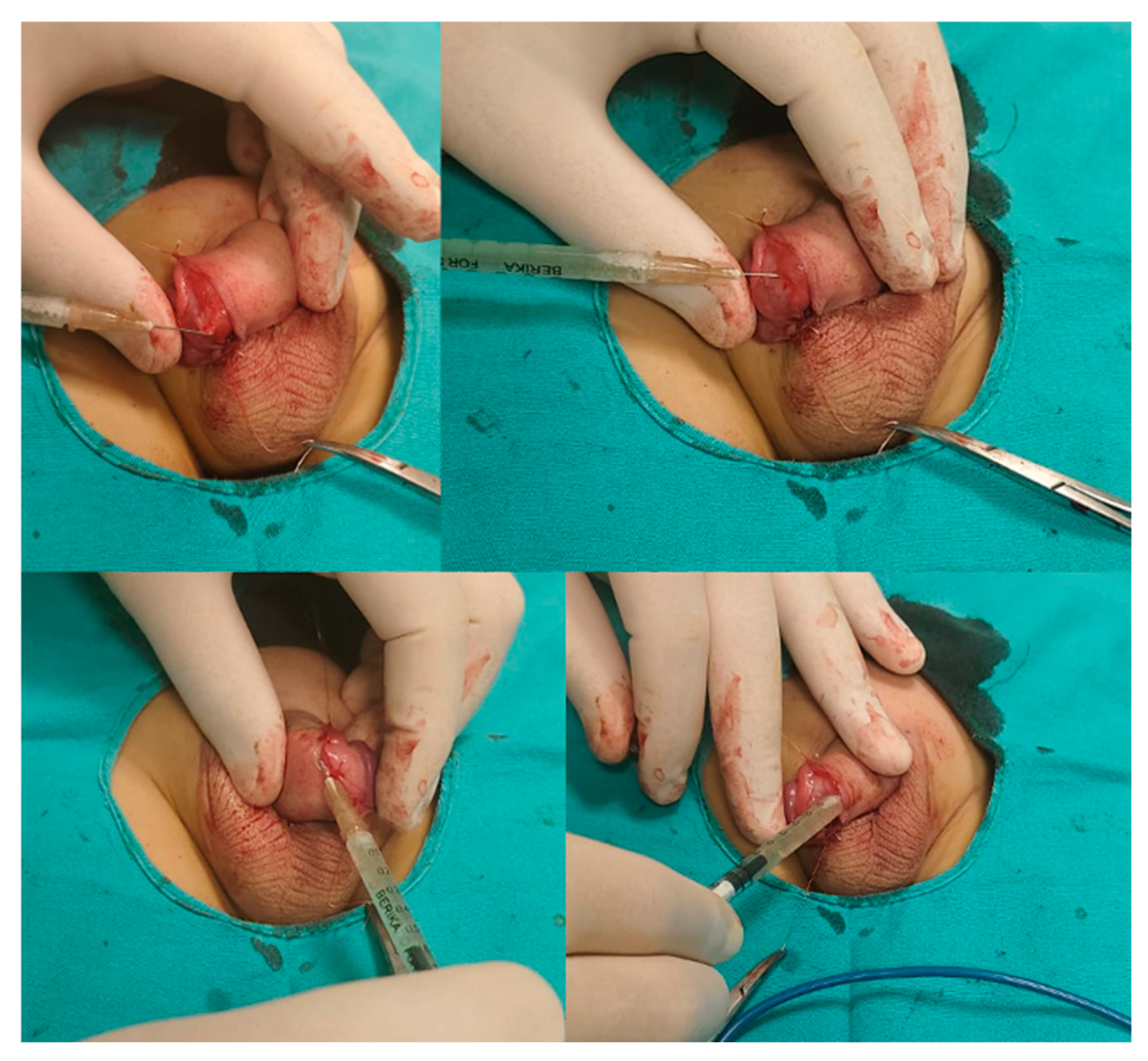
2.5. Application Technique of PRP
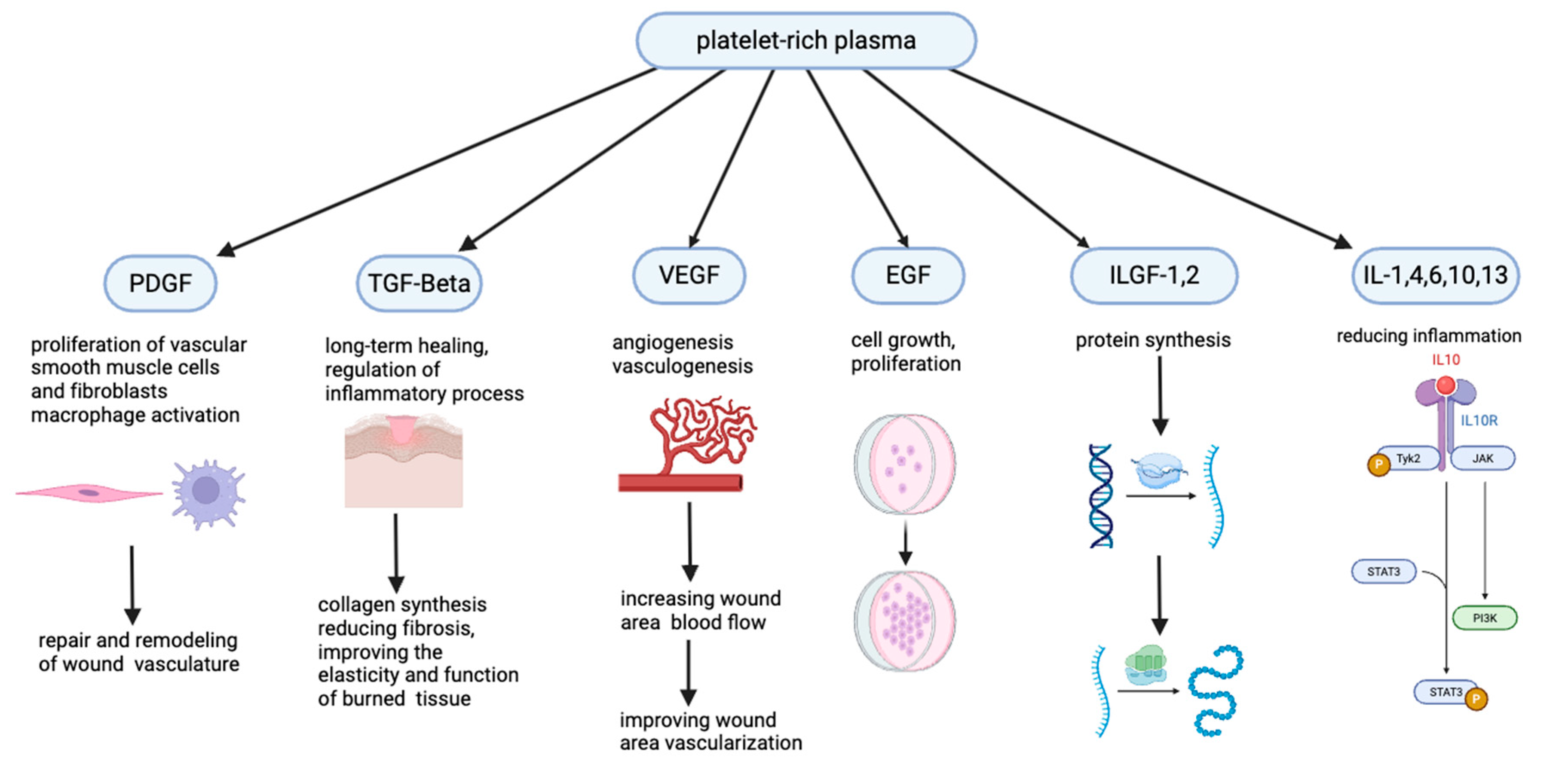
2.6. Potential PRP Mechanism of Action
2.7. Sample Size Calculation
2.8. Statistical Analysis
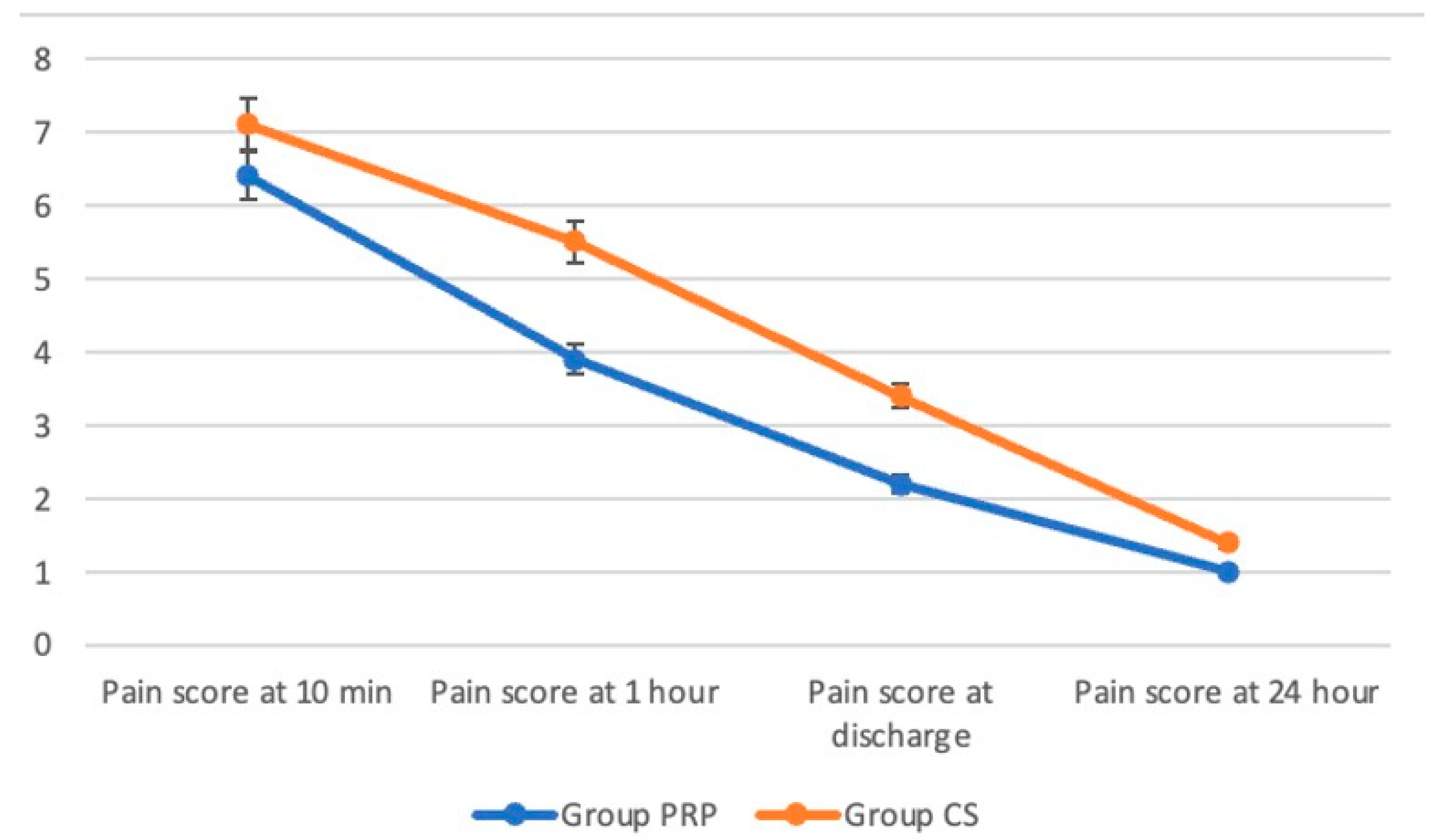
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiss, H.A.; Larke, N.; Halperin, D.; Schenker, I. Complications of circumcision in male neonates, infants and children: A systematic review. BMC Urol. 2010, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, S.; Ljuhar, D.; Coleman, R.; Nataraja, R.M. Circumcision in the paediatric patient: A review of indications, technique and complications. J. Paediatr. Child Health 2018, 54, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Madhivanan, P.; Krupp, K.; Chandrasekaran, V.; Karat, S.C.; Reingold, A.L.; Klausner, J.D. Acceptability of male circumcision among mothers with male children in Mysore, India. AIDS 2008, 22, 983–988. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elalfy, M.S.; Mostafa, S.; Elalfy, O.M.; Ghamry, I.R.E.; Meabed, M.; Zafar, T.; Tarawah, A.; Elekiaby, M. A survey on practice of circumcision in children with severe haemophilia in Eastern Mediterranean Region. Haemophilia 2021, 27, e617–e619. [Google Scholar] [CrossRef] [PubMed]
- Akman, M. What is world pediatric surgeons’ opinion on EMLA® cream induced local anaesthesia in circumcision? Turk. J. Pediatr. Surg. 2021, 35, 137–142. [Google Scholar]
- Azizoglu, M.; Risteski, T.; Klyuev, S. Alisklamp versus Conventional Dorsal Slit Circumcision: A Multicentric Randomized Controlled Trial. J. Clin. Med. 2024, 13, 4568. [Google Scholar] [CrossRef] [PubMed]
- Ferhatoglu, M.F.; Kartal, A.; Gurkan, A. Evaluation of Male Circumcision: Retrospective Analysis of One Hundred and Ninety-eight Patients. Cureus 2019, 11, e4555. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, M.A.B.; Sabra, T.A.; Abdelmohsen, S.M. Management of penile post-circumcision ischemia by pentoxifylline infusion and hyperbaric oxygen therapy. BMC Urol. 2023, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Paliczak, A.; Delgado, D. Evidence-based indications of platelet-rich plasma therapy. Expert. Rev. Hematol. 2021, 14, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Dachille, G.; Panunzio, A.; Bizzotto, L.; D’Agostino, M.V.; Greco, F.; Guglielmi, G.; Carbonara, U.; Spilotros, M.; Citarella, C.; Ostuni, A.; et al. Platelet-rich plasma intra-plaque injections rapidly reduce penile curvature and improve sexual function in Peyronie’s disease patients: Results from a prospective large-cohort study. World J. Urol. 2025, 43, 306. [Google Scholar] [CrossRef] [PubMed]
- Kurt, B.; Yildiz, C.; Koc, T.; Kurt-Ozkaya, N.; Celikgun, S. The effect of platelet-rich plasma on intra-abdominal adhesions in rabbit uterine horn model. Cir. Cir. 2023, 91, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Moreland, R.B.; Gupta, S.; Goldstein, I.; Traish, A. Cyclic AMP modulates TGF-beta 1-induced fibrillar collagen synthesis in cultured human corpus cavernosum smooth muscle cells. Int. J. Impot. Res. 1998, 10, 159–163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, Z.; Zhang, Y.; Tang, Z.; Song, J.; Gao, X.; Sun, T.; Liu, Y.; Yang, J.; Wang, T.; Liu, J. Intracavernosal Adeno-Associated Virus-Mediated S100A1 Gene Transfer Enhances Erectile Function in Diabetic Rats by Promoting Cavernous Angiogenesis via VEGF-A/VEGFR2 Signaling. J. Sex. Med. 2019, 16, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Sun, S.; Guo, F.; Liu, H. Efficacy of platelet-rich plasma in the treatment of erectile dysfunction: A meta-analysis of controlled and single-arm trials. PLoS ONE 2024, 19, e0313074. [Google Scholar] [CrossRef] [PubMed]
- Azizoglu, M.; Klyuev, S.; Kamci, T.O.; Okur, M.H. Platelet-rich Plasma as an Adjuvant Therapy to Crystallized Phenol in the Treatment of Pediatric Pilonidal Sinus Disease: A Prospective Randomized Controlled Trial. J. Pediatr. Surg. 2025, 60, 161934. [Google Scholar] [CrossRef] [PubMed]
- Poulios, E.; Mykoniatis, I.; Pyrgidis, N.; Zilotis, F.; Kapoteli, P.; Kotsiris, D.; Kalyvianakis, D.; Hatzichristou, D. Platelet-Rich Plasma (PRP) Improves Erectile Function: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. J. Sex. Med. 2021, 18, 926–935. [Google Scholar] [CrossRef]
- Wu, P.I.; Diaz, R.; Borg-Stein, J. Platelet-Rich Plasma. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 825–853. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, Z.; Alimohamadi, Y.; Janani, M.; Hejazi, P.; Kamali, M.; Goodarzi, A. Platelet-rich plasma in the treatment of scars, to suggest or not to suggest? A systematic review and meta-analysis. J. Tissue Eng. Regen. Med. 2022, 16, 875–899. [Google Scholar] [CrossRef]
- Phoebe, L.K.W.; Lee, K.W.A.; Chan, L.K.W.; Hung, L.C.; Wu, R.; Wong, S.; Wan, J.; Yi, K.H. Use of platelet rich plasma for skin rejuvenation. Skin. Res. Technol. 2024, 30, e13714. [Google Scholar] [CrossRef] [PubMed]


| Group CS (n = 44) | Group PRP (n = 36) | p-Value | |
|---|---|---|---|
| Age | 11 (9; 13.3) | 10 (9; 12.4) | 0.101 |
| Nausea | 3 (6.8%) | 2 (5.6%) | 0.816 |
| Vomiting | 2 (4.5%) | 1 (2.8%) | 0.679 |
| Penile edema | 8 (18.2%) | 2 (5.6%) | 0.089 |
| Edema degree | 0.335 | ||
| Mild | 1 (12.5%) | 1 (50%) | |
| Moderate | 3 (37.5%) | 1 (50%) | |
| Severe | 4 (50%) | 0 (0%) | |
| Postoperative bleeding | 3 (6.8%) | 0 (0%) | 0.110 |
| Necrosis | 0 (0%) | 0 (0%) | 1.000 |
| Local infection | 1 (2.3%) | 0 (0%) | 0.363 |
| Wound dehiscence | 1 (2.3%) | 0 (0%) | 0.363 |
| Skin tunnel | 3 (6.8%) | 1 (2.8%) | 0.409 |
| Chordee after procedure | 0 (0%) | 0 (0%) | 1.000 |
| Rotational anomaly after procedure | 0 (0%) | 0 (0%) | 1.000 |
| Meatal stenosis | 0 (0%) | 0 (0%) | 1.000 |
| Secondary phimosis | 0 (0%) | 0 (0%) | 1.000 |
| n (%) | |
|---|---|
| Complications during blood draw | |
| Hypotension | 1 (2.8%) |
| Allergic reaction | 0 (0%) |
| Local ecchymosis | 2 (5.6%) |
| Complications during PRP application | |
| Hypotension | 0 (0%) |
| Allergic reaction | 1 (2.8%) |
| Local ecchymosis | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamci, T.O.; Azizoglu, M.; Klyuev, S.; Okur, M.H.; Aydogdu, H.; Escolino, M.; Zorba Yildiz, A.P.; Esposito, C.; Shehata, S. The Safety and Efficacy of Platelet-Rich Plasma in Enhancing Outcomes Following Circumcision in Children. J. Clin. Med. 2025, 14, 7620. https://doi.org/10.3390/jcm14217620
Kamci TO, Azizoglu M, Klyuev S, Okur MH, Aydogdu H, Escolino M, Zorba Yildiz AP, Esposito C, Shehata S. The Safety and Efficacy of Platelet-Rich Plasma in Enhancing Outcomes Following Circumcision in Children. Journal of Clinical Medicine. 2025; 14(21):7620. https://doi.org/10.3390/jcm14217620
Chicago/Turabian StyleKamci, Tahsin Onat, Mustafa Azizoglu, Sergey Klyuev, Mehmet Hanifi Okur, Hakkari Aydogdu, Maria Escolino, Asli Pinar Zorba Yildiz, Ciro Esposito, and Sameh Shehata. 2025. "The Safety and Efficacy of Platelet-Rich Plasma in Enhancing Outcomes Following Circumcision in Children" Journal of Clinical Medicine 14, no. 21: 7620. https://doi.org/10.3390/jcm14217620
APA StyleKamci, T. O., Azizoglu, M., Klyuev, S., Okur, M. H., Aydogdu, H., Escolino, M., Zorba Yildiz, A. P., Esposito, C., & Shehata, S. (2025). The Safety and Efficacy of Platelet-Rich Plasma in Enhancing Outcomes Following Circumcision in Children. Journal of Clinical Medicine, 14(21), 7620. https://doi.org/10.3390/jcm14217620






