Serum Behavior of NT-3 and VEGFβ, Two Unstudied Growth Factors in Patients with Diabetes Mellitus and End-Stage Renal Disease
Abstract
1. Introduction
- Prolonged hyperglycemia induces glycation of proteins (including collagen), lipids, and the extracellular matrix [9]. This results in the formation of AGEs (advanced glycation end products), which generate receptor-mediated and non-receptor-mediated damage. AGEs activate RAGEs (receptors for advanced glycation end products), triggering signaling cascades that induce excessive production of ROS (reactive oxygen species) and activation of the transcription factor NF-kB [9,10]. Consequences: hypertrophy of renal cells incapable of mitosis, angiogenesis, ECM (extracellular matrix) synthesis, and inflammation induced and maintained by ROS that alters the structure and function of endothelial cells.
- b.
- Altered glucose metabolism induces the development of diabetic complications. Three biochemical pathways are most involved: polyols, hexosamine, and protein kinase C (PKC).
2. Purpose of Study
- It is known that the two pathologies, DM and ESRD, are characterized by a significant/severe decrease in tissue-regeneration capacity. We will try to quantify this change by studying the two growth factors, NT-3 and VEGFβ.
- Why NT-3?
- 3.
- Why VEGFβ?
- 4.
- Why IL-10?
- 5.
- We believe that this study can bring improvements in the understanding of the very complex pathophysiological mechanisms in ESRD, associated or not with DM.
- 6.
- Furthermore, it could lead to the identification of new prognostic and therapeutic targets and a deeper understanding of the exchange of biological information between growth factors.
3. Patients and Methods
3.1. Patients and Serum Markers
- -
- patients with ESRD, some of them with associated DM2;
- -
- undergoing hemodialysis, receiving treatment three times a week.
- -
- acute inflammatory and infectious diseases;
- -
- current immunosuppressing treatment;
- -
- acute organ failure (cardiac and hepatic);
- -
- malignancies;
- -
- acute vascular disease (stroke and myocardial infarction);
- -
- psychiatric conditions or impaired judgment;
- -
- blood transfusions in the last 3 months;
- -
- hemoglobinopathy or anemia from other causes than CKD.
3.2. Method
3.3. Statistical Analysis
3.4. Ethical Considerations
4. Results
4.1. NT-3
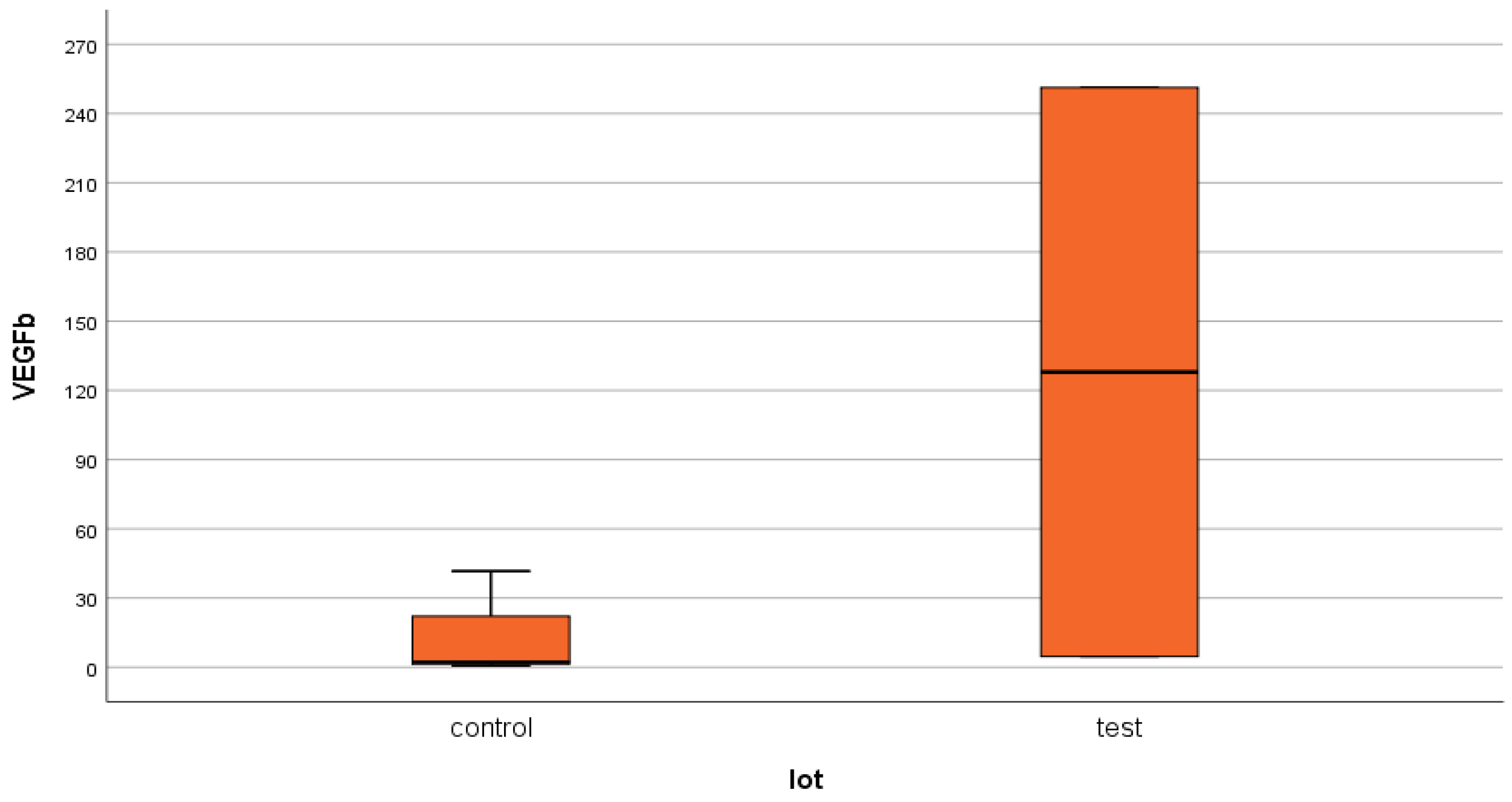
4.2. VEGFβ
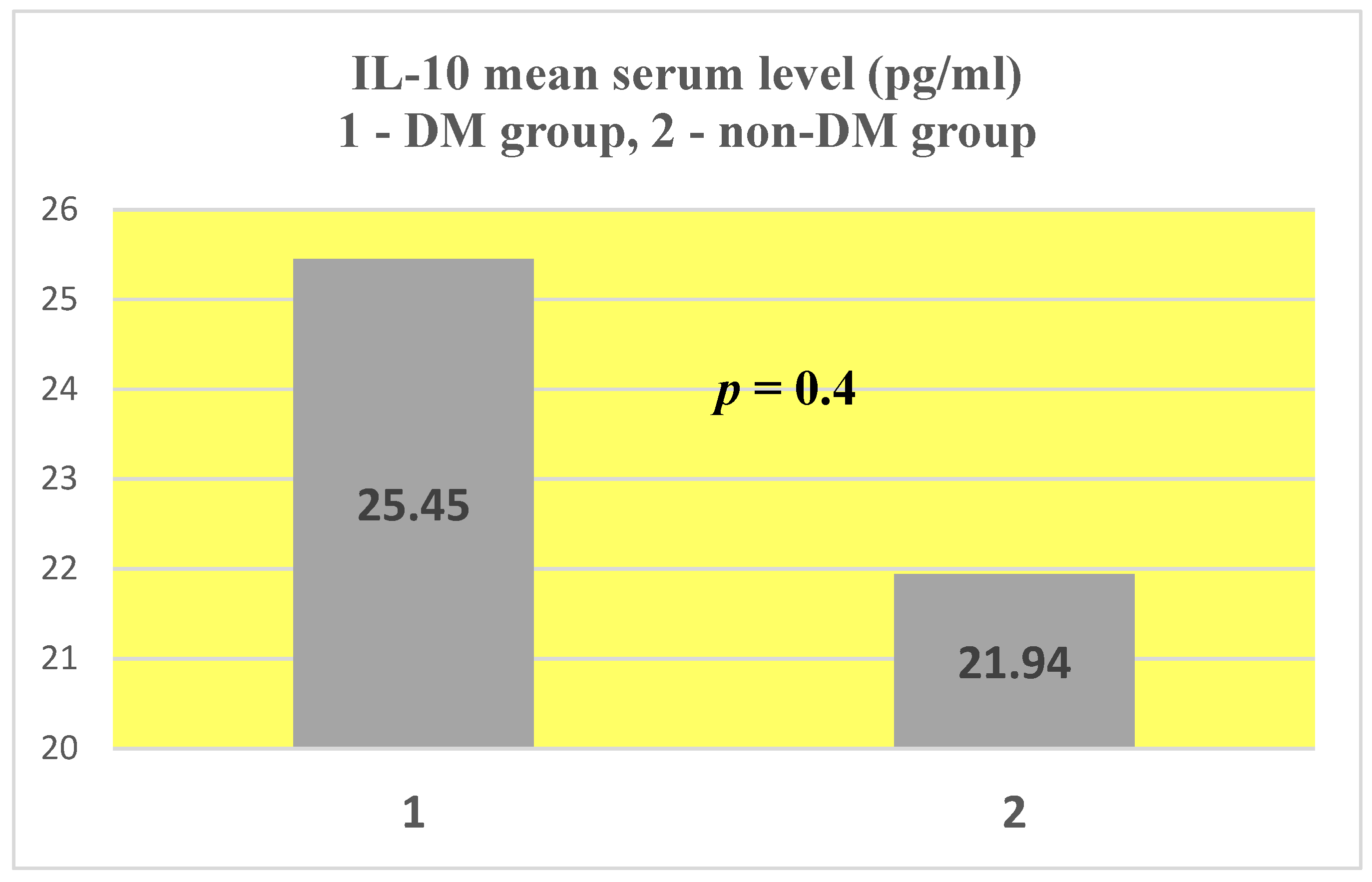
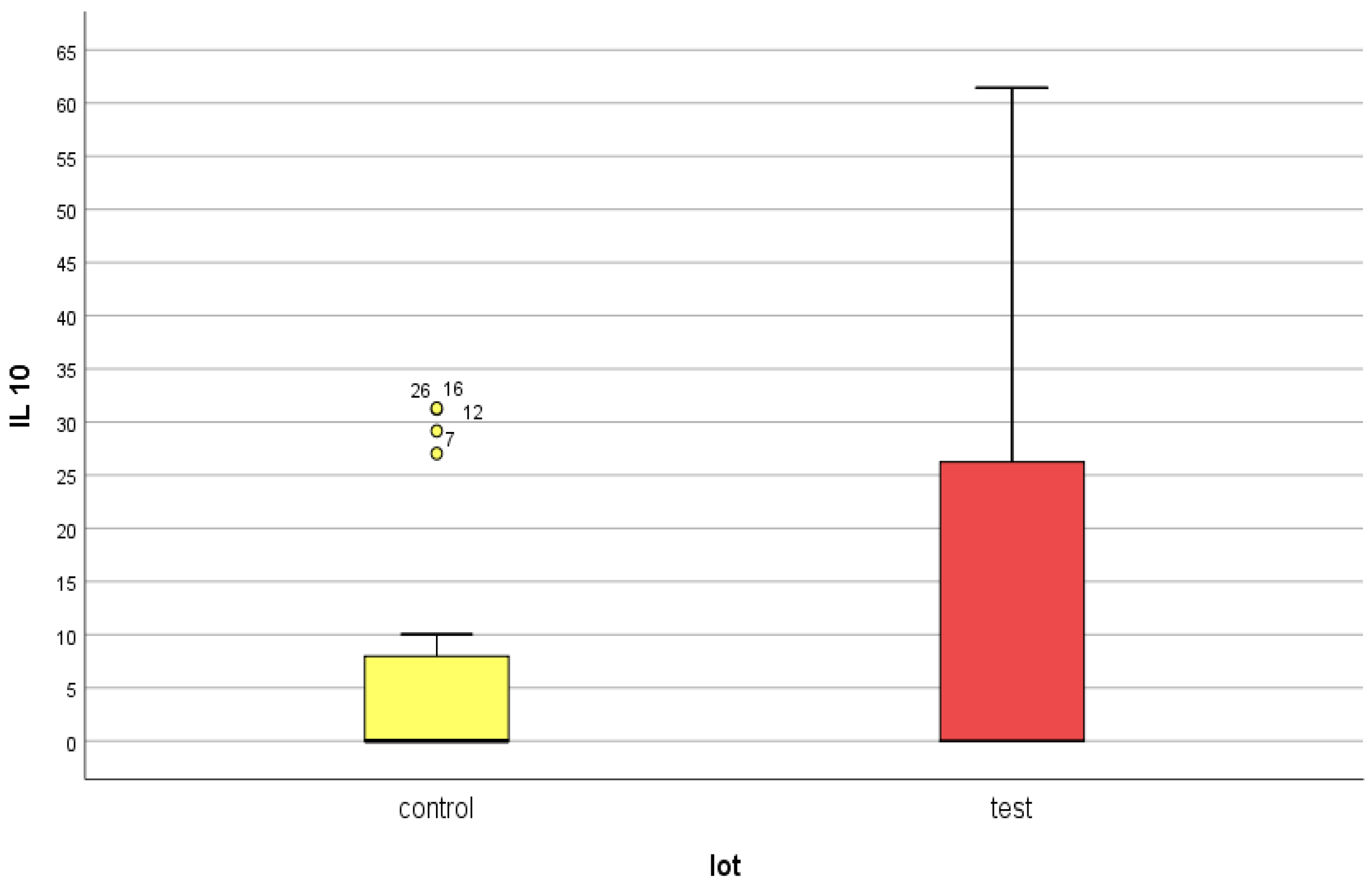
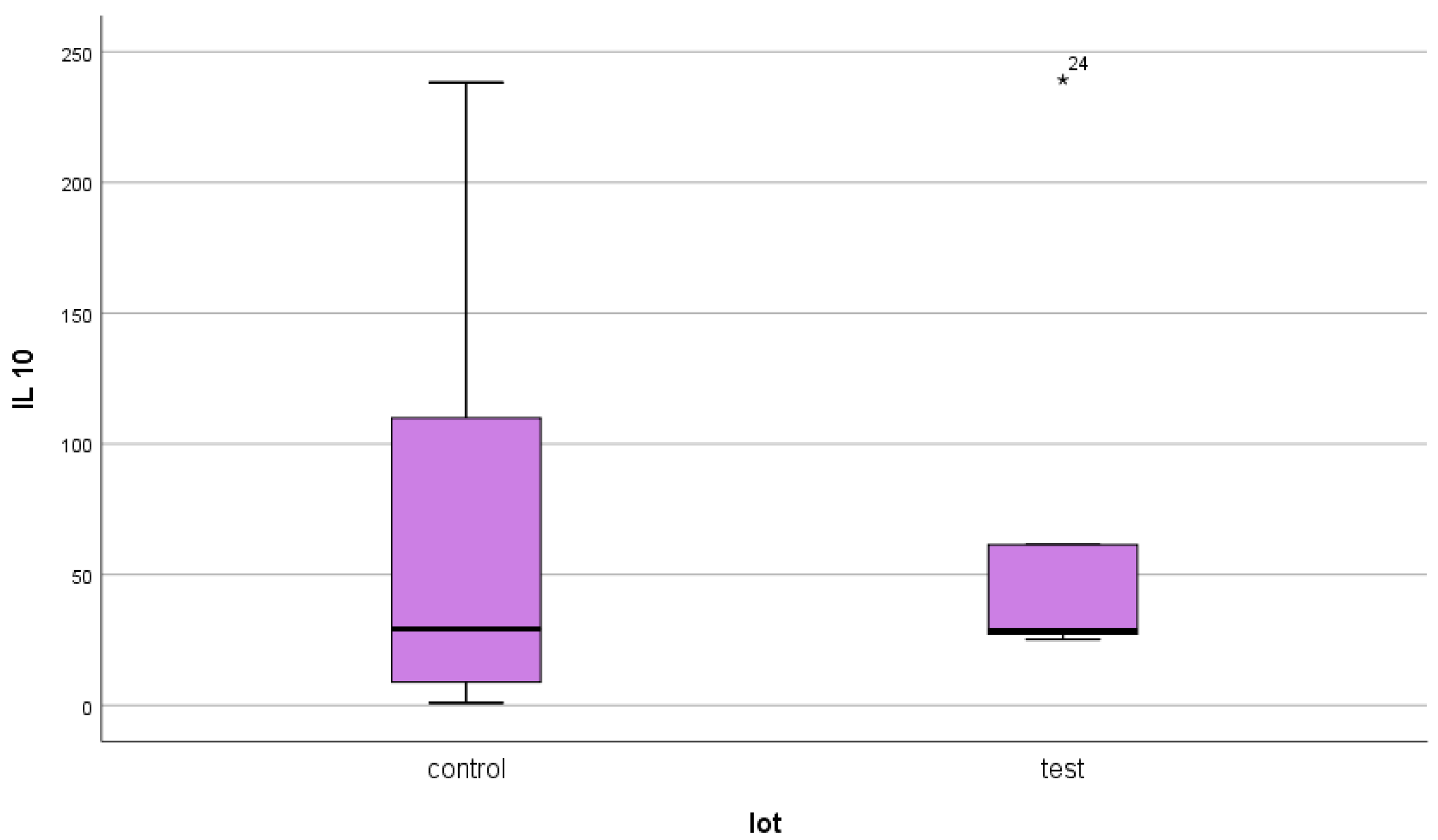
4.3. IL-10
5. Discussions
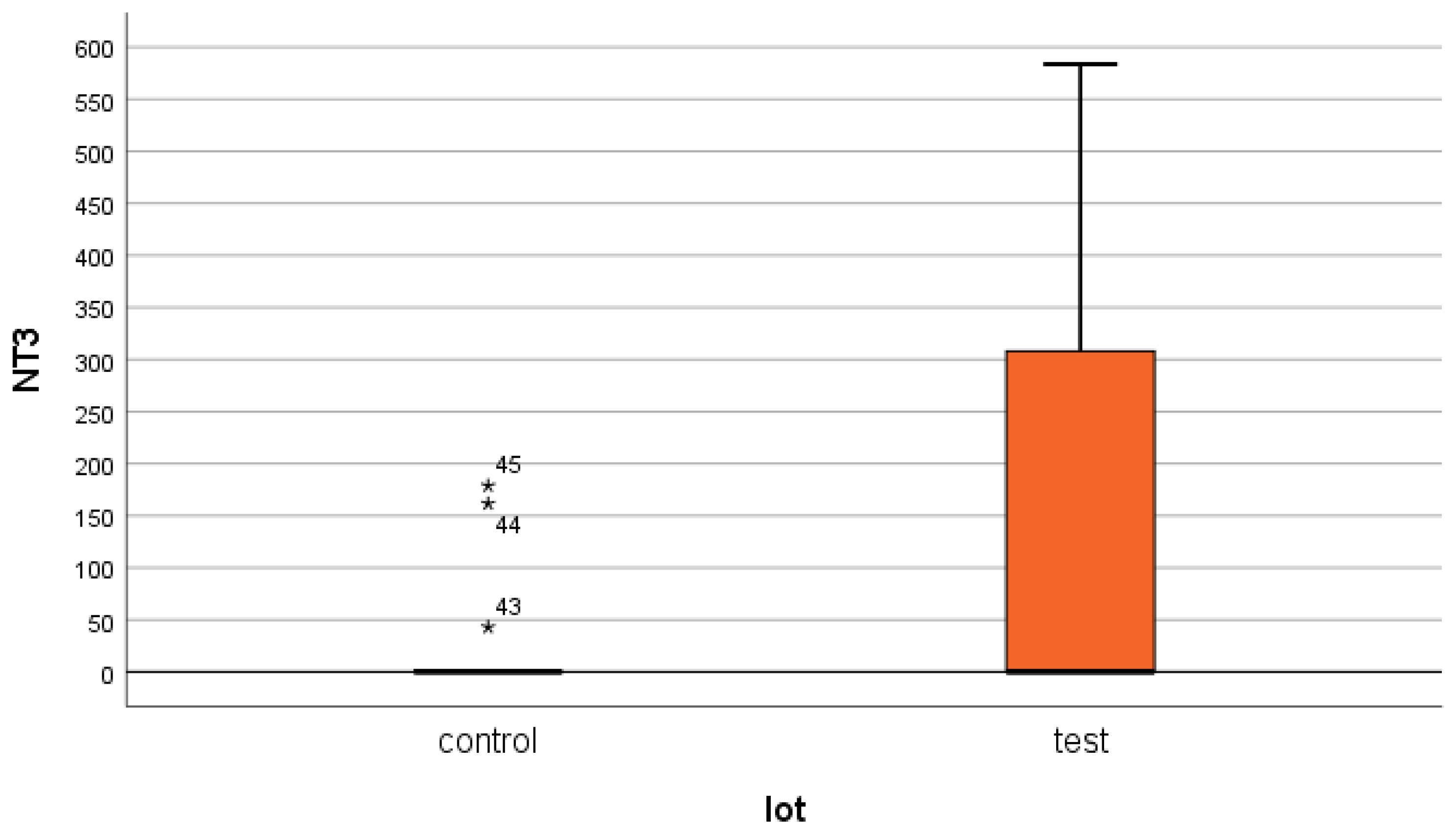
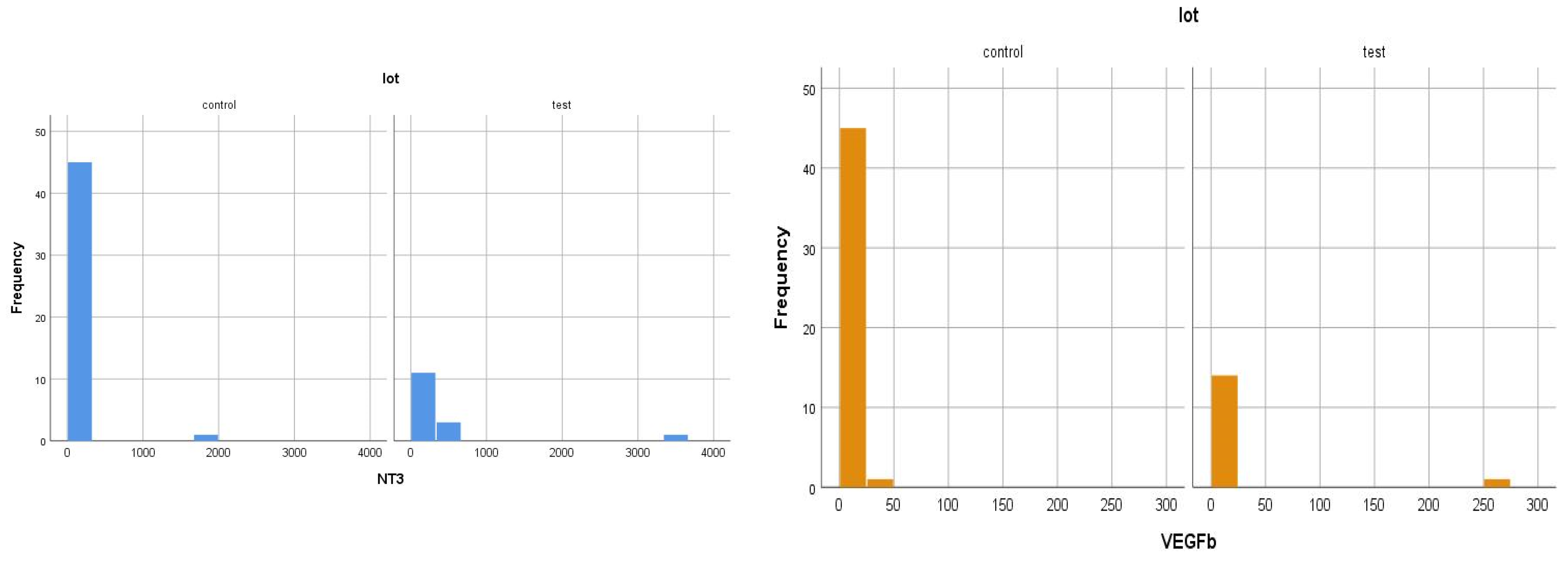
- most of the patients included in the study (60% of the test group and 92% of the control group) have very low serum NT-3 values (<4 pg/mL);
- only 40% of the patients in the test group and 8% of those in the control group had such high serum levels of NT-3 that they determined the average values to be so high, compared to normal levels;
- DM is a pathology that generates additional increases in serum NT-3 only in some of the patients, compared to ESRD.
- -
- very low overall capacity for neuroregeneration, neuronal survival, and functional control of synapses, even though these are essential in conditions of diabetic neuropathy;
- -
- reduced or inhibited angiogenic capacity through NT-3 antagonists, even in the presence of diabetic arteriopathy;
- -
- the canceling of kidney production of NT-3;
- -
- the existence of an intense inflammatory background in patients in the studied groups, with very high serum levels of IL-6 [28]. It is already proven that, in high concentrations, IL-6 inhibits BDNF synthesis [53]. We can state that our study confirms that chronic inflammation, namely, high serum levels of IL-6, also inhibits NT-3 secretion.
- -
- ESRD generates a decrease in serum NT-3 levels, the main explanation of which is the severe decrease in renal synthesis at the level of nerve endings and podocytes, through specific histological alterations;
- -
- even if DM is associated, diabetic neuropathy and angiopathy can only counteract the renal influence on serum NT-3 levels in 40% of the patients in the test group;
- -
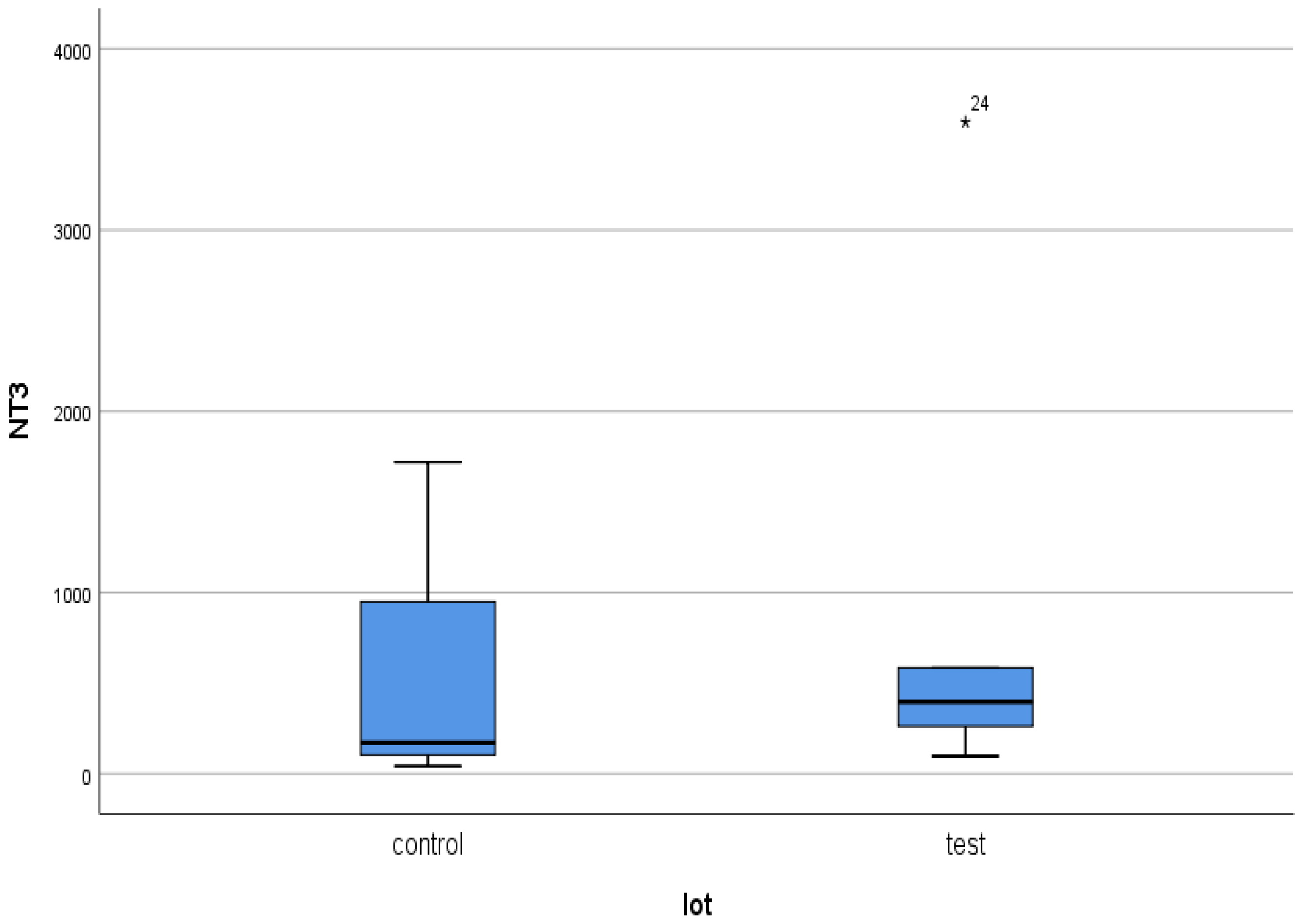
- the very high serum levels presented by a small proportion of patients in both groups indicate chronic extrarenal production of NT-3, most likely from degenerated nervous and cardiovascular structures. However, it is possible that these levels precede the onset of acute ischemia in different areas of the body. We allow ourselves to advance this idea because the 82-year-old patient in the test group who presented the maximum serum values of the three markers [42] passed away a few months after the end of our study. Relatives could not be contacted, but dialysis colleagues had information about a fatal stroke.
- -
- there is still active renal tissue with inflammatory reaction, fibrosis, and eventual angiogenesis;
- -
- there is DM-induced angiopathy, which the body fights against through the hyperproduction of VEGFβ, with an antiapoptotic action on damaged vessels;
- -
- there are associated cardiovascular and neurodegenerative pathologies in which the affected tissues require the antiapoptotic and proregenerative actions of VEGFβ;
- -
- in both pathologies, there is an intense inflammatory background proven by our previous studies [28,42]. Under these conditions, IL-1β, TNFα, and IL-17 stimulate the VEGFβ synthesis from activated macrophages. But the same macrophages also secrete IL-10, an anti-inflammatory cytokine, which inhibits the VEGF synthesis [74].
Functional Interrelationships
- We cannot state that there is a mutual stimulation mechanism between NT-3 and VEGFβ.
- -
- Of the fifteen patients in the test group, only two (13.33%) showed elevated values for both NT-3 and VEGFβ.
- -
- In the control group (forty-five patients), only one (2.22%) showed this aspect.
- Can we talk about a negative influence that each of the two growth factors exerts on the other?
- In both groups’ patients with maximum values of NT-3 and/or VEGFβ, well above the non-null values of the others, can a structural alteration of the receptors be considered as the cause? That is, is it possible that the huge values are induced by the inability to signal through these receptors? Is it possible that the intracellular signaling of NT-3 and/or VEGFβ is extremely reduced despite the very high serum values?
- IL-10 stimulates the synthesis of NT-3, especially in adult neural stem cells. In this way, IL-10 indirectly stimulates the synthesis of neurons, oligodendrocytes, axonal growth, and myelin repair [97]. We can confirm this aspect based on the results obtained in the test group. Of the patients with normal or elevated NT-3 values, 83.33% also showed elevated IL-10 values. All patients (100%) in the control group with normal or elevated NT-3 values showed elevated IL-10 values. We can state that the presence of DM slightly decreases the positive correlation between serum NT-3 and IL-10 values.
- Regarding the scientific data describing the functional interrelationship between IL-10 and VEGFβ, the information is very limited. The existing works consulted describe either the interrelationship between IL-10 and total VEGF, or that between IL-10 and VEGFα. In the patients we studied, we found that all those in the test group (ESRD + DM) with elevated VEGFβ values had associated elevated serum levels of IL-10 (100%). Of the patients in the test group who showed elevated VEGFβ levels, 75% also had elevated IL-10 levels.
- Although the anti-inflammatory, anti-atherosclerotic, and antithrombotic effects of IL-10 are known, a prospective study suggested that elevated serum levels of IL-10 are associated with an increased risk of cardiovascular events in patients with CKD [98]. Under these conditions, the simultaneous presence of elevated serum levels of NT-3, VEGFβ, and IL-10 in patients from both groups could be considered a very important prognostic marker for acute cardiac and vascular events, including at the level of the central and peripheral nervous system.
6. Conclusions
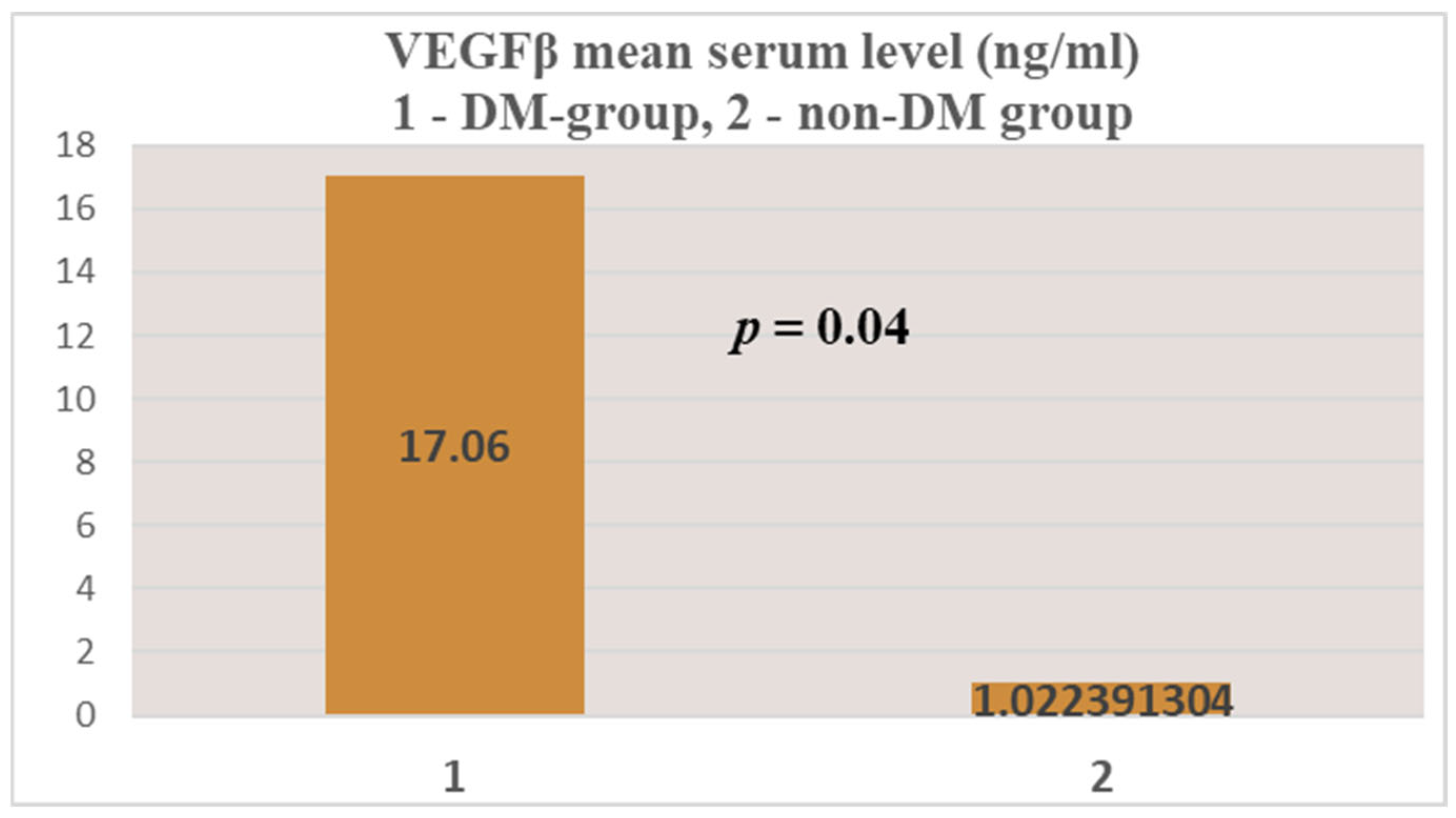
- Significantly increased mean serum levels were obtained for the two growth factors (NT-3 and VEGFβ) in the test group patients (ESRD + DM) compared to those in the control group (ESRD only).
- Compared to the internationally accepted upper limits of normal, the mean serum levels of NT-3 and VEGFβ were much higher.
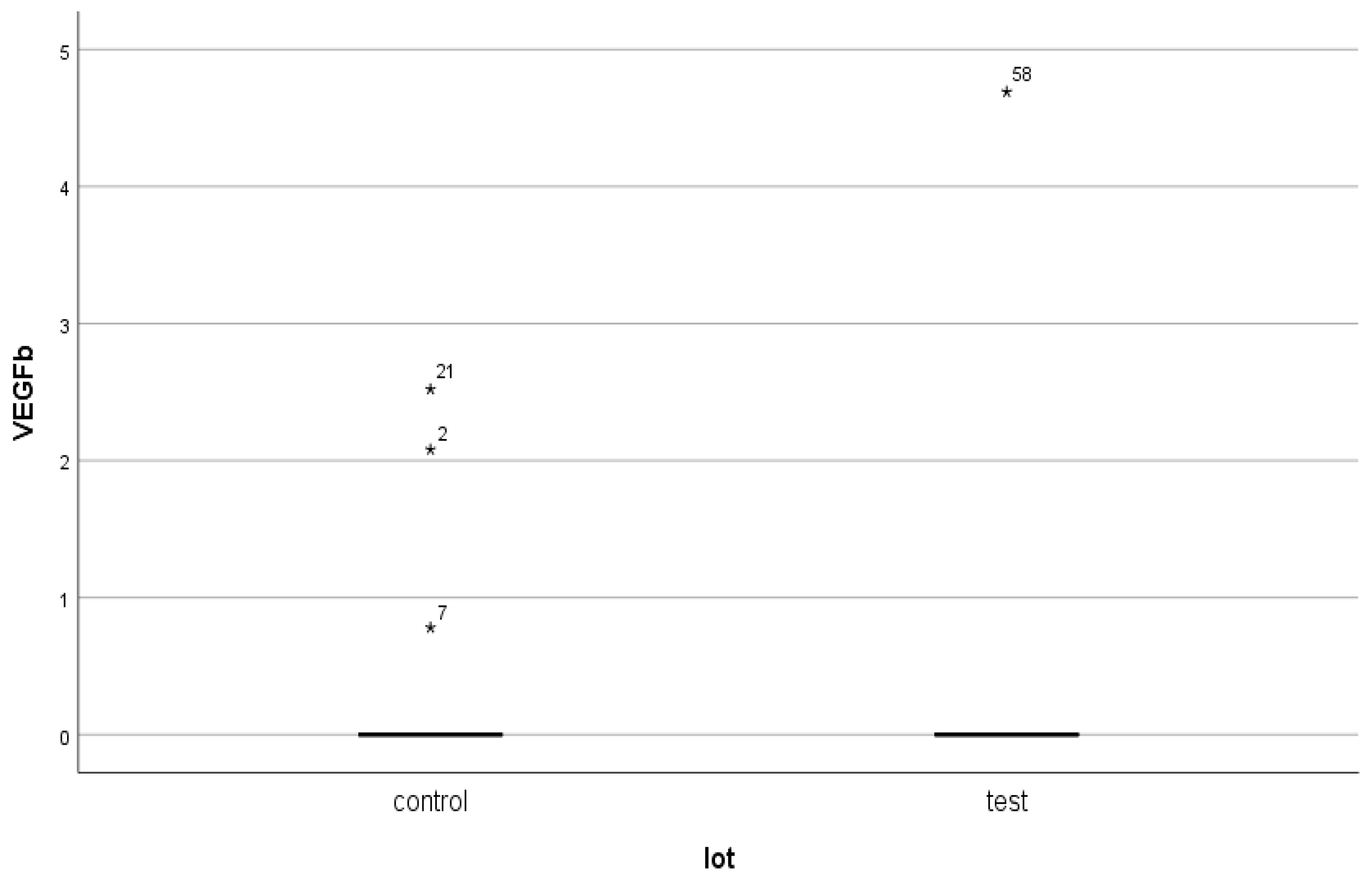
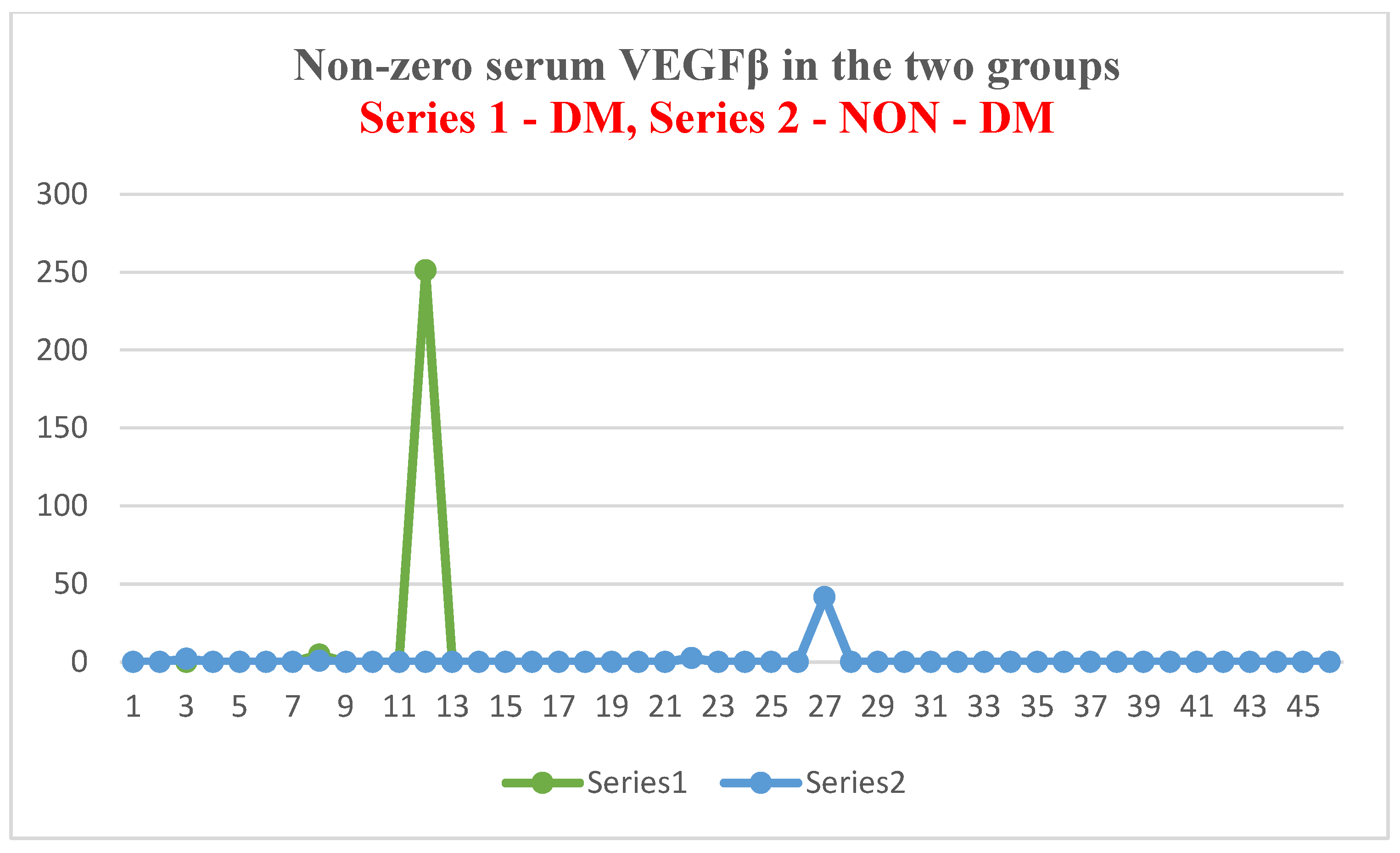
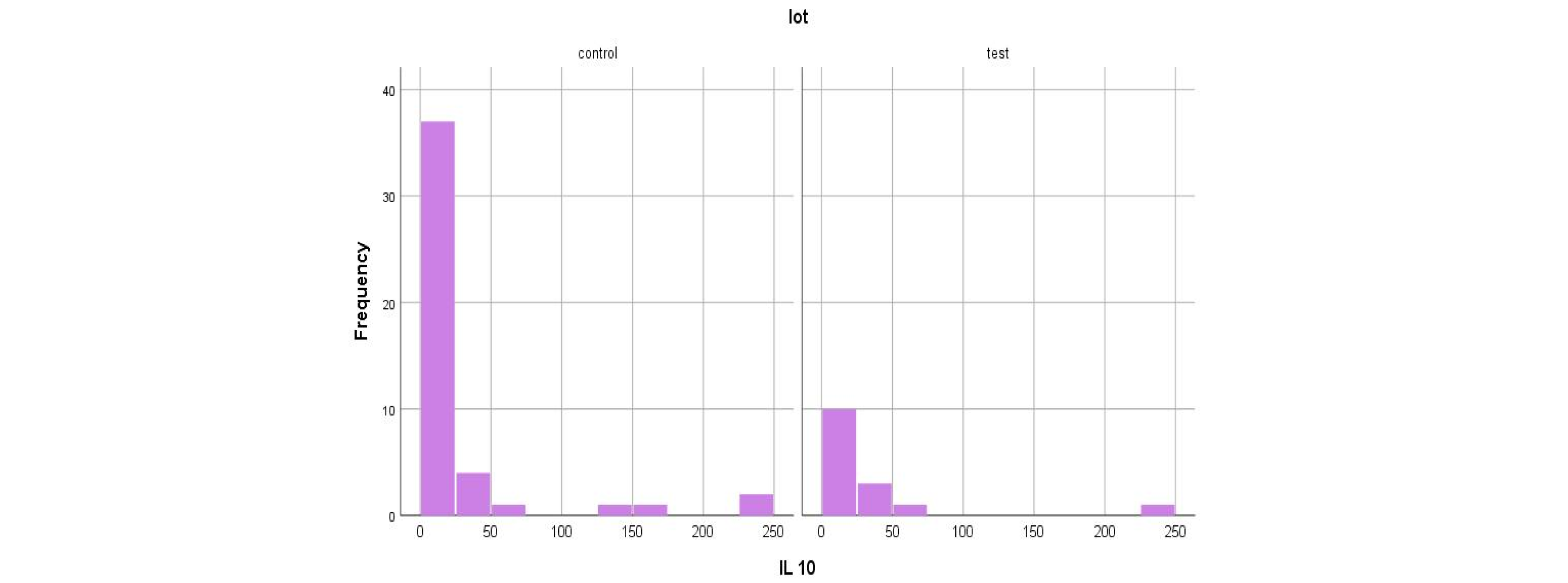
- The major polarization of serum NT-3 and VEGFβ values was very surprising. Although most patients had very low serum levels (most frequently below the detection limit of the kits), some of them had huge levels, which generated an elevated mean value.
- This polarization cannot be attributed to the quality of the kits or the conditions of collection, preservation, and processing of biological samples. The results presented in this article belong to a very wide range of studied parameters. Although kits of the same origin were used for other markers, and the dosages were performed on the same day for all the markers studied, polarizations were present only for NT-3 and VEGFβ, and to a lesser extent for IL-10.
- In patients of both groups with very high NT-3 and VEGFβ values, we consider that their source is most likely extrarenal, from areas affected by diabetic neuropathy and angiopathy but also from the multiple inflammatory foci generated in the body by chronic uremia and hemodialysis. This does not exclude the possibility of low renal production.
- The very low levels of NT-3 in most of the studied patients indicate a severe decrease in its renal production at the level of nerve endings and podocytes. We believe that the kidney should be reconsidered as an important source of this growth factor.
- Very low VEGFβ values, below the detection limit of the kit which is very close to the upper limit of normal serum levels, actually mean normal or low values. It is possible that these values obtained in most patients in the two groups signify an inhibition of angiogenesis induced by ESRD, as well as an intense proapoptotic tendency of the cells.
- IL-10 presented similar mean serum values in the two groups at approximately double the upper limit of normal. This aspect leads us to conclude that, on the one hand, DM does not have a major influence on the serum level of IL-10, and on the other hand, even if this anti-inflammatory cytokine has elevated values, it cannot counteract the pronounced inflammatory background existing in both categories of patients.
- We can discuss the existence of a negative/inhibitory influence that each of the two growth factors exerts on the other, predominantly on the signaling pathway. Each of the two growth factors utilizes cytosolic tyrosine kinases, between which substrate competition can occur.
- In patients with maximum NT-3 and VEGFβ values, we can consider both the existence of an imminent ischemic-neurodegenerative event, as well as the structural and functional alteration of the specific receptor (the decrease/absence of intracellular signaling triggers positive feedback on the ligand synthesis and release into the serum).
- Positive correlations were obtained between NT-3 and IL-10, as well as between VEGFβ and IL-10. Especially for the latter, the information is extremely limited, so these correlations may represent an unexplored area of research.
- IL-10 is not only an anti-inflammatory, anti-atherosclerotic, and antithrombotic cytokine, but also a profibrotic one. Furthermore, experimental data have shown that elevated serum levels of IL-10 are associated with an increased risk of cardiovascular events in patients with KCD.
- Under these conditions, the simultaneous presence of elevated serum levels of NT-3, VEGFβ, and IL-10 could announce the imminence of acute cardiac and/or neurovascular events. It is certainly a phenomenon that requires very careful further research. If this aspect is confirmed, it may be a very important predictive marker that requires the introduction of intensive preventive therapy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasri, H.; Rafieian-Kopaei, M. Diabetes mellitus and renal failure: Prevention and management. J. Res. Med. Sci. 2015, 20, 1112–1120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahimi, Z.; Mansouri Zaveleh, O.; Rahimi, Z.; Abbasi, A. AT2R-1332 G: A polymorphism and diabetic nephropathy in type 2 diabetes mellitus patients. J. Ren. Inj. Prev. 2013, 2, 97–101. [Google Scholar] [CrossRef]
- Einollahi, B. Are acquired cystic kidney disease and autosomal dominant polycystic kidney disease risk factors for renal cell carcinoma in kidney transplant patients? J. Nephropathol. 2012, 1, 65–68. [Google Scholar] [CrossRef]
- Pavkov, M.E.; Miyamoto, Y. IDF Diabetes Atlas Reports: Diabetes and Kidney Disease. Available online: https://diabetesatlas.org/atlas/diabetes-and-kidney-disease/ (accessed on 23 September 2025).
- De Boer, I.H.; Rue, T.C.; Hall, Y.N.; Heagerty, P.J.; Weiss, N.S.; Himmelfarb, J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011, 305, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, L.; Kang, A.; Hockham, C.; Young, T.; Jun, M.; Foote, C.; Pecoits-Filho, R.; Neuen, B.; Rogers, K.; Pollock, C.; et al. Incidence and Associations of Chronic Kidney Disease in Community Participants with Diabetes: A 5-Year Prospective Analysis of the EXTEND45 Study. Diabetes Care 2020, 43, 982–990. [Google Scholar] [CrossRef]
- Wen, H.; Yang, D.; Xie, C.; Shi, F.; Liu, Y.; Zhang, J.; Yu, C. Comparison of trend in chronic kidney disease burden between China, Japan, the United Kingdom, and the United States. Front. Public Health 2022, 10, 999848. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Lopez-Trevino, S.; Kankanamalage, H.R.; Jha, J.C. Diabetes and Renal Complications: An Overview on Pathophysiology, Biomarkers and Therapeutic Interventions. Biomedicines 2024, 12, 1098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, R. Pathogenesis of Diabetic Nephropathy. In Chronic Kidney Disease and Type 2 Diabetes; American Diabetes Association: Arlington, VA, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK571720/ (accessed on 23 September 2025).
- Prasad, K.; Mishra, M. AGE-RAGE Stress, Stressors, and Antistressors in Health and Disease. Int. J. Angiol. 2018, 27, 1–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prasad, K. Involvement of AGE and Its Receptors in the Pathogenesis of Hypertension in Elderly People and Its Treatment. Int. J. Angiol. 2022, 31, 213–221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawanami, D.; Matoba, K.; Utsunomiya, K. Signaling pathways in diabetic nephropathy. Histol. Histopathol. 2016, 31, 1059–1067. [Google Scholar]
- Srikanth, K.K.; Orrick, J.A. Biochemistry, Polyol or Sorbitol Pathways. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK576381/ (accessed on 23 September 2025).
- Marshall, S.; Bacote, V.; Traxinger, R.R. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J. Biol. Chem. 1991, 266, 4706–4712. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Garvey, W.T.; Traxinger, R.R. New insights into the nutrient metabolic regulation of insulin action and insulin resistance: Role of glucose and amino acids. FASEB J. 1991, 5, 3031–3036. [Google Scholar] [CrossRef]
- Nerlich, A.; Schleicher, E. Immunohistochemical localization of extracellular matrix components in human diabetic glomerular lesions. Am. J. Pathol. 1991, 139, 889–899. [Google Scholar]
- Kim, Y.; Butkowski, R.; Burke, B. Differential expression of basement membrane collagen in membranous nephropathy. Am. J. Pathol. 1991, 139, 1381–1388. [Google Scholar]
- Mohan, P.S.; Carter, W.G.; Spiro, R.G. Occurrence of type VI collagen in extracellular matrix of renal glomeruli and its increase in diabetes. Diabetes 1990, 39, 31–37. [Google Scholar] [CrossRef]
- Ceol, M.; Nerlich, A.; Baggio, B. Increased glomerular α1 (IV) expression and deposition in long-term diabetic rats is prevented by chronic glycosaminoglycan treatment. Lab. Investig. 1996, 74, 484–495. [Google Scholar] [PubMed]
- Ziyadeh, F.N. The extracellular matrix in diabetic nephropathy. Am. J. Kidney Dis. 1993, 22, 736–744. [Google Scholar] [CrossRef]
- Fukui, M.; Nakamura, T.; Ebihara, I. ECM gene expression and its modulation by insulin in diabetic rats. Diabetes 1992, 41, 1520–1527. [Google Scholar] [CrossRef]
- Lim, P.S.; Sutton, C.R.; Rao, S. Protein kinase C in the immune system: From signalling to chromatin regulation. Immunology 2015, 146, 508–522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kazanietz, M.G.; Cooke, M. Protein kinase C signaling “in” and “to” the nucleus: Master kinases in transcriptional regulation. J. Biol. Chem. 2024, 300, 105692. [Google Scholar] [CrossRef] [PubMed]
- Satriano, J.; Vallon, V. Primary kidney growth and its consequences at the onset of diabetes mellitus. Amino Acids 2006, 31, 1–9. [Google Scholar] [CrossRef]
- Fitchett, D.; Zinman, B.; Wanner, C.; Lachin, J.M.; Hantel, S.; Salsali, A.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Inzucchi, S.E. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: Results of the EMPA-REG OUTCOME® trial. Eur. Heart J. 2016, 37, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Reidy, K.; Kang, H.M.; Hostetter, T.; Susztak, K. Molecular mechanisms of diabetic kidney disease. J. Clin. Investig. 2014, 124, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Russell McEvoy, M.G.; Wells, N.B.; Kiley, E.M.; Shogan, H.; Fraser, M.G. Skeletal muscle microvascular hemodynamic responses during hyperinsulinemic-euglycemic clamp in a Zucker Diabetic Sprague Dawley rat model of type 2 diabetes. Front. Physiol. 2025, 16, 1568145. [Google Scholar] [CrossRef]
- Trandafir, M.F.; Savu, O.; Pasarica, D.; Bleotu, C.; Gheorghiu, M. Interleukin-6 as a Director of Immunological Events and Tissue Regenerative Capacity in Hemodialyzed Diabetes Patients. Med. Sci. 2024, 12, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, Y.; Yan, H.R.; Wang, B.; Liu, B.C. Macrophage Heterogeneity in Kidney Injury and Fibrosis. Front. Immunol. 2021, 12, 681748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, Q.; He, J.; Cao, Y.; Liu, X.; Nie, W.; Han, F.; Shi, P.; Shen, X.Z. Analysis of mononuclear phagocytes disclosed the establishment processes of two macrophage subsets in the adult murine kidney. Front. Immunol. 2022, 13, 805420. [Google Scholar] [CrossRef]
- Luther, J.M.; Fogo, A.B. The role of mineralocorticoid receptor activation in kidney inflammation and fibrosis. Kidney Int. Suppl. 2022, 12, 63–68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lendeckel, U.; Venz, S.; Wolke, C. Macrophages: Shapes and functions. ChemTexts 2022, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Cao, Q.; Wang, Y.; Harris, D.C.H. M2 Macrophages in Kidney Disease Biology, Therapies, and Perspectives. Kidney Int. 2019, 95, 760–773. [Google Scholar] [CrossRef]
- Wu, Y.S.; Liang, S.; Li, D.Y.; Wen, J.H.; Tang, J.X.; Liu, H.F. Cell Cycle Dysregulation and Renal Fibrosis. Front. Cell Dev. Biol. 2021, 9, 714320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Youngman, O. The insulin-like growth factor system in chronic kidney disease: Pathophysiology and therapeutic opportunities. Kidney Res. Clin. Pract. 2012, 31, 26–37. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, X.H.; Ma, G.B. Role of serum β2-microglobulin, glycosylated hemoglobin, and vascular endothelial growth factor levels in diabetic nephropathy. World J. Clin. Cases 2022, 10, 8205–8211. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.; Tarnow, L.; Jorsal, A.; Oliver, N.; Roestenberg, P.; Ito, Y.; Parving, H.H.; Rossing, P.; van Nieuwenhoven, F.A.; Goldschmeding, R. Plasma connective tissue growth factor is an independent predictor of end-stage renal disease and mortality in type 1 diabetic nephropathy. Diabetes Care 2008, 31, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Boor, P.; Floege, J. Chronic Kidney Disease Growth Factors in Renal Fibrosis. Clin. Exp. Pharmacol. Physiol. 2011, 38, 441–450. [Google Scholar] [CrossRef]
- Boor, P.; Ostendorf, T.; Floege, J. Renal Fibrosis: Novel Insights into Mechanisms and Therapeutic Targets. Nat. Rev. Nephrol. 2010, 6, 643–656. [Google Scholar] [CrossRef]
- Available online: https://legislatie.just.ro/Public/DetaliiDocument/274779 (accessed on 23 September 2025).
- Tonyan, S.; Pospelova, M.; Krasnikova, V.; Fionik, O.; Alekseeva, T.; Samochernykh, K.; Ivanova, N.; Vavilova, T.; Vasilieva, E.; Makhanova, A.; et al. Neurotrophin-3 (NT-3) as a Potential Biomarker of the Peripheral Nervous System Damage Following Breast Cancer Treatment. Pathophysiology 2023, 30, 110–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gheorghiu, M.; Trandafir, M.-F.; Savu, O.; Pasarica, D.; Bleotu, C. Unexpectedly High and Difficult-to-Explain Regenerative Capacity in an 82-Year-Old Patient with Insulin-Requiring Type 2 Diabetes and End-Stage Renal Disease. J. Clin. Med. 2025, 14, 2556. [Google Scholar] [CrossRef]
- Keeler, A.B.; Suo, D.; Park, J.; Deppmann, C.D. Delineating neurotrophin-3 dependent signaling pathways underlying sympathetic axon growth along intermediate targets. Mol. Cell Neurosci. 2017, 82, 66–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernández-Echeagaray, E. Chapter Four—Neurotrophin-3 modulates synaptic transmission, Editor(s): Gerald Litwack. Vitam. Horm. 2020, 114, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Bové, M.; Monto, F.; Guillem-Llobat, P.; Ivorra, M.D.; Noguera, M.A.; Zambrano, A.; Sirerol-Piquer, M.S.; Requena, A.C.; García-Alonso, M.; Tejerina, T.; et al. NT3/TrkC Pathway Modulates the Expression of UCP-1 and Adipocyte Size in Human and Rodent Adipose Tissue. Front. Endocrinol. 2021, 12, 630097. [Google Scholar] [CrossRef]
- Cristofaro, B.; Stone, O.A.; Caporali, A.; Dawbarn, D.; Ieronimakis, N.; Reyes, M.; Madeddu, P.; Bates, D.O.; Emanueli, C. Neurotrophin-3 is a novel angiogenic factor capable of therapeutic neovascularization in a mouse model of limb ischemia. Arter. Thromb. Vasc. Biol. 2010, 30, 1143–1150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trinh, V.Q.; Lee, T.F.; Lemoinne, S.; Ray, K.C.; Ybanez, M.D.; Tsuchida, T.; Carter, J.K.; Agudo, J.; Brown, B.D.; Akat, K.M.; et al. Hepatic stellate cells maintain liver homeostasis through paracrine neurotrophin-3 signaling that induces hepatocyte proliferation. Sci. Signal. 2023, 16, eadf6696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cacialli, P.; Lucini, C. Analysis of the Expression of Neurotrophins and Their Receptors in Adult Zebrafish Kidney. Vet. Sci. 2022, 9, 296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karavanov, A.; Sainio, K.; Palgi, J.; Saarma, M.; Saxen, L.; Sariola, H. Neurotrophin 3 rescues neuronal precursors from apoptosis and promotes neuronal differentiation in the embryonic metanephric kidney. Proc. Natl. Acad Sci. USA 1995, 92, 11279–11283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Good, W.D.; George, T. Neurotrophin-3 inhibits HCO 3—Absorption via a cAMP-dependent pathway in renal thick ascending limb. Am. J. Physiol. Cell Physiol. 2001, 281, C1804–C1811. [Google Scholar] [CrossRef] [PubMed]
- Lepa, C.; Hoppe, S.; Stöber, A.; Skryabin, B.V.; Sievers, L.K.; Heitplatz, B.; Ciarimboli, G.; Neugebauer, U.; Lindenmeyer, M.T.; Cohen, C.D.; et al. TrkC Is Essential for Nephron Function and Trans-Activates Igf1R Signaling. J. Am. Soc. Nephrol. 2021, 32, 357–374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, Y.; Yanagita, M. Resident fibroblasts in the kidney: A major driver of fibrosis and inflammation. Inflamm. Regen. 2017, 37, 17. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.J.; Cai, L.L.; Na, W.Q.; Jia, R.L.; Zhu, J.L.; Pan, X. Interaction between serum inflammatory cytokines and brain-derived neurotrophic factor in cognitive function among first-episode schizophrenia patients. World J. Psychiatry. 2024, 14, 1804–1814. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silvestre, J.S.; Tamarat, R.; Ebrahimian, T.G.; Le-Roux, A.; Clergue, M.; Emmanuel, F.; Duriez, M.; Schwartz, B.; Branellec, D.; Lévy, B.I. Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ. Res. 2003, 93, 114–123. [Google Scholar] [CrossRef]
- Wright, C.E. Effects of vascular endothelial growth factor (VEGF)A and VEGFB gene transfer on vascular reserve in a conscious rabbit hindlimb ischaemia model. Clin. Exp. Pharmacol. Physiol. 2002, 29, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Mould, A.W.; Greco, S.A.; Cahill, M.M.; Tonks, I.D.; Bellomo, D.; Patterson, C.; Zournazi, A.; Nash, A.; Scotney, P.; Hayward, N.K.; et al. Transgenic overexpression of vascular endothelial growth factor-B isoforms by endothelial cells potentiates postnatal vessel growth in vivo and in vitro. Circ. Res. 2005, 97, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Wafai, R.; Tudor, E.M.; Angus, J.A.; Wright, C.E. Vascular effects of FGF-2 and VEGF-B in rabbits with bilateral hind limb ischemia. J. Vasc. Res. 2008, 46, 45–54. [Google Scholar] [CrossRef]
- Muhl, L.; Moessinger, C.; Adzemovic, M.Z.; Dijkstra, M.H.; Nilsson, I.; Zeitelhofer, M.; Hagberg, C.E.; Huusko, J.; Falkevall, A.; Ylä-Herttuala, S.; et al. Expression of vascular endothelial growth factor (VEGF)-B and its receptor (VEGFR1) in murine heart, lung and kidney. Cell Tissue Res. 2016, 365, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, B.; Korpelainen, E.; Pepper, M.S.; Mandriota, S.J.; Aase, K.; Kumar, V.; Gunji, Y.; Jeltsch, M.M.; Shibuya, M.; Alitalo, K.; et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc. Natl. Acad Sci. USA 1998, 95, 11709–11714. [Google Scholar] [CrossRef]
- Cao, Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci. Signal. 2009, 2, re1. [Google Scholar] [CrossRef]
- Jin, J.; Sison, K.; Li, C.; Tian, R.; Wnuk, M.; Sung, H.K.; Jeansson, M.; Zhang, C.; Tucholska, M.; Jones, N.; et al. Soluble FLT1 Binds Lipid Microdomains in Podocytes to Control Cell Morphology and Glomerular Barrier Function. Cell 2012, 151, 384–399. [Google Scholar] [CrossRef]
- Li, X.; Tjwa, M.; Van Hove, I.; Enholm, B.; Neven, E.; Paavonen, K.; Jeltsch, M.; Juan, T.D.; Sievers, R.E.; Chorianopoulos, E.; et al. Reevaluation of the role of VEGF-B suggests a restricted role in the revascularization of the ischemic myocardium. Arter. Thromb. Vasc. Biol. 2008, 28, 1614–1620. [Google Scholar] [CrossRef]
- Claesson-Welsh, L. VEGF-B taken to our hearts: Specific effect of VEGF-B in myocardial ischemia. Arter. Thromb. Vasc. Biol. 2008, 28, 1575–1576. [Google Scholar] [CrossRef]
- Lähteenvuo, J.E.; Lähteenvuo, M.T.; Kivelä, A.; Rosenlew, C.; Falkevall, A.; Klar, J.; Heikura, T.; Rissanen, T.T.; Vähäkangas, E.; Korpisalo, P.; et al. Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1- and neuropilin receptor-1-dependent mechanisms. Circulation 2009, 119, 845–856. [Google Scholar] [CrossRef]
- Li, X.; Lee, C.; Tang, Z.; Zhang, F.; Arjunan, P.; Li, Y.; Hou, X.; Kumar, A.; Dong, L. VEGF-B: A survival, or an angiogenic factor? Cell Adhes. Migr. 2009, 3, 322–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Aase, K.; Li, H.; von Euler, G.; Eriksson, U. Isoform-specific expression of VEGF-B in normal tissues and tumors. Growth Factors 2001, 19, 49–59. [Google Scholar] [CrossRef]
- Li, X.; Eriksson, U. Novel VEGF family members: VEGF-B, VEGF-C and VEGF-D. Int. J. Biochem. Cell Biol. 2001, 33, 421–426. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, K.; Childs, J.T.; Xie, L.; Mao, X.O.; Greenberg, D.A. Increased severity of cerebral ischemic injury in vascular endothelial growth factor-B-deficient mice. J. Cereb. Blood Flow. Metab. 2004, 24, 1146–1152. [Google Scholar] [CrossRef]
- Poesen, K.; Lambrechts, D.; Van Damme, P.; Dhondt, J.; Bender, F.; Frank, N.; Bogaert, E.; Claes, B.; Heylen, L.; Verheyen, A.; et al. Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its Ligand VEGF-B in motor neuron degeneration. J. Neurosci. 2008, 28, 10451–10459. [Google Scholar] [CrossRef]
- Wei, Y.; Han, S.; Zhou, R.; Xu, P.; Zhou, L.; Zhu, Z.; Kan, Y.; Yang, X.; Xiang, Y.; Cao, Y.; et al. Increased Serum VEGF-B Level Is Associated with Renal Function Impairment in Patients with Type 2 Diabetes. Front. Endocrinol. 2022, 13, 862545. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaydarski, L.; Petrova, K.; Angushev, I.; Stanchev, S.; Iliev, A.; Stamenov, N.; Kirkov, V.; Landzhov, B. Exploring the Molecular Modalities in the Pathogenesis of Diabetic Kidney Disease with a Focus on the Potential Therapeutic Implications. Biomedicines 2025, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhao, H.; Fan, J.; Shen, Y.; Han, L.; Jing, G.; Zeng, X.; Jin, X.; Zhu, Z.; Bian, Q.; et al. Simultaneous blockade of VEGF-B and IL-17A ameliorated diabetic kidney disease by reducing ectopic lipid deposition and alleviating inflammation response. Cell Death Discov. 2023, 9, 8. [Google Scholar] [CrossRef]
- Lee, C.; Chen, R.; Sun, G.; Liu, X.; Lin, X.; He, C.; Xing, L.; Liu, L.; Jensen, L.D.; Kumar, A.; et al. VEGF-B prevents excessive angiogenesis by inhibiting FGF2/FGFR1 pathway. Signal Transduct. Target. Ther. 2023, 8, 305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, W.K.; Llewellyn, O.P.; Bates, D.O.; Nicholson, L.B.; Dick, A.D. IL-10 regulation of macrophage VEGF production is dependent on macrophage polarisation and hypoxia. Immunobiology 2010, 215, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhao, Y.; Zhang, Y.; Jin, H.; Shou, S. The role of IL-10 in kidney disease. Int. Immunopharmacol. 2022, 108, 108917. [Google Scholar] [CrossRef]
- Commins, S.; Steinke, J.W.; Borish, L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J. Allergy Clin. Immunol. 2008, 121, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, R.; Xie, J.; Xiong, H.; He, J.C.; Chen, N. Interleukin-10 deficiency aggravates kidney inflammation and fibrosis in the unilateral ureteral obstruction mouse model. Lab. Investig. 2013, 93, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wang, C.; Benedict, C.; Huang, G.; Truongcao, M.; Roy, R.; Cimini, M.; Garikipati, V.N.S.; Cheng, Z.; Koch, W.J.; et al. Interleukin-10 Deficiency Alters Endothelial Progenitor Cell-Derived Exosome Reparative Effect on Myocardial Repair via Integrin-Linked Kinase Enrichment. Circ. Res. 2020, 126, 315–329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sinuani, I.; Averbukh, Z.; Gitelman, I.; Rapoport, M.J.; Sandbank, J.; Albeck, M.; Sredni, B.; Weissgarten, J. Mesangial cells initiate compensatory renal tubular hypertrophy via IL-10-induced TGF-beta secretion: Effect of the immunomodulator AS101 on this process. Am. J. Physiol. Ren. Physiol. 2006, 291, F384–F394. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, K. Über den bau des glomerulus der säugerniere. Weitere mittleillungen. Z. Mikrosk. Anat. Forsch. 1933, 32, 176–278. [Google Scholar]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Sabat, R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010, 21, 315–324. [Google Scholar] [CrossRef]
- Sinuani, I.; Beberashvili, I.; Averbukh, Z.; Sandbank, J. Role of IL-10 in the progression of kidney disease. World J. Transplant. 2013, 3, 91–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haney, L.B.; Hussaini, I.M.; Karns, L.R.; Glass, W.F. Regulation of smooth muscle α-actin expression and hypertrophy in cultured mesangial cells. Kidney Int. 1998, 54, 1175–1187. [Google Scholar] [CrossRef]
- Dubus, I.; Vendrely, B.; Christophe, I.; Labouyrie, J.P.; Delmas, Y.; Bonnet, J.; Combe, C. Mycophenolic acid antagonizes the activation of cultured human mesangial cells. Kidney Int. 2002, 62, 857–867. [Google Scholar] [CrossRef]
- Schena, F.P.; Strippoli, G.F.; Wankelmuth, P. Renal growth factors: Past, present and future. Am. J. Nephrol. 1999, 19, 308–312. [Google Scholar] [CrossRef]
- Schlöndorff, D.; Banas, B. The mesangial cell revisited: No cell is an island. J. Am. Soc. Nephrol. 2009, 20, 1179–1187. [Google Scholar] [CrossRef]
- Mene, P.; Simonson, M.S.; Dunn, M.J. Physiology of the mesangial cell. Physiol. Rev. 1989, 69, 1347–1411. [Google Scholar] [CrossRef]
- Thomas, H.Y.; Ford Versypt, A.N. Pathophysiology of mesangial expansion in diabetic nephropathy: Mesangial structure, glomerular biomechanics, and biochemical signaling and regulation. J. Biol. Eng. 2022, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.H. The glomerular basement membrane. Exp. Cell Res. 2012, 318, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Makino, H.; Shikata, K.; Wieslander, J.; Wada, J.; Kashihara, N.; Yoshioka, K.; Ota, Z. Localization of fibril/microfibril and basement membrane collagens in diabetic glomerulosclerosis in type 2 diabetes. Diabet. Med. 1994, 11, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Sakai, F.; Kriz, W. The structural relationship between mesangial cells and basement membrane of the renal glomerulus. Anat. Embryol. 1987, 176, 373–386. [Google Scholar] [CrossRef]
- Lemley, K.V.; Elger, M.; Koeppen-Hagemann, I.; Kretzler, M.; Nagata, M.; Sakai, T.; Uiker, S.; Kriz, W. The glomerular mesangium: Capillary support function and its failure under experimental conditions. Clin. Investig. 1992, 70, 843–856. [Google Scholar] [CrossRef]
- Kriz, W.; Elger, M.; Lemley, K.V.; Sakai, T. Mesangial cell-glomerular basement membrane connections counteract glomerular capillary and mesangium expansion. Am. J. Nephrol. 1990, 10, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Volinsky, N.; Kholodenko, B.N. Complexity of receptor tyrosine kinase signal processing. Cold Spring Harb. Perspect. Biol. 2013, 5, a009043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, J.; Jiang, Z.; Fitzgerald, D.C.; Ma, C.; Yu, S.; Li, H.; Zhao, Z.; Li, Y.; Ciric, B.; Curtis, M.; et al. Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J. Clin. Investig. 2009, 119, 3678–3691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carney, E. Paradoxical association between serum IL-10 levels and risk of cardiovascular events. Nat. Rev. Nephrol. 2014, 10, 362. [Google Scholar] [CrossRef] [PubMed]



| Hemodialysis Patients Included in the Study | Average Age | Sex | CKD Duration | Hemodialysis Duration |
|---|---|---|---|---|
| Non-DM (45) | 61.5 ± 15.1 yrs. | 33 ♂, 12 ♀ | 1–24 yrs. | 5.29 ± 4.71 yrs. |
| DM (15) | 63 ± 11.8 yrs. | 9 ♂, 6 ♀ | 1–14 yrs. | 2.53 ± 2.69 yrs. |
| Reduced Group | NT-3 | VEGFβ | IL-10 | |
|---|---|---|---|---|
| Control (ESRD) | Numer of Patients | 4 | 4 | 15 |
| Minimum | 43.00 | 0.78 | 0.89 | |
| Maximum | 1718.71 | 41.65 | 238.30 | |
| Mean | 525.49 | 11.75 | 67.45 | |
| Std. Deviation | 797.76 | 19.94 | 85.00 | |
| Median | 170.14 | 2.30 | 29.16 | |
| Test (DM + ESRD) | Number of Patients | 6 | 2 | 5 |
| Minimum | 95.86 | 4.69 | 25.23 | |
| Maximum | 3594.43 | 251.21 | 239.32 | |
| Mean | 888.09 | 127.95 | 76.35 | |
| Std. Deviation | 1335.95 | 174.31 | 92.31 | |
| Median | 396.57 | 127.95 | 28.5 | |
| Total | Number of Patients | 10 | 6 | 20 |
| Minimum | 43.00 | 0.78 | 0.89 | |
| Maximum | 3594.43 | 251.21 | 239.32 | |
| Mean | 743.0560 | 50.4883 | 69.6815 | |
| Std. Deviation | 1112.98942 | 99.5795 | 84.4646 | |
| Median | 308.0000 | 3.6050 | 28.8450 | |
| Measured Marker | Mean Serum Level and Standard Deviation | Maximum Value, Age, Gender | Minimum Value, Age, Gender | Number of Patients with Non-Zero Level | Number of Patients with Zero Level |
|---|---|---|---|---|---|
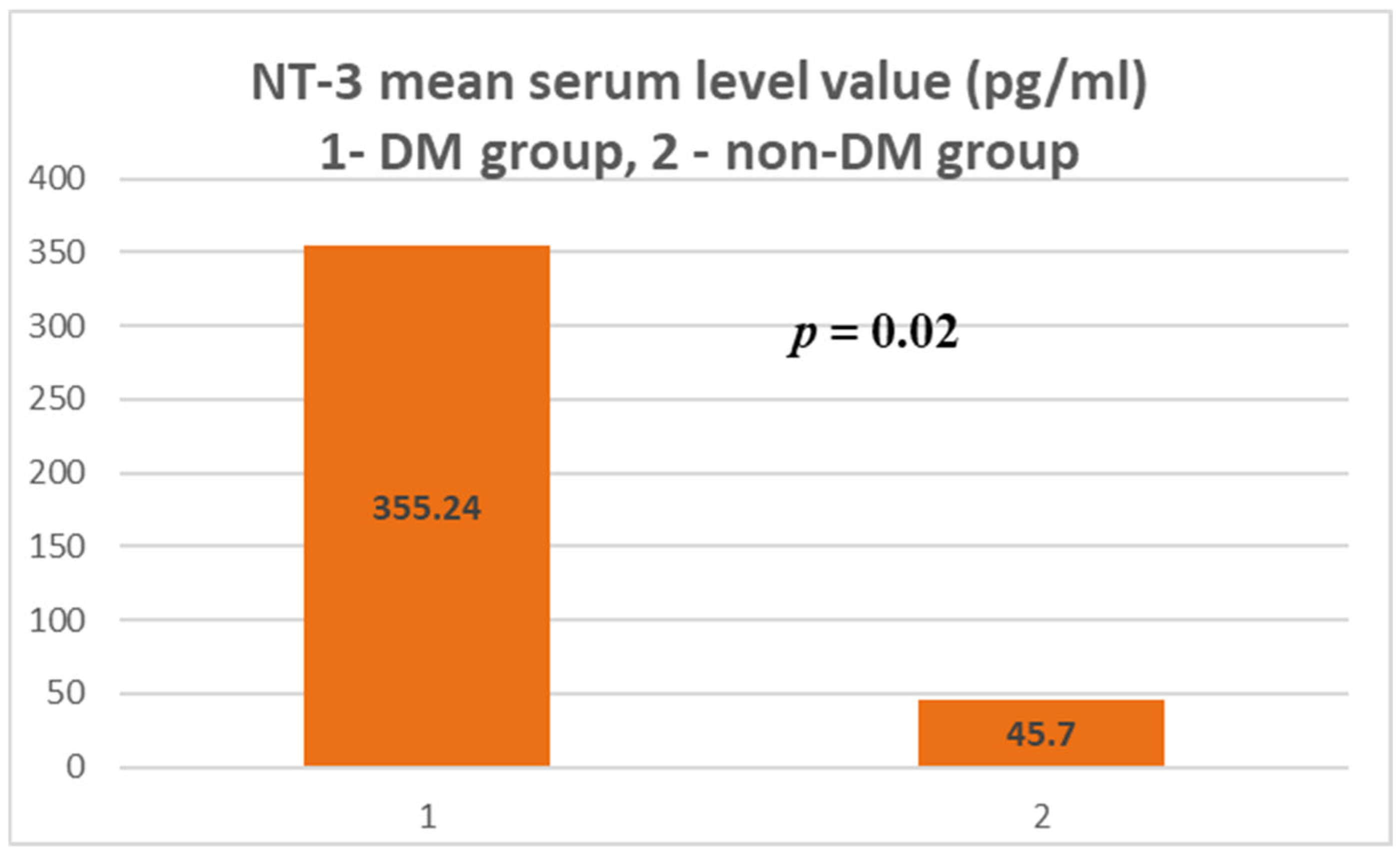
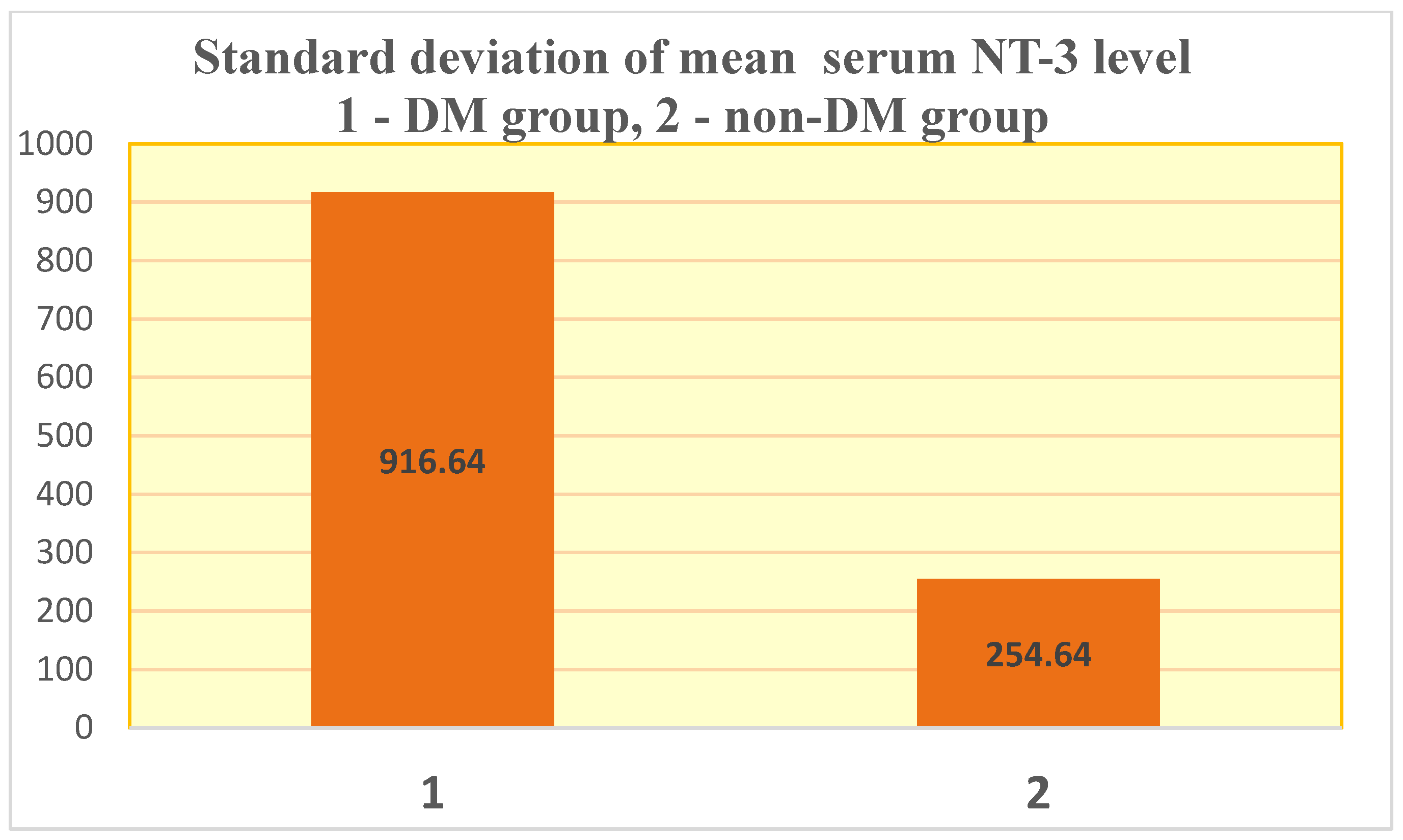
| Test group (DM + ESRD) NT-3 | 354.24 ± 916.64 pg/mL | 3594.43 pg/mL 82 yrs., ♀ | 0 (<4 pg/mL) 50–83 yrs., 6 ♂, 9 ♀ | 6 | 9 |
| Control group (ESRD) NT-3 | 45.7 ± 254.64 pg/mL | 1718.71 pg/mL 83 yrs., ♀ | 0 (<4 pg/mL) 28–88 yrs., 30 ♂, 12 ♀ | 4 | 41 |
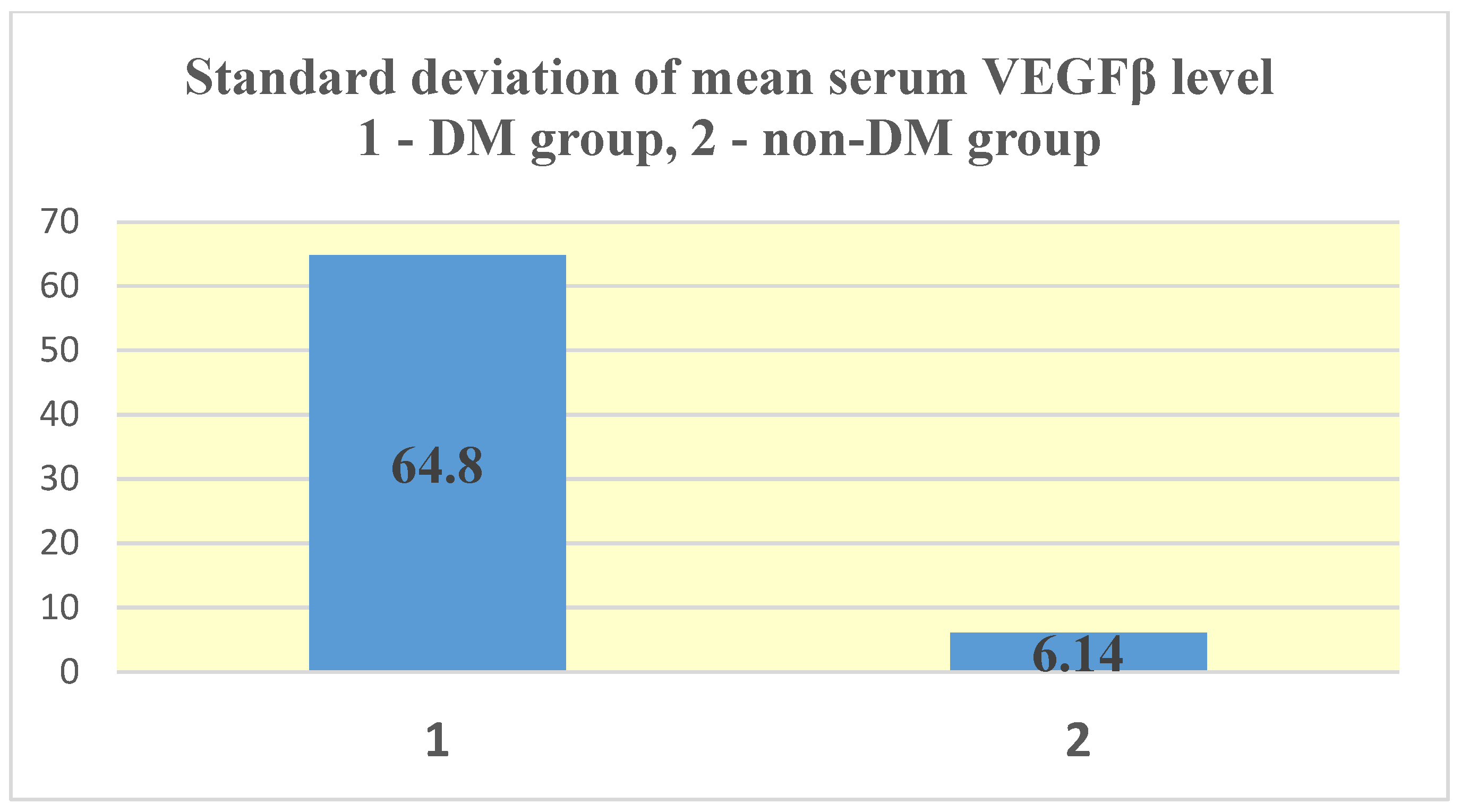
| Test group (DM + ESRD) VEGFβ | 17.06 ± 64.8 ng/mL | 251.21 ng/mL 82 yrs., ♀ | 0 (<0.4 ng/mL) 50–83 yrs., 7 ♂, 5 ♀ | 2 | 13 |
| Control group (ESRD) VEGFβ | 1.02 ± 6.14 ng/mL | 41.65 ng/mL 83 yrs., ♀ | 0 (<0.4 ng/mL) 28–88 yrs., 30 ♂, 11 ♀ | 4 | 41 |
| Test group (DM + ESRD) IL-10 | 25.45 ± 61.83 pg/mL | 239.32 pg/mL 82 yrs., ♀ | 0 (<1 pg/mL) 50–83 yrs., 7 ♂, 3 ♀ | 5 | 10 |
| Control group (ESRD) IL-10 | 21.99 ± 57.18 pg/mL | 238.3 pg/mL 52 yrs., ♂ | 0 (<1 pg/mL), 28–88 yrs., 9 ♀, 22 ♂ | 14 | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghiu, M.; Trandafir, M.-F.; Savu, O.; Pasarica, D.; Bleotu, C. Serum Behavior of NT-3 and VEGFβ, Two Unstudied Growth Factors in Patients with Diabetes Mellitus and End-Stage Renal Disease. J. Clin. Med. 2025, 14, 7585. https://doi.org/10.3390/jcm14217585
Gheorghiu M, Trandafir M-F, Savu O, Pasarica D, Bleotu C. Serum Behavior of NT-3 and VEGFβ, Two Unstudied Growth Factors in Patients with Diabetes Mellitus and End-Stage Renal Disease. Journal of Clinical Medicine. 2025; 14(21):7585. https://doi.org/10.3390/jcm14217585
Chicago/Turabian StyleGheorghiu, Mihaela, Maria-Florina Trandafir, Octavian Savu, Daniela Pasarica, and Coralia Bleotu. 2025. "Serum Behavior of NT-3 and VEGFβ, Two Unstudied Growth Factors in Patients with Diabetes Mellitus and End-Stage Renal Disease" Journal of Clinical Medicine 14, no. 21: 7585. https://doi.org/10.3390/jcm14217585
APA StyleGheorghiu, M., Trandafir, M.-F., Savu, O., Pasarica, D., & Bleotu, C. (2025). Serum Behavior of NT-3 and VEGFβ, Two Unstudied Growth Factors in Patients with Diabetes Mellitus and End-Stage Renal Disease. Journal of Clinical Medicine, 14(21), 7585. https://doi.org/10.3390/jcm14217585






