Increased Serum Angiopoietin-like Peptide 4 in Impaired Glucose Tolerance and Diabetes Subjects with or Without Hepatic Steatosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Review
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
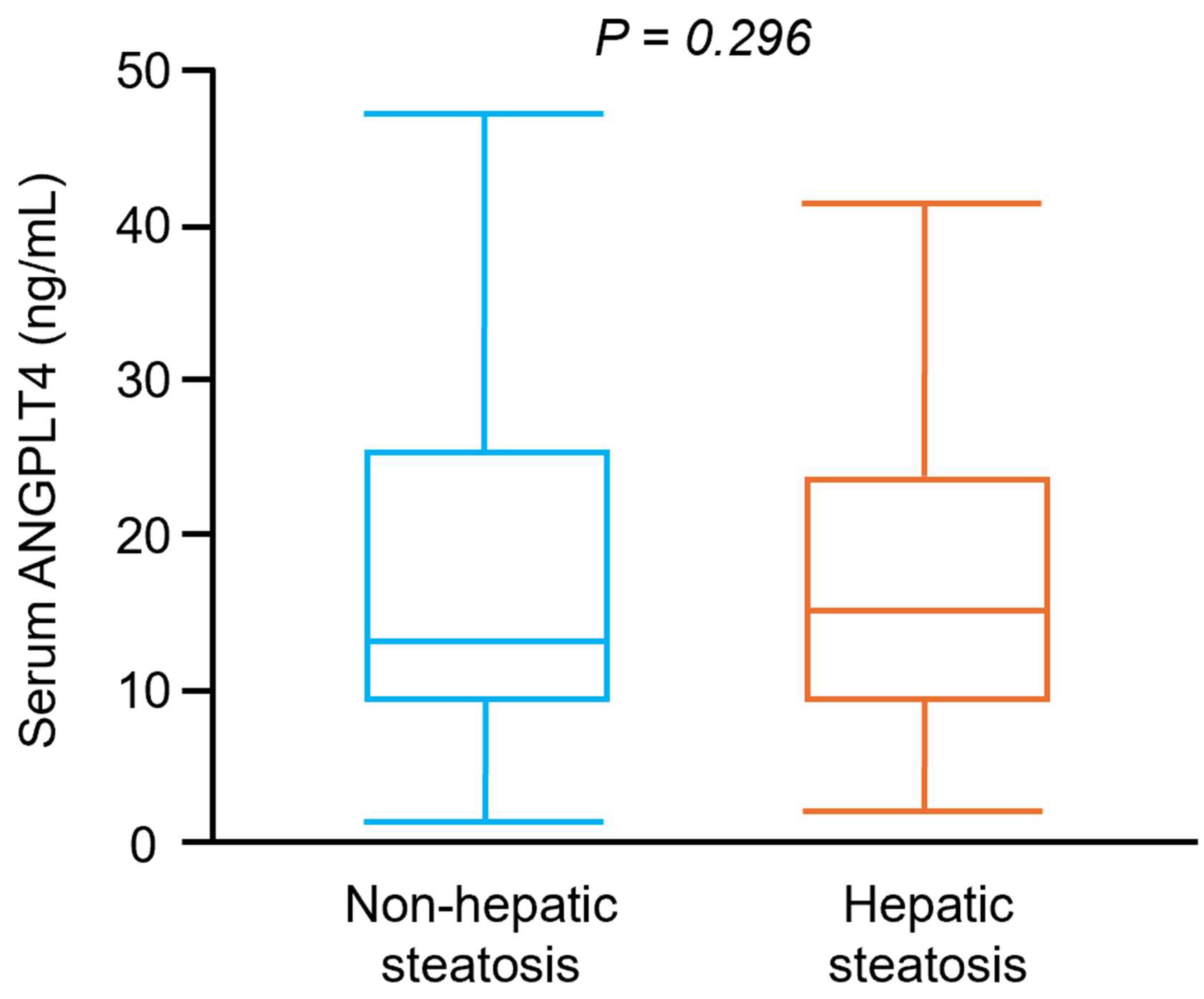
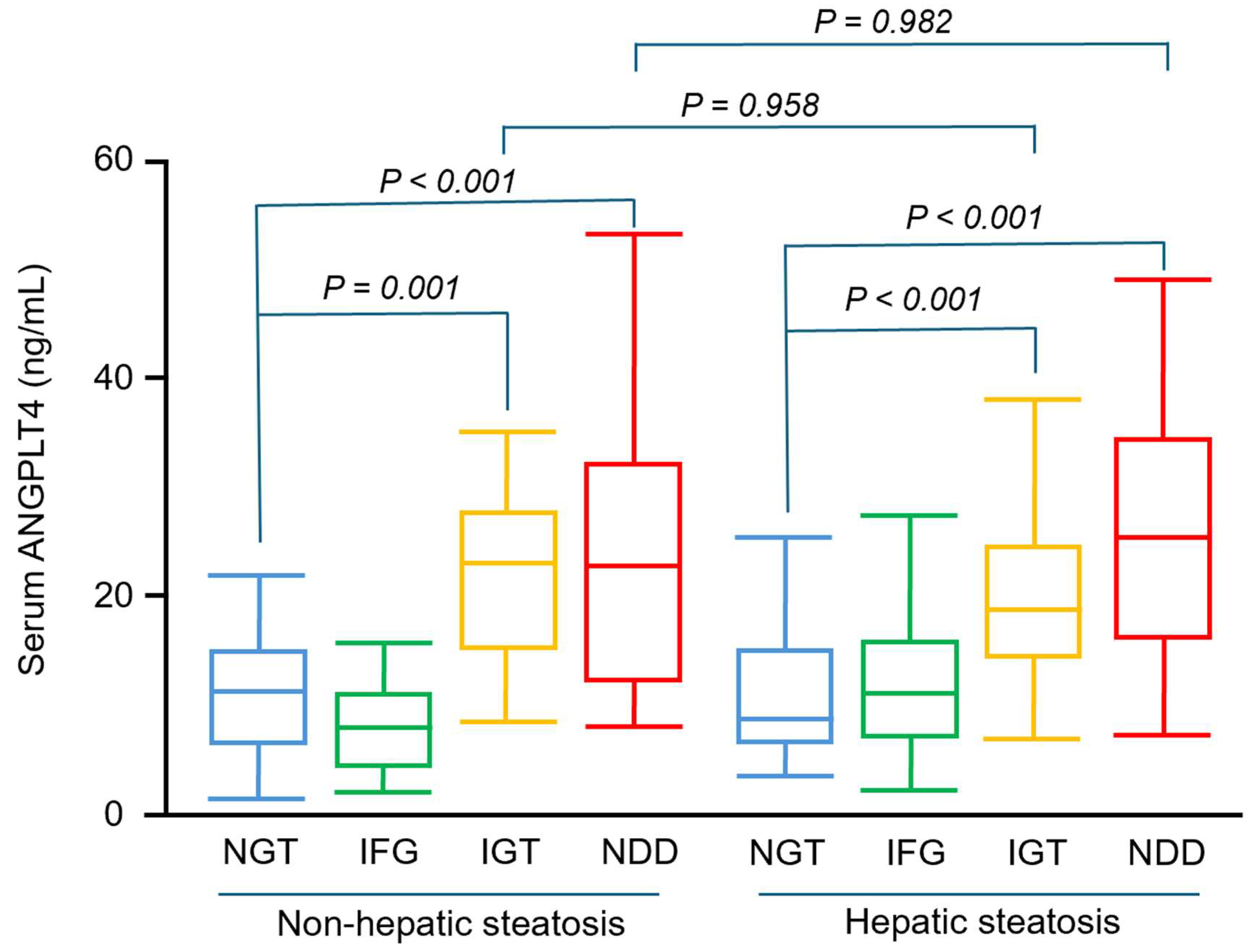
3.2. Participants with IGT and NDD Had Significantly Higher Serum ANGPTL4 Concentrations than Those with NGT, Irrespective of Hepatic Steatosis Status
3.3. Prediabetes and Diabetes Were Independently Associated with Higher Serum ANGPTL4 Concentrations, Whereas Hepatic Steatosis Was Not
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanwal, F.; Neuschwander-Tetri, B.A.; Loomba, R.; Rinella, M.E. Metabolic dysfunction-associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology 2024, 79, 1212–1219. [Google Scholar] [CrossRef]
- Huang, Y.H.; Chan, C.; Lee, H.W.; Huang, C.; Chen, Y.J.; Liu, P.C.; Lu, S.N.; Chuang, W.L.; Huang, J.F.; Yu, M.L.; et al. Influence of Nonalcoholic Fatty Liver Disease With Increased Liver Enzyme Levels on the Risk of Cirrhosis and Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2023, 21, 960–969.e961. [Google Scholar] [CrossRef] [PubMed]
- En Li Cho, E.; Ang, C.Z.; Quek, J.; Fu, C.E.; Lim, L.K.E.; Heng, Z.E.Q.; Tan, D.J.H.; Lim, W.H.; Yong, J.N.; Zeng, R.; et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: An updated systematic review and meta-analysis. Gut 2023, 72, 2138–2148. [Google Scholar] [CrossRef]
- Beaulant, A.; Dia, M.; Pillot, B.; Chauvin, M.A.; Ji-Cao, J.; Durand, C.; Bendridi, N.; Chanon, S.; Vieille-Marchiset, A.; Da Silva, C.C.; et al. Endoplasmic reticulum-mitochondria miscommunication is an early and causal trigger of hepatic insulin resistance and steatosis. J. Hepatol. 2022, 77, 710–722. [Google Scholar] [CrossRef]
- Tubbs, E.; Chanon, S.; Robert, M.; Bendridi, N.; Bidaux, G.; Chauvin, M.A.; Ji-Cao, J.; Durand, C.; Gauvrit-Ramette, D.; Vidal, H.; et al. Disruption of Mitochondria-Associated Endoplasmic Reticulum Membrane (MAM) Integrity Contributes to Muscle Insulin Resistance in Mice and Humans. Diabetes 2018, 67, 636–650. [Google Scholar] [CrossRef]
- Wang, J.; He, W.; Tsai, P.J.; Chen, P.H.; Ye, M.; Guo, J.; Su, Z. Mutual interaction between endoplasmic reticulum and mitochondria in nonalcoholic fatty liver disease. Lipids Health Dis. 2020, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S.; Mandard, S.; Tan, N.S.; Escher, P.; Metzger, D.; Chambon, P.; Gonzalez, F.J.; Desvergne, B.; Wahli, W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 2000, 275, 28488–28493. [Google Scholar] [CrossRef] [PubMed]
- Sukonina, V.; Lookene, A.; Olivecrona, T.; Olivecrona, G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Natl. Acad. Sci. USA 2006, 103, 17450–17455. [Google Scholar] [CrossRef]
- Kaddatz, K.; Adhikary, T.; Finkernagel, F.; Meissner, W.; Müller-Brüsselbach, S.; Müller, R. Transcriptional profiling identifies functional interactions of TGF β and PPAR β/δ signaling: Synergistic induction of ANGPTL4 transcription. J. Biol. Chem. 2010, 285, 29469–29479. [Google Scholar] [CrossRef]
- Mandard, S.; Zandbergen, F.; Tan, N.S.; Escher, P.; Patsouris, D.; Koenig, W.; Kleemann, R.; Bakker, A.; Veenman, F.; Wahli, W.; et al. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J. Biol. Chem. 2004, 279, 34411–34420. [Google Scholar] [CrossRef]
- Ramos, P.; Shi, Q.; Kleberg, J.; Maharjan, C.K.; Zhang, W.; Kolb, R. ANGPTL4: A Comprehensive Review of 25 Years of Research. Cancers 2025, 17, 2364. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Merkel, M. Lipoprotein lipase: Physiology, biochemistry, and molecular biology. Front. Biosci. 2001, 6, D388–D405. [Google Scholar] [CrossRef]
- Lichtenstein, L.; Berbée, J.F.; van Dijk, S.J.; van Dijk, K.W.; Bensadoun, A.; Kema, I.P.; Voshol, P.J.; Müller, M.; Rensen, P.C.; Kersten, S. Angptl4 upregulates cholesterol synthesis in liver via inhibition of LPL- and HL-dependent hepatic cholesterol uptake. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2420–2427. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed]
- Gusarova, V.; O’Dushlaine, C.; Teslovich, T.M.; Benotti, P.N.; Mirshahi, T.; Gottesman, O.; Van Hout, C.V.; Murray, M.F.; Mahajan, A.; Nielsen, J.B.; et al. Genetic inactivation of ANGPTL4 improves glucose homeostasis and is associated with reduced risk of diabetes. Nat. Commun. 2018, 9, 2252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.M.; Wei, L.; Ye, W.W.; Meng, X.Y.; Chen, F.; Xiao, Q.; Chen, J.Y.; Zhou, Y. Angiopoietin-like protein 4 improves glucose tolerance and insulin resistance but induces liver steatosis in high-fat-diet mice. Mol. Med. Rep. 2016, 14, 3293–3300. [Google Scholar] [CrossRef]
- Hezarkhani, S.; Hajighaderi, A.; Hosseinzadeh, S.; Behnampour, N.; Veghari, G.; Fathabadi, F.; Hesari, Z.; Joshaghani, H.R. The serum levels of angiopoietin-like protein 3 and 4 in type 2 diabetic patients with and without metabolic syndrome compared to the control group. Endocrinol. Diabetes Metab. 2024, 7, e466. [Google Scholar] [CrossRef]
- Yen, I.W.; Lin, S.Y.; Lin, M.W.; Lee, C.N.; Kuo, C.H.; Chen, S.C.; Tai, Y.Y.; Kuo, C.H.; Kuo, H.C.; Lin, H.H.; et al. The association between plasma angiopoietin-like protein 4, glucose and lipid metabolism during pregnancy, placental function, and risk of delivering large-for-gestational-age neonates. Clin. Chim. Acta 2024, 554, 117775. [Google Scholar] [CrossRef]
- Altun, Ö.; Dikker, O.; Arman, Y.; Ugurlukisi, B.; Kutlu, O.; Ozgun Cil, E.; Aydin Yoldemir, S.; Akarsu, M.; Ozcan, M.; Kalyon, S.; et al. Serum Angiopoietin-like peptide 4 levels in patients with hepatic steatosis. Cytokine 2018, 111, 496–499. [Google Scholar] [CrossRef]
- Muzica, C.M.; Sfarti, C.; Trifan, A.; Zenovia, S.; Cuciureanu, T.; Nastasa, R.; Huiban, L.; Cojocariu, C.; Singeap, A.M.; Girleanu, I.; et al. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: A Bidirectional Relationship. Can. J. Gastroenterol. Hepatol. 2020, 2020, 6638306. [Google Scholar] [CrossRef]
- 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47, S20–S42. [CrossRef] [PubMed]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e1264. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.D.; Chiang, C.E.; Chao, T.H.; Cheng, H.M.; Wu, Y.W.; Wu, Y.J.; Lin, Y.H.; Chen, M.Y.; Ueng, K.C.; Chang, W.T.; et al. 2022 Guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the Management of Hypertension. Acta Cardiol. Sin. 2022, 38, 225–325. [Google Scholar] [CrossRef] [PubMed]
- Schwenzer, N.F.; Springer, F.; Schraml, C.; Stefan, N.; Machann, J.; Schick, F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J. Hepatol. 2009, 51, 433–445. [Google Scholar] [CrossRef]
- Ohkura, T.; Shiochi, H.; Fujioka, Y.; Sumi, K.; Yamamoto, N.; Matsuzawa, K.; Izawa, S.; Kinoshita, H.; Ohkura, H.; Kato, M.; et al. 20/(fasting C-peptide × fasting plasma glucose) is a simple and effective index of insulin resistance in patients with type 2 diabetes mellitus: A preliminary report. Cardiovasc. Diabetol. 2013, 12, 21. [Google Scholar] [CrossRef]
- Aryal, B.; Singh, A.K.; Zhang, X.; Varela, L.; Rotllan, N.; Goedeke, L.; Chaube, B.; Camporez, J.P.; Vatner, D.F.; Horvath, T.L.; et al. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. JCI Insight 2018, 3, e97918. [Google Scholar] [CrossRef]
- Xu, A.; Lam, M.C.; Chan, K.W.; Wang, Y.; Zhang, J.; Hoo, R.L.; Xu, J.Y.; Chen, B.; Chow, W.S.; Tso, A.W.; et al. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 6086–6091. [Google Scholar] [CrossRef]
- Cummings, B.B.; Bouchard, P.R.; Milton, M.N.; Moesta, P.F.; Ramanan, V.; Trauger, J.W.; Maratos-Flier, E.; Voznesensky, A.; Splawski, I.; Nimonkar, A.V.; et al. An ANGPTL4 inhibitory antibody safely improves lipid profiles in non-human primates. eBioMedicine 2025, 117, 105748. [Google Scholar] [CrossRef]
- van der Kolk, B.W.; Goossens, G.H.; Jocken, J.W.; Kersten, S.; Blaak, E.E. Angiopoietin-Like Protein 4 and Postprandial Skeletal Muscle Lipid Metabolism in Overweight and Obese Prediabetics. J. Clin. Endocrinol. Metab. 2016, 101, 2332–2339. [Google Scholar] [CrossRef]
- Tong, Z.; Peng, J.; Lan, H.; Sai, W.; Li, Y.; Xie, J.; Tan, Y.; Zhang, W.; Zhong, M.; Wang, Z. Cross-talk between ANGPTL4 gene SNP Rs1044250 and weight management is a risk factor of metabolic syndrome. J. Transl. Med. 2021, 19, 72. [Google Scholar] [CrossRef]
- Tjeerdema, N.; Georgiadi, A.; Jonker, J.T.; van Glabbeek, M.; Alizadeh Dehnavi, R.; Tamsma, J.T.; Smit, J.W.; Kersten, S.; Rensen, P.C. Inflammation increases plasma angiopoietin-like protein 4 in patients with the metabolic syndrome and type 2 diabetes. BMJ Open Diabetes Res. Care 2014, 2, e000034. [Google Scholar] [CrossRef]
- Lichtenstein, L.; Mattijssen, F.; de Wit, N.J.; Georgiadi, A.; Hooiveld, G.J.; van der Meer, R.; He, Y.; Qi, L.; Köster, A.; Tamsma, J.T.; et al. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab. 2010, 12, 580–592. [Google Scholar] [CrossRef]
- Kersten, S.; Lichtenstein, L.; Steenbergen, E.; Mudde, K.; Hendriks, H.F.; Hesselink, M.K.; Schrauwen, P.; Müller, M. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 969–974. [Google Scholar] [CrossRef]
- Jonker, J.T.; Smit, J.W.; Hammer, S.; Snel, M.; van der Meer, R.W.; Lamb, H.J.; Mattijssen, F.; Mudde, K.; Jazet, I.M.; Dekkers, O.M.; et al. Dietary modulation of plasma angiopoietin-like protein 4 concentrations in healthy volunteers and in patients with type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Dijk, W.; Kersten, S. Regulation of lipid metabolism by angiopoietin-like proteins. Curr. Opin. Lipidol. 2016, 27, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Role and mechanism of the action of angiopoietin-like protein ANGPTL4 in plasma lipid metabolism. J. Lipid Res. 2021, 62, 100150. [Google Scholar] [CrossRef]
- Dikker, O.; Çetin Dağ, N.; Şahin, M.; Türkkan, E.; Dağ, H. The association of angiopoietin-like peptide 4 levels with obesity and hepatosteatosis in adolescents. Cytokine 2020, 125, 154802. [Google Scholar] [CrossRef]
- Gomes, D.; Sobolewski, C.; Conzelmann, S.; Schaer, T.; Lefai, E.; Alfaiate, D.; Tseligka, E.D.; Goossens, N.; Tapparel, C.; Negro, F.; et al. ANGPTL4 is a potential driver of HCV-induced peripheral insulin resistance. Sci. Rep. 2023, 13, 6767. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, L.J.; Bramwell, L.R.; Knight, B.; Kos, K. Circulating and tissue specific transcription of angiopoietin-like protein 4 in human Type 2 diabetes. Metabolism 2020, 106, 154192. [Google Scholar] [CrossRef]
- Sylvers-Davie, K.L.; Davies, B.S.J. Regulation of lipoprotein metabolism by ANGPTL3, ANGPTL4, and ANGPTL8. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E493–E508. [Google Scholar] [CrossRef]
- Gaudet, D.; Gonciarz, M.; Shen, X.; Leohr, J.K.; Beyer, T.P.; Day, J.W.; Mullins, G.R.; Zhen, E.Y.; Hartley, M.; Larouche, M.; et al. Targeting the angiopoietin-like protein 3/8 complex with a monoclonal antibody in patients with mixed hyperlipidemia: A phase 1 trial. Nat. Med. 2025, 31, 2632–2639. [Google Scholar] [CrossRef]
- Ghosh, A.; Chénier, I.; Leung, Y.H.; Oppong, A.K.; Peyot, M.L.; Madiraju, S.R.M.; Al-Khairi, I.; Abubaker, J.; Al-Mulla, F.; Prentki, M.; et al. Adipocyte Angptl8 deletion improves glucose and energy metabolism and obesity associated inflammation in mice. iScience 2024, 27, 111292. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Yamakawa, T.; Kashiwagi, R.; Ohira, A.; Sugiyama, M.; Sugiura, Y.; Kondo, Y.; Terauchi, Y. Association between ANGPTL3, 4, and 8 and lipid and glucose metabolism markers in patients with diabetes. PLoS ONE 2021, 16, e0255147. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Su, X.; Lai, M.; Liu, X.; Cheng, Y. Angiopoietin-Like Protein Family-Mediated Functions in Modulating Triglyceride Metabolism and Related Metabolic Diseases. Front. Biosci. (Landmark Ed.) 2025, 30, 25862. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Ou, H.Y.; Hung, H.C.; Su, Y.C.; Lu, F.H.; Wu, J.S.; Yang, Y.C.; Wu, C.L.; Chang, C.J. A novel hepatokine, HFREP1, plays a crucial role in the development of insulin resistance and type 2 diabetes. Diabetologia 2016, 59, 1732–1742. [Google Scholar] [CrossRef]
- Personnaz, J.; Guillou, H.; Kautz, L. Fibrinogen-like 1: A hepatokine linking liver physiology to hematology. Hemasphere 2024, 8, e115. [Google Scholar] [CrossRef]
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M.K. The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef]
- Wu, H.T.; Lu, F.H.; Ou, H.Y.; Su, Y.C.; Hung, H.C.; Wu, J.S.; Yang, Y.C.; Wu, C.L.; Chang, C.J. The role of hepassocin in the development of non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 1065–1072. [Google Scholar] [CrossRef]
- Ou, H.Y.; Yang, Y.C.; Wu, H.T.; Wu, J.S.; Lu, F.H.; Chang, C.J. Increased fetuin-A concentrations in impaired glucose tolerance with or without nonalcoholic fatty liver disease, but not impaired fasting glucose. J. Clin. Endocrinol. Metab. 2012, 97, 4717–4723. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Mey, J.; Varady, K.A. Fetuin-A: A novel link between obesity and related complications. Int. J. Obes. 2015, 39, 734–741. [Google Scholar] [CrossRef]
- Al Ali, L.; van de Vegte, Y.J.; Said, M.A.; Groot, H.E.; Hendriks, T.; Yeung, M.W.; Lipsic, E.; van der Harst, P. Fetuin-A and its genetic association with cardiometabolic disease. Sci. Rep. 2023, 13, 21469. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.Y.; Lu, F.H.; Wu, H.T.; Hung, H.C.; Wu, J.S.; Yang, Y.C.; Chang, C.J. Both diabetes and fetuin-A are independently associated with increased risk of arterial stiffness. Clin. Chim. Acta 2015, 445, 133–138. [Google Scholar] [CrossRef]
- Saaddine, J.B.; Cadwell, B.; Gregg, E.W.; Engelgau, M.M.; Vinicor, F.; Imperatore, G.; Narayan, K.M. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988-2002. Ann. Intern. Med. 2006, 144, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Hoerger, T.J.; Segel, J.E.; Gregg, E.W.; Saaddine, J.B. Is glycemic control improving in U.S. adults? Diabetes Care 2008, 31, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Absetz, P.; Valve, R.; Oldenburg, B.; Heinonen, H.; Nissinen, A.; Fogelholm, M.; Ilvesmäki, V.; Talja, M.; Uutela, A. Type 2 diabetes prevention in the “real world”: One-year results of the GOAL Implementation Trial. Diabetes Care 2007, 30, 2465–2470. [Google Scholar] [CrossRef]
- Phillips, L.S.; Branch, W.T.; Cook, C.B.; Doyle, J.P.; El-Kebbi, I.M.; Gallina, D.L.; Miller, C.D.; Ziemer, D.C.; Barnes, C.S. Clinical inertia. Ann. Intern. Med. 2001, 135, 825–834. [Google Scholar] [CrossRef]
- Holman, R.R. Assessing the potential for alpha-glucosidase inhibitors in prediabetic states. Diabetes Res. Clin. Pract. 1998, 40 (Suppl. S1), S21–S25. [Google Scholar] [CrossRef]
- Buchanan, T.A. (How) can we prevent type 2 diabetes? Diabetes 2007, 56, 1502–1507. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Blackburn, G.; Brancati, F.L.; Bray, G.A.; Bright, R.; Clark, J.M.; Curtis, J.M.; Espeland, M.A.; Foreyt, J.P.; Graves, K.; et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: One-year results of the look AHEAD trial. Diabetes Care 2007, 30, 1374–1383. [Google Scholar] [CrossRef]
- Kuo, F.Y.; Cheng, K.C.; Li, Y.; Cheng, J.T. Oral glucose tolerance test in diabetes, the old method revisited. World J. Diabetes 2021, 12, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; McFann, K.; Newnes, L.; Nadeau, K.J.; Zeitler, P.S.; Kelsey, M. Hemoglobin A1c assay variations and implications for diabetes screening in obese youth. Pediatr. Diabetes 2014, 15, 557–563. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | NGT (n = 45) | IFG (n = 38) | IGT (n = 42) | NDD (n = 46) | NGT + Hepatic Steatosis (n = 45) | IFG + Hepatic Steatosis (n = 43) | IGT + Hepatic Steatosis (n = 47) | NDD + Hepatic Steatosis (n = 42) | p |
|---|---|---|---|---|---|---|---|---|---|
| Male, % | 64.4 | 63.2 | 57.1 | 58.7 | 60.0 | 62.8 | 61.7 | 61.9 | 0.998 |
| Age, year | 61.3 ± 11.7 | 61.0 ± 11.9 | 62.7 ± 11.5 | 61.7 ± 11.3 | 61.4 ± 10.4 | 60.8 ± 10.2 | 61.0 ± 11.0 | 61.2 ± 10.7 | 0.681 |
| BMI, kg/m2 | 22.9 ± 2.8 | 23.9 ± 2.2 | 23.7 ± 3.4 | 23.5 ± 3.1 | 26.1 ± 3.2 | 26.7 ± 2.8 | 26.8 ± 2.8 | 27.5 ± 2.9 | <0.001 |
| HTN, % | 28.9 | 18/4 | 21.4 | 21.7 | 13.3 | 30.2 | 29.8 | 31.0 | 0.423 |
| CKD, % | 3.3 | 5.0 | 1.9 | 4.8 | 5.3 | 4.2 | 4.1 | 5.1 | 0.235 |
| Obesity, % | 4.5 | 11.4 | 14.3 | 15.2 | 42.2 | 36.6 | 46.8 | 45.2 | <0.001 |
| HbA1c, % | 5.5 ± 0.4 | 5.9 ± 0.4 | 5.9 ± 0.3 | 7.2 ± 2.1 | 5.5 ± 0.3 | 5.9 ± 0.4 | 5.9 ± 0.4 | 7.6 ± 1.7 | <0.001 |
| HOMA-IR | 0.6 ± 0.6 | 0.8 ± 0.6 | 1.3 ± 0.5 | 1.5 ± 0.9 | 0.7 ± 0.5 | 1.2 ± 0.6 | 1.3 ± 0.9 | 2.9 ± 2.4 | <0.001 |
| ALT, U/L | 24.0 ± 11.1 | 24.8 ± 19.9 | 20.7 ± 6.9 | 24.9 ± 9.3 | 30.1 ± 11.4 | 32.2 ± 19.9 | 36.2 ± 20.4 | 43.3 ± 29.9 | <0.001 |
| AST, U/L | 25.6 ± 6.8 | 26.0 ± 8.7 | 23.4 ± 4.6 | 24.9 ± 6.9 | 27.7 ± 7.7 | 26.6 ± 7.9 | 30.1 ± 9.6 | 34.3 ± 16.6 | <0.001 |
| SBP, mmHg | 123.6 ± 17.3 | 126.6 ± 17.3 | 128.5 ± 17.7 | 126.5 ± 16.6 | 124.8 ± 14.8 | 131.0 ± 16.1 | 132.3 ± 13.5 | 130.0 ± 12.6 | 0.247 |
| Creatinine, μmol/L | 76.5 ± 15.2 | 77.3 ± 17.1 | 78.8 ± 16.3 | 75.3 ± 21.1 | 77.1 ± 14.5 | 77.1 ± 13.7 | 76.3 ± 18.5 | 78.5 ± 19.2 | 0.126 |
| Albumin, µmol/L | 654.7 ± 36.5 | 669.2 ± 32.9 | 650.0 ± 38.9 | 656.2 ± 49.7 | 653.4 ± 35.5 | 671.6 ± 39.9 | 676.2 ± 35.4 | 667.6 ± 41.9 | 0.226 |
| hs-CRP, nmol/L | 27.1 ± 40.7 | 26.9 ± 16.7 | 28.0 ± 46.4 | 62.5 ± 93.2 | 26.3 ± 81.6 | 33.1 ± 41.5 | 33.2 ± 31.8 | 62.9 ± 77.4 | 0.002 |
| Total cholesterol, mmol/L | 5.4 ± 1.1 | 5.4 ± 1.0 | 5.3 ± 1.0 | 5.4 ± 1.2 | 5.5 ± 1.1 | 5.4 ± 1.0 | 5.5 ± 1.0 | 5.3 ± 1.0 | 0.981 |
| TG, mmol/L a | 1.1 ± 0.4 | 1.3 ± 0.9 | 1.3 ± 0.7 | 1.6 ± 1.3 | 1.8 ± 0.8 | 1.7 ± 1.3 | 1.9 ± 1.0 | 2.1 ± 1.8 | 0.007 |
| HDL-cholesterol, mmol/L | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.3 | 1.2 ± 0.2 | 1.2 ± 0.4 | 1.2 ± 0.3 | 1.2 ± 0.3 | <0.001 |
| LDL-cholesterol, mmol/L | 3.3 ± 1.0 | 3.3 ± 1.0 | 3.3 ± 0.9 | 3.4 ± 1.0 | 3.4 ± 1.0 | 3.4 ± 0.9 | 3.4 ± 0.8 | 3.3 ± 0.8 | 0.894 |
| Adiponectin, μmol/dL | 12.9 ± 12.8 | 14.4 ± 9.3 | 10.5 ± 4.9 | 10.1 ± 5.6 | 10.0 ± 9.5 | 10.2 ± 3.4 | 8.9 ± 4.8 | 7.4 ± 3.7 | <0.001 |
| Smoking, % | 4.4 | 5.3 | 7.1 | 8.7 | 8.9 | 7.0 | 12.8 | 2.4 | 0.706 |
| Habitual exercise, % | 4.4 | 5.3 | 9.5 | 2.2 | 2.2 | 0.0 | 4.3 | 0.0 | 0.267 |
| Alcohol use, % | 2.2 | 0.0 | 2.4 | 2.2 | 0.0 | 0.0 | 6.4 | 0.0 | 0.239 |
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| Sex | 0.56 (−4.30, 5.42) | 0.821 | 3.15 (−0.12, 6.42) | 0.059 |
| Age | −0.03 (−0.20, 0.14) | 0.734 | 0.02 (−0.15, 0.19) | 0.962 |
| HOMA-IR | 1.57 (0.10, 3.03) | 0.036 | ||
| Body-mass index | 0.89 (0.32, 1.46) | 0.002 | ||
| Adiponectin | −0.08 (−0.32, 0.16) | 0.498 | ||
| hs-CRP | 0.02 (−0.01, 0.04) | 0.247 | 0.01 (−0.02, 0.04) | 0.477 |
| Triacylglycerol | −0.89 (−2.53, 0.76) | 0.292 | ||
| HDL-cholesterol | −2.66 (−8.26, 2.94) | 0.351 | ||
| SBP | 0.01 (−0.11, 0.12) | 0.934 | ||
| ALT | 0.04 (−0.06, 0.13) | 0.434 | ||
| Albumin | −7.32 (−13.986, −0.644) | 0.032 | ||
| Creatinine | 0.11 (−0.02, 0.25) | 0.097 | ||
| IFG vs. NGT | 2.34 (−2.07, 6.75) | 0.297 | ||
| IGT vs. NGT | 11.76 (7.55, 15.98) | <0.001 | ||
| NDD vs. NGT | 16.49 (12.24, 20.73) | <0.001 | ||
| Hepatic steatosis | −0.86 (−4.12, 2.40) | 0.605 | ||
| Obesity | −2.91 (−6.59, 0.77) | 0.121 | ||
| HTN | −0.02 (−3.64, 3.61) | 0.994 | ||
| CKD | 1.32 (−1.94, 4.57) | 0.427 | ||
| Smoking | 1.69 (−4.84, 8.23) | 0.510 | ||
| Habitual exercise | −5.03 (−14.51, 4.45) | 0.297 | ||
| Alcohol use | −3.98 (−16.86, 8.90) | 0.544 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-W.; Li, C.-H.; Wu, H.-T.; Lee, C.-T.; Chen, H.-P.; Ou, H.-Y.; Kuo, H.-Y. Increased Serum Angiopoietin-like Peptide 4 in Impaired Glucose Tolerance and Diabetes Subjects with or Without Hepatic Steatosis. J. Clin. Med. 2025, 14, 7599. https://doi.org/10.3390/jcm14217599
Lin M-W, Li C-H, Wu H-T, Lee C-T, Chen H-P, Ou H-Y, Kuo H-Y. Increased Serum Angiopoietin-like Peptide 4 in Impaired Glucose Tolerance and Diabetes Subjects with or Without Hepatic Steatosis. Journal of Clinical Medicine. 2025; 14(21):7599. https://doi.org/10.3390/jcm14217599
Chicago/Turabian StyleLin, Meng-Wei, Chung-Hao Li, Hung-Tsung Wu, Chun-Te Lee, Huang-Pin Chen, Horng-Yih Ou, and Hsin-Yu Kuo. 2025. "Increased Serum Angiopoietin-like Peptide 4 in Impaired Glucose Tolerance and Diabetes Subjects with or Without Hepatic Steatosis" Journal of Clinical Medicine 14, no. 21: 7599. https://doi.org/10.3390/jcm14217599
APA StyleLin, M.-W., Li, C.-H., Wu, H.-T., Lee, C.-T., Chen, H.-P., Ou, H.-Y., & Kuo, H.-Y. (2025). Increased Serum Angiopoietin-like Peptide 4 in Impaired Glucose Tolerance and Diabetes Subjects with or Without Hepatic Steatosis. Journal of Clinical Medicine, 14(21), 7599. https://doi.org/10.3390/jcm14217599








