Dumbbell Spinal Desmoid Tumor Mimicking a Giant Schwannoma: Case Report and Literature Review
Abstract
1. Introduction
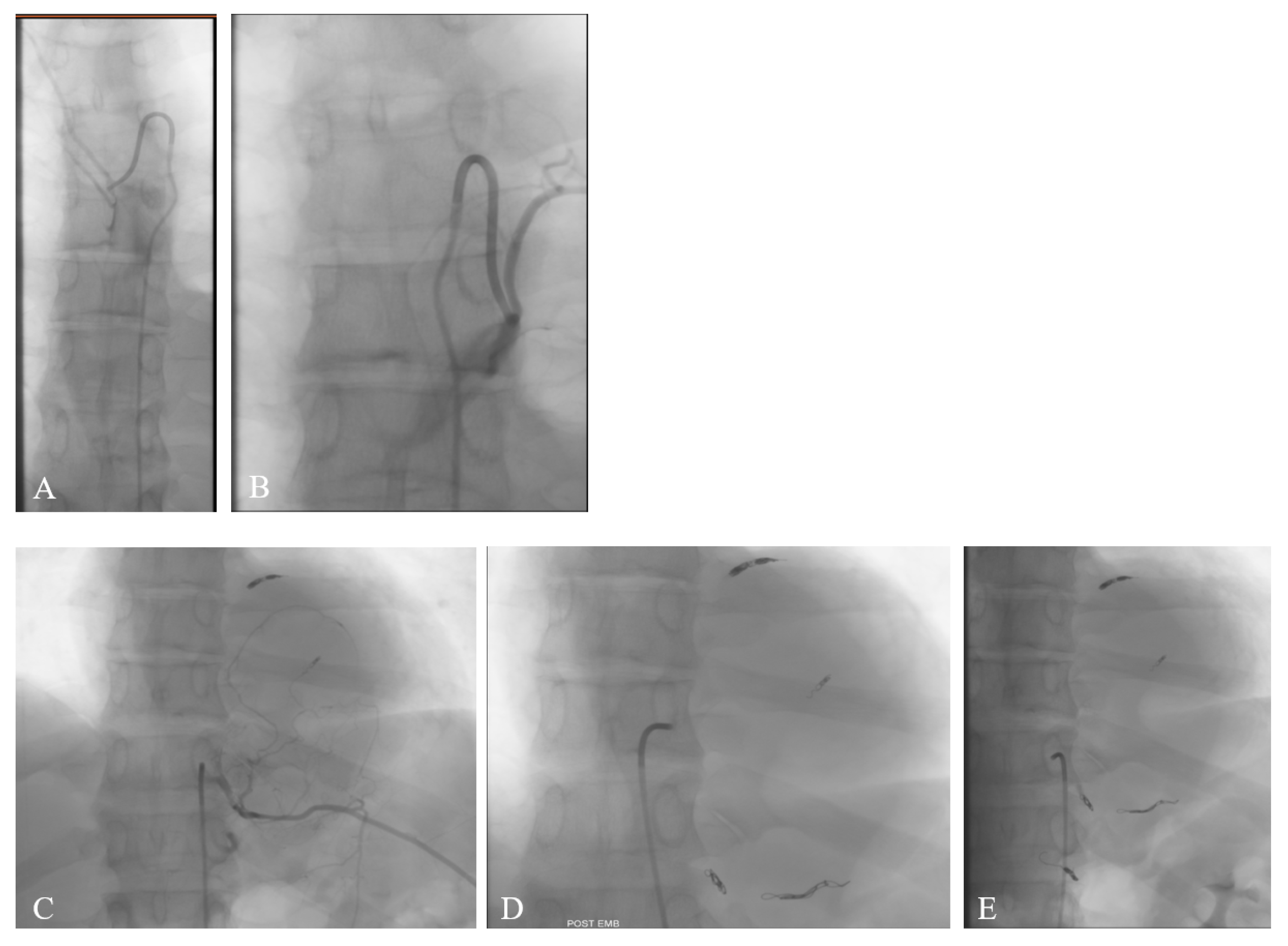
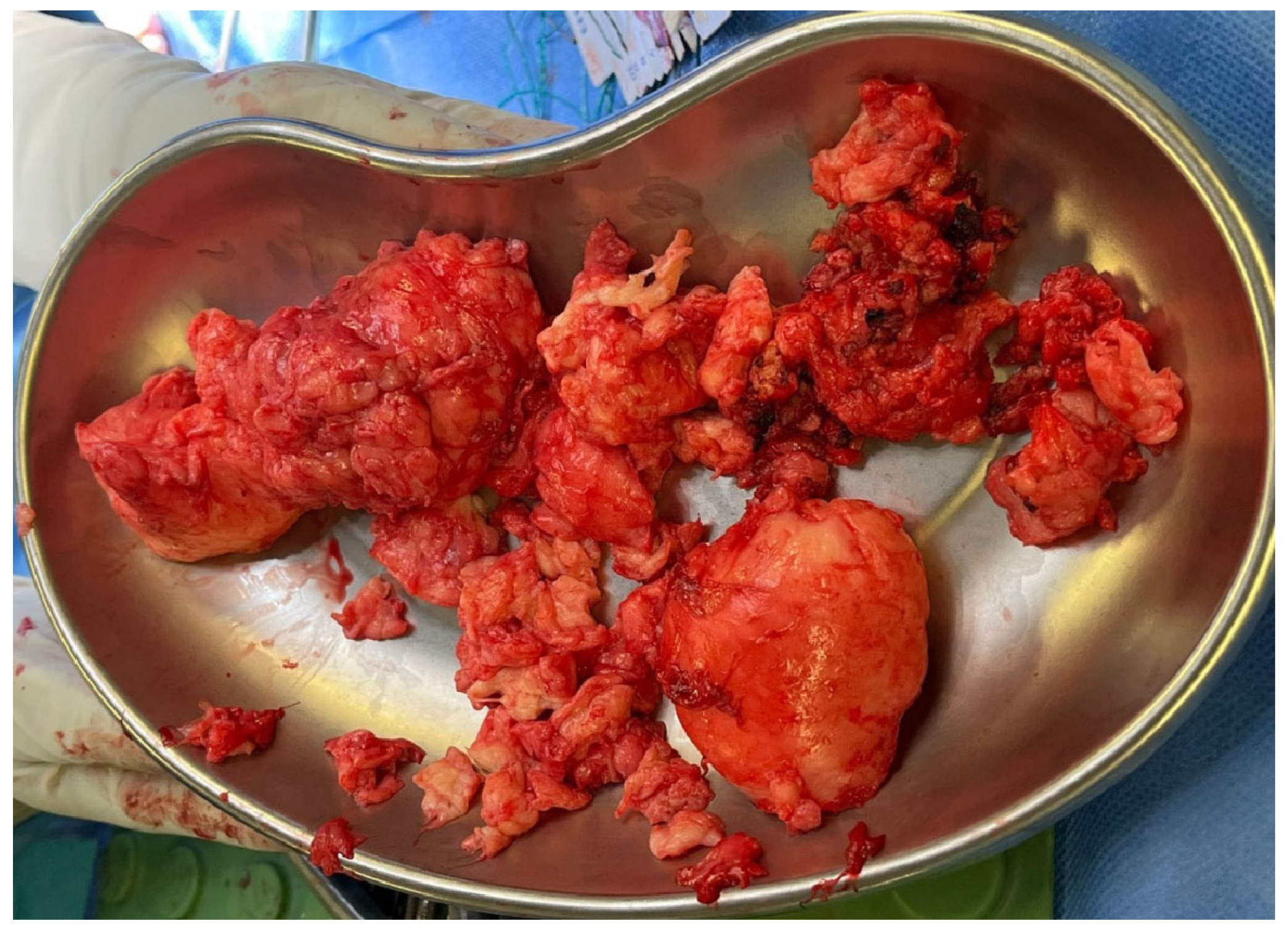
2. Case Report
3. Discussion
3.1. Diagnosis
3.1.1. Definition, Historical Background, and Epidemiology
3.1.2. Classification
3.1.3. Review of Reported Spinal Cases
3.1.4. Pathogenesis and Molecular Mechanisms
3.1.5. Clinical Presentation
3.1.6. Radiological Features and Differential Diagnosis
3.1.7. Histopathological and Immunohistochemical Findings
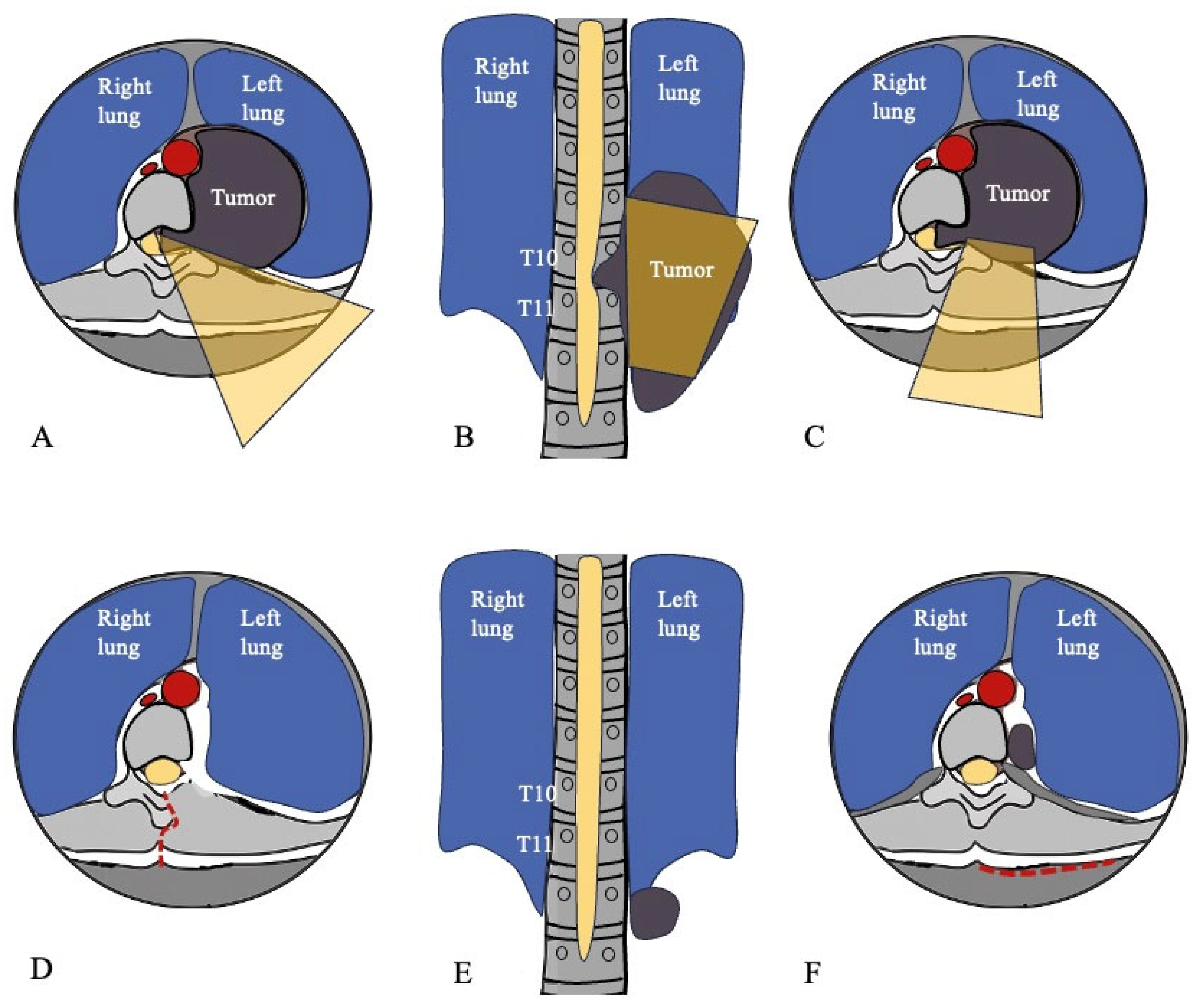
3.2. Surgical Management
3.3. Postoperative Adjuvant Therapy
3.4. Recurrence
3.5. Management of Recurrence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.E.M.; Arantes, E.C.; Villela, R.F.; Soares, C.B.G.; Costa, R.B.D.C.; de Andrade, M.A.P. Extra-abdominal desmoid tumor: Local recurrence and treatment options. Acta Ortop. Bras. 2016, 24, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Papagelopoulos, P.J.; Mavrogenis, A.F.; Mitsiokapa, E.A.; Papaparaskeva, K.T.; Galanis, E.C.; Soucacos, P.N. Current trends in the management of extra-abdominal desmoid tumours. World J. Surg. Oncol. 2006, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.J.; Boland, P.J.; Leung, D.H.Y.; Woodruff, J.M.; Brennan, M.F. The enigma of desmoid tumors. Ann. Surg. 1999, 229, 866. [Google Scholar] [CrossRef]
- Bohl, M.A.; Leveque, J.C.; Bayles, S.; Sethi, R. Postoperative Development of Desmoid Tumor After Surgical Correction of Adult Spinal Deformity: Case Report and Review of Literature. World Neurosurg. 2019, 128, 4–10. [Google Scholar] [CrossRef]
- Yogesh Kumar, B.; Vidyadhara, S.; Vadhiraja, B.M. Pediatric recurrent aggressive spinal fibromatosis with progressive kyphosis and neurological deficits. J. Orthop. Surg. 2019, 27, 2309499019846618. [Google Scholar] [CrossRef]
- Luo, N.; He, X.; Li, G.; Liao, Y.; Tang, Q.; Ye, R.; Zhong, D. Desmoid Tumor Presenting as a Typical Cervical Dumbbell Tumor: Case Report and Review of the Literature. World Neurosurg. 2019, 124, 151–156. [Google Scholar] [CrossRef]
- Mujtaba, B.; Call, C.; Rowland, F.; Spear, R.P.; Amini, B.; Valenzuela, R.; Nassar, S. Desmoid fibromatosis following surgical resection of spinal meningioma. Radiol. Case Rep. 2020, 15, 697–701. [Google Scholar] [CrossRef]
- Schlag, H.; Neuhoff, J.; Castein, J.; Hoffmann, C.; Kandziora, F. Sporadic desmoid fibromatosis of the neck after dorsal spondylodesis of the cervical spine. Surg. Neurol. Int. 2022, 13, 64. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, G.; Liu, S.; Li, T.; Guan, Y.; Yu, B.; Ding, J. Aggressive fibromatosis near the incision after cervical spinal cord ependymoma: A case report. Exp. Ther. Med. 2023, 26, 1–6. [Google Scholar] [CrossRef]
- De Vloo, P.; De Vlieger, J.; Vander Poorten, V.; Sciot, R.; van Loon, J.; Van Calenbergh, F. Desmoid tumors in neurosurgery: A review of the literature. Clin. Neurol. Neurosurg. 2015, 129, 78–84. [Google Scholar] [CrossRef]
- Johannes, M. Ueber den Feinern Bau und Die Formen der Krankhaften Geschwulste [About the Fine Structure and Types of Pathological Tumours]. Reimer 1838, 178, 209–216. [Google Scholar]
- World Health Organization. Pathology & Genetics Tumours of Soft Tissue and Bone; IARC Press: Geneva, Switzerland, 2006. [Google Scholar]
- Bauer, B.M.; Williams, N.L.; Zuckerman, L.M. Development of multifocal extra-abdominal desmoid fibromatosis after surgical resection. Clin. Case Rep. 2019, 7, 2515–2519. [Google Scholar] [CrossRef] [PubMed]
- Bektas, M.; Bell, T.; Khan, S.; Tumminello, B.; Fernandez, M.M.; Heyes, C.; Oton, A.B. Desmoid Tumors: A Comprehensive Review. Adv. Ther. 2023, 40, 3697–3722. [Google Scholar] [CrossRef] [PubMed]
- El-Mahdy, W.; Kane, P.J.; Powell, M.P.; Crockard, H.A. Spinal intradural tumours: Part I—Extramedullary. Br. J. Neurosurg. 1999, 13, 550–557. [Google Scholar] [CrossRef]
- Ballo, M.T.; Zagars, G.K.; Pollack, A.; Pisters, P.W.T.; Pollock, R.A. Desmoid tumor: Prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J. Clin. Oncol. 1999, 17, 158. [Google Scholar] [CrossRef]
- Smith, M.E.F. Atlas of Tumor Pathology. Tumors of the Soft Tissues. Richard L. Kempson, Christopher D. M. Fletcher, Harry L. Evans, Michael R. Hendrickson, and Richard K. Sibley. AFIP, Bethesda, MD 2001. ISBN: 18810416. J. Pathol. 2002, 197, 136–137. [Google Scholar] [CrossRef]
- Mindell, E.R. Enzinger and Weiss’s Soft Tissue Tumors. 4th ed. J. Bone Jt. Surg.-Am. 2001, 83, 1778. [Google Scholar] [CrossRef]
- Dinauer, P.A.; Brixey, C.J.; Moncur, J.T.; Fanburg-Smith, J.C.; Murphey, M.D. Pathologic and MR imaging features of benign fibrous soft-tissue tumors in adults. RadioGraphics 2007, 27, 173–187. [Google Scholar] [CrossRef]
- de Bree, E.; Zoras, O.; Hunt, J.L.; Takes, R.P.; Suárez, C.; Mendenhall, W.M.; Hinni, M.L.; Rodrigo, J.P.; Shaha, A.R.; Rinaldo, A.; et al. Desmoid tumors of the head and neck: A therapeutic challenge. Head Neck 2014, 36, 1517–1526. [Google Scholar] [CrossRef]
- Gonatas, N.K. Extra-abdominal desmoid tumors. Report of six cases. Arch. Path. 1961, 71, 214–221. [Google Scholar]
- Wyler, A.R.; Harris, A.B. Recurrent desmoid tumor following cervical laminectomy: Case report. J. Neurosurg. 1973, 39, 114–116. [Google Scholar] [CrossRef]
- Friede, R.L.; Pollak, A. Neurosurgical desmoid tumors Presentation of four cases with a review of the differential diagnoses. J. Neurosurg. 1979, 50, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Oberthaler, W.; Rhomberg, W. Aggressive fibromatosis a rare cause for lumbar bulging. Arch. Orthop. Trauma Surg. 1988, 107, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Kriss, T.C.; Warf, B.C. Cervical paraspinous desmoid tumor in a child: Case report. Neurosurgery 1994, 35, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.F.; Ng, S.H.; Hsiao, C.C.; Hsieh, C.S.; Lin, J.W.; Huang, C.C.; Shih, T.Y. Juvenile fibromatosis of the posterior mediastinum with intraspinal extension. Am. J. Neuroradiol. 1996, 17, 522–524. [Google Scholar]
- Maurer, F.; Horst, F.; Pfannenberg, C.; Wehrmann, M. Multifocal extra-abdominal desmoid tumor—Diagnostic and therapeutic problems. Arch. Orthop. Trauma Surg. 1996, 115, 359–362. [Google Scholar] [CrossRef]
- Lynch, J.J.; Parvizi, J.; Scheithauer, B.W.; Krauss, W.E. Development of postoperative fibromatosis after resection of an intraspinal meningioma. Case report. J. Neurosurg. Spine 1999, 90, 121–124. [Google Scholar] [CrossRef]
- Shindle, M.K.; Khanna, A.J.; McCarthy, E.F.; O’Neill, P.J.; Sponseller, P.D. Desmoid tumor of the spinal canal causing scoliosis and paralysis. Spine 2002, 27, E304–E307. [Google Scholar] [CrossRef]
- Güzey, F.K.; Emel, E.; Bas, N.S.; Ozkan, N.; Turgut, H.; Sel, B. Aggressive postoperative lumbar fibromatosis after the placement of instrumentation for treatment of spondylolisthesis: Case report. J. Neurosurg. Spine 2006, 4, 338–341. [Google Scholar] [CrossRef]
- Hara, R.; Matsuguma, H.; Suzuki, H.; Ishikawa, Y.; Nakahara, R.; Yamaguchi, T.; Hirabayashi, K. Desmoid Tumor Presenting as a Superior Sulcus Tumor: A Unique Bone Change in the Vertebral Body. Ann. Thorac. Surg. 2007, 84, 1752–1754. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Ad-El, D.; Benjaminov, O.; Gutman, H. Post-traumatic soft tissue tumors: Case report and review of the literature a propos a Post-traumatic paraspinal desmoid tumor. World J. Surg. Oncol. 2008, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.H.C.; Charlton, A.; Arbuckle, S.; Chaseling, R.; Owler, B.K. Metachronous multifocal desmoid-type fibromatoses along the neuraxis with adenomatous polyposis syndrome: Case report. J. Neurosurg. Pediatr. 2010, 6, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, E.; Altinors, N.; Gulsen, S.; Ozen, O. Arisen of extraabdominal desmoid tumor following resection of thoracolumbar schwannoma. Turk. Neurosurg. 2011, 21, 246–248. [Google Scholar] [CrossRef]
- Sevak, S.; Blount, A.L.; Cottingham, S.; DeLano, M.; Vander Woude, D.L.; Stevenson, J.; Chung, M.H. Fibromatosis of the cervical region following laminectomy: A case report and literature review. Spine 2012, 37, E456–E459. [Google Scholar] [CrossRef]
- Shakur, S.F.; Takagi, I.; Jacobsohn, J.A.; Golden, B.M.; Karahalios, D.G. Spinal fibromatosis: A report of two cases and review of the literature. Spine J. 2013, 13, e1–e6. [Google Scholar] [CrossRef]
- Butel-Simoes, G.I.; Spigelman, A.D. Case report: Metachronous central nervous system desmoid tumours and thyroid carcinoma in a young familial adenomatous polyposis patient. Fam. Cancer 2013, 12, 647–649. [Google Scholar] [CrossRef]
- Furlan, J.C.; Valiante, T.; Dickson, B.; Kiehl, T.R. Paraspinal desmoid-type fibromatosis as a cause of low back pain. Spine J. 2013, 13, 1958–1959. [Google Scholar] [CrossRef]
- Hood, B.; Benglis, D.M.; Levi, A.D.; Vanni, S. Occiput to thoracic fusion after surgical resection of desmoid tumor. World Neurosurg. 2013, 79, 207.e15–207.e18. [Google Scholar] [CrossRef]
- Kim, S.J.; Ha, D.H.; Lee, S.M.; Kang, H. Desmoid type fibromatosis in the facet joint of lumbar spine: Case report and review of literature. Korean J. Radiol. 2013, 14, 818–822. [Google Scholar] [CrossRef]
- Puvanesarajah, V.; Lina, I.; Liauw, J.; Hsu, W.; Burger, P.; Witham, T. Desmoid Tumor Formation following Posterior Spinal Instrumentation Placement. Evid.-Based Spine-Care J. 2013, 04, 137–142. [Google Scholar] [CrossRef]
- Eksi, M.S.; Turkoz, H.K.; Ozcan Eksi, E.E.; Akakin, A.; Toktas, Z.O.; Konya, D. Locally aggressive de novo spinal fibromatosis: Case report and review of the literature. Turk. Neurosurg. 2015, 25, 818–823. [Google Scholar] [CrossRef]
- Rispoli, R.; Di Chirico, A.; Tinella, E.; Peciarolo, A.; Ascani, S.; Caputo, N.; Carletti, S. Desmoid tumor following dorsal laminectomy: Case report. Clin. Case Rep. Rev. 2016, 1, 251–252. [Google Scholar] [CrossRef]
- Lacayo, E.A.; Glastonbury, C.M.; Hoang, J.K.; Magliocca, K.R.; Hill, K.L.; Hudgins, P.A. Deep neck fibromatosis after diskectomy and cervical fusion: Case series and review of the literature. Am. J. Roentgenol. 2016, 206, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Avinçsal, Ö.M.; Shinomiya, H.; Otsuki, N.; Sasaki, R.; Nibu, K.I. Successful management of aggressive fibromatosis of the neck: A case report. Balk. Med J. 2018, 35, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.; Hoang, S.; Miller, D.C.; Mesfin, F.B. Extra-abdominal Desmoid Tumor Mimicking Cervical Spine Schwannoma. Cureus 2018, 10, e3145. [Google Scholar] [CrossRef]
- Reitamo, J.J.; Hayry, P.; Nykyri, E.; Saxen, E. The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the Finnish population. Am. J. Clin. Pathol. 1982, 77, 665–673. [Google Scholar] [CrossRef]
- Dahn, I.; Jonsson, N.; Lundh, G. DESMOID TUMOURS. A SERIES OF 33 CASES. Acta Chir. Scand. 1963, 126, 305–314. [Google Scholar]
- Ozawa, H.; Kokubun, S.; Aizawa, T.; Hoshikawa, T.; Kawahara, C. Spinal dumbbell tumors: An analysis of a series of 118 cases. J. Neurosurg. Spine 2007, 7, 587–593. [Google Scholar] [CrossRef]
- Bhat, J.A.; Mir, A. Aggressive Fibromatosis: A Case of Bone Involvement. JK Sci. 2002, 4, 208–209. [Google Scholar]
- Alman, B.; Attia, S.; Baumgarten, C.; Benson, C.; Blay, J.-Y.; Bonvalot, S.; Breuing, J.; Cardona, K.; Casali, P.G.; van Coevorden, F.; et al. The management of desmoid tumours: A joint global consensus-based guideline approach for adult and paediatric patients. Eur. J. Cancer 2020, 127, 96–107. [Google Scholar] [CrossRef]
- Lazar, A.J.; Tuvin, D.; Hajibashi, S.; Habeeb, S.; Bolshakov, S.; Mayordomo-Aranda, E.; Warneke, C.L.; Lopez-Terrada, D.; Pollock, R.E.; Lev, D. Specific mutations in the β-Catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am. J. Pathol. 2008, 173, 1518–1527. [Google Scholar] [CrossRef]
- Dempke, W.; Rie, C.; Grothey, A.; Schmoll, H.J. Cyclooxygenase-2: A novel target for cancer chemotherapy? J. Cancer Res. Clin. Oncol. 2001, 127, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Easter, D.W.; Halasz, N.A. Recent trends in the management of desmoid tumors. Summary of 19 cases and review of the literature. Ann. Surg. 1989, 210, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Enzinger, F.M.; Shiraki, M. Musculo-aponeurotic fibromatosis of the shoulder girdle (extra-abdominal desmoid). Analysis of thirty cases followed up for ten or more years. Cancer 1967, 20, 1131–1140. [Google Scholar] [CrossRef]
- Lee, J.C.; Thomas, J.M.; Phillips, S.; Fisher, C.; Moskovic, E. Aggressive fibromatosis: MRI features with pathologic correlation. Am. J. Roentgenol. 2006, 186, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, S.; Sedaghat, M.; Krohn, S.; Jansen, O.; Freund, K.; Streitbürger, A.; Reichardt, B. Long-term diagnostic value of MRI in detecting recurrent aggressive fibromatosis at two multidisciplinary sarcoma centers. Eur. J. Radiol. 2021, 134, 109406. [Google Scholar] [CrossRef]
- Vandevenne, J.E.; De Schepper, A.M.; De Beuckeleer, L.; Van Marck, E.; Aparisi, F.; Bloem, J.L.; Erkorkmaz, Z.; Brijs, S. New concepts in understanding evolution of desmoid tumors: MR imaging of 30 lesions. Eur. Radiol. 1997, 7, 1013–1019. [Google Scholar] [CrossRef]
- Binatli, Ö. Intramedullary schwannoma of the spinal cord: A case report and review of the literature. J. Neurosurg. Sci. 1999, 43, 163. [Google Scholar]
- Coffin, C.M.; Watterson, J.; Priest, J.R.; Dehner, L.P. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor): A clinicopathologic and immunohistochemical study of 84 cases. Am. J. Surg. Pathol. 1995, 19, 859–872. [Google Scholar] [CrossRef]
- Bernstein, K.E.; Lattes, R. Nodular (Pseudosarcomatous) fasciitis, a nonrecurrent lesion: Clinicopathologic study of 134 cases. Cancer 1982, 49, 1668–1678. [Google Scholar] [CrossRef]
- Gluck, I.; Griffith, K.A.; Biermann, J.S.; Feng, F.Y.; Lucas, D.R.; Ben-Josef, E. Role of radiotherapy in the management of desmoid tumors. Int. J. Radiat. Oncol. 2011, 80, 787–792. [Google Scholar] [CrossRef]
- Buitendijk, S.; Van De Ven, C.P.; Dumans, T.G.; Den Hollander, J.C.; Nowak, P.J.; Tissing, W.J.; Pieters, R.; van den Heuvel-Eibrink, M.M. Pediatric aggressive fibromatosis: A retrospective analysis of 13 patients and review of the literature. Cancer 2005, 104, 1090–1099. [Google Scholar] [CrossRef]
- Kiel, K.D.; Suit, H.D. Radiation therapy in the treatment of aggressive fibromatoses (desmoid tumors). Cancer 1984, 54, 2051–2055. [Google Scholar] [CrossRef]
- Shin, K.H.; Shin, S.J.; Lee, D.H.; Kang, E.S.; Suh, C.O. The Role of Radiotherapy in the Treatment of Aggressive Fibromatosis. Yonsei Med. J. 1999, 40, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Zlotecki, R.A.; Morris, C.G.; Hochwald, S.N.; Scarborough, M.T. Aggressive Fibromatosis. Am. J. Clin. Oncol. 2005, 28, 211–215. [Google Scholar] [CrossRef] [PubMed]
- A Jelinek, J.; Stelzer, K.J.; Conrad, E.; Bruckner, J.; Kliot, M.; Koh, W.-J.; E Laramore, G. The efficacy of radiotherapy as postoperative treatment for desmoid tumors. Int. J. Radiat. Oncol. 2001, 50, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Nuyttens, J.J.; Rust, P.F.; Thomas, C.R.; Turrisi, A.T. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: A comparative review of 22 articles. Cancer 2000, 88, 1517–1523. [Google Scholar] [CrossRef]
- Greenberg, H.M.; Goebel, R.; Weichselbaum, R.R.; Greenberger, J.S.; Chaffey, J.T.; Cassady, J.R. Radiation therapy in the treatment of aggressive fibromatoses. Int. J. Radiat. Oncol. 1981, 7, 305–310. [Google Scholar] [CrossRef]
- Wang, C.P.; Chang, Y.L.; Ko, J.Y.; Cheng, C.H.; Yeh, C.F.; Lou, P.J. Desmoid tumor of the head and neck. Head Neck 2006, 28, 1008–1013. [Google Scholar] [CrossRef]
- Hendriks, M.P.; Driessen, C.M.L.; van Laarhoven, H.W.M.; Janssens, G.O.R.J.; Verbist, B.M.; van der Graaf, W.T.A.; Slootweg, P.J.; Merkx, M.A.W.; van Herpen, C.M.L. Aggressive fibromatosis in the head and neck region: Benign tumor with often mutilating effects. Head Neck 2013, 35, E246–E250. [Google Scholar] [CrossRef]
- Riedel, R.F.; Agulnik, M. Evolving strategies for management of desmoid tumor. Cancer 2022, 128, 3027–3040. [Google Scholar] [CrossRef]
- Eastley, N.; McCulloch, T.; Esler, C.; Hennig, I.; Fairbairn, J.; Gronchi, A.; Ashford, R. Extra-abdominal desmoid fibromatosis: A review of management, current guidance and unanswered questions. Eur. J. Surg. Oncol. (EJSO) 2016, 42, 1071–1083. [Google Scholar] [CrossRef]
- Gega, M.; Yanagi, H.; Yoshikawa, R.; Noda, M.; Ikeuchi, H.; Tsukamoto, K.; Oshima, T.; Fujiwara, Y.; Gondo, N.; Tamura, K.; et al. Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J. Clin. Oncol. 2006, 24, 102–105. [Google Scholar] [CrossRef]
- Goy, B.W.; Lee, S.P.; Eilber, F.; Dorey, F.; Eckardt, J.; Fu, Y.-S.; Juillard, G.J.; Selch, M.T. The role of adjuvant radiotherapy in the treatment of resectable desmoid tumors. Int. J. Radiat. Oncol. 1997, 39, 659–665. [Google Scholar] [CrossRef]



| Author and Year | Age | Gender | Presentation | Etiology | Period Between Surgery and Diagnosis of DT | Tumor Location | Treatment | Follow Up (Months) | Recurrence | Time to Recurrence | Recurrence Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| * Gonatas et al., 1961 [22] | 45 | F | painful mass | post-op | years | cervical paraspinal | marginal resection | 14 | no | / | / |
| Wyler et al., 1973 [23] | 39 | F | painful mass | post-op | 12 | cervicothoracic junction paraspinal | wide marginal resection | 15 | yes | 8 | wide marginal resection |
| * Friede et al., 1979 [24] | 11 | F | hypoesthesia, spasticity and paraparesis | de novo | / | T2–T5 intramedullary | intralesional resection | / | / | / | / |
| Oberthaler et al., 1988 [25] | 21 | M | painful mass | de novo | / | T10–L4 (bone) | intralesional resection + radiotherapy | 36 | no | / | / |
| Kriss et al., 1994 [26] | 1.5 | F | painless mass | de novo | / | cervical C2–C5 paraspinal | wide marginal resection | 17 | no | / | / |
| Ko et al., 1996 [27] | 3 | F | painless mass | de novo | / | T9–T10 intraspinal + mediastinal and chest wall | wide marginal resection | / | / | / | / |
| Maurer et al., 1996 [28] | 18 | F | painless mass | post-op | 12 | lumbar paraspinal | intralesional resection | 6 | yes | 4 | no |
| Lynch et al., 1999 [29] | 49 | F | painful mass | post-op | 18 | thoracic paraspinal | wide marginal resection | / | / | / | / |
| Shindle et al., 2002 [30] | 12 | F | pain, scoliosis and paraparesis | de novo | / | T7–T10 intraspinal, T9 and T10 root encasement, bone erosion + chest mass | Intralesional resection + radiotherapy | 108 | no | / | / |
| Guzey et al., 2006 [31] | 50 | F | painful mass | post-op | 14 | thoracolumbar junction paraspinal | wide marginal resection | 14 | no | / | / |
| Hara et al., 2007 [32] | 50 | M | shoulder pain | de novo | / | T3–T4 paraspinal | chemotherapy + radiotherapy | 12 | no | / | / |
| Cohen et al., 2008 [33] | 27 | F | painless mass | de novo | / | cervicothoracic paraspinal | chemotherapy + wide marginal resection | 24 | no | / | / |
| Chung et al., 2010 [34] | 14 | M | asymptomatic finding on routine imaging | Gardner syndrome | / | C7 vertebral body | marginal resection | 12 | no | / | / |
| Sonmez et al., 2011 [35] | 55 | F | pailful mass | post-op | 6 | thoracolumbar paraspinal | wide marginal resection | / | / | / | / |
| Sevak et al., 2012 [36] | 48 | F | painless mass | post-op | 24 | C7–T1 paraspinal | wide marginal resection | 6 | no | / | / |
| Shakur et al., 2013 [37] | 45 | F | neck and arm pain and paraesthesia | de novo | / | C5–T1 intraspinal- paraspinal | intralesional resection | 40 | no | / | / |
| 38 | F | midthoracic back pain and paraesthesia | de novo | / | T9–T10 intraspinal-paraspinal | marginal resection + chemotherapy (tamoxifen) | 10 | no | / | / | |
| Butel-Simoes et al., 2013 [38] | 13 | M | asymptomatic finding on routine imaging | Gardner syndrome | / | C7–T1 paraspinal | marginal resection + chemotherapy | 48 | no | / | / |
| Furlan et al., 2013 [39] | 41 | F | painful mass and low back pain | de novo | / | lumbar | / | / | / | / | / |
| Hood et al., 2013 [40] | 25 | F | painful mass | de novo | / | occipitocervical | subtotal resection + chemotherapy (imatinib) | 27 | yes | 7 | wide marginal resection |
| Kim et al., 2013 [41] | 31 | M | painful mass with sciatica | de novo | L3–L4 bone + paraspinal mass | marginal resection | / | / | / | / | |
| Puvanesarajah et al., 2013 [42] | 57 | F | painful mass | post-op | 10 | thoracic paraspinal | wide marginal resection | 24 | no | / | / |
| * Eksi et al., 2015 [43] | 63 | F | low back pain, claudication and hypoesthesia | de novo | / | lumbar paraspinal | intralesional resection | 24 | yes | 6 | wide marginal resection + radiotherapy |
| Rispoli et al., 2015 [44] | 57 | F | painful mass | post-op | 24 | thoracic paraspinal | wide marginal resection | 24 | no | / | / |
| * Lacayo et al., 2016 [45] | 48 | M | painful mass | post-op | 60 | cervical paraspinal | radiotherapy | / | / | / | / |
| 53 | M | painful mass | post-op | 12 | cervical paraspinal | wide marginal resection | / | / | / | / | |
| 25 | M | painful mass | post-op | 48 | cervical paraspinal | wide marginal resection | / | / | / | / | |
| 61 | M | painful mass | post-op | 48 | cervical paraspinal | / | / | / | / | / | |
| Avincsal et al., 2018 [46] | 71 | M | painless mass | de novo | / | cervical paraspinal | wide marginal resection | 84 | yes | 3 | radiotherapy |
| * Goldstein et al., 2018 [47] | 67 | F | neck and bilateral shoulder pain | post-op | / | C2–C4 paraspinal | wide marginal resection | / | / | / | / |
| Bohl et al., 2019 [5] | 56 | F | painless mass | post-op | 24 | cervical paraspinal | wide marginal resection | 1 | no | / | / |
| Kumar et al., 2019 [6] | 13 | F | paraparesis | de novo | / | thoracic T2–T5 spinous process | marginal resection | 48 | yes | 12 | wide marginal resection + radiotherapy |
| * Luo et al., 2019 [7] | 47 | F | painless mass and bilateral cervico-brachialgia | de novo | / | cervical intraspinal-paraspinal | marginal resection + radiotherapy + chemotherapy | 12 | no | / | / |
| Mujtaba et al., 2020 [8] | 42 | F | neck pain and paraparesis | post-op | 72 | C7–T2 intraspinal-paraspinal | wide marginal resection | / | / | / | / |
| Schlag et al., 2022 [9] | 58 | M | painful mass | post-op | 24 | cervical paraspinal | wide marginal resection | 12 | no | / | / |
| Zheng et al., 2023 [10] | 36 | F | painless mass | post-op | 11 | cervical paraspinal | marginal resection | 6 | yes | 6 | wide marginal resection + radiotherapy |
| Recurrence Rate | N. Patients with Recurrence | N. Patients with Follow-Up | N. Patients | Extent of Excision |
|---|---|---|---|---|
| 20% | 2 | 10 | 17 | Wide |
| 29% | 2 | 7 | 8 | Marginal |
| 50% | 3 | 6 | 7 | Subtotal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nafidi, H.; Rispoli, R.; Pizzolitto, S.; Iaccarino, C.; Pavesi, G.; Cappelletto, B. Dumbbell Spinal Desmoid Tumor Mimicking a Giant Schwannoma: Case Report and Literature Review. J. Clin. Med. 2025, 14, 7596. https://doi.org/10.3390/jcm14217596
Nafidi H, Rispoli R, Pizzolitto S, Iaccarino C, Pavesi G, Cappelletto B. Dumbbell Spinal Desmoid Tumor Mimicking a Giant Schwannoma: Case Report and Literature Review. Journal of Clinical Medicine. 2025; 14(21):7596. https://doi.org/10.3390/jcm14217596
Chicago/Turabian StyleNafidi, Hajar, Rossella Rispoli, Stefano Pizzolitto, Corrado Iaccarino, Giacomo Pavesi, and Barbara Cappelletto. 2025. "Dumbbell Spinal Desmoid Tumor Mimicking a Giant Schwannoma: Case Report and Literature Review" Journal of Clinical Medicine 14, no. 21: 7596. https://doi.org/10.3390/jcm14217596
APA StyleNafidi, H., Rispoli, R., Pizzolitto, S., Iaccarino, C., Pavesi, G., & Cappelletto, B. (2025). Dumbbell Spinal Desmoid Tumor Mimicking a Giant Schwannoma: Case Report and Literature Review. Journal of Clinical Medicine, 14(21), 7596. https://doi.org/10.3390/jcm14217596






